Abstract
Background
Malignant biliary obstruction, which requires endoscopic stenting as palliative therapy, is often complicated by clogging of the stent with subsequent jaundice and/or cholangitis. Stent clogging may be caused by microbiological adhesion and biliary stasis. Therefore, antibiotics and choleretic agents like ursodeoxycholic acid (UDCA) have been investigated to see whether they prolong stent patency.
Objectives
To evaluate if UDCA and/or antibiotics may prolong stent patency and survival in patients with strictures of the biliary tract and endoscopically inserted stents.
Search methods
The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Library, MEDLINE, Current Contents, EMBASE, and CancerLit were searched until June 2001. Reference lists of the identified articles were checked for further trials.
Selection criteria
All randomised or quasi‐randomised clinical trials investigating UDCA and/or antibiotics in patients with biliary stents were considered for inclusion, regardless of blinding, language, and publication status.
Data collection and analysis
Trial inclusion, quality assessment, and data extraction were performed independently by two reviewers. Principal investigators were contacted for further information. Survival data were combined by using hazard ratios (with 95% confidence interval (95% CI)).
Main results
Five non‐blinded randomised trials with 258 patients with malignant strictures treated with polyethylene stents were included. Three trials, including 152 patients, investigated a combination of UDCA and antibiotics versus no treatment. The meta‐analysis of these three trials does not show a significant treatment effect on the duration of stent patency (hazard ratio (random effects model) 0.58, 95% CI 0.22 to 1.54) or mortality (hazard ratio (fixed effect model) 0.99, 95% CI 0.68 to 1.43). Two trials with 106 patients compared antibiotics with no treatment, one of these trials used a combination of antibiotics and rowachol (an 'alternative' drug of the 'mint' family). The pooled results of these two trials do not show significant effects of antibiotics on the duration of stent patency (hazard ratio (fixed effect model) 0.69 (95% CI 0.37 to 1.30)) or mortality (hazard ratio (fixed effect model) 1.23 (95% CI 0.72 to 2.08). Data concerning duration of hospital stay, frequency of cholangitis, and rate of infectious complications due to selection of antibiotic resistant bacteria strains were not available.
Authors' conclusions
Treatment with UDCA and/or antibiotics to prevent clogging of biliary stents in patients with malignant stricture of the biliary tract cannot be recommended routinely on the basis of the existing randomised clinical trials. Further trials are needed with rigorous methodology and sufficient statistical power.
Plain language summary
Still awaiting evidence for effect of ursodeoxycholic acid and/or antibiotics in the prevention of biliary stent occlusion
Malignant occlusion of the biliary tract can be relieved by insertion of a stent, which allows passage of the biliary fluid. However, stents often clog. This Review examines if ursodeoxycholic acid (a bile acid) and/or antibiotics may prevent clogging of biliary stents. At present there is not sufficient evidence to recommend ursodeoxycholic acid and/or antibiotics for biliary stented patients.
Background
Stenoses of the biliary tract cause major problems in patients with pancreatic carcinoma, cholangiocarcinoma, ampullary carcinoma, or other malignant disorders where the bile duct is compressed by the primary tumour or metastases. When a surgical cure is not attainable, endoscopic stenting is the preferred palliative procedure for patients with bile duct stenosis of malignant origin (Hagenmuller 1983; Huibregtse 1986; Siegel 1986; Smith 1994). Compared with bile duct surgery, endoscopically placed stents are less invasive and seems to achieve similar median survival times (Andersen 1989). In addition, some benign causes of cholestasis (e.g., bile duct strictures due to primary sclerosing cholangitis) require the insertion of biliary stents.
The major drawback of biliary stents is the occlusion of the prostheses with recurrence of jaundice and/or cholangitis. Stent clogging usually occurs 20 to 30 weeks after insertion when polyethylene stents are used (Andersen 1989). Self expanding metal stents reach significantly longer periods of patency, but their use is limited because of high costs (about 20 times more expensive than polyethylene stents) (Davids 1992). In fact, fewer ERCP‐investigations would be necessary if metal stents are used and this may outweigh the higher price of the prosthesis (particularly in patients with good clinical performance status and a life expectancy of at least six months).
There is evidence that stent clogging is caused by microbiological adhesion and biliary stasis leading to encrustation of the bile components (Groen 1987; Leung 1988; Speer 1988; Smit 1989; Moesch 1991). As a consequence of these findings it was hypothesised that antibacterial and choleretic agents could delay the occlusion of biliary stents. In an animal model, Libby et al. were able to reach four times longer periods of stent patency by administration of ciprofloxacin (Libby 1996). Some randomised trials have been conducted to evaluate the effect of the administration of antibiotics alone or in combination with choleretic agents like ursodeoxycholic acid (UDCA) on the duration of stent patency in patients with malignant diseases leading to compression of the biliary tract (Barrioz 1994; Ghosh 1994; Luman 1999). However, the evidence has not yet been systematically reviewed and the effect of these interventions on clinically relevant outcomes is not clear. The aim of this systematic Review was to evaluate this evidence and to integrate these findings in a comprehensive evaluation of these interventions.
Objectives
The aims of this systematic Review were to evaluate the beneficial and harmful effects of UDCA and/or antibiotics in patients with extra hepatic biliary stenosis and endoscopically inserted stents on
the duration of stent patency,
mortality (in patients with malignant diseases), quality of life, costs, duration of hospitalisation, cholangitis, and infectious complications due to selection of antibiotic resistant bacteria strains.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised clinical trials were considered for inclusion irrespective of blinding. Unpublished trials were included as well, and no language limitations were applied.
Types of participants
All patients with endoscopically inserted biliary stents (to treat jaundice and/or cholangitis due to occlusion of the bile duct). Whenever possible, the analysis was stratified according to stent material (metal versus plastic) and cause of jaundice (malignant versus others).
Types of interventions
UDCA alone or combined with antibiotics or antibiotics alone compared with no treatment or placebo administration.
The planned comparisons encompassed:
UDCA and antibiotics versus placebo or no intervention;
UDCA versus placebo or no intervention;
Antibiotics versus placebo or no intervention;
UDCA versus antibiotics;
UDCA and antibiotics versus UDCA alone;
UDCA and antibiotics versus antibiotics alone.
Types of outcome measures
The primary measure of outcome was the duration of stent patency measured by clinical parameters (jaundice, cholangitis).
Secondary outcomes were (a) overall mortality in patients with malignant extrahepatic biliary stenosis, (b) duration of hospital stay, (c) frequency of cholangitis, and (d) rate of infectious complications due to selection of antibiotic resistant bacteria strains.
Search methods for identification of studies
See: Collaborative Review Group search strategy.
Electronic search strategy: #1 biliary OR (bile AND duct) #2 stent* OR prosthes* #3 1 AND 2 #4 occlu* OR block* OR clogg* #5 ursodeoxycholic acid OR UDCA #6 antibiotic* OR antimicrobi* OR ciprofloxacin OR norfloxacin OR ampicillin OR amoxicillin OR metronidazole OR gentamicin OR piperacillin #7 5 OR 6 #8 3 AND 4 AND 7
This strategy was combined with the highly sensitive search strategy (HSSS) developed by Dickersin et al. (Dickersin 1994). The Controlled Trials Register of The Cochrane Hepato‐Biliary Group and The Cochrane Library were searched without the HSSS.
The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Library (including CENTRAL and the Medical Editors Trial Amnesty), MEDLINE, EMBASE, Cancer Lit, and Current Contents were searched electronically until June 2001, and further handsearch was conducted in two German journals (Zeitschrift für Gastroenterologie, Endoskopie heute) and conference proceedings. In databases which provide a controlled vocabulary (e.g., MEDLINE, EMBASE) the free text‐search was combined with a comparable search strategy adapted to the thesaurus of the database. The detailed search strategies for each database are available within the 'Published notes' section.
The reference lists of the identified trials and review articles were checked for further trial references.
The principal authors of the included trials were contacted and asked about additional trials they might had known of.
Pharmaceutical companies producing UDCA and the antibiotics most commonly used were asked for results of further (unpublished) trials.
Data collection and analysis
The systematic Review and its meta‐analysis were performed according to the formerly published protocol (Galandi 2001).
Trial selection One reviewer (DG) collected the complete publication of all citations that seem to be clearly or potentially relevant trials for this Review. Based on the full text of the trial two reviewers (DG and GS) independently applied the inclusion and exclusion criteria. Disagreements were solved by discussion and by asking a third party (HPA).
Quality assessment All trials included in the Review were assessed for their methodological quality. Two reviewers (DG and GS) independently assessed the trial quality and disagreements were solved by discussion, and if necessary by asking a third party (HPA).
Quality was assessed according to the 0‐5 point Jadad scale described below (Jadad 1996). Additionally, data about concealment of treatment allocation was collected (Schulz 1995).
Jadad scale: 1 point for randomisation of trial participants; 1 point for description of an adequate method for generation of the randomised sequence; 1 point for double blinding; 1 point for description of an adequate blinding method; 1 point for description of drop outs and withdrawals.
(If the randomisation or blinding method is described but inadequate, one point was deducted.)
According to allocation concealment the trials were classified as follows:
a) Adequate method of allocation concealment; b) Uncertain because of lacking data; c) Inadequate method of allocation concealment.
Data extraction Two reviewers (DG and GS) independently extracted the complete data from all included trials. Disagreements were solved by discussion. Principal authors of the trials were contacted to retrieve missing data. Statistical analyses Analyses were conducted with The Cochrane Collaboration's software package (RevMan 4.1). Additional analyses (like hazard ratios to combine survival data), which cannot be calculated with RevMan 4.1, were conducted using the STATA software package (StataCorp. 2001. Stata statistical software: Release 7.0. College station, TX: Stata Corporation).
The analyses were performed according to the intention‐to‐treat principle. All patients were analysed as randomised, and missing patient data for binary outcomes were considered as failures. In case of lacking data on the outcome of excluded patients, the principal author was asked to provide the original data in order to perform an intention‐to‐treat analysis.
Heterogeneity between trials was analysed by a Chi‐squared test; a P‐value of < 0.1 was considered significant. In the case of statistical heterogeneity between trials, a random effects model was used (DerSimonian 1986). Potential causes of heterogeneity were explored by performing sensitivity analyses if possible. When there was no evidence for heterogeneity, the data were analysed using a fixed effect model. Hazard ratios were used to combine survival data (Parmar 1998).
The presence of bias (e.g., publication bias) was investigated by a funnel plot (Egger 1997). Furthermore, a rank correlation test was conducted, a P‐value of < 0.1 was considered significant (Begg 1994).
Results
Description of studies
Using the described search methods, we identified five trials fulfilling the inclusion criteria of this Review with a total of 258 patients (range 20 to 70 patients per trial). All were randomised, non‐blinded trials that were investigating patients with malignant biliary stricture and endoscopically inserted polyethylene stents. All trials compared the investigated treatment versus a no treatment‐control group. No quasi‐randomised studies have been identified.
Three trials investigated a combination of UDCA and an antibiotic (two trials used norfloxacin, in the third trial monthly cycles of ampicillin, metronidazole, and ciprofloxacin were used). Two trials compared an antibiotic (ciprofloxacin) versus no treatment. In one of these trials, ciprofloxacin was combined with rowachol (contains chemicals from several members of the 'mint family' and has been used as an 'alternative treatment' for gallstones). We decided to include this trial in the analysis as well since there was no significant heterogeneity in the outcomes of the trials and we know of no randomised trials demonstrating the efficacy of rowachol.
The main outcome of all trials was the duration of stent patency. Mortality was the secondary outcome in all trials but Barrioz et al. (Barrioz 1994), which did not report sufficient data to extract hazard ratios for mortality nor did they answer our query concerning additional information about this outcome. The Barrioz et al. trial provided some information with respect to the length of hospital stay.
Concerning the other investigated outcomes of this Review (frequency of cholangitis and the selection of antibiotic resistant bacteria strains) no data were available. Data about costs or quality of life were also missing.
Except Sung et al. (Sung 1999), none of the investigators responded to our queries, therefore, the analysis is based on the published information of the remaining trials.
The distribution of the trials in the funnel plot does not show evidence of publication bias or other biases. Due to the small number of trials in this Review the power of this test is limited (Sterne 2000).
Risk of bias in included studies
The identified trials were of intermediate to weak methological quality. All trials were described as randomised trials but none of them gave adequate description on how the allocation sequence was generated and only three trials gave detailed information about the method of allocation concealment (sealed envelopes) (De Ledinghen 2000; Luman 1999; Sung 1999). No trial described the sealed envelopes as 'opaque'. All trials were non‐blinded although blinding would have been possible. Information about dropouts or losses to follow up was given in four trials.
Assessed by the Jadad‐scale, the trials obtained 1 to 3 points on the 5 point scale (see table 'Characteristics of included studies').
Effects of interventions
UDCA plus antibiotics versus no intervention Three trials could be identified investigating this comparison. The trial of Barrioz et al., although very small, with only 10 patients in each group, reports a statistically significant benefit of the drug combination with respect to median stent patency and mortality (Barrioz 1994). The remaining two trials (with 29‐39 patients in each treatment group) did not identify any positive effect of the investigated therapy on these two outcomes (De Ledinghen 2000; Ghosh 1994). Thus the test of heterogeneity showed evidence for statistical heterogeneity (Chi‐square‐test 7.68, P = 0.022) and we decided to combine these trials using a random effects model (as planned in the protocol of this Review). The hazard ratio for stent occlusion calculated on the basis of the three trials in the random effects model is 0.58 (95% CI 0.22 to 1.54), P = 0.27. The analysis does not show a significant extension of stent patency in patients treated with UDCA plus antibiotics versus no intervention. The random effects analysis of the hazard ratios cannot be displayed by MetaView but the results of the single trials are presented.
With regard to mortality the data given by Barrioz et al. were not sufficient to be included in the meta‐analysis. Neither the report of the trial gives information which would allow the calculation of hazard ratios nor the investigators replied to our inquiry. The meta‐analysis is based on the remaining two trials (132 patients) only and showed no significant difference between the survival of the two groups: hazard ratio 0.99 (95% CI 0.68 to 1.43) (De Ledinghen 2000; Ghosh 1994).
Antibiotics versus no intervention
Two trials with 106 patients were identified which investigated this comparison (Luman 1999; Sung 1999). There was no evidence for heterogeneity within these two trials (Chi‐square‐test 0.65, P = 0.42) so we combined the trials using a fixed effect model. With respect to the duration of stent patency, the meta‐analysis results in a hazard ratio of 0.69 (95% CI 0.37 to 1.30) which does not indicate a significant difference in duration of stent patency between the treatment and the control group.
With respect to mortality, the two trials do not show a significant difference between treatment and control group: hazard ratio 1.23 (95% CI 0.72 to 2.08).
We were not able to find any trial investigating the following comparisons:
UDCA versus placebo or no intervention
UDCA versus antibiotics
UDCA and antibiotics versus UDCA alone
UDCA and antibiotics versus antibiotics alone.
Data concerning duration of hospital stay, frequency of cholangitis, and rate of infectious complications due to selection of antibiotic resistant bacteria strains were not available.
Subgroup analysis according to stent material (metal versus plastic), cause of jaundice (malignant versus others), or the impact of different plastic stent material on the basis of the identified data were not feasible, because all identified trials investigated patients with malignant cause of jaundice treated with polyethylene stents.
Sensitivity analyses according to method of allocation concealment, blinding, and randomisation (randomised trials versus quasi‐randomised ) could not be conducted because all of the included five trials were randomised, open trials with unclear allocation concealment.
Discussion
Although almost every patient with an endoscopically inserted polyethylene stent for the treatment of a malignant biliary stricture experiences a relapse of jaundice and/or cholangitis, only five randomised trials evaluating UDCA and/or antibiotics as prevention of early stent occlusion could be identified. Furthermore, these trials are small with a maximum of 70 patients per trial. Four of these trials did not show a significant effect of the treatment on the duration of stent patency and survival. The remaining trial has to be interpreted with caution because this very small trial has some serious limitations like a very short time of stent patency in the control group and evidence of unbalanced randomisation of patients due to the small number of participants. Cholangiocarcinoma was most common in the active treatment group. This unbalanced distribution of malignancies between the two intervention groups may explain the longer survival in the active treatment group because cholangiocarcinoma show a rather slow progression when compared to pancreatic carcinoma (Nagakawa 1989).
Investigating the cause of heterogeneity in the comparison 'UDCA and antibiotics versus no intervention', it must be recognised that a very short duration of stent patency was observed in the control group of the trial of Barrioz et al. with a median time of stent patency of six weeks for the first stent and seven weeks for all stents. This is clearly a shorter patency time than in all other trials which gave information about the duration of stent patency in the untreated group of patients. Ghosh et al. report a median time to stent occlusion of 28 weeks and De Ledinghen et al. of 21 weeks in the control group. Patients with polyethylene stents may potentially be helped by undergoing routine changes of the stent before cholangitis or jaundice reappear. An appropriate interval could be three months, which is below the median time to stent occlusion reported in the identified RCTs. However, we need high quality randomised trials examining the beneficial and harmful effects of such a strategy. Patients in good clinical status and a life expectancy of more than six months may be potentially helped by the insertion of metal stents that tend to stay patent a longer time as demonstrated in a RCT comparing metal and polyethylene stents (Davids 1992). However, we also need more high quality RCTs on beneficial and harmful effects of metal stents.
The systematic Review and meta‐analyses of the five included trials do not show any significant effect of antibiotics alone or in combination with UDCA on the duration of stent patency or survival.
On the basis of the existing evidence a clinically relevant treatment effect cannot be precluded.
Authors' conclusions
Implications for practice.
On the basis of the trials pooled in this systematic Review, prophylactic treatment with UDCA and/or antibiotics to prevent clogging of polyethylene stents in patients with malignant biliary strictures cannot be recommended routinely.
Implications for research.
Further properly designed RCTs with sufficient statistical power should be initiated to evaluate the use of ursodeoxycholic acid and/or antibiotics in patients with endoscopically inserted biliary stents in the treatment of biliary strictures and such RCTs should stratify patients at randomisation according to the cause of stricture. Additionally, new substances should be considered for this indication since it is to be expected that UDCA and/or antibiotics are not able to prevent stent clogging completely. Furthermore, future trials should also provide data concerning additional, clinically relevant aspects like duration of hospital stay, frequency of cholangitis, and rate of infectious complications due to selection of antibiotic resistant bacteria strains. The use of metal stents should be investigated to identify situations in which these stents with longer patency times may be superior to plastic stents.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2008 | Amended | Converted to new review format. |
Notes
I. MEDLINE (Ovid)
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized controlled trials.sh. 4 random allocation.sh. 5 double blind method.sh. 6 single blind method.sh. 7 or/1‐6 8 clinical trial.pt. 9 exp clinical trials/ 10 (clin$ adj25 trial$).ti,ab. 11 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 12 placebos.sh. 13 placebo$.ti,ab. 14 random$.ti,ab. 15 research design.sh. 16 or/8‐15 17 comparative study.sh. 18 exp evaluation studies/ 19 follow up studies.sh. 20 prospective studies.sh. 21 (control$ or prospectiv$ or volunteer$).ti,ab. 22 or/17‐21 23 7 or 16 or 22 24 "human"/ 25 "animal"/ 26 24 and 25 27 25 not 26 28 23 not 27 29 exp bile ducts, extrahepatic/ or Bile ducts/ 30 exp bile duct neoplasms/ or Bile duct diseases/ or Biliary tract diseases/ 31 Common bile duct diseases/ or Common bile duct neoplasms/ 32 Stents/ 33 "Prostheses and implants"/ 34 Bile duct obstruction, extrahepatic/ or Cholestasis/ 35 exp "Cholagogues and choleretics"/ 36 exp Deoxycholic acid/ 37 exp Antibiotics/ 38 Antibiotic prophylaxis/ 39 37 or 38 40 29 or 30 or 31 or 34 41 32 or 33 42 35 or 36 or 39 43 40 and 41 and 42 44 rando$.mp,pt. 45 43 and 44 46 Metronidazole/ 47 exp Gentamicins/ 48 exp anti‐infective agents, quinolone/ 49 46 or 47 or 48 50 42 or 49 51 40 and 41 and 44 and 50 52 exp Anti‐infective agents/ 53 38 or 52 54 35 or 36 or 53 55 40 and 41 and 44 and 54 (specific) 56 40 and 41 and 28 and 54 57 (bile adj2 duct$).tw. 58 biliar$.tw. 59 (bili$ adj2 duct$).tw. 60 or/57‐59 61 prothe$.tw. 62 prosthe$.tw. 63 *Prosthesis Implantation/ 64 stent$.tw. 65 obstruct$.tw. 66 (norfloxacin$ or ampicillin$ or amoxicillin$ or metronidazole$ or gentamycin$ or piperacillin$).tw,rw. 67 anti‐biotic$.tw,rw. 68 antibiotic$.tw,rw. 69 anti‐microbi$.tw,rw. 70 antimicrobi$.tw,rw. 71 ciprofloxacin$.tw,rw. 72 ciprofloxacin.tw. 73 or/66‐71 74 udca.tw,rw. 75 ursodeox$.tw,rw. 76 udc$.tw. 77 urso‐deox$.tw,rw. 78 deoxychol$.tw,rw. 79 deoxichol$.tw,rw. 80 or/74‐79 81 73 or 80 82 or/61‐64 83 23 or 44 84 83 not 27 85 cholestas$.tw. 86 60 or 85 87 (occlu$ or block$ or clogg$).tw. 88 65 or 87 89 81 and 82 and 84 and 86 and 88 90 56 or 89 91 90 not 55 92 from 91 keep 1‐18
II. Web of Science/Science Citation Index
(Cholesta* or ((Bile or biliar*) and (duct or tract*))) and (Stent* or prothe* or prosthe*) and (occlu* or block* or clogg* or obstruct*) and (norfloxacin* or ampicillin* or amoxicillin* or metronidazole* or gentamycin* or piperacillin* or anti‐biotic* or antibiotic* or anti‐microbi* or antimicrobi* or ciprofloxacin* or ursodeox* or udc* or urso‐deox* or deoxychol* or deoxichol*) and (rando* or control* or (clinical and (trial* or stud*)) or ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or placebo* or research design or compar* or prospectiv* or volunteer*)
III. Cancerlit (via Dimdi)
39 S=38 AND S=22 38 S=37 NOT S=18 37 S=36 AND S=35 AND S=34 AND S=32 36 norfloxacin? or ampicillin? or amoxicillin? or metronidazole? or gentamycin? or piperacillin? or anti‐biotic? or antibiotic? or anti‐microbi? or antimicrobi? or ciprofloxacin? or ursodeox? or udc? or urso‐deox? or deoxychol? or deoxichol? 35 (((occlu? OR block?) OR clogg?) OR obstruct?) 34 ((stent? OR ?prothe?) OR ?prosthe?) 33 ((stent? OR prothe?) OR prosthe?) 32 S=31 OR S=30 31 cholesta? 30 S=29 AND S=28 29 (bile OR biliar?) 28 (duct? OR tract?) 24 S=18 NOT S=23 23 S=22 AND S=18 22 S=21 OR S=20 OR S=19 21 DT=?"rando"? 20 CT=?"rando"? 19 rando? 18 S=17 AND S=8 AND S=5 10 (entspr. MeSH‐Terms, ohne rando) 17 S=16 OR S=14 OR S=13 OR S=12 OR S=11 OR S=10 OR S=9 16 CT DOWN "anti‐infective agents" 15 CT DOWN "anti‐infective agents, quinolone" 14 CT DOWN "gentamicins" 13 CT="metronidazole" 12 CT="antibiotic prophylaxis" 11 CT DOWN "antibiotics" 10 CT DOWN "deoxycholic acid" 9 CT DOWN "cholagogues and choleretics" 8 S=7 OR S=6 7 CT="PROSTHESES AND IMPLANTS" 6 CT="stents" 5 S=4 OR S=3 OR S=2 4 (CT="bile duct obstruction, extrahepatic" OR CT="cholestasis") 3 ((CT DOWN "bile duct neoplasms" OR CT="bile duct diseases") OR CT="biliary tract diseases") 2 (((CT DOWN "bile ducts, extrahepatic" OR CT="bile ducts") OR CT="common bile duct diseases") OR CT="common bile duct neoplasms") 1 CL63
IV: EMBASE (Dimdi)
1 EM74 2 EM74 3 CT D RANDOMIZATION 5 CT D RANDOMIZED CONTROLLED TRIAL 6 CT D CONTROLLED STUDY 7 CT D CLINICAL TRIAL 8 CT D DRUG COMPARISON 9 CT D MAJOR CLINICAL STUDY 10 CC D D40 11 CT D DOUBLE BLIND PROCEDURE 12 CT D SINGLE BLIND PROCEDURE 13 CT D CROSSOVER PROCEDURE 14 PLACEBO? 15 RANDO? 16 SINGL? OR DOUBL? OR TREBL? OR TRIPL? 17 BLIND? OR MASK? 18 16 AND 17 19 COMPAR? OR PROSPECTIV? OR VOLUNTEER? 20 RESEARCH DESIGN 21 3 TO 13 22 CLINICAL TRIAL? 23 14 OR 15 OR 18 OR 19 OR 20 OR 22 24 21 OR 23 25 EM74 26 CT D ANTIBIOTIC PROPHYLAXIS 27 CT D ANTIBIOTIC AGENT 28 CT D METRONIDAZOLE 30 CC=D20 31 CT=CIPROFLOXACIN 38 NORFLOXACIN? OR AMPICILLIN? OR AMOXICILLIN? OR METRONIDAZOLE? OR GENTAMYCIN? OR PIPERACILLIN? OR ANTI BIOTIC? OR ANTIBIOTIC? OR ANTI MICROBI? OR ANTIMICROBI? OR CIPROFLOXACIN? OR URSODEOX? OR UDC? OR URSO DEOX? OR DEOXYCHOL? OR DEOXICHOL? 40 CT D ANTIINFECTIVE AGENT 41 TE=NORFLOXACIN? 42 TE=AMPICILLIN? 43 TE=AMOXICILLIN? 44 TE=METRONIDAZOLE? 45 TE=GENTAMYCIN? 46 TE=PIPERACILLIN? 47 TE=ANTIBIOTIC? 48 TE=ANTIMICROBI? 49 TE=CIPROFLOXACIN? 50 TE=URSODEOX? 51 TE=DEOXYCHOL? 52 CT=UDCA 53 CT=URSODEOXYCHOLIC ACID 58 52 OR 53 59 26 TO 58 60 EM74 61 CT D BILE DUCT 62 CT D BILE DUCT OBSTRUCTION 63 CT D BILE DUCT NEOPLASMS 64 CT D BILIARY TRACT DISEASES 65 CT D BILE DUCT DILATATION 66 CT D BILIARY TRACT DRAINAGE 68 CT D COMMON BILE DUCT DISEASES 69 CT D COMMON BILE DUCT NEOPLASMS 70 CT D CHOLESTASIS 71 BILE? ?, DUCT?. 72 BILE? ?, TRACT?. 73 BILIARY ?, TRACT?. 74 BILIARY?, DUCT?. 75 71 TO 74 76 61 OR 62 OR 63 OR 64 OR 68 OR 69 OR 70 77 65 OR 66 78 75 OR 76 83 CT D STENT 84 CT="PROSTHESES AND ORTHOSES" 85 CT=ENDOPROSTHESIS 86 CT=IMPLANT 88 STENT? 89 PROSTHE? 90 PROTHE? 91 IMPLANT? 93 CT D IMPLANTATION 95 88 TO 91 99 83 OR 84 OR 85 OR 86 OR 93 100 95 OR 99 101 77 OR 100 102 78 AND 101 103 CT D BLOCKING 106 CT D OCCLUSION 108 CC=C6.835.650 109 CLOGG? 110 BLOCK? 111 OCCLU? 112 OBSTRUCT? 113 CC=C6.835 114 CC=C6.835.645 115 113 OR 114 117 103 OR 108 OR 115 118 109 TO 112 119 117 OR 118 120 102 AND 119 121 59 AND 120 122 24 AND 121 123 CT D HUMAN 124 CT D ANIMAL 125 123 AND 124 126 124 NOT 125 127 122 NOT 126
Acknowledgements
We thank Edith Motschall for her help in conducting the electronic literature searches. We thank Prof. J. Sung and his colleagues for the readiness to cooperate and for providing additional data on the results of their trial.
Data and analyses
Comparison 1. UDCA and antibiotics versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stent patency | 3 | Peto Odds Ratio (95% CI) | Subtotals only | |
| 2 Overall survival | 2 | 132 | Peto Odds Ratio (95% CI) | 0.99 [0.68, 1.43] |
1.1. Analysis.
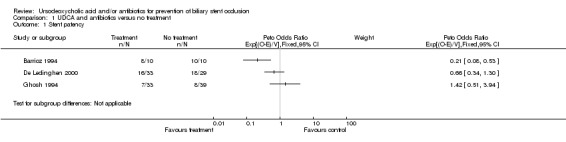
Comparison 1 UDCA and antibiotics versus no treatment, Outcome 1 Stent patency.
1.2. Analysis.
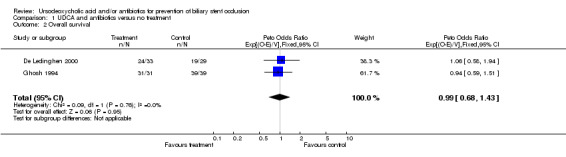
Comparison 1 UDCA and antibiotics versus no treatment, Outcome 2 Overall survival.
Comparison 2. Antibiotics versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stent patency | 2 | 92 | Peto Odds Ratio (95% CI) | 0.69 [0.37, 1.30] |
| 2 Overall survival | 2 | 92 | Peto Odds Ratio (95% CI) | 1.23 [0.72, 2.08] |
2.1. Analysis.
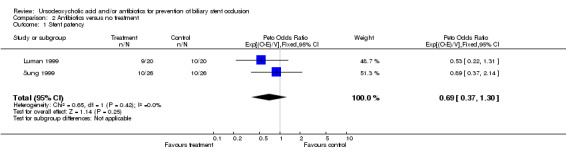
Comparison 2 Antibiotics versus no treatment, Outcome 1 Stent patency.
2.2. Analysis.
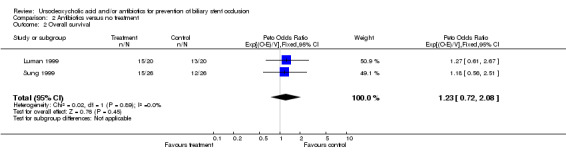
Comparison 2 Antibiotics versus no treatment, Outcome 2 Overall survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrioz 1994.
| Methods | Generation of the allocation sequence: method unclear. Allocation concealment: method unclear. Blinding: Not blinded. Intention‐to‐treat: not stated. Exclusions from analysis: not stated. Follow up period: unclear. Jadad‐score: 2. | |
| Participants | Twenty patients with malignant biliary stricture and endoscopically inserted biliary polyethylene stent. | |
| Interventions | Ursodeoxycholic acid (13‐15 mg/kg/d) and norfloxacin (400 mg/d) versus no treatment. | |
| Outcomes | Duration of stent patency. Mortality. Duration of hospitalisation. | |
| Notes | Cholangiocarcinoma was more common in the drug treated group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
De Ledinghen 2000.
| Methods | Generation of the allocation sequence: method unclear. Allocation concealment: consecutively numbered series of sealed envelopes. Blinding: not blinded. Intention‐to‐treat: yes. Exclusions from analysis: no. Follow up period: 240 days. Jadad‐score: 3. | |
| Participants | Sixty‐two patients with malignant biliary stricture and endoscopically inserted biliary polyethylene stent. | |
| Interventions | Ursodeoxycholic acid (13‐15 mg/kg/d) and norfloxacin (400 mg/d) versus no treatment. | |
| Outcomes | Duration of stent patency. Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ghosh 1994.
| Methods | Generation of the allocation sequence: method unclear. Allocation concealment: method unclear. Blinding: not blinded. Intention‐to‐treat: not stated. Exclusions from analysis: not stated. Follow up period: median 238 days (35‐336 days). Jadad‐score: 2, | |
| Participants | Seventy patients with malignant biliary stricture and endoscopically inserted biliary polyethylene stent. | |
| Interventions | Ursodeoxycholic acid (10 mg/kg/d) and ciprofloxacin (250 mg twice a day) or ampicillin (500 mg four times a day) or metronidazole (400 mg twice a day) in monthly cycles versus no treatment. | |
| Outcomes | Duration of stent patency, Mortality, | |
| Notes | Rotating regimen of three different antibiotics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Luman 1999.
| Methods | Generation of the allocation sequence: method unclear. Allocation concealment: sealed envelopes. Blinding: Not blinded. Intention‐to‐treat: No. Exclusions from analysis: five in the control group and three in the intervention group because of death within one month after randomisation. Follow up period: Unclear. Jadad‐score: 3. | |
| Participants | Forty‐eight patients with malignant biliary stricture and endoscopically inserted biliary polyethylene stent. | |
| Interventions | Ciprofloxacin (500 mg twice a day) and Rowachol (2 tablets three times a day) versus no treatment. | |
| Outcomes | Duration of stent patency. Mortality. | |
| Notes | Rowachol was given as additional treatment to the antibiotics group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sung 1999.
| Methods | Generation of the allocation sequence: method unclear. Allocation concealment: sealed envelopes. Blinding: Not blinded. Intention‐to‐treat: Yes. Exclusions from analysis: three in each group (four ‐ surgery, one lost to follow up and one technical failure of stenting). Follow up period: 140 days. Jadad‐score: 3. | |
| Participants | Fifty‐eight patients with malignant biliary stricture and endoscopically inserted biliary polyethylene stent. | |
| Interventions | Ciprofloxacin (250 mg twice a day) versus no treatment. | |
| Outcomes | Duration of stent patency. Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davids 1992 | Comparison of metal versus polyethylene stents, no prophylactic treatment for stent occlusion evaluated. |
| Smit 1989 | Stents were removed after two months in each patient and no follow‐up data were available. |
Contributions of authors
DG: ‐ development of the concept of the Review, ‐ collection and assessment of trials, ‐ data extraction, ‐ contacting authors, and ‐ conducting the Review.
DB: ‐ trial assessment, and ‐ data extraction.
GS: ‐ trial assessment, ‐ statistical analyses, and ‐ data extraction.
HPA: ‐ advice in hepatological problems, ‐ trial assessment, and ‐ data extraction.
Sources of support
Internal sources
No sources of support supplied
External sources
The Copenhagen Hospital Corporation's Research Council's Grant on Getting Research into Practice (GRIP), Denmark.
The Danish Medical Research Council's Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Barrioz 1994 {published data only}
- Barrioz T, Ingrand P, Besson I, Ledinghen V, Silvain C, Beauchant M. Randomised trial of prevention of biliary stent occlusion by ursodeoxycholic acid plus norfloxacin. Lancet 1994;344(8922):581‐2. [DOI] [PubMed] [Google Scholar]
De Ledinghen 2000 {published data only}
- Ledinghen V, Person B, Legoux JL, Sidaner A, Desaint B, Greff M, et al. Prevention of biliary stent occlusion by ursodeoxycholic acid plus norfloxacin. A multicenter randomized trial. Digestive Diseases and Sciences 2000;45(1):145‐150. [DOI] [PubMed] [Google Scholar]
Ghosh 1994 {published data only}
- Ghosh S, Palmer KR. Prevention of biliary stent occlusion using cyclical antibiotics and ursodeoxycholic acid. Gut 1994;35(12):1757‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luman 1999 {published data only}
- Luman W, Ghosh S, Palmer KR. A combination of ciprofloxacin and rowachol does not prevent biliary stent occlusion. Gastrointestinal Endoscopy 1999;49(3):316‐21. [DOI] [PubMed] [Google Scholar]
Sung 1999 {published and unpublished data}
- Sung JJY, Sollano JD, Wai Lai C, Ismael A, Yung MY, Tumala I, et al. Long‐term ciprofloxacin treatment for the prevention of biliary stent blockage: a prospective randomized study. American Journal of Gastroenterology 1999;94(11):3197‐3201. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Davids 1992 {published data only}
- Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self‐expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992;340(8834‐8835):1488‐92. [DOI] [PubMed] [Google Scholar]
Smit 1989 {published data only}
- Smit JM, Out MM, Groen AK, Huibregtse K, Jansen PL, Marle J, et al. A placebo‐controlled study on the efficacy of aspirin and doxycycline in preventing clogging of biliary endoprostheses. Gastrointestinal Endoscopy 1989;35(6):485‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Andersen 1989
- Andersen JR, Sørensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut 1989;30(8):1132‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Davids 1992
- Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self‐expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992;340(8834‐8835):1488‐92. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. British Medical Journal 1994;309:1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(9):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galandi 2001
- Galandi D, Bassler D, Schwarzer G, Allgaier HP. Ursodeoxycholic acid and/or antibiotics for prevention of biliary stent occlusion (Protocol for a Cochrane Review). The Cochrane Library 2001, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groen 1987
- Groen AK, Out T, Huibregtse K, Delzenne B, Hoek FJ, Tytgat GN. Characterization of the content of occluded biliary endoprostheses. Endoscopy 1987;19(2):57‐9. [DOI] [PubMed] [Google Scholar]
Hagenmuller 1983
- Hagenmuller F, Soehendra N. Non‐surgical biliary drainage. Clinics in Gastroenterology 1983;12(1):297‐316. [PubMed] [Google Scholar]
Huibregtse 1986
- Huibregtse K, Katon RM, Coene PP, Tytgat GN. Endoscopic palliative treatment in pancreatic cancer. Gastrointestinal Endoscopy 1986;32(5):334‐8. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Leung 1988
- Leung JW, Ling TK, Kung JL, Vallance‐Owen J. The role of bacteria in the blockage of biliary stents. Gastrointestinal Endoscopy 1988;34(1):19‐22. [DOI] [PubMed] [Google Scholar]
Libby 1996
- Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. American Journal of Gastroenterology 1996;91(7):1301‐8. [PubMed] [Google Scholar]
Moesch 1991
- Moesch C, Sautereau D, Cessot F, Berry P, Mounier M, Gainant A, et al. Physicochemical and bacteriological analysis of the contents of occluded biliary endoprostheses. Hepatology 1991;14(6):1142‐6. [PubMed] [Google Scholar]
Nagakawa 1989
- Nagakawa T, Konishi I, Higashino, Y, Ueno K, Ohta T, Kayahara M, et al. The spread and prognosis of carcinoma in the region of the pancreatic head. Japanese Journal of Surgery 1989;19(5):510‐8. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐2834. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Siegel 1986
- Siegel JH, Snady H. The significance of endoscopically placed prostheses in the management of biliary obstruction due to carcinoma of the pancreas: results of nonoperative decompression in 277 patients. American Journal of Gastroenterology 1986;81(8):634‐41. [PubMed] [Google Scholar]
Smith 1994
- Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet 1994;344(8938):1655‐60. [DOI] [PubMed] [Google Scholar]
Speer 1988
- Speer AG, Cotton PB, Rode J, Seddon AM, Neal CR, Holton J, et al. Biliary stent blockage with bacterial biofilm. A light and electron microscopy study. Annals of Internal Medicine 1988;108(4):546‐53. [DOI] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JAC, Gavaghan DJ, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53:1119‐1129. [DOI] [PubMed] [Google Scholar]


