Abstract
The discovery of Helicobacter pylori infection in 1984 revolutionised the management of several common upper gastrointestinal diseases. However, some of the clinical practices that were adopted following discovery of this organism have become less appropriate over the intervening years. This article discusses five ‘myths and misconceptions’ that we believe have now emerged and which we argue need re-evaluation. Although the prevalence of H. pylori infection is decreasing in some developed countries, it remains a huge global problem and the most serious consequence of infection, gastric adenocarcinoma, is still a major cause of mortality. The epidemiology of H. pylori-related diseases is also changing and careful testing remains crucially important, especially in patients with peptic ulceration. Eradication of H. pylori infection has also become much more difficult over recent years as a result of the widespread acquisition of antibiotic resistance. Routine assessment of the success of eradication should therefore now be performed. Finally, there has been increased awareness about the role of H. pylori in the multistep pathway of gastric carcinogenesis, about the opportunities to prevent cancer development by eradicating this infection in some individuals and about detecting high-risk preneoplastic changes via endoscopic surveillance. The discovery of H. pylori was rightly honoured by the award of the Nobel prize for Physiology and Medicine in 2005. However, unless we re-evaluate and update the ways in which we manage H. pylori infection, much of the fantastic progress that has been made in this field of medicine may tragically be lost once again.
Keywords: helicobacter pylori, gastric carcinoma, duodenal ulcer, helicobacter therapy
Key points.
Although Helicobacter pylori prevalence has been decreasing in many Western countries over recent years, it remains an important cause of serious diseases such as peptic ulcer and gastric cancer.
Assessment of H. pylori infection may give false-negative results, especially in patients who are hypochlorhydric as a result of atrophic gastritis or proton pump inhibitor consumption.
Biopsies from both the gastric corpus as well as antrum should be assessed for H. pylori colonisation, especially when there are factors that affect gastric acid secretion.
Many peptic ulcers are not caused by H. pylori infection, so careful evaluation of the cause is recommended and empirical eradication regimes should not be used.
The prevalence of antibiotic-resistant H. pylori strains is increasing in most countries. Assessment of the success of eradication therapy is therefore usually required and many patients will need more than one attempt at eradication.
H. pylori infection is the major cause of gastric cancer and eradicating this infection in patients who have preneoplastic gastric pathology can prevent cancer development in some patients.
Periodic endoscopic surveillance for detection of early gastric cancers is now recommended for people who have extensive atrophic gastritis or intestinal metaplasia.
Introduction
Following the discovery of Helicobacter pylori by Marshall and Warren in 1984,1 this organism and the consequences of its infection were intensely researched. Any gastroenterologist who attended a major gastroenterology conference in the late 1980s or early 1990s will remember the vast amount of attention that was devoted to H. pylori infection on the scientific programme and the huge amount of sponsorship provided by the pharmaceutical companies who manufactured H2 receptor antagonist and proton pump inhibitor (PPI) drugs. Great scientific and clinical progress was made over a very short period of time in understanding the pathogenesis, prevention and treatment of H. pylori-related diseases, in particular peptic ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (figure 1). This research also led to a number of clinical practices that were evidence-based at the time, such as the use of empirical H. pylori eradication therapy for patients who had a duodenal ulcer.
Figure 1.
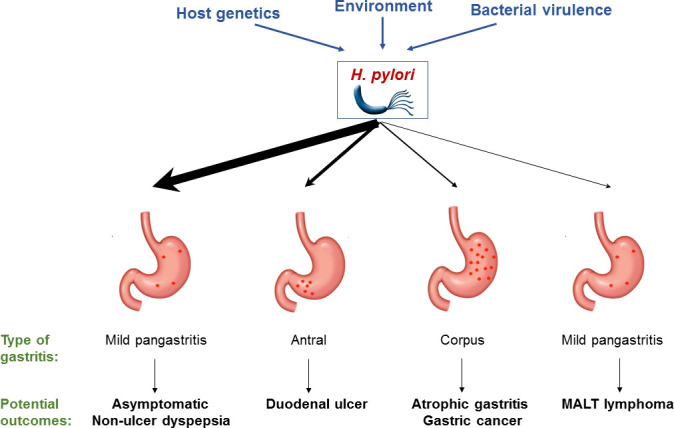
Potential outcomes of H. pylori infection in the upper GI tract. The thickness of the arrows is intended to give some indication of the likelihood of the various outcomes.
However, over the intervening 35 years, the global situation has changed considerably. In particular, the prevalence of H. pylori infection has decreased in some geographical areas, testing approaches for H. pylori have generally become less thorough, traditional triple therapy eradication regimes have become less effective as a result of increasing bacterial antibiotic resistance and H. pylori infection has become a topic of much less scientific and clinical research. These issues mean that some of the historical approaches that were once useful for evaluating and treating H. pylori-associated diseases are no longer appropriate. In this article, we will develop some of the themes that were recently outlined in a paper in this journal2 and discuss five topics which we refer to as ‘current myths and misconceptions’ regarding H. pylori infection and which we argue need re-evaluation in 2021. We encourage our readers to reassess their beliefs in these myths, so that they can hopefully modify and improve their current management of H. pylori-infected patients.
Myth 1: H. pylori infection is now rare and therefore no longer a major clinical problem
Several studies have shown that the prevalence of H. pylori infection has reduced over recent years, especially in some developed countries.3 4 This has led to the current widely held myth that H. pylori infection is no longer a major clinical problem and that consequently H. pylori-related diseases warrant less clinical interest and research. Although this statement contains a modicum of truth, it unfortunately fails to recognise the full persisting extent of H. pylori-related diseases. Recent meta-analyses have reported that the global prevalence of H. pylori infection is still 44.3% (95% CI 40.9% to 47.7%)4 and that this infection affected an estimated 4.4 billion people worldwide in 2015.5 There is, however, considerable geographical variation in infection rates, with much lower prevalence being reported in many developed compared with developing countries.4 5 The reductions in prevalence that have been observed in some European countries have not yet been witnessed to the same extent in many other locations.6
Studies have also shown that the prevalence of gastric adenocarcinoma, the most serious consequence of H. pylori infection, has reduced considerably in many countries over recent years.3 7 However, stomach cancer still remains a very significant cause of mortality in many countries, and this disease was estimated to affect 1.22 million people and cause 865 000 deaths globally in 2017.8 Even in countries such as the UK, where gastric cancer is perceived as no longer causing a major problem, it is still one of the top 15 leading causes of cancer-associated death.
As a result of the decreasing prevalence of H. pylori infection in some Western countries over the last few decades, the extent to which age now influences the likelihood of infection is probably also not fully appreciated. In many developed countries, older individuals have an at least twofold to threefold higher prevalence of infection than young adults.3 Other factors, particularly socioeconomic deprivation, are also well established independent risk factors for the infection.9
H. pylori infection therefore remains very prevalent globally and the conditions which it causes, such as dyspepsia, peptic ulceration and gastric adenocarcinoma, remain major causes of morbidity and mortality.10 Although in some developed countries, H. pylori-related diseases are less of a problem than they once were, clinical suspicion of this infection should still remain very high. In countries such as the UK, the index of suspicion should be particularly high in immigrants from higher prevalence areas of the world, as well as in older and more socioeconomically deprived members of the community.
Myth 2: The tests that are used to detect H. pylori infection are accurate in most clinical situations
Most gastroenterologists will be familiar with the range of invasive and non-invasive investigations that can be used to detect H. pylori infection. Endoscopic biopsy-based tests include histology, rapid urease (Campylobacter like organisms, CLO) test and bacterial culture, whereas non-invasive investigations include 13C-urea breath test,11 faecal antigen test and serology (table 1). These investigations have all been reported to show high sensitivities and specificities in multiple clinical studies. This has led to the myth that all these tests are equivalent and provide accurate results in most clinical settings. However, many clinicians may not appreciate that the majority of these studies were conducted in predominantly young patients with dyspepsia who were not taking acid-suppressing medications and who were also unlikely to have atrophic gastritis. The actual performance of the various tests in routine clinical practice is much more complex. False-negative tests occur frequently and clinicians should therefore maintain a high degree of suspicion about the possibility of undetected H. pylori infection, especially in high-risk individuals.
Table 1.
Diagnostic tests for H. pylori infection (adapted from Fischbach et al 39 and using primary data from Gisbert JP, Pajares, Cutler et al, Thijs et al, Laheij et al, Gisbert et al, Calvet et al, Deguchi et al, Korkmaz et al 11 40–46)
| Sensitivity (%) | Specificity (%) | Pros | Cons | |
| Invasive tests | ||||
| Culture | 70–90 | 100 | High specificity | Invasive, expensive, low sensitivity, requires lab expertise |
| Histology | 80–98 | 90–98 | High specificity | Invasive |
| Rapid urease test (CLO) | 90–95 | 90–95 | Cheap, quick result, high accuracy | Invasive |
| PCR | 90–95 | 90–95 | High accuracy | Expensive, no routine application |
| Non-invasive tests | ||||
| 13C-Urea breath test | 85–95 | 85–95 | Non-invasive, reasonable cost | Compliance, specific clinic set-up required |
| Faecal antigen test * | ||||
| ELISA Quick Test |
85–95 70–95 |
85–95 85–95 |
Cheap, convenient (no clinic attendance required) | Patient acceptance (collection of faecal matter) |
| IgG serology† | >95 | >90 | Cheap, easy to obtain | High risk of false positives |
Apart from serology, all test modalities require proton pump inhibitor treatment to be stopped 2 weeks prior to testing due to a high likelihood of false-negative results on acid suppression.
*Monoclonal antibodies only.
†After exclusion of patients who have had previous eradication treatment.
CLO, Campylobacter-like organisms.
False-negative H. pylori tests are more likely to be found in situations in which the density of gastric bacterial infection is reduced such as recent antibiotic, acid suppressant or bismuth-containing drug use, the hypochlorhydria associated with atrophic gastritis or the presence of blood in the stomach. Efforts can be made to reduce the risk of such false-negative tests. In particular acid-suppressing drugs should be stopped for at least 2 weeks prior to conducting the test. However, this is often not routinely done, particularly in patients attending for diagnostic endoscopy. Other measures that can be employed to increase the likelihood of detecting a true infection are to test both gastric body (corpus) and antral biopsies (for urease as well as histology-based tests), to request immunohistochemical analysis of gastric biopsies as well as routine histology in high-risk patients, to perform more than one type of test and to repeat the analysis on a separate occasion after stopping any potentially interfering medication. Accurate assessment of H. pylori status is most crucial in high-risk patients, such as those who have had a complicated peptic ulcer, in whom failure to eradicate the infection may lead to potentially serious clinical consequences.
A clinician’s ability to assess for the presence of H. pylori infection may also be affected by local test availability. For example, in the UK, many hospitals now only offer one type of non-invasive test, the most frequent being a faecal antigen test.12 In many hospitals, 13C-urea breath test labs have been decommissioned over recent years. H. pylori IgG serology is no longer widely available in many hospitals, probably because this test is not helpful in certain clinical scenarios, such as testing the success of eradication therapy. Serology can remain positive for years following successful eradication, so if it is positive, it may not indicate the presence of current active infection and in this scenario it may constitute a false-positive result. The majority of centres in the UK now offer faecal antigen testing as the only option for non-invasive testing. However, serological tests can still be helpful for baseline assessment in some clinical situations, for example, in patients with bleeding peptic ulcers or who are reluctant or unable to stop taking PPIs. Lack of access to specific tests in certain situations is therefore unhelpful.
As discussed below, the chances of successfully eradicating H. pylori infection are nowadays much lower than they used to be, especially when using conventional 7-day triple therapy eradication regimes. The high effectiveness of H. pylori eradication in the past led many gastroenterologists to stop routinely testing whether eradication had been successful and instead they used symptomatic improvement as a surrogate indicator of treatment success. However, this approach can no longer be safely advocated and we strongly suggest that a non-invasive test should now be used to check the success of eradication in the vast majority of patients. This is particularly pertinent in patients who have a serious diagnosis such as a peptic ulcer, as this pathology is likely to recur if H. pylori eradication has been unsuccessful.
Myth 3: H. pylori infection is still the main cause of peptic ulceration, so empirical eradication therapy is justified in patients who have a duodenal ulcer
When it was first discovered, H. pylori was globally very prevalent and was responsible for the pathogenesis of a very large proportion of peptic ulcers. This subsequently led to the widely held view that H. pylori infection was the cause of all duodenal ulcers and consequently that H. pylori testing was not required in this situation and empirical eradication therapy could reasonably be prescribed. While there was some justification for this practice in an era when H. pylori-induced ulcers were extremely common, the current epidemiology of peptic ulcer disease means that such an approach is no longer appropriate. The pathogenesis of gastric and duodenal ulcers also differs and it remains essential to biopsy all gastric ulcers to ensure that they are not malignant and to repeat an endoscopy after a course of PPIs to check for ulcer healing.13
It is now clear that there are multiple causes of peptic ulceration in addition to H. pylori infection and that these account for a significant proportion of cases. In particular, aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) are responsible for a large number of peptic ulcers.14 Other possible causes include diseases such as Zollinger-Ellison syndrome, Crohn’s disease and Behҫet’s disease as well as rare malignancies and infections. Peptic ulcers are also increasingly being detected where there is no obvious aetiological agent, so are called true idiopathic peptic ulcers. These have been shown to account for more than 10% of peptic ulcers in some case series.15 While some are possibly H. pylori-induced ulcers in which the infection has not been accurately detected, idiopathic peptic ulceration does appear to be a genuine entity.
In view of the multiple aetiologies of peptic ulcer and the increasingly lower relative proportion of H. pylori-induced ulcers, we believe that the historic practice of prescribing empirical eradication to patients who have a duodenal ulcer is no longer justified. Antibiotic stewardship is also increasingly important and measures should be adopted to try to prevent the unnecessary use of antibiotics, as this can lead to the emergence of antibiotic-resistant bacterial strains. Rigorous testing of H. pylori status is therefore to be encouraged in all patients who have a gastric or duodenal ulcer. In patients who have a bleeding ulcer, gastric antral and corpus biopsies can be taken at the time of endoscopy after haemostasis has been achieved (and blood will not prejudice histological assessment, only potentially the result of a urease test) or a non-invasive test can be considered after completion of a course of PPI to heal the ulcer. In those patients who have no evidence of H. pylori infection after adequate testing and who are not taking aspirin or NSAIDs, additional tests such as taking biopsies of the ulcer (if persistent) and measuring fasting serum gastrin concentrations should be considered.14 It should also be noted that the manifestations of Zollinger-Ellison syndrome can be partly masked by PPI consumption.16
Myth 4: H. pylori eradication is straightforward and standard triple therapy is usually effective
Soon after H. pylori had been discovered, effective eradication regimes were developed, leading to a revolution in the way in which patients with dyspepsia and peptic ulceration were managed. In 2015, the members of the Kyoto Consensus Conference went a step further and defined H. pylori ‘as an infectious disease, even when patients have no symptoms and irrespective of complications such as peptic ulcers and gastric cancer’.17 This statement was confirmed as the opening statement of the most recent European Maastricht V/Florence consensus from 2017,18 leading to a paradigm shift from defining indications for treatment of H. pylori towards defining indications for testing for H. pylori. This has since been adapted in clinical practice and respective guidelines. Thus, the indication for H. pylori eradication treatment should now be clear at the time of testing. There is then no good reason to leave the infection untreated, so treatment should be prescribed following detection. Key indications to investigate include peptic ulcer disease, dyspepsia, symptomatic gastritis, idiopathic thrombocytopenic purpura, (otherwise investigated and unexplained) iron deficiency anaemia, as well as more serious conditions such as gastric adenocarcinoma or gastric MALT lymphoma.18 A general population-based ‘screen-and-treat’ is not recommended in low-prevalence countries such as the UK. Potential positive consequences of H. pylori infection (eg, reduction of the incidence of asthma or atopic eczema) are usually only relevant in children and are less relevant in adults, so they do not represent a reason to withhold eradication therapy.
The controversies about whether H. pylori eradication exacerbates gastro-oesophageal reflux disease (GORD) or promotes the development of oesophageal adenocarcinoma (OAC) have never been conclusively resolved. While a recent meta-analysis confirmed a 30% risk reduction for OAC in H. pylori-positive patients, some other studies in large Western populations have not confirmed this effect.19 20 A nationwide population-based cohort study on more than 80 000 individuals in Sweden confirmed a significant association between H. pylori status and the presence of Barrett’s oesophagus, but this effect decreased with longer follow-up time after eradication, and there was also no statistically significant association with oesophageal cancer in this study.21 Another meta-analysis documented an increased risk of erosive reflux oesophagitis after H. pylori eradication, but not of GORD-related symptoms.22 The Barrett’s and Esophageal Adenocarcinoma Consortium confirmed a reduced risk of Barrett’s oesophagus in H. pylori-positive patients, but only when cases were compared with population-based controls, not when cases were compared with GORD controls.23 Other conditions that are potentially influenced by H. pylori infection are metabolic disorders including non-alcoholic fatty liver disease or obesity, with around a 20% risk increase for these conditions in H. pylori-positive patients. For further details on the link between H. pylori infection and gastric and extragastric conditions we suggest consulting an excellent recent review by O’Connor et al.24
The biological properties of H. pylori as well as its niche within the stomach make treatment more difficult compared with other bacteria and requires a combination of (at least) two antibiotics and a PPI. The latter enhances the treatment effect by reducing the local bacterial colonisation density. Classic eradication regimens include the ‘French’ triple therapy regime of clarithromycin, amoxicillin and PPI and the ‘Italian’ version with clarithromycin, metronidazole and PPI in patients who are allergic to penicillin (figure 2). While these regimens showed very high efficacy when first introduced, increasing bacterial resistance rates have led to significant problems of eradication failure with a considerable proportion of patients now requiring second-line or third-line treatment attempts.25 Recent data from the European Registry on Helicobacter pylori management, including data on 21 533 patients from 27 European countries showed pretreatment resistance against clarithromycin in 22.7% and against metronidazole in 32.0% (13.4% to both).26 The primary treatment success rate of the ‘French’ triple therapy regimen in this cohort was only 81.5% in the modified intention-to-treat analysis. Resistance rates against second-line drugs such as quinolones are lower, but are also increasing. Data from 2012 showed that this phenomenon also affects the UK, but with considerable regional variation.27
Figure 2.
Suggested treatment algorithm for H. pylori infection in the UK. The algorithm is mostly in line with current guidance published by Public Health England (PHE), apart from some minor changes. In patients without penicillin allergy, PHE does not state a preference for the use of either clarithromycin or metronidazole as first-line agent and leaves this to the discretion of the prescriber (*). PHE also suggests use of tetracycline as an alternative to levofloxacin in empirical third-line therapy (#) in these patients. In patients with penicillin allergy, bismuth-based quadruple therapy is recommended as second-line treatment, and levofloxacin-containing triple therapy as empirical third-line regimen. However, due to the limited availability of bismuth within the UK, we suggest using bismuth-based quadruple therapy rather as a third-line option. Second-line failure should be followed by specialist referral and, ideally, bacterial culture and resistance testing. BT, 13C-urea breath test; FAT, faecal antigen test; OGD, oesophagogastroduodenoscopy.
Means to overcome eradication therapy failure include prolonging the course of treatment (10 days or 14 days instead of 7 days) or prescribing alternative treatment regimens. In most countries, the recommended first-line alternative is bismuth-based quadruple therapy which has had a renaissance during the last decade. This has mainly been due to the availability of a combination drug (Pylera) which also encourages compliance and thus improves treatment success. The European Maastricht V Consensus suggested that clarithromycin-based treatment regimens should be abandoned when the local resistance rate exceeds 15%.18 However, this is somewhat difficult in the UK, where antibiotic susceptibility data are not routinely generated and the recommended alternative is not easily available. Bismuth-containing quadruple therapy can still be an option for selected UK patients, but the bismuth component needs to be sourced individually using compounds that are usually used for different indications (eg, Pepto-Bismol). A pragmatic approach is shown in figure 2. Most importantly though, the same regimen should never be administered twice!
Referral to secondary care should always be considered after second-line eradication failure, as this allows access to biopsy-based culture and susceptibility testing. Ideally, third-line treatment should be individually tailored based on the resulting resistogram and empirical treatment should only be considered if a gastroscopy is not feasible or contraindicated. Successful culture of H. pylori from gastric biopsies, however, requires expertise and specialised techniques and is best performed in high-volume centres which have the appropriate skills. For example, this service is offered in the UK at the Public Health England reference laboratories in Colindale, London. PPIs should also be stopped prior to attempted culture to maximise the chances of success. As mentioned above, a confirmative test of eradication success is required after each line of treatment, for which the patient needs to be off any PPI for at least 2 weeks and off any antibiotics for at least 4 weeks. Once a patient has been tested negative, no further tests will be required as re-infection rates in adults are negligible (<1%).
In rare cases, eradication of H. pylori infection is not successful, even after administration of tailored, culture-based treatment regimens. In these patients a pragmatic approach is required that considers both the symptom burden and the potential risk of developing future serious disease including gastric cancer. PPI use often remains the mainstay for symptomatic relief, but short-term and long-term side effects need to be monitored. Other symptomatic treatments such as low-dose amitriptyline in patients who present with epigastric pain as their dominant complaint can also be considered. If not already done at the index presentation, a repeat endoscopy with biopsy sampling of the gastric mucosa is advised to allow stratification for further endoscopic surveillance as per the national British Society of Gastroenterology (BSG) and European Management of precancerous conditions and lesions in the stomach (MAPS) guidelines.28–30
Myth 5: Detection and eradication of H. pylori in patients who have an increased risk of developing gastric cancer is not worthwhile
Although the association between H. pylori infection and gastric carcinogenesis was initially recognised several decades ago, the concept that this allows opportunities to prevent the development of some stomach cancers and detect other tumours at an earlier stage in their evolution has only been appreciated more recently. Endoscopic surveillance approaches and treatment of preneoplastic conditions of the gastrointestinal tract are not new. Colonoscopy surveillance with polyp resection as well as endoscopic surveillance of Barrett’s oesophagus are well-accepted approaches.31 Compared with the colon and oesophagus, however, the stomach is somewhat at a disadvantage, since preneoplastic changes are often more subtle, and adequate assessment requires time, expertise and appropriate equipment.
Pelayo Correa was the first to propose the concept of stepwise changes leading from chronic-active H. pylori-induced gastritis via glandular atrophy and intestinal metaplasia (IM) towards dysplastic lesions and, finally, invasive gastric neoplasia of the intestinal type.32 In contrast, diffuse-type gastric cancers are considered to be a direct result of genetic alterations and arise without any precursor lesions. However, recent studies confirmed that a substantial number of patients with diffuse-type cancer also present with IM and atrophy which can therefore still serve as a risk indicator.33 H. pylori-induced inflammation is usually the driver of gastric mucosal transformation, and eradication has been shown to be effective in preventing gastric cancer development in a large proportion of patients.34 Since some patients still develop cancer even after successful eradication, there is ongoing debate as to the point at which H. pylori treatment can prevent further disease progression. After some initial controversy, the presence of IM is now considered to mark the so-called ‘point of no return’.35 This does not mean that eradication attempts should be abandoned once IM is established, since H. pylori eradication can prevent metachronous gastric cancer even in patients that have been endoscopically treated for early gastric cancer, that is, at a late stage of mucosal transformation.36
The definition of a ‘point of no return’ delivers the rationale for keeping a close eye on patients who have preneoplastic changes and are therefore at higher risk for gastric cancer development. This has long been established in the Far East, mainly Japan and Korea, where the gastric cancer incidence is considerably higher compared with the West. In Europe, the first promising data on the cost-effectiveness of endoscopic surveillance were generated in Portugal and the Baltic states, also regions where the gastric cancer incidence is above the European average, and demonstrated a higher detection rate of early neoplasia and hence lower overall mortality following surveillance. While population-based endoscopic screening of the upper GI tract is too expensive and not feasible in the West, these results support endoscopic surveillance of patients in whom extensive atrophy and IM has been diagnosed by gastroscopy that was undertaken for other reasons.
The first European guidelines on MAPS were published in 2012 and have recently been revised.28 29 The proposed strategy includes 3-yearly endoscopic surveillance in patients who have extensive atrophy/IM in both the antrum and corpus, with shorter intervals being proposed for those who have additional risk factors (eg, positive first-degree family history of gastric cancer). These recommendations have now also been adopted by the British and German Societies of Gastroenterology30 37 (figure 3).
Figure 3.
Inflammatory and preneoplastic changes of the gastric mucosa and opportunities for intervention. The bottom left shows the sequential mucosal changes induced by H. pylori infection in some patients and the associated chronic inflammation, the so-called Correa sequence. The likelihood for regression of the mucosal changes is lower the later H. pylori eradication is given and the further advanced the changes are. However, some effect still remains even at the stage of early neoplasia. Patients with a mucosal risk profile should be offered endoscopic surveillance. Extensive glandular atrophy and/or IM in the antrum and corpus are considered as high-risk factors for further progression. (Adapted from Banks et al [30] and modified). CAG, chronic atrophic gastritis; EGC, early gastric cancer; IM, intestinal metaplasia; MDT, multidisciplinary team meeting.
While considerable progress has been made in the field of endoscopic imaging, risk stratification still relies on histopathological assessment of biopsies taken according to the updated Sydney protocol, that is, from the prepyloric antrum (x2), incisura (x1) and lesser (x1) and greater (x1) curves of the gastric corpus.38 Thus, it is of utmost importance that biopsies from both the gastric antrum and the corpus are obtained for adequate risk assessment, and these biopsies should be taken from areas with the most marked macroscopic changes. The BSG has also published guidance on quality standards for upper GI endoscopy including requirements for the average time that should be spent inspecting the mucosa and the need for appropriate photographic documentation.13 The establishment of national surveillance registries would be desirable to allow a systematic evaluation of the effectiveness of these measures and comparison to the results obtained in other parts of the world.
Conclusions
The discovery of H. pylori and the establishment of effective and relatively straightforward treatment regimens for this infection have revolutionised the management of several important upper GI diseases over the last three decades. Although the prevalence of H. pylori infection and its consequent diseases are now decreasing (at least in some countries), humanity’s battle against this infection is by no means yet won, and thorough and appropriate management of this infection remains imperative. Some clinicians might have become a little complacent about their management of H. pylori infection over recent years. If this trend continues unabated, the extent of the problems that H. pylori causes may escalate once more. We therefore encourage clinicians who treat H. pylori-infected patients to carefully re-evaluate the ways in which they test for and treat this infection, in the light of the current changing epidemiology of peptic ulcer disease, gastric adenocarcinoma and antibiotic resistance. Finally, there remain huge opportunities to prevent the development of gastric adenocarcinoma by means of H. pylori eradication and surveillance of patients who have high-risk gastric preneoplastic conditions. If these opportunities are successfully grasped, the global impact of this otherwise devastating disease will hopefully continue to decrease. We therefore make a plea for ongoing clinical research to identify the best ways to manage H. pylori-infected patients in the future.
Footnotes
Twitter: @gastrolivuni
Contributors: JB and DMP wrote sections of the first draft of this review and revised the final article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: DMP has received consultancy funding from Ipsen, Advanced Accelerator Applications and Mayoly Spindler laboratories and research funding to investigate gastric NETs from Trio Medicines Ltd. JB received consultancy funding from Mayoly Spindler laboratories.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311–5. 10.1016/S0140-6736(84)91816-6 [DOI] [PubMed] [Google Scholar]
- 2. Wands DIF, El-Omar EM, Hansen R. Helicobacter pylori: getting to grips with the guidance. Frontline Gastroenterol 2020;204:flgastro-2020-101571. 10.1136/flgastro-2020-101571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts SE, Morrison-Rees S, Samuel DG, et al. Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol Ther 2016;43:334–45. 10.1111/apt.13474 [DOI] [PubMed] [Google Scholar]
- 4. Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018;47:868–76. 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
- 5. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–9. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 6. Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 2016;43:514–33. 10.1111/apt.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin Y, Zheng Y, Wang H-L, et al. Global patterns and trends in gastric cancer incidence rates (1988-2012) and predictions to 2030. Gastroenterology 2021. 10.1053/j.gastro.2021.03.023. [Epub ahead of print: 18 Mar 2021]. [DOI] [PubMed] [Google Scholar]
- 8.. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 2020;5:42–54. 10.1016/S2468-1253(19)30328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moayyedi P, Axon ATR, Feltbower R, et al. Relation of adult lifestyle and socioeconomic factors to the prevalence of Helicobacter pylori infection. Int J Epidemiol 2002;31:624–31. 10.1093/ije/31.3.624 [DOI] [PubMed] [Google Scholar]
- 10. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607–15. 10.1016/S1470-2045(12)70137-7 [DOI] [PubMed] [Google Scholar]
- 11. Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther 2004;20:1001–17. 10.1111/j.1365-2036.2004.02203.x [DOI] [PubMed] [Google Scholar]
- 12. Allison R, Lecky DM, Bull M, et al. Audit of Helicobacter pylori testing in microbiology laboratories in England: to inform compliance with NICE guidance and the feasibility of routine antimicrobial resistance surveillance. Int J Microbiol 2016;2016:1–6. 10.1155/2016/8540904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beg S, Ragunath K, Wyman A, et al. Quality Standards in upper gastrointestinal endoscopy: a position statement of the British Society of gastroenterology (Bsg) and association of upper gastrointestinal surgeons of great britain and ireland (AUGIS). Gut 2017;66:1886–99. 10.1136/gutjnl-2017-314109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malfertheiner P, Chan FKL, McColl KEL. Peptic ulcer disease. Lancet 2009;374:1449–61. 10.1016/S0140-6736(09)60938-7 [DOI] [PubMed] [Google Scholar]
- 15. Musumba C, Jorgensen A, Sutton L, et al. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment Pharmacol Ther 2012;36:48–56. 10.1111/j.1365-2036.2012.05118.x [DOI] [PubMed] [Google Scholar]
- 16. Murugesan SVM, Varro A, Pritchard DM. Review article: strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther 2009;29:1055–68. 10.1111/j.1365-2036.2009.03976.x [DOI] [PubMed] [Google Scholar]
- 17. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017;66:6–30. 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]
- 19. Erőss B, Farkas N, Vincze Áron, et al. Helicobacter pylori infection reduces the risk of Barrett's esophagus: a meta-analysis and systematic review. Helicobacter 2018;23:e12504. 10.1111/hel.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar S, Metz DC, Ginsberg GG, et al. Oesophageal and proximal gastric adenocarcinomas are rare after detection of Helicobacter pylori infection. Aliment Pharmacol Ther 2020;51:781–8. 10.1111/apt.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doorakkers E, Lagergren J, Santoni G, et al. Helicobacter pylori eradication treatment and the risk of Barrett's esophagus and esophageal adenocarcinoma. Helicobacter 2020;25:e12688. 10.1111/hel.12688 [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Li Y, Hu J, et al. The effect of Helicobacter pylori eradication in patients with gastroesophageal reflux disease: a meta-analysis of randomized controlled studies. Dig Dis 2020;38:261–8. 10.1159/000504086 [DOI] [PubMed] [Google Scholar]
- 23. Wang Z, Shaheen NJ, Whiteman DC, et al. Helicobacter pylori infection is associated with reduced risk of Barrett's esophagus: an analysis of the Barrett's and esophageal adenocarcinoma Consortium. Am J Gastroenterol 2018;113:1148–55. 10.1038/s41395-018-0070-3 [DOI] [PubMed] [Google Scholar]
- 24. O'Connor A, O'Morain CA, Ford AC. Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol 2017;14:230–40. 10.1038/nrgastro.2016.195 [DOI] [PubMed] [Google Scholar]
- 25. De Francesco V, Zullo A, Manta R, et al. Helicobacter pylori eradication following first-line treatment failure in Europe: what, how and when chose among different standard regimens? A systematic review. Eur J Gastroenterol Hepatol 2021. 10.1097/MEG.0000000000002100. [Epub ahead of print: 19 Mar 2021]. [DOI] [PubMed] [Google Scholar]
- 26. Nyssen OP, Bordin D, Tepes B, et al. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021;70:40–54. 10.1136/gutjnl-2020-321372 [DOI] [PubMed] [Google Scholar]
- 27. McNulty CAM, Lasseter G, Shaw I, et al. Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment Pharmacol Ther 2012;35:1221–30. 10.1111/j.1365-2036.2012.05083.x [DOI] [PubMed] [Google Scholar]
- 28. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (maps): guideline from the European Society of gastrointestinal endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94. 10.1055/s-0031-1291491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (maps II): European Society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota Study Group (EHMSG), European Society of pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019;51:365–88. 10.1055/a-0859-1883 [DOI] [PubMed] [Google Scholar]
- 30. Banks M, Graham D, Jansen M, et al. British Society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019;68:1545–75. 10.1136/gutjnl-2018-318126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiao Y, Hyder A, Bae SJ, et al. Surveillance in patients with Barrett's esophagus for early detection of esophageal adenocarcinoma: a systematic review and meta-analysis. Clin Transl Gastroenterol 2015;6:e131. 10.1038/ctg.2015.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 33. Bornschein J, Dingwerth A, Selgrad M, et al. Adenocarcinomas at different positions at the gastro-oesophageal junction show distinct association with gastritis and gastric preneoplastic conditions. Eur J Gastroenterol Hepatol 2015;27:492–500. 10.1097/MEG.0000000000000299 [DOI] [PubMed] [Google Scholar]
- 34. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020;69:2113–21. 10.1136/gutjnl-2020-320839 [DOI] [PubMed] [Google Scholar]
- 35. Chen H-N, Wang Z, Li X, et al. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer 2016;19:166–75. 10.1007/s10120-015-0462-7 [DOI] [PubMed] [Google Scholar]
- 36. Ford AC, Yuan Y, Forman D, et al. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev 2020;7:CD005583. 10.1002/14651858.CD005583.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moehler M, Al-Batran S-E, Andus T, et al. Z Gastroenterol 2019;57:1517–632. 10.1055/a-1018-2516 [DOI] [PubMed] [Google Scholar]
- 38. Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. 10.1097/00000478-199610000-00001 [DOI] [PubMed] [Google Scholar]
- 39. Fischbach W, Zerl A, Klassert C. [How do primary care physicians manage their patients with Helicobacter pylori infection? Results of a survey and their implementation into the German S2k guideline 2016]. Z Gastroenterol 2017;55:136–9. 10.1055/s-0042-119453 [DOI] [PubMed] [Google Scholar]
- 40. Cutler AF, Havstad S, Ma CK, et al. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology 1995;109:136–41. 10.1016/0016-5085(95)90278-3 [DOI] [PubMed] [Google Scholar]
- 41. Thijs JC, van Zwet AA, Thijs WJ, et al. Diagnostic tests for Helicobacter pylori: a prospective evaluation of their accuracy, without selecting a single test as the gold standard. Am J Gastroenterol 1996;91:2125–9. [PubMed] [Google Scholar]
- 42. Laheij RJ, de Boer WA, Jansen JB, et al. Diagnostic performance of biopsy-based methods for determination of Helicobacter pylori infection without a reference standard. J Clin Epidemiol 2000;53:742–6. 10.1016/S0895-4356(99)00222-X [DOI] [PubMed] [Google Scholar]
- 43. Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol 2006;101:1921–30. 10.1111/j.1572-0241.2006.00668.x [DOI] [PubMed] [Google Scholar]
- 44. Calvet X, Lario S, Ramírez-Lázaro MJ, et al. Comparative accuracy of 3 monoclonal stool tests for diagnosis of Helicobacter pylori infection among patients with dyspepsia. Clin Infect Dis 2010;50:323–8. 10.1086/649860 [DOI] [PubMed] [Google Scholar]
- 45. Deguchi R, Matsushima M, Suzuki T, et al. Comparison of a monoclonal with a polyclonal antibody-based enzyme immunoassay stool test in diagnosing Helicobacter pylori infection after eradication therapy. J Gastroenterol 2009;44:713–6. 10.1007/s00535-009-0069-z [DOI] [PubMed] [Google Scholar]
- 46. Korkmaz H, Kesli R, Karabagli P, et al. Comparison of the diagnostic accuracy of five different stool antigen tests for the diagnosis of Helicobacter pylori infection. Helicobacter 2013;18:384–91. 10.1111/hel.12053 [DOI] [PubMed] [Google Scholar]




