Abstract
BRAF V600E mutations are detected in 3%–10% of patients with multiple myeloma (MM) and are associated with more aggressive disease, higher frequency of extramedullary growth and shorter survival. Monotherapy with the BRAF inhibitor vemurafenib has been disappointing in MM. In patients with BRAF-mutated melanoma, MEK and BRAF inhibition has been a successful approach. Here we describe a very good partial response and possible mechanisms of resistance to a combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib in a patient with BRAF V600E-mutant refractory MM.
Keywords: Haematology (incl blood transfusion), Oncology
Background
Multiple myeloma (MM) is a malignancy of terminally differentiated B-lymphocytes, but its pathogenesis is only partly understood.1 The disease is still considered incurable in most cases. Current treatment strategies are based on agents without tumour cell specificity, such as proteasome inhibitors (eg, bortezomib, carfilzomib), immunomodulatory drugs (eg, thalidomide, lenalidomide, pomalidomide) monoclonal antibodies (eg, daratumumab, elotuzumab) and conventional chemotherapy.
Genome sequencing of myeloma cells revealed a wide spectrum of potentially actionable mutations, including BRAF, KRAS, NRAS, TP53, FAM46C, DIS3 and others.2
The mitogen-activated protein kinase ERK pathway is the major signal transduction cascade that regulates cell growth. In myeloma the most frequently observed recurrent mutations involve this pathway.3 An emerging therapeutic target is the BRAF V600E mutation that leads to constitutional activation of the RAS-RAF-MEK-ERK signalling pathway and results in tumour cell growth, differentiation and survival.4
Oral inhibitors of BRAF-mutant kinase are approved and widely used for the treatment of BRAF-mutant melanoma.5 In MM, BRAF V600E mutations are detected in 3%–10% of patients, and have been associated with rapidly progressing, relapsed or refractory disease, extramedullary disease and IgA MM, with short PFS and overall survival.6
The activating BRAF V600E mutation was reported to be of therapeutic relevance in MM: The BRAF inhibitor vemurafenib as a single agent was evaluated in a small study of patients with BRAF-mutant MM. Although the overall response rate was 33%, median PFS of 4.3 months was short and suggested early resistance.7 In experimental tumour models and in patients with malignant melanoma, MEK activation was shown to compensate for pharmacological BRAF inhibition, and combined MEK and BRAF inhibition was highly synergistic and therefore able to delay resistance.8 Our hypothesis was that MM harbouring an activating mutation of BRAF could be highly vulnerable to dual BRAF and downstream MEK inhibition. Here, we describe successful treatment with a combination of dabrafenib and the MEK inhibitor trametinib in a patient with refractory BRAF-mutant MM.
Case presentation
A man in his 70s presented with an osteolytic skull lesion, headache, bone pain, and pancytopenia without fever. Biopsy of the skull lesion revealed IgA-kappa MM with an extraordinarily high proliferation index (Ki67=70%). Cytogenetic studies indicated high-risk disease with gain of 1q and IGH-FGFR3 fusion t(4;14); serum free light chain concentrations were kappa 195 mg/L and lambda 8 mg/L, with elevated kappa/lambda ratio (24.4). At diagnosis, serum paraprotein was 13.2 g/L.
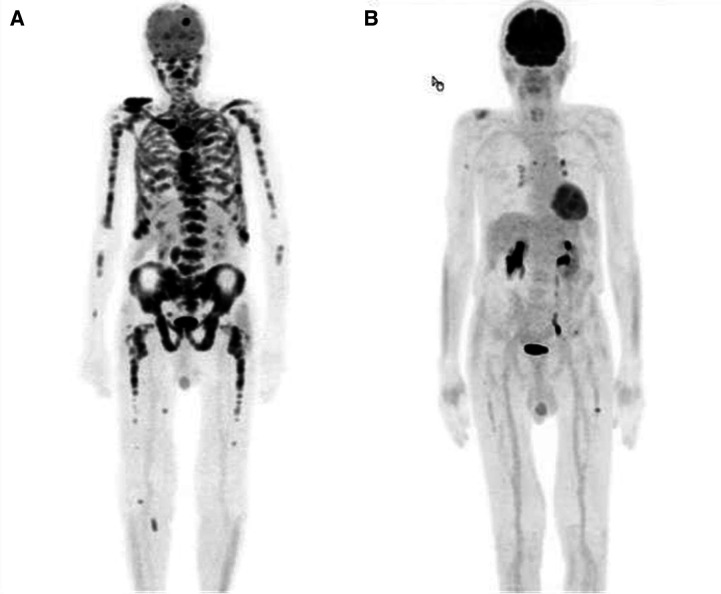
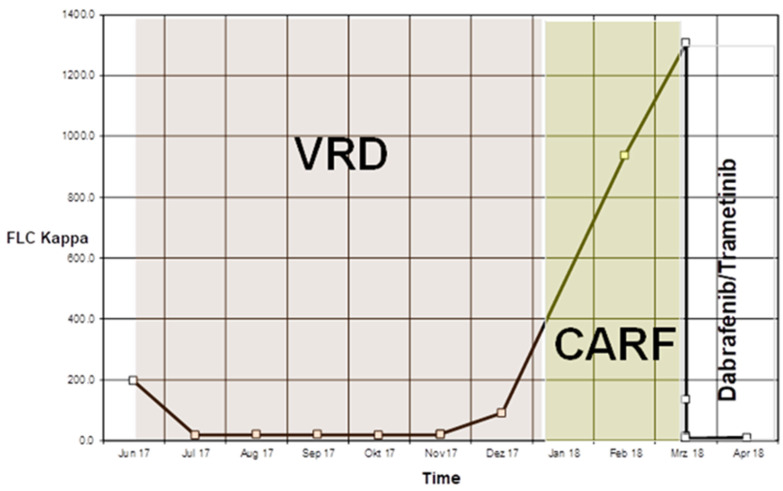
Standard systemic therapy with bortezomib, lenalidomide and dexamethasone (VRd) was initiated, along with palliative radiotherapy to the skull (15×2.5 Gy) and to an osteolytic vertebral body (12×3 Gy). After five cycles, the patient met the criteria for very good partial response (VGPR). After two further cycles of treatment, rapid clinical and serological relapse was evident, with regrowth of the skull lesion and increased kappa light chains (936 mg/L). Carfilzomib was initiated as a salvage treatment but was unsuccessful, and kappa light chains rose to 1307 mg/L. Positron Emission Tomography (PET)-CT indicated a metabolically active MM with osteolytic bone lesions, extramedullary soft tissue lesions and pleural effusion (figure 1). Severe fatigue, bone pain requiring opioid analgesics, anaemia and fever were present, with deterioration of performance status to ECOG 3 (Eastern Cooperative Oncology Group Performance Status).
Figure 1.
PET scan before (A) and after 4 weeks of treatment with dabrafenib and trametinib (B), (A) Initial PET scan, (B) PET scan after 4 weeks. PET, Positron Emission Tomography.
Investigations
In addition to the analysis of the bone marrow biopsy, we performed next-generation sequencing of circulating tumour DNA (‘liquid biopsy’) at the time of the relapse to detect genomic alterations. Evaluation of the primary biopsy specimen, using PCR and sequencing of exon 15, revealed BRAF V600E mutation (c.1799T>A). In the liquid biopsy, we identified dominant newly emerging mutation clusters in RAS genes and altered MAP kinase (MAP2K1) (table 1). None of these activating mutations was identified in the primary biopsy.
Table 1.
Mutation status at progression (NGS)
| Gene | Gene alteration | Change in protein | Allele frequency |
| KRAS | c35G>T | p.G12V | 7.53% |
| KRAS | c.34G>C | p.G12R | 1.10% |
| NRAS | c.181C>A | p.Q61K | 3.11% |
| MAP2K1 | c.167A>C | p.Q56P | 1.77% |
NGS, Next Generation Sequencing.
Treatment
Given the lack of standard therapy for relapsing myeloma and the newly detected mutation, we obtained the informed consent of the patient for initiating off-label treatment with dabrafenib and trametinib as approved for melanoma treatment (dabrafenib 150 mg two times daily and trametinib 2 mg two times daily).
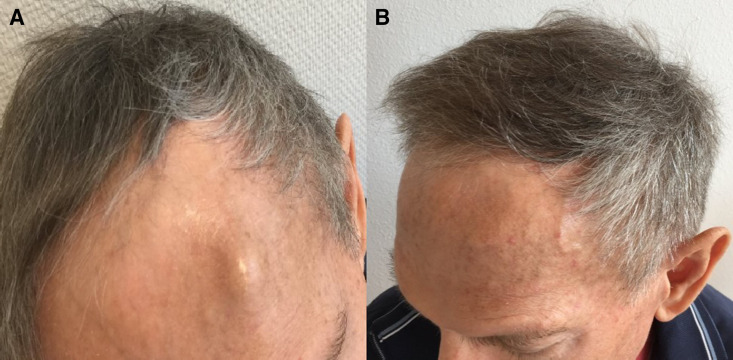
The patient’s general health status improved rapidly (to ECOG 0) within a few days, and kappa free light chains normalised (13.2 mg/L) within 12 days (figure 2). 18FDG-PET (F18-Fluorodeoxyglucose Positron Emission Tomography) scan after 4 weeks showed a metabolic response, with small residual lesions in the right clavicle and humerus (figure 1) and complete resolution of the extramedullary, frontal tumour bulk (figure 3).
Figure 2.
Time course of free light chain kappa during successive treatments. CARF, carfilzomib; VRD, bortezomib, lenalidomide and dexamethasone.
Figure 3.
View showing extramedullary tumour (A) before and after (B) treatment with dabrafenib and trametinib.
Treatment was tolerated very well without relevant toxicities. Based on serum free light chain concentrations without bone marrow evaluation, the patient achieved VGPR according to standard criteria.9 Four months after starting dabrafenib and trametinib disease progression was noted, with a sharp rise in kappa free light chains to 417 mg/L.
Outcome and follow-up
Based on serum free light chain concentrations without bone marrow evaluation, the patient achieved VGPR according to standard criteria after only 4 weeks of therapy.9 Four months after starting dabrafenib and trametinib disease progression was noted, with a sharp rise in kappa free light chains to 417 mg/L. The patient died 11 months after the beginning of treatment with BRAF/MEK inhibition.
Discussion
Our patient had highly aggressive disease responding poorly to prior standard therapy. Combination therapy with dabrafenib and trametinib was well tolerated, and led to rapid clinical, radiographic and laboratory response. This observation is consistent with other preliminary reports on dual inhibition of BRAF/MEK in MM.10 11
Raab et al presented results of 12 patients with BRAFV600 mutant relapsed MM treated with encorafenib (BRAF-inhibitor) and binimetinib (MEK-inhibitor). Overall response was achieved in ten of the twelve patients (83%), six of whom had a VGPR and three had complete remission. No new safety signals could be identified.12 Interestingly, two patients under dual BRAF/MEK inhibition had new KRAS mutations at time of progression—one with the same KRAS p.G12V mutation we found in our patient.
In another study of 180 paired tissue biopsies of newly diagnosed and relapsed MM Xu et al showed enrichment of RAS and BRAF mutations in relapsed MM compared with newly diagnosed MM. As expected, BRAF mutations were significantly associated with activated downstream signalling while only KRAS and not NRAS mutations were associated with pathway activation.3 These findings correspond well with our observations.
MM follows multiple evolutionary pathways over a patient’s course of disease. Keats et al used serial genomic analysis of samples collected at different points of progression in 28 patients. They found in a high-risk patient with t(4;14)—similar to our patient—a higher mutation rate than patients without high-risk genetic abnormalities. Different clones appeared to compete with each other depending on the selection pressure caused by therapy.13
These observations suggest that resistance to BRAF/MEK inhibition can be mediated by selection of genetically distinct resistant clones characterised by activating mutations of RAS genes. A better understanding of resistance mechanisms could lead to the development of new therapies acting on the RAS/RAF pathway (eg, highly selective KRAS or pan-KRAS inhibitors).
Sequencing of BRAF provides a rapid method for detecting BRAF V600E mutations.14 The presence of this mutation should be considered particularly in patients with limited treatment options such as rapidly progressive disease, IgA MM and extramedullary disease.15 While the primary biopsy can be re-examined, a new bone marrow/extramedullary biopsy should be preferred at relapse, as mutations are more common in the recurrent setting. A ‘liquid biopsy’ may be an alternative to repeat bone marrow sampling to characterise the mutational landscape that developed during clonal selection in response to the specific treatment.
Combined BRAF and MEK inhibition represents a promising strategy for treating BRAF V600-mutated MM. More information is required regarding the long-term success rate and optimal treatment regimen at relapse. Clinical trials with BRAF/MEK inhibition in patients with BRAF V600E mutations are ongoing in Germany (encorafenib +binimetinib NCT02834364) and the US (dabrafenib +trametinib NCT03091257) and results are eagerly awaited.
Learning points.
This is a case of a highly aggressive IgA-kappa multiple myeloma (MM) with poor response to standard therapy.
Having progressed after two therapy lines, evaluation of the primary biopsy specimen revealed a BRAV V600E mutation and treatment with BRAF/MEK inhibition was initiated (off label use).
There was an excellent response to BRAF/MEK inhibition, but duration to this therapeutic approach was only short.
Combined BRAF and MEK inhibition represents a promising strategy for treating BRAF V600-mutated MM, especially when high-risk features such as IgA MM and extramedullary disease is present.
Rebiopsy or liquid biopsy is helpful to find treatable mutations.
Acknowledgments
We acknowledge Professor Oliver Gautschi and Profesor Joachim Diebold for their expert opinion and proofreading support.
Footnotes
Twitter: @ThiloZander
Contributors: TE and TZ treated the patient, conceived the idea and design of the article, drafted the initial manuscript and reviewed it critically. TZ did the final adjustments. SA contributed to the molecular details of the article and reviewed its final version. AZ adjudged the scans.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Raab MS, Podar K, Breitkreutz I, et al. Multiple myeloma. Lancet 2009;374:324–39. 10.1016/S0140-6736(09)60221-X [DOI] [PubMed] [Google Scholar]
- 2.Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell 2014;25:91–101. 10.1016/j.ccr.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Pfarr N, Endris V, et al. Molecular signaling in multiple myeloma: association of RAS/RAF mutations and MEK/ERK pathway activation. Oncogenesis 2017;6:e337. 10.1038/oncsis.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263–84. 10.1016/j.bbamcr.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358–65. 10.1016/S0140-6736(12)60868-X [DOI] [PubMed] [Google Scholar]
- 6.Andrulis M, Lehners N, Capper D, et al. Targeting the BRAF V600E mutation in multiple myeloma. Cancer Discov 2013;3:862–9. 10.1158/2159-8290.CD-13-0014 [DOI] [PubMed] [Google Scholar]
- 7.Raje N, Chau I, Hyman DM, et al. Vemurafenib in Patients With Relapsed Refractory Multiple Myeloma Harboring BRAF V600 Mutations: A Cohort of the Histology-Independent VE-BASKET Study. JCO Precis Oncol 2018;2. 10.1200/PO.18.00070. [Epub ahead of print: 31 Aug 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867–76. 10.1056/NEJMoa1408868 [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Paiva B, Anderson KC, et al. International myeloma Working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328–46. 10.1016/S1470-2045(16)30206-6 [DOI] [PubMed] [Google Scholar]
- 10.Mey UJM, Renner C, von Moos R. Vemurafenib in combination with cobimetinib in relapsed and refractory extramedullary multiple myeloma harboring the BRAF V600E mutation. Hematol Oncol 2017;35:890–3. 10.1002/hon.2353 [DOI] [PubMed] [Google Scholar]
- 11.Čepulytė R, Žučenka A, Pečeliūnas V. Combination of dabrafenib and trametinib for the treatment of relapsed and refractory multiple myeloma harboring BRAF V600E mutation. Case Rep Hematol 2020;2020:8894031. 10.1155/2020/8894031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raab MS, Giesen N, Scheid C, et al. Safety and preliminary efficacy results from a phase II study evaluating combined BRAF and MEK inhibition in relapsed/refractory multiple myeloma (rrMM) patients with activating BRAF V600E mutations: the GMMG-Birma trial. Blood 2020;136:44–5. 10.1182/blood-2020-142600 [DOI] [Google Scholar]
- 13.Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012;120:1067–76. 10.1182/blood-2012-01-405985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisschop C, Ter Elst A, Bosman LJ, et al. Rapid BRAF mutation tests in patients with advanced melanoma: comparison of immunohistochemistry, droplet digital PCR, and the Idylla mutation platform. Melanoma Res 2018;28:96–104. 10.1097/CMR.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danu A, Cotteret S, Lacroix L. BRAF V600E targetable mutation in relapsed/refractory multiple myeloma (R/R Mm) patients: a high incidence in R/R mm detected using cell sorting screening. DC: American Society of Hematology Washington, 2016. [Google Scholar]