Abstract
Background
Hormone therapy for early prostate cancer has demonstrated an improvement in clinical and pathological variables, but not always an improvement in overall survival. We performed a systematic review of both adjuvant and neo‐adjuvant hormone therapy combined with surgery or radiotherapy in localised or locally advanced prostate cancer.
Objectives
The objective of this review was to undertake a systematic review and, if possible, a meta‐analysis of neo‐adjuvant and adjuvant hormone therapy in localised or locally advanced prostate cancer.
Search methods
We searched MEDLINE (1966 to 2006), EMBASE, The Cochrane Library, Science Citation Index, LILACS, and SIGLE for relevant randomised trials. Handsearching of appropriate publications was also undertaken.
Selection criteria
Randomised or quasi‐randomised controlled trials of patients with localised or locally advanced prostate cancer, that is, stages T1 to T4, any N, M0, comparing neo‐adjuvant or adjuvant hormonal deprivation in combination with primary therapy (radical radiotherapy or radical prostatectomy) versus primary therapy alone were included in this review.
Data collection and analysis
Data were extracted from eligible studies and assessed for quality, and included information on study design, participants, interventions, and outcomes. Comparable data were pooled together for meta‐analysis with intention‐to treat principle.
Main results
Men with prostate cancer have different clinical outcomes based on their risk (T1 to T2, T3 to T4, PSA levels and Gleason score). However, the majority of studies included in this review did not report results by risk groups; therefore, it was not possible to perform sub‐group analysis.
Neo‐adjuvant hormonal therapy prior to prostatectomy did not improve overall survival (OR 1.11, 95% CI 0.67 to 1.85, P = 0.69). However, there was a significant reduction in the positive surgical margin rate (OR 0.34, 95% CI 0.27 to 0.42, P < 0.00001) and a significant improvement in other pathological variables such as lymph node involvement, pathological staging and organ confined rates. There was a borderline significant reduction of disease recurrence rates (OR 0.74, 95% CI 0.55 to 1.0, P = 0.05), in favour of treatment. The use of longer duration of neo‐adjuvant hormones, that is either 6 or 8 months prior to prostatectomy, was associated with a significant reduction in positive surgical margins (OR 0.56, 95% CI 0.39 to 0.80, P = 0.002).
In one study, neo‐adjuvant hormones prior to radiotherapy significantly improved overall survival for Gleason 2 to 6 patients; although, in two studies, there was no improvement in disease‐specific survival (OR 0.99, 95% CI 0.75 to 1.32, P = 0.97). However, there was a significant improvement in both clinical disease‐free survival (OR 1.86, 95% CI 1.93 to 2.40, P < 0.00001) and biochemical disease‐free survival (OR 1.93, 95% CI 1.45 to 2.56, P < 0.00001).
Adjuvant androgen deprivation following prostatectomy did not significantly improve overall survival at 5 years (OR 1.50, 95% CI 0.79 to 2.85, P = 0.2); although one study reported a significant disease‐specific survival advantage with adjuvant therapy (P = 0.001). In addition, there was a significant improvement in disease‐free survival at both 5 years (OR 3.73, 95%CI 2.30 to 6.03, P < 0.00001) and 10 years (OR 2.06, 95% CI 1.34 to 3.15, P = 0.0009).
Adjuvant therapy following radiotherapy resulted in a significant overall survival gain apparent at 5 (OR 1.46, 95% CI 1.17 to 1.83, P = 0.0009) and 10 years (OR 1.44, 95% CI 1.13 to 1.84, P = 0.003); although there was significant heterogeneity (P = 0.09 and P = 0.07, respectively). There was also a significant improvement in disease‐specific survival (OR 2.10, 95% CI 1.53 to 2.88, P = 0.00001) and disease‐free survival (OR 2.53, 95% CI 2.05 to 3.12, P < 0.00001) at 5 years.
Authors' conclusions
Hormone therapy combined with either prostatectomy or radiotherapy is associated with significant clinical benefits in patients with local or locally advanced prostate cancer. Significant local control may be achieved when given prior to prostatectomy or radiotherapy, which may improve patient's quality of life. When given adjuvant to these primary therapies, hormone therapy, not only provides a method for local control, but there is also evidence for a significant survival advantage. However, hormone therapy is associated with significant side effects, such as hot flushes and gynaecomastia, as well as cost implications. The decision to use hormone therapy should, therefore, be taken at a local level, between the patient, clinician and policy maker, taking into account the clinical benefits, toxicity and cost. More research is needed to guide the choice, the duration, and the schedule of hormonal deprivation therapy, and the impact of long‐term hormone therapy with regard to toxicity and the patient's quality of life.
Plain language summary
Neo‐adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer
The management of early prostate cancer is one of the most controversial areas in the field of cancer medicine with surgery, radiotherapy, primary hormonal therapy (achieved either by medication or by the surgical removal of the testes ‐ orchidectomy) and watchful waiting, all being acceptable forms of initial treatment. Treatment decision making is often based on patient and provider preferences taking into account the risks and benefits of therapies and disease progression. Since prostate cancer is driven, in part by male sex hormones, the use of hormonal treatment to reduce the level of circulating male hormones is a potentially very useful method of treating all stages of this disease. Recently, research on the use of such hormonal therapy in combination with both surgery and radiotherapy has increased. This systematic review combines the results of all the important trials looking at the role of hormones in combination with surgery and radiotherapy for localised and locally advanced prostate cancer.
The results of this review indicate that neo‐adjuvant hormone therapy administered three to six months before the primary curative therapy (radical prostatectomy radical radiotherapy) did not, as yet, result in a detectable improvement in overall survival or disease‐specific survival. There was, however, a significant improvement in disease‐free survival (approximately 90%) when given before radiotherapy. Neo‐adjuvant hormone therapy prior to radical prostatectomy also significantly improved pathological variables associated with poor prognosis, such as the positive surgical margin rate and the proportion of patients with positive lymph nodes. Adjuvant hormone therapy following prostatectomy did not change overall or disease‐specific survival compared to prostatectomy alone. However, adjuvant therapy following radiotherapy significantly improved overall survival and disease‐specific survival up to 10 years post‐treatment. Disease‐free survival was also significantly improved at 10 years. Hormone therapy is associated with a number of side effects including hot flushes and gynaecomastia. The decision to use these agents has to be made after a full discussion between the patient and the physician regarding the disease risk of the patient, the benefits from the use of additional hormones and the side effects of hormonal therapy.
Background
Since the advent of routine PSA screening in the mid 1990s the incidence of early prostate cancer has increased. The management of patients with early stage disease has remained one of the most controversial areas in oncological practice.
The hormone responsive nature of prostate cancer was first demonstrated by Huggins and Hodges in 1941 (Huggins 1941). Since then the rationale for combined modality treatment, such as radiotherapy with hormone therapy or surgery with hormone therapy, has gained prominence, although not yet established in routine clinical practice. For patients undergoing prostatectomy, neo‐adjuvant hormonal treatment (that is, hormone therapy given for various lengths of time prior to definitive therapy) has been shown to cause both clinical as well as pathological downstaging and thereby reduces the positive margin rates (Witjes 1997). This, however, does not translate into an improvement in overall and disease‐free survival (Aus 1997; Baert 1998; Klotz 1999).
The rationale for neo‐adjuvant treatment prior to radical radiotherapy is to shrink the tumour thus enabling optimal doses to be administered to the prostate, whilst reducing surrounding tissue exposure. Another rationale is that it may reduce margin positivity prior to prostatectomy and thereby allow adequate coverage of the cancerous area by surgery. Adjuvant hormones are given so as to eliminate micrometastatic disease and thereby reduce both local recurrence and distant metastasis. Several studies have demonstrated an improvement in clinical and pathologic endpoints, but not always an improvement in overall survival (Hanks 2000; Lawton 1997; Laverdiere 1997; Pilepich 2000). The EORTC 22863, which was a phase 3 trial evaluating the role of long term therapy, ‐ that is, 3 years of adjuvant hormones immediately after radiotherapy ‐ did however, show an improvement in overall survival. These studies have primarily investigated patients with bulky and locally advanced disease, and indeed adjuvant hormonal treatment has become the standard of care for locally advanced disease.
It is, however, unclear whether hormonal treatment combined with surgery or radiotherapy will impact on earlier stage disease, i.e. stage T1 and T2a, and is currently being evaluated in RTOG trial 94‐08. No randomised trials have been performed to look at the role of hormones in patients receiving brachytherapy alone. However, Stone and Stock (Stone 1999) report an improvement in freedom from biochemical relapse with the addition of hormones to brachytherapy for intermediate risk patients (i.e. stage T2b, Gleason grade > 6, and PSA > 10). There is, therefore, a need to evaluate the role of hormone therapy in combination with either radical prostatectomy, external beam radiotherapy, interstitial brachytherapy or cryotherapy for localised or locally advanced prostate cancer.
Objectives
The objective of this review was to conduct a systematic review and, if possible, a meta‐analysis of neo‐adjuvant and adjuvant hormone therapy in localised or locally advanced prostate cancer to assess the effectiveness and adverse effects of these treatments.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials or quasi‐randomised trials reporting on neo‐adjuvant and adjuvant hormone therapy for localised or locally advanced prostate cancer. Randomised controlled trials that compare schedules of neo‐adjuvant or adjuvant hormone therapy will also be eligible for inclusion. Only full peer‐reviewed published articles will be included for analysis.
Types of participants
Men with stage T1 to T4, N1, M0 according to the WHO 1997 TNM classification will be used. Alternative systems of staging such as the Jewett system will also be accepted. Clinically or histologically confirmed local or locally advanced prostate cancer with or without lymph node involvement, but not with bone or visceral metastasis, will be the included in the review. Stage A1 and A2 in the Jewett staging system roughly corresponds to stage T1 in the 1997 TNM staging system. Similarly, stage B corresponds to stage T2, stage C to stage T3 and T4. Stage D1 of the Jewett system corresponds to N1 of the TNM system and stage D2 corresponds to M1 disease in the 1997 TNM system. There was no limitation on age or ethnicity of study participants.
Types of interventions
Primary therapies included either radical prostatectomy, radical radiotherapy, brachytherapy or cryotherapy. Neo‐adjuvant (hormonal manipulation started 3 to 6 months prior to and including the duration of radiotherapy or until the time of radical prostatectomy) prior to the primary treatment of either surgery or radiotherapy, or adjuvant hormone therapies (hormonal manipulation given after completion of radical prostatectomy and for radiotherapy studies, beginning either at the start or at completion of radiotherapy), may consist of combination hormone therapy with LHRH agonists plus anti‐androgens, or single agent hormone deprivation therapies. Hormone therapies of any duration were considered. Only studies of either adjuvant or neo‐adjuvant hormones were included and not those that are looking at both. For the purposes of this review, neo‐adjuvant and adjuvant hormonal therapies were taken to include those that overlap or were concurrent with radiotherapy treatment.
Types of outcome measures
For neo‐adjuvant studies, the primary outcome was overall survival, with secondary outcomes of disease‐specific survival, disease‐free survival, disease recurrence, pT staging, surgical margin status, seminal vesicle invasion rate, the presence of lymph node involvement (radical prostatectomy patients only). Overall survival was defined from the date of randomisation to the date of death, due to whatever cause, or date of last contact. Disease‐specific survival was also defined from the date of randomisation to the date of death, caused by prostate cancer. Disease recurrence was either local, distant metastasis or biochemical, that is, PSA progression. Pathological staging was expressed in a number of ways either as organ‐confined rates, pathological overstaging or downstaging compared to clinical staging.
For adjuvant hormone therapy the primary outcome of interest was overall survival with secondary outcomes of disease‐free survival, disease recurrence and time‐to‐progression. Disease‐free survival, time‐to‐progression or progression‐free survival were determined from the date of randomisation, or from the completion of protocol treatment to relapse either clinical or biochemical. Treatment‐related side effects were assessed in all studies as well as any quality of life measures reported.
For a definition of the outcomes used in this review, refer to the additional Table 1.
1. Definition of outcomes used in this review.
| Outcomes | Definition |
| Time‐to‐progression | A measure of the time after a disease is treated until the disease starts to get worse |
| Progression‐Free Survival | The probability that a patient will remain alive, without the disease getting worse |
| Disease progression | Cancer that continues to grow or spread |
| Overall Survival | Percentage of subjects in a study who have survived for a defined period of time. A.K.A. Survival rate. |
| Recurrence (Disease Recurrence/ Relapse) | Cancer that has returned after a period of time during which the cancer could not be detected. The cancer may come back to the same place as the original (primary) tumour or to another place in the body. |
| Disease‐Free Survival | Length of time after treatment during which no cancer is found |
| Disease‐Specific Survival | The percentage of subjects in a study who have survived a particular disease for a defined period of time. In calculating the percentage, only deaths from the disease being studied are counted |
| Surgical Margins | Surgical removal of a tumor with an additional margin of normal tissue, and the outer edge is coated with ink. |
| Positive (+) Surgical Margins | Cancer cells that are in contact with the inked outer surface (margin) are described as positive. |
| Negative (‐) Surgical Margins | Cancer cells not found at the edge of the inked tissue (Margin) are described as negative |
| Pathological (pT) Staging | The staging of cancer cells determined by tissue sample removed during surgery or a biopsy. |
| Seminal Vesicle Rate | The proportion of patients that have tumour infiltrating the seminal vesicles. |
| Positive Lymph Nodes | Tumour has spread to the lymph nodes ‐ generally the pelvic lymph nodes in prostate cancer. |
Search methods for identification of studies
Our search strategy included an electronic search of MEDLINE from 1966 to February 2006 to identify all relevant published randomised trials of neo‐adjuvant and adjuvant hormone therapy with prostatectomy or radiotherapy for localised or locally advanced prostate cancer.
The search strategy was as follows:
randomized controlled trial.pt.
controlled clinical trial.pt.
exp randomized controlled trials/
exp random allocation/
exp double blind method/
exp single‐blind method/
or/1‐6
(animal not human).sh.
7 not 8
clinical trial.pt.
exp clinical trials/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw.
exp placebos/
placebo$.tw.
random$.tw.
exp research design/
or/10‐17
18 not 8
19 not 9
exp Comparative Study/
exp evaluation studies/
exp follow up studies/
exp prospective studies/
(control$ or prospectiv$ or volunteer$).tw.
or/21‐25
26 not 8
26 not (9 or 20)
9 or 20 or 28
exp Prostatic Neoplasms/
prostatic intraepithelial neoplasia/
(prostat$ adj3 (cancer$ or carcinoma$ or malignan$ or tumo?r$ or neoplas$ or intraepithelial or adeno$)).tw.
or/30‐32
Chemotherapy, Adjuvant/
exp Neoadjuvant Therapy/
(mulitmodal$ or adjuvant or neoadjuvant or adjunct$ or prophylact$).mp.
primary chemotherapy.mp.
(together or plus or concurrent or combin$ or add$ or conjuct$ or without).tw.
exp Antineoplastic Agents, Hormonal/tu [Therapeutic Use]
exp Androgen Antagonists/
antiandrogens.mp.
((androgen$ or hormon$) adj3 (ablat$ or block$ or withdraw$or depriv$ or suppress$)).mp.
gonadotrophin releasing hormone analogue$.mp.
grha.tw.
exp goserelin/
exp Cyproterone/
bicalutamide.mp.
exp Estrogens/
oestrogen.mp.
exp Leuprolide/
(leuprorelin or enatone or a‐43818 or lupron or tap‐144).mp.
exp flutamide/
niftolid$.mp.
zoladex.mp.
eulexin.mp.
casodex.mp.
nilutamide.mp.
nilandrone.mp.
exp diethylstilbestrol/
exp Gonadorelin/
(luteinizing hormone releasing hormone or LHRH).mp.
exp progestins/
megastrol.mp.
exp Finasteride/
proscar.mp.
or/34‐38
or/39‐65
29 and 33 and 66 and 67
Additional electronic searches of the following databases were conducted: EMBASE, The Cochrane Library, Science Citation Index, LILACS, SIGLE, as well as searching for Meeting Abstracts and Trials via the National Library of Medicine Gateway website. Handsearching of recent Proceedings of the American Society of Clinical Oncology 1996 to 2005 were undertaken. In addition, the reference list in each primary reference was scrutinised for additional randomised studies. Other major journals handsearched included Cancer, Journal of Clinical Oncology, Journal of the National Cancer Institute, European Journal of Cancer, Lancet, Lancet Oncology and Cancer Chemotherapy and Pharmacology.
Data collection and analysis
Trial Selection
The electronic literature listed the titles and abstracts of potentially relevant trials reporting on neo‐adjuvant or adjuvant hormone therapy for localised or locally advanced prostate cancer. Two authors searched the list from each search independently, and by consensus, relevant articles were retrieved. Data was extracted from each identified paper independently by two or more reviewers, and included information on trial design, participants, the type of intervention, and outcome measures. Randomised trials that had more than two arms but include one of the previous outlined comparisons were eligible for review.
Assessment of Trial Quality
The assessment of trial quality followed the guidelines described in the Cochrane Handbook for Systematic Review of Interventions. Each study was evaluated for quality using Jadad's five‐point scale (Jadad 1996; Moher 1999). These scores were not used to select studies for inclusion or weight studies for analysis. The five points were assigned by the following criteria:
Randomised trials were to receive one point. Randomisation was considered adequate if a central randomised centre was used, allocation by sealed envelopes, or coded numbers by the pharmacy. Trials that described an appropriate method of randomisation, such as table of random numbers or computer generated randomisation, received an additional point. However, if the report described the trial as randomised and used an inappropriate method, such as the date of birth or hospital numbers, a point was deducted.
Studies reporting that the trial was double‐blind received one point. Those that described the method of double‐blinding, such as identical placebo, active placebo, received an additional point. However, if the report described the trial as double‐blind and it was inappropriate, such as comparison of tablets versus injection with no double dummy, a point was deducted.
Trials reporting the number and reasons for patient dropouts and withdrawals for each group received one point. However, if there was no statement for withdrawals, no point was given.
Other quality issues included the comparability of patients in the treatment and control groups, the employment of appropriate statistical methods and whether an intention‐to‐treat analysis was performed.
Data Synthesis
Data analysis compared neo‐adjuvant therapy plus primary therapy with placebo plus primary therapy or primary therapy alone. The analysis for adjuvant therapy compared primary therapy plus adjuvant therapy with primary therapy plus placebo or primary therapy alone. The data analysis was performed using Review Manager version 4.2.8 supplied by the Cochrane Collaboration. Comparable data from each trial was combined in a meta‐analysis where possible. For dichotomous data the odds ratio was calculated with the 95% confidence interval according to the Peto method. The results were tested for homogeneity at a significance level of P < 0.1 according to the methods outlined by DerSimonian and Laird (DerSimonian 1986). A fixed‐effect model was used if there was no evidence of heterogeneity between studies. Attempts were made to identify possible causes of heterogeneity when observed in the data.
The data derived from different primary therapies were not pooled and were analysed separately. If sufficient data were available, the effect of hormone therapy duration, stage of disease, and the type of hormone intervention on disease outcomes were explored using sensitivity analyses.
Results
Description of studies
A total of 93 studies were identified as potentially suitable for inclusion in the review. However, 71 trials were excluded from the review. Thirty‐seven studies were initial or preliminary reports or updated results while ten were not randomised controlled trials. Fourteen studies did not fit the protocol criteria and there were 12 reviews. A full list of these studies and reasons for exclusion are given in the 'Table of excluded studies'.
A total of 21 studies fulfilled all the inclusion criteria and were the subject of this review, and included a total of 11,149 patients. One study, Lamb 2003, was an assessment of quality of life in the Denham 2005 study. They were divided into the following categories:
1. Studies on neo‐adjuvant hormones and prostatectomy
There were a total of 10 studies that included seven studies of 0 versus 3 months of neo‐adjuvant hormones with a total of 1534 patients (Dalkin 1996; Aus 2002; Klotz 2003; Labrie 1997; Prezioso 2004; Schulman 2000; Soloway 2002). The remaining three studies, Selli 2002, Van Der Kwast 1999, and Gleave 2001, evaluated three versus a longer duration ‐ ie six or eight months in the case of the Gleave 2001 study. Included patients had predominantly localised T1 and T2 disease, low and intermediate grade with Gleason scores up to and including 7, and PSA < 20 in most patients. Thus study participants were predominantly of low and intermediate risk categories by conventional definitions with no study having stringent restrictions on serum PSA or grade of tumour. The mean age ranged from 64 to 67 years.
Three studies reported on overall survival (Klotz 2003; Schulman 2000; Aus 2002) and only two studies analysed disease‐free survival (Klotz 2003; Soloway 2002). All studies analysed positive surgical margins as one of the primary outcomes and some studies assessed other pathological variables such as organ confined rates, lymph node status and seminal vesicle invasion rate. Additionally, some papers gave information on disease recurrence either biochemical (Klotz 2003; Prezioso 2004; Schulman 2000) or clinical recurrence (Prezioso 2004; Schulman 2000). Three studies examined the issue of duration of neo‐adjuvant therapy (Selli 2002; Van Der Kwast 1999) and compared 3 versus 6 months of neo‐adjuvant hormones, whereas Gleave 2001 compared 3 versus 8 months of hormonal therapy. The follow up of these studies were as follows: Schulman 2000 ‐ four years; Klotz 2003 ‐ six years; Aus 2002 ‐ seven years; and Prezioso 2004 ‐ six months.
2. Studies of neo‐adjuvant hormones and radiotherapy
Four studies with a total of 1865 patients were included in this review (Pilepich 2001; Denham 2005; Laverdiere 2004; Crook 2004). All patients had non‐metastatic, localised or locally advanced prostate cancer (T1 to T3). The vast majority of patients included in these studies had T3 or T4 disease, locally advanced with lymph node involvement, or moderate to high Gleason scores of 7 or higher (those patients with intermediate to high‐risk disease by conventional criteria). The mean age ranged from 67 to 72 years and the radiotherapy doses were between 65 and 70 Gy given over 6 to 7 weeks.
Three studies (Pilepich 2001; Denham 2005; Laverdiere 2004) evaluated the addition of neo‐adjuvant hormones prior to radiotherapy. One study, Crook 2004, compared two different durations of hormone therapy prior to radiotherapy.
3. Studies of adjuvant hormones and prostatectomy
There were three studies with 4906 patients in this category (Messing 1999; Wirth 2004a; McLeod 2005). Eligible patients had a mean age ranging from 64 to 66 years, and stages of T3, T4, N0 or N1 disease with any PSA level and grade (Messing 1999; Wirth 2004a). Patients included in the McLeod 2005 Early Prostate Cancer programme with adjuvant bicalutamide were men with clinically or pathologically localised (T1 to T2, N0/Nx) or locally advanced (T3 to T4, any N; or any T, N+) prostate cancer and no distant metastases. All studies analysed overall survival and disease‐free survival. The Messing 1999 study also presented disease‐specific survival, and all studies reported information on adverse events.
4. Studies of adjuvant hormones and radiotherapy
There were four studies with a total of 2844 patients in this category (Bolla 2002; Pilepich 2005; Zagars 1988; Tyrrell 2005). Bolla 2002 was the only study which characterised the subject population and 80% of the study population had T3 disease of intermediate and high grade. The radiation dose used in all the studies was 70 Gy given over 7 weeks. All studies reported overall survival and disease‐free survival as primary outcomes and, additionally Bolla 2002 and Tyrrell 2005 reported information on toxicity.
Adverse Outcomes/Toxicity
Very few studies gave any data on adverse events and toxicity and they will be referred to at the end of the results section.
Risk of bias in included studies
All the studies included in this review were randomised. However, only six studies described the method of randomisation. Three were centrally generated by telephone (Dalkin 1996; Tyrrell 2005; Klotz 2003), one by centralised sealed envelopes (Crook 2004). One was centralised using the minimisation method after patient stratification (Denham 2005), and one utilised the method of Zelen (Pilepich 2005). All but two of the studies adequately described the number and reasons for patients' withdrawals and dropouts (Lamb 2003; Laverdiere 2004). None of the studies reported blinding the allocation of interventions or the assessment of outcomes, apart from the Tyrrell 2005. There were no obvious differences in the characteristics of patients recruited in control groups compared to treatment groups. The quality scores of included studies are summarised in the table 'Characteristics of included studies'.
Effects of interventions
The results are discussed based on primary treatment modalities of either prostatectomy or radiotherapy and whether the hormonal therapy was administered as neo‐adjuvant or adjuvant. There were no relevant studies using brachytherapy or cryotherapy. The studies comparing different durations of hormonal therapy will be discussed separately.
NEO‐ADJUVANT HORMONAL THERAPY IN LOCALISED PROSTATE CANCER
Neo‐adjuvant hormone therapy and prostatectomy
There were 10 studies of neo‐adjuvant hormones prior to prostatectomy, which were heterogeneous for the type of hormonal therapy used and the staging of disease. Three of the 10 studies (Dalkin 1996; Aus 2002; Klotz 2003) investigating neo‐adjuvant hormones and prostatectomy, included single agents either as an anti‐androgen or LHRH analogue. One study, Prezioso 2004, allowed either an LHRH analogue or bilateral orchidectomy, whereas the remaining six studies used combined androgen blockade as the hormonal therapy of choice (Gleave 2001; Labrie 1997; Schulman 2000; Selli 2002; Soloway 2002; Van Der Kwast 1999). The study participants included patients with T1 to T3 disease with or without lymph node involvement and no evidence of distant metastatic disease, but the patients were predominantly those with T1 and T2 disease. All but two studies (which specified PSA levels of < 50 and < 100 ng/mL) had no limits on PSA or grade of tumour.
Studies comparing zero versus three months of hormonal therapy
Hormonal therapy consisted of an anti‐androgen alone, a LHRH analogue alone or a combination of the two. As per study protocol, the outcomes for the neo‐adjuvant studies included the positive surgical margins, the seminal vesicle invasion rate, lymph node positivity, pathological stage and disease‐free‐survival. All analyses were performed on an intention‐to‐treat basis and, in addition, evaluable patient data were analysed to look for differences in results.
Overall Survival
Three studies provided information on overall survival (Klotz 2003; Schulman 2000; Aus 2002). With a 4‐year follow‐up period, Schulman 2000 reported no difference in overall survival rates, with 93% and 95% of patients alive in the treatment and control groups, respectively (P = 0.64). Similarly, Klotz 2003, had a 6‐year follow‐up period and also reported no difference in overall survival (P = 0.38), with 5‐year overall survival rates of 88.4% and 93.9%, respectively, for the neo‐adjuvant hormonal and surgery only arms. The third study, Aus 2002, recruited 126 patients, 63 randomised to both arms and a follow up of 7 years, and again indicated no significant difference in overall survival (P = 0.513). These three studies all show that neo‐adjuvant hormone therapy before prostatectomy does not provide a significant survival advantage over prostatectomy alone (pooled OR 1.11, 95% CI 0.67 to 1.85, P = 0.69, comparison 1: outcome 1).
There were no data available for disease‐specific survival.
Disease‐free survival
Five studies analysed disease‐free survival, defined by either biochemical progression (PSA) or clinical progression (an enlarged prostate or distant metastasis) (Klotz 2003; Prezioso 2004; Schulman 2000; Soloway 2002; Aus 2002). The follow‐up period varied from approximately six months (Prezioso 2004) to seven years (Aus 2002). The study with the longest follow up, Aus 2002, showed no significant difference in PSA progression‐free survival rates (49.8% for the neo‐adjuvant arm and 51.5% for the prostatectomy arm, P = 0.588). The Klotz 2003 study had the second longest follow‐up period with a median of 5.75 years, but showed no difference in 5‐year biochemical free survival between treatment arms (60.2% and 68.2%, respectively, for the hormonal and surgery only arms, P = 0.73). One study, Soloway 2002, recruited patients with clinical stage T2bNXMO, and also reported a 5‐year biochemical‐free survival that was not significantly different between treatment arms (64.8% for the hormonal arm versus 67.6% for the surgery only arm, P = 0.663). A further study, Schulman 2000, evaluated the 4‐year PSA progression rate of patients with T2 to T3,NO,MO disease and showed no difference in disease‐free survival (26% versus 33%, respectively, P = 0.18). The main aim of another study, Prezioso 2004, was to evaluate the effect of neo‐adjuvant hormone therapy on pathological variables and PSA compared to prostatectomy alone, but also reported data on PSA relapse. However, the median follow‐up period was less than 7 months and the data were considered to be immature (90% treatment group versus 84% surgery, no P value given). None of the above studies showed a statistical difference in disease‐free survival between the control and the treatment arms. Data for disease‐free survival at 5 years was extracted from four studies (Klotz 2003; Aus 2002; Schulman 2000; Soloway 2002) and gave a pooled OR of 1.24 (95% CI, 0.97 to 1.57), which did not achieve statistically significance (P = 0.13) (comparison 1: outcome 2).
Pathological outcomes
Treatment with neo‐adjuvant hormones was shown to substantially improve local pathological variables such as organ confined rates (overall OR 2.30, 95% CI 1.72 to 3.08, P < 0.00001, comparison 1: outcomes 3 and 4), pathological down‐staging (overall OR 2.42, 95% CI 1.50 to 3.90, P = 0.0003, comparison 1: outcomes 5 and 6), positive surgical margins (overall OR 0.34, 95% CI 0.27 to 0.42, P< 0.00001, comparison 1: outcomes 7 and 8) and rate of lymph node involvement (overall OR 0.63, 95% CI 0.42 to 0.93, P = 0.02, comparison 1: outcomes 11 and 12). One study, Soloway 2002, reported a decrease in seminal vesicle invasion rate with neo‐adjuvant therapy, whereas one study, Klotz 2003, showed no difference (comparison 1; outcomes 9 and 10).
Different durations of neo‐adjuvant hormonal treatment
Two studies evaluated six versus three months of neo‐adjuvant hormonal therapy before radical prostatectomy (Selli 2002; Van Der Kwast 1999) and one study, Gleave 2001, evaluated three versus eight months of hormonal therapy. When evaluable cases from these three studies with a total of 805 patients were pooled, there was a significant improvement in positive surgical margin rate with the longer duration of therapy (overall OR 0.56, 95% CI 0.39 to 0.80, P = 0.002, comparison 2: outcome 1). Data from the two studies (case analysis only) without significant heterogeneity, with a total of 805 patients were pooled for organ‐confined rates, and shown to have a significant improvement with 6 months of neo‐adjuvant hormonal therapy (overall OR 1.41, 95% CI 1.05 to 1.89, P = 0.02, comparison 2 : outcome 2).
Neo‐adjuvant hormonal therapy and radiotherapy
There were four eligible studies included in this section of the review that investigated the role of neo‐adjuvant hormones before radiotherapy (Pilepich 2001; Denham 2005; Crook 2004; Laverdiere 2004). The Crook 2004 study compared three versus eight months of neo‐adjuvant hormones prior to radiotherapy, whereas Denham 2005 examined zero, three and six months of neo‐adjuvant concurrent hormonal therapy and radiotherapy. The duration of hormonal therapy (all were of approximately three to four months in duration), the radiotherapy techniques used and the definitions of biochemical relapse varied between studies.
The Laverdiere 2004 study included patients with T2 to T3 disease and neo‐adjuvant hormones were given for 3 months prior to radiotherapy. The Pilepich 2001 study included those patients with bulky T2 to T4 disease with or without pelvic lymph node involvement, with hormones being administered for three months before and during radiotherapy. Similarly, the Denham 2005 study evaluated the use of either three months or six months of neo‐adjuvant therapy in a group of patients with T2b, T3, T4 disease without lymph node involvement and no limit on initial PSA levels.
Overall survival
The Pilepich 2001 study provided data on overall survival at both five and eight years. At 8 years, there was no significant improvement of overall survival between neo‐adjuvant and control groups (53% versus 44%, P = 0.10). Sub‐group analysis, however, showed that there was a significant survival benefit for those patients with Gleason 2 to 6 tumours (70% versus 52%, P = 0.015).
Disease‐specific survival
Two studies reported on this endpoint, Denham 2005 and Pilepich 2001. In one study, 3 months of hormonal therapy had no impact on disease‐specific survival at 5 years (HR 0.91, P = 0.91), whereas, 6 months of neo‐adjuvant therapy had a significant effect on disease‐specific survival (HR 0.58, P = 0.04) (Denham 2005). There was a trend of increasing benefit with increasing Gleason scores, PSA levels and high risk groups. Pilepich 2001 reported the 8‐year disease‐specific mortality as being significantly lower in the neo‐adjuvant arm compared to the radiotherapy only arm (23% versus 33%, P = 0.05). Both studies, with a total of 991 patients were available for analysis of 5‐year disease‐specific survival (heterogeneity, P = 0.68). The overall OR of 0.99 (95% CI, 0.75 to 1.32) was not statistically significant (P = 0.97) between the two arms (comparison 3: outcome 1).
Biochemical disease‐free survival
The 3 studies with a total of 1097 patients were eligible for analysis of biochemical disease‐free survival (Denham 2005; Laverdiere 2004; Pilepich 2001). The first study reported an improvement in 5‐year biochemical failure‐free survival for those patients receiving 3 months of androgen deprivation compared to no treatment ( HR 0.70, P = 0.002). Laverdiere 2004 reported the 7‐year biochemical disease‐free survival as 42% for the control arm and 66% for the neo‐adjuvant treatment arm (P = 0.009). Similarly, the third study reported the 8‐year biochemical disease‐free survival (that is, PSA < 1.5) as 24% and 10% for the treatment and control arms, respectively (P < 0.0001) (Pilepich 2001). Pooling these three studies resulted in an overall odds ratio of 1.93 which was highly statistically significant in favour of neo‐adjuvant hormones (P < 0.00001) (comparison 3: outcome 2). Heterogeneity was observed between these studies (P = 0.05) and variations in PSA methodology, subject population and differences in sequencing treatment, may contribute to this, and are described in the discussion.
Clinical disease‐free survival
Two studies reported results for clinical disease‐free survival of neo‐adjuvant hormone therapy prior to radiotherapy (Denham 2005; Pilepich 2001). Denham 2005 reported that for those receiving three months of neo‐adjuvant hormones, there was an improvement of five‐year disease‐free survival (HR 0.65, P = 0.0001) compared to the control arm. The study did not show a significant interaction between treatment effect and prognostic subgroups such as tumour stage, grade and PSA levels; however, there was a trend of increasing treatment effect with increasing Gleason score, PSA levels and high risk. The RTOG trial 86‐10 by Pilepich 2001 also reported a statistically significant improvement in disease‐free survival at 8 years in favour of the neo‐adjuvant arm (33% versus 21%, P = 0.004). Subset analysis in this study showed a preferential benefit in Gleason score 2 to 6 patients in whom there was an improvement in all end‐points including survival. The two studies with a total of 991 patients were available for a pooled analysis (heterogeneity P = 0.39). The overall OR was 1.86 (95% CI, 1.93 to 2.40) and was highly significant in favour of the treatment arm (P < 0.00001) (comparison 3: outcome 3).
Different durations of hormonal therapy prior to radiotherapy
Two studies investigated the duration of neo‐adjuvant therapy prior to radiotherapy (Crook 2004; Denham 2005). The Denham 2005 study evaluated 0, 3 and 6 months of combined androgen blockade in patients with T2b ‐ T4,NO prostate cancer. The Crook 2004 study evaluated 3 versus 6 months of hormonal therapy in patients with clinically localised prostate cancer (T1c ‐ T4) and all levels of PSA.
Overall survival
The Crook 2004 paper reported the actuarial survival rate at 5 years for the 3 and 8 months as 85% and 88%, respectively (P = 0.13). No overall survival data were reported in the Denham 2005.
Prostate cancer‐specific survival
Three months of androgen deprivation before radiotherapy did not reduce the probability of deaths from prostate cancer (Denham 2005). However, 6 months of hormone therapy significantly reduced this probability (HR 0.56, 95% CI, 0.32 to 0.98, P = 0.04).
Disease‐free‐survival The data presented by Crook 2004 indicated that after a follow up of 44 months, there was an improvement of 5‐year disease free survival for the 8 month arm in the high risk group (39% versus 52%), that is, those with stages T3 and T4, Gleason score 8 to 10 and PSA > 20 ng/mL, but did not reach statistical significance. The Denham 2005 study evaluated combined androgen blockade in patients with T2b ‐ T4,NO prostate cancer, and reported that in the 6 month treatment arm there was significant improvement in disease‐free survival (HR 0.56, 05% CI, 0.45 to 0.69, P < 0.00001).
Biochemical disease‐free survival
The same two studies reported biochemical disease‐free survival (Crook 2004; Denham 2005). Crook et al reported the actuarial biochemical disease‐free survival at 3 years as 66% for the 3‐month arm and 68% for the 8‐month arm, and by 5 years was 61% versus 62%, respectively (P = 0.88). Denham et al reported a significant improvement in biochemical disease‐free survival for 6 months of neo‐adjuvant therapy compared to 3 months (HR 0.83, 95% CI, 0.64 to 1.07, P = 0.155). The two studies with a total of 893 patients were available for analysis of 3 and 5‐year biochemical disease‐free survival. Pooling the data at 3 years (heterogeneity P = 0.87) resulted in an overall OR of 1.06, 95% CI 0.81 to 1.39, which was not statistically significant (P = 0.67) (comparison 4: outcome 1). At 5 years (heterogeneity P = 0.72) the overall OR of 0.91, 95% CI 0.67 to 1.24 and again was not statistically significant (P = 0.55) (comparison 4: outcome 2).
ADJUVANT HORMONAL THERAPY IN LOCALISED PROSTATE CANCER
There were a total of six randomised studies reporting adjuvant hormone therapy; three of them combined hormone therapy with prostatectomy (Messing 1999; Wirth 2004a; McLeod 2005) and four with radiotherapy (Bolla 2002; Pilepich 2005; Zagars 1988; Tyrrell 2005). (Note: The McLeod 2005 and Tyrrell 2005 reports were the same study, The Early Prostate Cancer Programme. However, data on adjuvant therapy with prostatectomy were extracted from the McLeod 2005 paper, and data for adjuvant therapy with radiotherapy were extracted from both papers). The prostatectomy studies used different hormonal treatments; Wirth 2004a used an anti‐androgen, whereas Messing 1999 used either an LHRH analogue or bilateral orchidectomy. The largest of the adjuvant studies is the Early Prostate Cancer Programme (McLeod 2005; Tyrrell 2005), which used adjuvant bicalutamide or placebo in addition to standard therapy and included surgery, radiotherapy and watchful waiting. An important difference between trials was that while the Messing 1999 study included T1, T2 and node positive patients, the Wirth 2004a study included pT3 and pT4 (only 2 pT4) node‐negative patients. Similarly in the radiotherapy studies, different drugs were used which also differed in the timing of treatment. Zagars 1988 used adjuvant estrogen beginning at completion of radiotherapy, whereas the other two trials used an LHRH analogue. Bolla 2002 started adjuvant hormones at the beginning of radiotherapy whilst Pilepich 2005 started adjuvant hormones during the last week of radiation therapy.
Adjuvant hormonal therapy and prostatectomy
Overall survival The three randomised studies evaluated overall survival and included a total of 4861 patients (Messing 1999; Wirth 2004a; McLeod 2005). The Messing 1999 study reported a median follow up of 7.1 years and showed that overall survival was better in the immediate hormonal treatment arm compared to the control arm (7 out of 47 deaths compared to 18 of 51 in the observation arm, P = 0.02). On the other hand the Wirth 2004a data showed after a median follow up of 6.1 years no significant improvement in overall survival in the hormonal arm compared to surgery only arm (P = 0.92). The bicalutamide study, McLeod 2005, included 4454 patients who underwent radical prostatectomy, and reported no improvement in overall survival in both the local (HR 1.0, 95% CI 0.80 to 1.26) or the locally advanced (HR 1.09, 95% CI 0.85 to 1.39, P = 0.51) subgroups. This study, however, could not be included in the meta‐analysis, as the number of events for the treatment and control arms was not reported, rather the total number of events was given and the overall results expressed as hazard ratios. The pooled data for five years for the other two studies was not significantly heterogeneous (P = 0.35) yielding an overall OR 1.50 95% CI 0.79 to 2.84 that was not statistically significant, although there was a trend favouring adjuvant hormonal therapy (comparison 5: outcome 1). Similarly, there was no survival advantage at 10 years with adjuvant hormone therapy following prostatectomy (comparison 5: outcome 2).
Disease‐specific survival
Messing 1999 reported that at a median follow up of 7.3 years, there was a statistically significant improvement in disease specific survival in favour of the addition of adjuvant hormones with radiotherapy (P = 0.001).
Disease‐free survival
Three studies with a total of 486 patients were evaluated for disease‐free survival (Messing 1999; Wirth 2004a; McLeod 2005). The Messing 1999 data indicated a significant improvement in disease‐free survival with adjuvant hormone therapy (P < 0.001). The Wirth 2004a study also reported a significant improvement in disease‐free survival in the hormonal arm after median follow up of 7.1 years (P = 0.0041). The Early Prostate Cancer Programme (McLeod 2005) reported an improvement in progression‐free survival after a median of 7.4 years (HR 0.75, 95% CI 0.61 to 0.91, P = 0.004). This result was for a combined group of local and locally advanced patients and was highly influenced by the much better response observed in patients with locally advanced disease. However, this study could not be pooled with the other two because the number of events in the two arms has not been published separately (personal communication with the author to provide these data; awaiting response). Therefore, combining these data for the two remaining studies (heterogeneity P = 0.11) gave an overall OR of 3.73 (95% CI 2.30 to 6.03). The overall effect estimate was highly statistically significant (P < 0.00001) in favour of the hormonal arm (comparison 5: outcome 3). The improvement in disease‐free survival extended to 10 years with a similar high statistical significance (P = 0.0009) (comparison 5: outcome 4). Adjuvant hormone therapy and radiotherapy
Four studies evaluated adjuvant hormones following radiotherapy (Bolla 2002; Pilepich 2005; Zagars 1988; McLeod 2005). The Bolla 2002 study recruited patients with T1 ‐ T2 tumours of WHO histological grade 3 or T3 and T4N0M0 disease and given adjuvant hormones for a period of 3 years. The Pilepich 2005 RTOG 85‐31 trial included patients with lymph node positive disease of all T stages or T3 disease irrespective of lymph node status. Adjuvant hormones were initiated in the last week of radiation therapy and continued indefinitely or until signs of disease progression. The Zagars 1988 study recruited patients with stage C (T3 and T4) disease, although bone scans were not performed to exclude metastatic disease.
Overall survival
Four studies above all evaluated overall survival for patients receiving adjuvant hormones following radiotherapy (Bolla 2002; Pilepich 2005; Zagars 1988; Tyrrell 2005; (McLeod 2005 same study)). The Bolla 2002 study reported a significant improvement in 5‐year overall survival of 78% versus 62% (P = 0.0002) for the combined therapy and radiotherapy arms, respectively. Pilepich 2005 reported the 5 and 9‐year absolute survival rates as 72% and 62% for those receiving adjuvant treatment and 50% and 38% for those not receiving adjuvant therapy, respectively (P = 0.23). The Zagars 1988 study also showed no significant gain in overall survival rates at 5, 10 and 15 years for the adjuvant oestrogen arm (68%, 42% and 25% for the combined arm and 73%, 53% and 33% for the radiotherapy arm alone, P = 0.58). After a median follow up of 7.4 years, the use of adjuvant bicalutamide resulted in a significant improvement in overall survival for the locally advanced patients (HR 0.65, 95% CI 1.13 to 1.84, P = 0.03), but not for the overall population undergoing radiotherapy (McLeod 2005). From these four studies, a total of 2844 patients were included in the pooled analysis and survival data were analysed at 5 and 10 years. At both 5 years (comparison 6: outcome 1) and 10 years (comparison 6: outcome 2), the test for overall effect was significantly in favour of adjuvant hormones (OR 1.29, 95% CI 1.07 to 1.56, P = 0.007 for 5 years and OR 1.44, 95% CI 1.13 to 1.84, P = 0.003 for 10 years). Heterogeneity was P = 0.03 and P = 0.07, respectively.
Disease‐specific survival
Three studies, including a total of 1392 patients, were pooled for disease‐specific survival (Pilepich 2005; Bolla 2002; McLeod 2005). The Bolla 2002 study reported 5‐year disease‐specific survival significantly in favour of the adjuvant hormonal arm (94% versus 79%, P = 0.0001). However, the Pilepich 2005 study did not report a significant benefit of adjuvant hormones in terms of disease‐specific survival between groups (16% and 22% for the adjuvant and control arms, respectively, at 12 years from randomisation) The EPCP reported that there were fewer deaths in those patients receiving bicalutamide (16.1% versus 24.3%) compared to the standard treatment alone, although this was only evident in patients with locally advanced disease (McLeod 2005). No data were extractable for the whole patient population receiving radiotherapy in this study. On pooling the data for the other two studies, the overall treatment effect was significantly in favour of adjuvant therapy (OR 2.10, 95% CI 1.53 to 2.88, P < 0.00001); however, significant heterogeneity was evident (P = 0.01) (comparison 6: outcome 3).
Disease‐free survival
Four studies provided data on disease‐free survival for adjuvant hormones with radiotherapy, and included a total of 1474 patients (Bolla 2002; Pilepich 2005; Zagars 1988; McLeod 2005). One study reported a 5‐year clinical disease‐free survival of 74% in the combined treatment group which was significantly better than the 40% in the RT only group (P = 0.0001) (Bolla 2002). With a median follow up of 6.5 years for all patients and 9.5 years for living patients, Pilepich 2005 reported a significant improvement in disease‐free survival at 5 and 9 years in the adjuvant treatment group compared to RT alone (54% and 10%, respectively versus 33% and 4% P < 0.0001). Additionally, there was a significant improvement in disease‐free survival at 5, 10 and 15 years in favour of the adjuvant oestrogen arm (71%, 63% and 63% versus 49%, 43% and 35% for the radiotherapy arm alone, P = 0.008) (Zagars 1988). The EPCP reporting after a follow up of 7.4 years, showed a statistically significant improvement in progression‐free survival for the bicalutamide group for patients with locally advanced disease (HR 0.56, 95% CI 0.61 to 0.91, P = 0.04) (McLeod 2005). At 5 years the results of four studies with a total of 2844 patients were pooled. The overall OR of 0.91 (95% CI 1.1.6 to 2.23) was significantly in favour of treatment (P < 0.0001) though there was heterogeneity (P < 0.0001). Pooling the 10‐year survival data available for two studies with a total of 1059 patients (Zagars 1988; Pilepich 2005) (heterogeneity P = 0.69) gave an overall OR of 1.96 (95% CI 1.49 to 2.56) and was highly statistically significant (P < 0.00001) in favour of the treatment arm (comparison 6: outcome 4).
Disease recurrence A significant improvement was reported for loco‐regional failure (16.4% versus 1.7%, P < 0.0001) and in the incidence of distant metastasis (29.2% versus 9.8%, P < 0.0001), in favour of adjuvant hormone therapy plus radiotherapy (Bolla 2002). Similarly, biochemical control rates at both 5 and 9 years were significantly better for the combined treatment group (54% and 10% versus 33% and 4%, P < 0.0001) (Pilepich 2005). In addition, in this study the incidence of distant metastasis was statistically better for the adjuvant treatment group (P = 0.026).
Side effects/ toxicity of hormonal treatment
Four studies reporting on neo‐adjuvant therapy with prostatectomy gave information on adverse events, but it was not possible to pool the data as specific numbers of patients in the individual arms of the each study were not available (Klotz 2003; Prezioso 2004; Soloway 2002; Gleave 2001). The Prezioso 2004 study suggested an increase in adverse events in the neo‐adjuvant treatment arm compared to control (17 in the hormonal arm and 5 in the control arm), with the main adverse event being hot flushes. There were two deaths in the hormonal arm, one due to myocardial infarction and the other was of unknown cause. There was no difference in the incidence of deep vein thrombosis between study arms in the Klotz 2003 study (P = 0.263), and one case per arm of pulmonary embolism; the case in the hormonal arm being the only fatality in the entire study. The most common adverse events in the 101 patients receiving the hormonal treatment were asthenia (n = 24), dyspnoea on exertion (n = 16), hot flushes (n = 10) and depression (n = 7). The frequency of adverse events in the 147 patients receiving neo‐adjuvant hormones in the Soloway 2002 study were hot flushes (25%), diarrhoea (14%), nausea with or without vomiting (6%) and abnormalities in liver function tests (5%). There were five withdrawals from the androgen deprivation group due to abdominal pain in two, ECG changes in one, angina in one, and myocardial infarction in one.
The fourth study, comparing 3 versus 8 months of neo‐adjuvant therapy prior to prostatectomy, reported no fatal adverse events (Gleave 2001). In addition, there were no differences between the two patient groups in the severity or the causality of the adverse events (P = 0.287 and P = 0.0564, respectively), or for the incidence of increased liver enzymes or diarrhoea (P = 0.691 and P = 0.288, respectively). However, more men in the 8‐month treatment group reported new adverse events (4.5 versus 2.9, P < 0.0001) and a higher proportion of hot flushes (87% versus 72%, respectively, P < 0.0001).
Three studies on neo‐adjuvant hormone therapy and radiotherapy presented data on adverse events, but again it was not possible to combine these in a meta‐analysis due to the lack of extractable data (Fellows 1992; Pilepich 2001; Lamb 2003). In the Fellows 1992 study, 16% (31 patients) had side effects attributable to hormones with the main adverse event being hot flushes. The Pilepich 2001 study provided hormone compliance information. Ninety‐four per cent of 225 patients completed goserelin, of whom 188 (84%) completed flutamide as well. The reasons for terminating flutamide in 23 patients included diarrhoea in 11 patients, hot flushes in three patients and miscellaneous problems such as liver function abnormalities, rash, and nausea in the remaining patients. The Lamb 2003 study was an acceptability or quality‐of‐life study in patients receiving maximal androgen blockade in the Denham 2005 study of 0, 3 or 6 months of neo‐adjuvant hormones prior to and concurrent with radiotherapy. This study reported that while nearly all patients completed therapy with goserelin, 27% in the 6‐month hormonal arm and 20% in the 3‐month arm had to stop flutamide early. This was due to liver function abnormality (17%) and bowel side effects (18%). In addition, of the 36% of patients who were sexually active prior to any therapy, all became inactive whilst on maximal androgen blockade. However, at 12 months post radiotherapy, sexual function was identical in all three groups
It was not possible to pool data for side effects for the studies of adjuvant hormone therapy and prostatectomy as the individual group data were not available for the Wirth 2004a dataset. Data from the Messing 1999 study suggested a statistically significant increase in grade 1 and 2 haematological, gastrointestinal, and non‐specific, genitourinary side effects (incontinence, frequency and nocturia) and a highly significant increase in hot flushes. The Wirth 2004a study concluded that documented discontinuation occurred twice as frequently in the study arm mainly attributed to adverse events but did not specify exact numbers of patients involved in both groups.
The adjuvant bicalutamide study (Tyrrell 2005; McLeod 2005) reported that the most common adverse events were breast pain (73.6%) and gynaecomastia (68.8%), which were mild to moderate in > 90% of patients. Other adverse events such as impotence, decreased libido, hot flushes and impaired liver function occurred with equally low frequency in both treatment groups. Withdrawal rates due to adverse events were 29.4% with bicalutamide and 10% with standard care alone.
Discussion
The hormone responsive nature of prostate cancer lends itself to effective treatment with agents that reduce the stimulation of the androgen receptor either by blocking the androgen receptor (mechanism of action of anti‐androgens, such as bicalutamide, nilutamide and cyproterone acetate) or by decreasing the levels of circulating male sex hormones (action of the LHRH analogues, such as goserelin and buserelin and orchidectomy). Neo‐adjuvant hormones are given to downstage the disease prior to surgical resection of the prostate and to augment cell kill with radiotherapy, though the biological mechanism is uncertain. Several trials included in this review have evaluated these agents both in the adjuvant and neo‐adjuvant settings and in relation to radiotherapy and prostatectomy in low risk, intermediate risk and high risk disease. The aim of this systematic review and meta‐analysis was to pool the results of all the available evidence to assess the full impact of the use of these agents with primary therapy.
In this review intention‐to‐treat analysis was used to minimise attrition bias, and quality assessment was performed to evaluate internal and external validity of the included studies. However, each of them has its own limitations. Intention‐to‐treat principle could not be followed if the original studies did not report the number of patients randomised to each treatment group. There is no consensus on the method of quality assessment and the extent to which it should be applied to meta‐analyses. It has been shown that the majority of meta‐analyses do not incorporate quality assessment as part of the method (Moher 1999). In the present review, included studies were assessed for quality using published methods. All included trials were considered to be of sufficient quality to be used for the assessment of neo‐adjuvant and adjuvant hormone therapy combined with prostatectomy or radiotherapy. The trials included in this review also enrolled clinically appropriate patients, with the surgical series having a younger median age compared to those treated with radiotherapy. All patients were eligible for definitive therapy, provided they had non‐metastatic prostate cancer as assessed by imaging such as bone scans, CT scans, and in the older trials, by chest X‐rays and lymph‐node mapping. The included studies also reported the primary outcomes of overall and disease‐specific survival, and secondary outcomes of disease‐free survival, both clinical and biochemical. In addition, the neo‐adjuvant surgical series assessed pathological outcomes such as positive surgical margin rates, organ confined rates, pathological downstaging and lymph‐node status.
When considering the results of this systematic review, it is important to note inter‐trial differences that may contribute to the observed heterogeneity of the results in some cases. These relate to variations of the specific hormonal treatments in the different studies, the duration of adjuvant treatment and timing of treatment with some radiotherapy studies using concurrent hormonal therapy and some studies where hormonal therapy and radiotherapy were not administered concurrently. In addition, there are differences in the baseline population demographics in relation to the disease stage and variation in radiotherapy techniques. There were baseline differences in PSA levels across studies with some studies applying strict criteria on the upper limit of PSA and whilst others having no such restrictions. There are also important differences between trials in the definitions of biochemical disease recurrence, that is PSA progression and variations in the TNM staging system used, as discussed in the results section. Some studies also used the Jewett system to stage their patients. These factors may have contributed to the lack of homogeneity of some studies included in the meta‐analysis and will be referred to in the following sections. One widely accepted definition of risk groups is as follows: 'low risk' patients are those with stage T1 to T2a, Gleason score 2 to 6 and PSA < 10; 'intermediate risk' are those with T2b to T3a, Gleason score 7 and PSA 10 to 20; and the 'high risk' group defined as those with > stage T3a, Gleason score > 7 and PSA > 20. These definitions have been used to describe the patient populations in the included studies.
The individual studies of neo‐adjuvant hormones and prostatectomy did not show any gain in overall survival, which was confirmed in the meta‐analysis. However, neo‐adjuvant therapy before prostatectomy led to a significant improvement in positive surgical margin rates, a reduction of positive lymph node involvement, an increase in organ confined rates and pathological downstaging. Although these latter outcomes were of secondary importance to overall survival, the significant benefit achieved with these pathological variables suggests that neo‐adjuvant therapy may be useful in attaining local control in non‐metastatic prostate cancer. However, seminal vesicle invasion rate, another important parameter of disease invasion, was not affected by neo‐adjuvant therapy. Also, there was no significant benefit in disease‐free survival, which may suggest that the pathological variables, such as positive margin status, may be a short‐term indication of the effectiveness of neo‐adjuvant therapy prior to prostatectomy. The discrepancy between disease‐free survival and these pathological variables could be due to a number of factors, such as the difficulty in assessing cell architecture for margin status following hormone therapy, the inability of some trials to detect disease progression due to low statistical power, too short a follow‐up period, or the inclusion of too many 'low risk' group men in some trials (Meng 2002).
There is some evidence to indicate that a longer duration of neo‐adjuvant therapy prior to radical prostatectomy could provide greater surgical downstaging, with one study, Gleave 2001, showing a significant improvement with 8‐month neo‐adjuvant therapy compared to 3 months. The two studies comparing 3 versus 6 months of neo‐adjuvant therapy (Selli 2002; Van Der Kwast 1999) showed a trend towards an improvement in positive surgical margin rates with 6 month therapy. A meta‐analysis of evaluable cases of the three studies (Gleave 2001; Selli 2002; Van Der Kwast 1999) again showed a significant improvement in the positive surgical margin rates in favour of the longer duration treatment (6 or 8 months). The studies examining the effect of different durations of neo‐adjuvant hormone therapy did not report survival data. Only one study, Gleave 2001, reported a significant increase in incidence of both hot flushes and newly reported adverse events for the longer durations of neo‐adjuvant therapy.
The one study reporting neo‐adjuvant hormone therapy with radiotherapy did not find a significant improvement in overall survival for the whole patient population studied (Pilepich 2001). However, the hormone therapy induced a significant survival benefit in patients with Gleason 2 to 6, and suggests that a short duration of neo‐adjuvant hormone treatment should be considered for these patients. There were two other trials that investigated neo‐adjuvant treatment with radiotherapy and reported overall survival data; however, they used orchidectomy as the method of hormone manipulation which implies permanent androgen deprivation and were, therefore, not considered neo‐adjuvant (Fellows 1992; Granfors 1998). Hormone treatment before radiotherapy also significantly improved clinical and biochemical disease‐free survival, although in the latter heterogeneity was observed on pooling, which may possibly relate to differences in hormone sequencing, variations in the patient populations and definitions of biochemical recurrence. (The Denham 2005 study assessed biochemical failure by the Houston method, which is an increase in PSA of at least 2 ng/mL above the post‐treatment nadir measure, defined as the last non‐rising measure. The Laverdiere 2004 study defined PSA failure according to the Vancouver rules, that is two consecutive increases with at least 1 of 1.5 ng/mL or greater. The Pilepich 2001 study reported on biochemical disease free survival as those with PSA < 1.5 and < 4 ng/mL). Disease‐free survival may or may not be a surrogate maker of overall survival. However, the benefit attained with neo‐adjuvant therapy with radiotherapy provides a significant enhancement of loco‐regional control in patients with intermediate to high‐risk disease. Six months of neo‐adjuvant treatment may be more effective in reducing deaths from prostate cancer and providing local control compared to three months of treatment (Denham 2005).
This systematic review showed that for patients undergoing prostatectomy as primary therapy, the addition of adjuvant hormones did not improve overall survival. The pooled data for the meta‐analysis, however, only combined two trials, one of which reported a significant overall survival benefit with adjuvant hormone therapy (Messing 1999). Adjuvant hormone therapy also showed a significant improvement in disease‐specific survival (Messing 1999), and resulted in a significant improvement in disease‐free survival at 5 years, which persisted until 10 years following prostatectomy. The included studies were on patients with intermediate to high‐risk disease and these clinical benefits need to be weighed against treatment costs, adverse events and possible quality‐of‐life differences.
The addition of adjuvant hormones with radiotherapy resulted in a significant improvement in overall survival, disease‐specific survival and disease‐free survival at 10 years. However, due to heterogeneity, caution should be exercised when considering the pooled estimate of the overall and disease‐specific survival data. The heterogeneity in the four studies presenting these data (Bolla 2002; Pilepich 2005; Zagars 1988; Tyrrell 2005) may possibly be due to differences in treatment schedule and method of androgen deprivation. Zagars 1988 used oestrogens initially at doses much higher than currently acceptable and did not exclude bone disease by means of bone scan. Bolla 2002 started total androgen deprivation hormones therapy at the commencement of radiotherapy and continued for 3 years, whereas Pilepich 2005 initiated goserelin hormone therapy in the last week of radiotherapy and continued indefinitely. Three studies included moderate to high‐risk patients, that is T3 and T4, T1 and T2 with grade 3 or lymph node positive patients (Bolla 2002; Pilepich 2005; Zagars 1988). In addition, the majority of patients in the EPCP study (Tyrrell 2005) had localised disease, but the overall survival benefit was restricted to patients with locally advanced disease. Longer follow‐up times may be required to establish a survival advantage in lower risk patients as the number of survival events is fewer and slower in this patient group. One of the limitations of this review is that most of the studies did not prospectively perform sub‐group analysis. This would have enabled analysis of the results by risk groups, thus further allowing physicians and patients to make optimum treatment decisions. Baseline demographic data does, however, provide some information, but this was often insufficient, as the older studies did not stratify patients or give precise estimates on the effectiveness of hormone therapy based on these prognostic subgroups. This review outlined the subgroups for the majority of patient in individual studies for the different categories of treatments (radiotherapy and surgical series and neo‐adjuvant versus adjuvant androgen deprivation), fully recognising that this is an imprecise method as most studies had patients of all risk groups. Describing the studies this way does reveal that the majority of patients in the radiotherapy series were in the intermediate and high‐risk groups and conversely, patient demographics in the surgical series indicate them to be in the early T1 and T2 stages with low and intermediate‐risk grades and PSA scores. It is important that future neo‐adjuvant and adjuvant trials in prostate cancer consider patient stratification and data analysis according to risk so that useful clinical information is not missed.
There is now an increasing body of evidence to suggest that hormonal therapy, particularly in the radiotherapy series, is cost effective. The Radiation Therapy Oncology Group trial, protocol 86‐10, which evaluated the addition of neo‐adjuvant total androgen suppression 2 months before and during radiotherapy, has been subjected to an economic analysis (Konski 2005). The authors used a Markov model, which models ongoing risk, and Monte Carlo simulations to generate survival distributions. The mean cost for radiotherapy alone was $29,240 associated with an effectiveness of 5.48 quality adjusted life years (QALYs). This compared to radiotherapy plus total androgen suppression of $31,286 and 6.43 QALY. The analysis implied that hormone therapy had a > 80% probability that it was cost‐effective. In the adjuvant setting, a cost‐analysis of trial RTOG 92‐02 of the use of 2 years of androgen suppression with radiotherapy after neo‐adjuvant hormonal cytoreduction, treatment was cost effective (90% probability) with gains in QALY (4.13 versus 3.68) (Konski 2006). Similarly the Bolla 2002 study has also been subjected to a cost analysis and reported the use of 3 years of adjuvant goserelin has been shown to improve overall survival at an acceptable cost (Samant 2003). These findings in addition to the wealth of evidence in the metastatic disease setting, suggests that hormonal deprivation therapy used long‐term has potential cost benefits.
Authors' conclusions
Implications for practice.
In patients with localised or locally advanced prostate cancer, the use of neo‐adjuvant and adjuvant hormone therapies combined with both prostatectomy and radiotherapy, are undoubtedly associated with important clinical benefits. There is a survival advantage with adjuvant hormone therapy combined with radiotherapy in moderate to high risk disease, and an indication of an improvement in disease‐specific survival when adjuvant to prostatectomy, and for neo‐adjuvant therapy plus radiotherapy in Gleason 2 to 6 patients. Local control is achieved with all therapeutic options which may lead to an improvement in the patients' quality of life. For example, using neo‐adjuvant hormone therapy with prostatectomy significantly improved pathological variables (positive surgical margin rates, organ confined rates, downstaging and positive pelvic lymph node rates). In addition, disease‐free survival was significantly improved with neo‐adjuvant therapy plus radiotherapy, and with adjuvant therapy combined with either primary treatments of radiotherapy or prostatectomy. However, hormone therapy is associated with common, and at times, significant acute and long‐term complications such as vasomotor symptoms, impotence, and impairment of cognitive function and increase the risk of osteoporosis. Additionally, hormone therapy will incur cost implications. Therefore, the decision to use neo‐adjuvant and adjuvant hormone therapy must be a local one between the patient and clinician (and possibly the policy maker) based on the potential clinical benefits, side effects and cost. The available evidence suggests that the use of long‐term hormonal deprivation is a reasonably cost effective method of treatment with gains in QALY scores.
Implications for research.
The majority of trials reporting survival data for hormone therapy combined with prostatectomy or radiotherapy, and consequently the number of patients recruited, were small and additional studies are required to confirm the current findings. Future studies should be conducted with primary outcomes of overall and disease‐specific survival, disease progression, adverse effects (including long‐term consequences of prolonged androgen deprivation) and quality of life. These studies should ideally stratify report outcomes by age, race and tumour risk groups (PSA, histological grade, tumour stage), as the EPCP study has done.
Additional questions remain about whether to use an LHRH analogue as a single agent or to use combined androgen blockade. In addition, the optimal schedule and duration of treatment continues to be the subject of further trials. Another important area of ongoing clinical research is to identify the role of these agents in the brachytherapy setting and IMRT, as well as its role following post‐surgical adjuvant radiotherapy. This review, for the sake of clarity, has only included studies looking at either adjuvant or neo‐adjuvant therapy, and not where both treatments methods are used in the same patient. Further studies are required to investigate the application of hormone therapy given as both neo‐adjuvant and adjuvant treatments and it's incorporation with chemotherapy in early disease. More information is also needed to evaluate these agents in terms of side effects and quality of life, which are lacking in the majority of studies presented in this review. Further cost analyses should be undertaken, in addition to those already published, so that contemporary data are available.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2009 | Amended | Minor copy editing mistakes fixed. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 29 May 2008 | Amended | Converted to new review format. |
| 8 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
None.
Data and analyses
Comparison 1. Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 827 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.67, 1.85] |
| 2 Disease‐free survival at 5 years (intention‐to‐treat analysis) | 4 | 1129 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.95, 1.52] |
| 3 pT Staging: Organ Confined (Intention‐to‐treat Analysis) | 4 | 922 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.72, 3.08] |
| 4 pT Staging: Organ Confined (Case Analysis) | 4 | 811 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.61 [1.93, 3.53] |
| 5 pT Staging: Downstaging (Intention‐to‐treat Analysis) | 2 | 648 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.50, 3.90] |
| 6 pT Staging: Downstaging (Case Analysis) | 2 | 563 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.61, 4.21] |
| 7 Positive Surgical Margin Status (Intention‐to‐treat Analysis) | 8 | 1802 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.27, 0.42] |
| 8 Positive Surgical Margin Status (Case Analysis) | 8 | 1600 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.26, 0.40] |
| 9 Seminal Vesicle Invasion Rate (Intention‐to‐treat Analysis) | 2 | 516 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.73] |
| 10 Seminal Vesicle Invasion Rate (Case Analysis) | 2 | 467 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.69, 1.71] |
| 11 Positive Lymph Nodes (Intention‐to‐treat Analysis) | 5 | 1247 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.93] |
| 12 Positive Lymph Nodes (Case Analysis) | 5 | 1070 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 1.00] |
1.1. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 1 Overall survival.
1.2. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 2 Disease‐free survival at 5 years (intention‐to‐treat analysis).
1.3. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 3 pT Staging: Organ Confined (Intention‐to‐treat Analysis).
1.4. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 4 pT Staging: Organ Confined (Case Analysis).
1.5. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 5 pT Staging: Downstaging (Intention‐to‐treat Analysis).
1.6. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 6 pT Staging: Downstaging (Case Analysis).
1.7. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 7 Positive Surgical Margin Status (Intention‐to‐treat Analysis).
1.8. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 8 Positive Surgical Margin Status (Case Analysis).
1.9. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 9 Seminal Vesicle Invasion Rate (Intention‐to‐treat Analysis).
1.10. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 10 Seminal Vesicle Invasion Rate (Case Analysis).
1.11. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 11 Positive Lymph Nodes (Intention‐to‐treat Analysis).
1.12. Analysis.

Comparison 1 Neo‐adjuvant hormone therapy and Prostatectomy Versus Prostatectomy alone, Outcome 12 Positive Lymph Nodes (Case Analysis).
Comparison 2. Short‐term versus Long‐term Neo‐adjuvant hormone therapy and prostatectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Positive Surgical Margin Status (Case analysis only) | 3 | 805 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.39, 0.80] |
| 1.1 6 months versus 3 months hormone therapy | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.17] |
| 1.2 8 months versus 3 months hormone therapy | 1 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.77] |
| 2 pT Staging: Organ confined (Case analysis only) | 3 | 805 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.05, 1.89] |
| 2.1 6 months versus 3 months hormone therapy | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.86, 2.18] |
| 2.2 8 months versus 3 months hormone therapy | 1 | 500 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.99, 2.09] |
2.1. Analysis.

Comparison 2 Short‐term versus Long‐term Neo‐adjuvant hormone therapy and prostatectomy, Outcome 1 Positive Surgical Margin Status (Case analysis only).
2.2. Analysis.

Comparison 2 Short‐term versus Long‐term Neo‐adjuvant hormone therapy and prostatectomy, Outcome 2 pT Staging: Organ confined (Case analysis only).
Comparison 3. Neo‐adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐specific survival | 2 | 991 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.75, 1.32] |
| 2 Biochemical disease‐free survival | 3 | 1097 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.45, 2.56] |
| 3 Clinical disease‐free survival | 2 | 991 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.43, 2.40] |
3.1. Analysis.
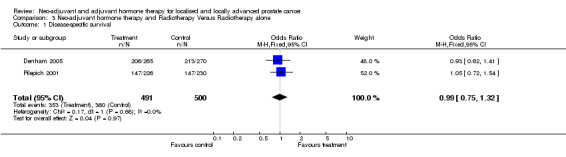
Comparison 3 Neo‐adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 1 Disease‐specific survival.
3.2. Analysis.

Comparison 3 Neo‐adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 2 Biochemical disease‐free survival.
3.3. Analysis.

Comparison 3 Neo‐adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 3 Clinical disease‐free survival.
Comparison 4. Short‐term versus long‐term neo‐adjuvant hormone therapy Radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 3 year biochemical disease‐free survival | 2 | 893 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 1.1 8 verses 3 months of neoadjuvant therapy and radiotherapy | 1 | 361 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| 1.2 6 versus 3 months of neo‐adjuvant therapy and radiotherapy | 1 | 532 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.76, 1.54] |
| 2 5‐year biochemical disease free survival | 2 | 893 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.24] |
| 2.1 8 months verses 3 months of neo‐adjuvant hormonal therapy and Radiotherapy | 1 | 361 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.85] |
| 2.2 6 months versus 3 months of neo‐adjuvant therapy and Radiotherapy | 1 | 532 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.26] |
4.1. Analysis.

Comparison 4 Short‐term versus long‐term neo‐adjuvant hormone therapy Radiotherapy, Outcome 1 3 year biochemical disease‐free survival.
4.2. Analysis.

Comparison 4 Short‐term versus long‐term neo‐adjuvant hormone therapy Radiotherapy, Outcome 2 5‐year biochemical disease free survival.
Comparison 5. Adjuvant hormone therapy and Prostatectomy Versus Prostatectomy Alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival: 5 Years | 2 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.79, 2.84] |
| 2 Overall Survival: 10 Years | 2 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.33, 0.84] |
| 3 Disease‐Free Survival: 5 Years | 2 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.73 [2.30, 6.03] |
| 4 Disease‐Free Survival: 10 Years | 2 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.34, 3.15] |
5.1. Analysis.

Comparison 5 Adjuvant hormone therapy and Prostatectomy Versus Prostatectomy Alone, Outcome 1 Overall Survival: 5 Years.
5.2. Analysis.
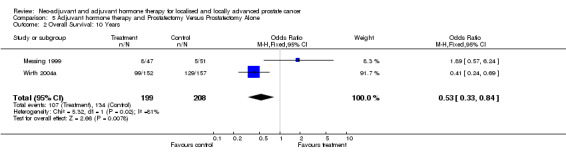
Comparison 5 Adjuvant hormone therapy and Prostatectomy Versus Prostatectomy Alone, Outcome 2 Overall Survival: 10 Years.
5.3. Analysis.

Comparison 5 Adjuvant hormone therapy and Prostatectomy Versus Prostatectomy Alone, Outcome 3 Disease‐Free Survival: 5 Years.
5.4. Analysis.

Comparison 5 Adjuvant hormone therapy and Prostatectomy Versus Prostatectomy Alone, Outcome 4 Disease‐Free Survival: 10 Years.
Comparison 6. Adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival: 5 Years | 4 | 2844 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.07, 1.56] |
| 2 Overall Survival: 10 Years | 2 | 1059 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.13, 1.84] |
| 3 Disease‐Specific Survival: 5 Years | 2 | 1392 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.53, 2.88] |
| 4 Disease‐Free Survival: 10 Years | 2 | 1059 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.49, 2.56] |
6.1. Analysis.

Comparison 6 Adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 1 Overall Survival: 5 Years.
6.2. Analysis.

Comparison 6 Adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 2 Overall Survival: 10 Years.
6.3. Analysis.

Comparison 6 Adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 3 Disease‐Specific Survival: 5 Years.
6.4. Analysis.
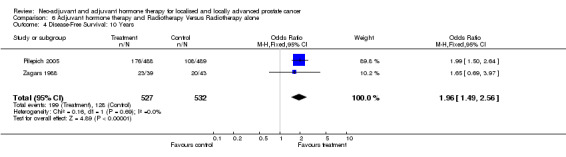
Comparison 6 Adjuvant hormone therapy and Radiotherapy Versus Radiotherapy alone, Outcome 4 Disease‐Free Survival: 10 Years.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aus 2002.
| Methods | Randomised study examining the use of hormone therapy neo‐adjuvant to prostatectomy in the treatment of prostate cancer (stage T1b‐3a, Nx, M0) Quality Score (1/0/1) | |
| Participants | Patients entered: 126
Patients evaluable: 111
Age (Mean): 67
Range: 50 ‐ 77 Eligibility Criteria: · Previously untreated prostate cancer · Histologically confirmed stage T1b‐3a, Nx, M0, G1‐3 · Aged = 75 years · Lifespan > 10 years · Diagnosis by histologically confirmed biopsies or TURP specimens Ineligibility Criteria: · Not Stated Exclusion Criteria: · 12 were excluded because of positive lymph nodes in frozen sections (9 in the control and 3 in the neo‐adjuvant group) · Another 3 patients not operated upon (1 moved abroad, 1 changed his mind in favour of radiotherapy, and 1 was disqualified from surgery owing to a newly detected aortic stenosis). |
|
| Interventions | Radical Prostatectomy alone Vs. 3 mths hormone treatment followed by Prostatectomy
Evaluable: 55 Vs. 56 Hormone Therapy: 3 months of Triptorelin (Decapeptyl Depot) administered as intra‐muscular injections (3.75mg) every fourth week N.B. To prevent tumour flare Cyproterone acetate (Androcur) was given 1 week before and 2 weeks after the first Triptorelin injection. |
|
| Outcomes | i. Histological grading ii. Positive surgical margin status iii. Crude overall survival iv. PSA progression free survival | |
| Notes | The main outcome of this study is surgical margins. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bolla 2002.
| Methods | Randomised study (centralised by the EORTC data centre) examining the use of luteinizing‐hormone releasing hormone (LHRH) adjuvant to Radiotherapy in the treatment of prostate cancer (tumour stages T1‐T4, N+, M0). Quality Score (2/0/1) | |
| Participants | Patients Entered: 415
Patients evaluable: 412
Age (Median): 71
Range:51‐80 Eligibility Criteria: · < 80 years old · T1‐2 prostatic adenocarcinoma of WHO histological grade 3 or T3‐4 prostatic adenocarcinoma of any histological grade · Have newly diagnosed prostate cancer · Given informed consent in writing Ineligibility Criteria: · Patients with a history of previous malignant disease, except for adequately treated basal‐cell carcinoma of the skin, or evidence of distant metastases, including involvement of common iliac or para‐aortic lymph nodes. Exclusion Criteria: · 11 patients were ineligible (7 radiation therapy & 4 combined treatment): ‐ 1 incomplete examination before randomisation ‐ 9 inadequate disease staging or histopathology ‐ 1 poor physical condition |
|
| Interventions | Radiation therapy only Vs. Radiation therapy with an analogue of LHRH
Entered: 208 Vs. 207
Evaluable: 198 Vs. 203 Hormone Therapy: · 3.6 mg Goserelin subcutaneously every 4 weeks administered on 1st day of irradiation and continued for 3 years. · Cyproterone acetate (150 mg orally) give for 1 month starting 1 week before the first Goserelin injection Radiotherapy: · Pelvic Region dose:50 Gy · Prostate dose:70 Gy Patients received a dose of 2 Gy over a period of 7 weeks to the relevant part of the body. Number of fractions: 35 |
|
| Outcomes | i. Disease‐Free Survival ii. Overall Survival iii. Progression‐Free Survival iv. Toxicity/ Side effects | |
| Notes | Patients were analysed on an "intention‐to‐treat analysis". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Crook 2004.
| Methods | Randomised study (centrally generated and sealed envelope method) comparing 3 months of neo‐adjuvant hormone therapy with 8 months before radiotherapy in the treatment of prostate cancer (stage T1c‐T4, M0) Quality Score (2/0/1) | |
| Participants | Patients entered:378
Patients evaluable: 361
Age (Median): 72
Range: 50 ‐ 84 Eligibility Criteria: · Histologic diagnosis of adenocarcinoma of the prostate · All gleason scores and prostate specific antigen (PSA) levels were acceptable · Clinical stages range from T1c‐T4 · Technetium‐99 bone scan and CT scan of the abdomen and pelvis were required for all patients with PSA level > 10 ng/ ml or G5=7 · Normal baseline hepatic and renal function required · Estimated life expectancy > 5 years · Diagnostic transrectal ultrasonography was required at baseline and again at completion of hormonal therapy before the start of radiotherapy · All patients provided written informed consent Ineligibility Criteria: · Not stated Exclusion Criteria: · 6 patients were lost to follow‐up · 11 did not complete the study protocol: ‐ 2 continued with hormonal therapy ‐ 1 chose radical prostatectomy ‐ 5 other malignant diagnosis ‐ 1 patient refused ‐ 2 unknown · 2 patients in the 8‐month arm had a truncated period of hormonal therapy, stopping after 4 months and preceding with radiotherapy and were analysed according to the arm of randomisation. · 16 patients discontinued with antiandrogen because of hepatic or gastrointestinal (GI) intoxicity, but remained in the analysis as per arm of randomisation. |
|
| Interventions | 3 mths hormone therapy and radiotherapy Vs. 8 mths hormone therapy and radiotherapy
Entered: 184 Vs. 184
Evaluable: 177 Vs. 184 Hormone Therapy: Goserelin acetate (Zoladex) was injected subcutaneously every 4 weeks. Flutamide was given (dose 250 mg) 2 weeks before the initial Zoladex injection and continued throughout the course of neo‐adjuvant therapy. Hepatic function was monitored every 2 months during hormonal therapy. Discontinuation of flutamide for reasons of hepatic of Gastrointestinal (GI) toxicity did not constitute a protocol violation. An alternative anti‐androgen could be substituted (nilutamide or bicalutamide) Radiotherapy: · Lymphatic dose:45 ‐ 46 Gy · Prostate dose:66 ‐ 67 Gy Patients received a dose of 1.8 ‐ 2 Gy over a period of 6.5 ‐ 7 weeks to the relevant part of the body. Number of fractions: 35 |
|
| Outcomes | i. Biochemical Failure ii. Disease‐free Survival iii. Histological grading iv. Overall Survival | |
| Notes | · This study compare 3 months of hormone treatment to 8 months treatment · The study provides information on disease free survival, but has two interpretations depending on the form of the ASTRO definition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dalkin 1996.
| Methods | Randomised study examining the use of luteinizing‐hormone releasing hormone (LHRH) neo‐adjuvant to prostatectomy in the treatment of prostate cancer (tumour stages T1C ‐ T2B). Quality Score (1/0/1) | |
| Participants | Patients entered:61
Patients evaluable: 56 Eligibility Criteria: · Patients diagnosed with clinically localised adenocarcinoma of the prostate · > 10 years projected survival · Participant had to have a prostate specific antigen (PSA) level of > 4.0ng/ ml and a clinical stage T1C, T2A or T2B. Ineligibility Criteria: · Not Stated Exclusion Criteria: · 5 patients (2 randomised to receive preoperative LHRH and 3 Surgery only) failed to undergo surgery because: ‐ 1 had a preoperative PSA level < 4.0 ng/ ml ‐ 2 failed preoperative cardiac clearance ‐ 1 refused surgical intervention ‐ 1 surgery aborted midway through the operation because of bleeding |
|
| Interventions | Radical prostatectomy (RP) alone Vs. Luteinizing hormone‐releasiiP
Evaluable: 28 Vs. 28 Hormone Therapy: 12 weeks of preoperative therapy with a Luteinizing hormone‐releasing hormone agonist. |
|
| Outcomes | i. Positive surgical margin status | |
| Notes | The only outcome reviewed was a positive surgical margin's. The author considered a larger sample size, but showed that such a sample needed to produce a slight improvement in pathological outcome would outweigh ant clinical significance in the study of that size. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Denham 2005.
| Methods | Randomised trial examining whether 3 or 6 months of during and before RT improves outcomes for patients with locally advanced prostate cancer. Quality score:2/0/1 | |
| Participants | Patients entered:818 Patients eligible:802 Median age: 67 in the 0 months, 68 in the 3 month group and 68 in the 6 month group , ranges from 51‐80 in the 0 months, 47‐80 in the 3 month group and 41‐87 in the 6 month group. Eligibility criteria: Men without substantial co‐morbidity defined as that of a severity to limit survival to < 5 years or previous malignant disease. Men with stage T2b, T2c, T3 and T4 adenocarcinoma of the prostate according to the 1992 TNM classification without evidence of lymph‐node involvement, bony metastasis or metastasis to other sites who had given written informed consent were eligible. Exclusion criteria: 3 were ineligible in the 0 month arm‐2 with malignant disease and 1 with metastasis. From this group 3 withdrew early‐2 chose alternative treatment and 1 had logistical concerns. In addition 3 patients from the 0 month group were excluded from analysis‐1 received 3 months androgen deprivation, 1 received 6 months androgen deprivation and 1 lost to follow up. From the 3 month group there were 2 ineligible due to previous malignant disease and 3 early withdrawals who chose alternative or no treatment. 2 were excluded as 1 received 6 months androgen deprivation and 1 was lost to follow up. In the 6 month group there was 1 ineligible pateint due to metastatic disease and 4 withdrawals‐3 choosing alternative or no treatment and 1 had logistical concerns. In addition there were 2 other exclusions from analysis‐1 received 3 months of androgen deprivation and 2 lost to follow up. | |
| Interventions | Radiation therapy only verses 3 months of androgen deprivation verses 6 months of androgen deprivation before and during radiotherapy. Patients randomised:Total of 818 patients‐276 allocated the no hormonal therapy arm, 270 allocated to the 3 months hormonal deprivation arm and 272 allocated to the 6 months treatment arm. Patients evaluable:270 in the 0 month arm, 265 in the 3 month arm and 267 in the 6 month arm. Hormonal therapy was with a combination of 3.6 mg of Zoladex every month given sub‐cutaneously and 250mg Flutamide three times a day given orally:one group was allocated 3 months androgen deprivation starting 2 months before radiotherapy and one group was allocated 6 months of androgen deprivation starting 5 months before radiotherapy. Radiation therapy was to the prostate and seminal vesicles at a dose of 66Gy given in 33 fractions of 2 Gy per day for 6.5‐7 weeks. | |
| Outcomes | i. time to local failure ii. prostate cancer specific survival iii. distant failure iv. disease free survival v. freedom from salvage treatment. | |
| Notes | Patients were analysed on an "intention‐to‐treat analysis". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gleave 2001.
| Methods | Randomised study comparing 3 months of neo‐adjuvant hormone therapy with 8 months before radical prostatectomy in the treatment of prostate cancer (stage T1b‐T2) Quality Score (1/0/1) | |
| Participants | Patients entered: 547
Patients evaluable: 500 Eligibility Criteria: · Patients requiring prostatectomy for previously untreated, histologically confirmed clinical stage T1b, T1c, T2 prostate carcinoma who · Gave informed consent Ineligibility Criteria: · Prior treatment with hormonal or radiotherapy · Concomitant use of medication with antiandrogenic activity · Prior history of cancer wit the exception of basal cell carcinoma of the skin, or severe renal or hepatic impairment. Exclusion Criteria: · 44 men withdrew from surgery and therefore non‐evaluable. · Additional 3 patients in the 3‐mth group in whom surgery aborted intra‐operatively because: ‐ metastatic or inoperable disease (2 patients), ‐ Intra‐operative myocardial infarction and no pathology was available (1 patient). |
|
| Interventions | 3 mths hormone therapy and surgery Vs. 8 mths hormone therapy and surgery
Entered: 273 Vs. 274
Evaluable: 253 Vs. 247 Hormone Therapy: Monthly injection of 7.5 mg leuprolide and 250 mg flutamide orally every 8 hours. |
|
| Outcomes | i. Positive surgical margin status ii. PSA Levels | |
| Notes | This is a dose study of neo‐adjuvant treatment. The only outcome that is reviewed which fits our protocol is positive surgical margins. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Klotz 2003.
| Methods | Randomised (centrally generated) study comparing prostatectomy alone to neo‐adjuvant hormone therapy and prostatectomy in the treatment of prostate cancer (stage T1b ‐ T2c) Quality Score (2/0/1) | |
| Participants | Patients entered: 213
Patients evaluable: 192
Age (Mean): 64 Eligibility Criteria: · Histologically confirmed and previously untreated adenocarcinoma of the prostate · Clinically localised to the prostate (stages T1 ‐ T2) · Negative bone scans (with complementary radiography, if indicated) · Enzymatic prostatic acid phosphatase less than twice normal (< 1.8 units per l.) · Prostate specific antigen (PSA) < 50 ng/ ml (normal < 4.0) · Patients provided informed consent Ineligibility Criteria: · Patients had hepatic disease · Renal dysfunction · History of other malignancies within the last 5 years · Received any concomitant medication with antiandrogenic activity or any investigational drug during the 30 days before entry into the study. Exclusion Criteria: · 13 excluded from analysis because they did not undergo prostatectomy · 7 (4 surgery & 3 hormone + surgery) who withdrew consent after randomisation · 2 who withdrew due to adverse effects of hormone treatment · 4 (1 surgery & 3 hormone + surgery) who aborted surgery for unrelated reasons · 5 (5 surgery) aborted surgery because of positive lymph nodes and considered treatment failures (and included in analysis) |
|
| Interventions | Prostatectomy alone Vs. 3 months Neo‐adjuvant hormone therapy and Prostatectomy
Entered: 101 Vs. 112
Evaluable: 91 Vs. 101 Hormone Therapy: 300 mg cyproterone acetate daily for 12 weeks |
|
| Outcomes | i. Adverse effects ii. Biochemical Relapse iii. Disease‐free Survival iv. Positive surgical margin status v. pT staging | |
| Notes | · It is 'Open labels' study, and therefore subjects to bias! · Possibly some discrepancies with the earlier papers about the number of exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Labrie 1997.
| Methods | Randomised study of 3 months neo‐adjuvant hormone therapy before prostatectomy compared with prostatectomy alone in the treatment of prostate cancer (Clinical Stages B0, B1, B2, C1, C2) Quality Score (1/0/1) | |
| Participants | Patients entered: 161
Range: 46 ‐ 72 Eligibility Criteria: · Histologically proven adenocarcinoma of the prostate · = 10 years life expectancy · Discovered as having localised prostate cancer in the first prospective study of screening for prostate cancer performed in a randomly selected population Ineligibility Criteria: · Not stated Exclusion Criteria: · 30 patients declined randomisation. |
|
| Interventions | Neoadjuvant therapy for 3 mths and Prostatectomy Vs. Prostatectomy alone
Evaluable: 90 Vs. 71 Hormone Therapy: Pure antiandrogen flutamide and a Luteinizing hormone‐releasing hormone (LHRH) agonist administered for 3 months before radical prostatectomy. |
|
| Outcomes | i. Positive surgical margin status | |
| Notes | · Poorly defined study characteristics. · Analysis included randomised as well as the non‐randomised patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lamb 2003.
| Methods | Randomised study examining the acceptability of radiotherapy and varying length neo‐adjuvant treatment with radiotherapy in the treatment of prostate cancer. Quality Score (1/0/0) | |
| Participants | Patients entered: 818
Patients evaluable: 800
Age (Median): 68 Eligibility Criteria: · Not Stated Ineligibility Criteria: · Not Stated Exclusion Criteria: · Not Stated |
|
| Interventions | Radiotherapy (RT) Vs. 3 mths hormone therapy + RT Vs. 6 mths hormone therapy + RT Entered: 278 Vs. 270 Vs. 272 Evaluable: 269 Vs. 264 Vs. 267 Hormone Therapy: Combination of LHRH analogue goserelin 3.6 mg subcutaneously once monthly, together with the non‐steroid anti‐androgen flutamide 750 mg daily by mouth. Dose reduction: Investigators followed a protocol of dose reduction in response to toxicity from flutamide. In the event of gastrointestinal side effects unacceptable to the patient, the dose of flutamide was initially reduced from 750 to 500 mg daily. If the gastrointestinal side effects improved on the reduced dosage, patients were maintained on the lower dose for the remainder of the allocated period of MAD. If gastrointestinal side effects persisted, flutamide was discontinued. The protocol also mandated close monitoring of liver enzyme levels. If one or more level rose to above 1.5 times the top of the normal range, dose reduction of flutamide was recommended. If the level(s) continued to rise to above twice the top of the normal range, discontinuation was recommended. Radiotherapy: · Prostate dose: 66 Gy Patients received a dose of Gy over a period of 6.5 weeks to the relevant part of the body. Number of fractions: 33 |
|
| Outcomes | i. Adverse side effects ii. Quality of Life | |
| Notes | Quality of life study ‐ May need to alter data collection form to get further detailed results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laverdiere 2004.
| Methods | Randomised study comparing radiotherapy with 3 month's androgen suppression and radiotherapy in the treatment of prostate cancer (stage T2 ‐ T3). Quality Score (1/0/0) | |
| Participants | Patients entered: 161
Patients evaluable: 148
Age (Mean): 69 Eligibility Criteria: · Diagnosed prostatic adenocarcinoma that was confirmed by histological analysis, and measurable lesions on digital examinations, and transrectal ultrasound · All patients had T2 or T3 clinical cancer. Clinical investigations were based on bone scan, chest x‐ray and abdominal and pelvic computerized tomography. Ineligibility Criteria: · Not stated Exclusion Criteria: · Not stated |
|
| Interventions | Radiotherapy alone Vs. 3 months of androgen suppression plus radiotherapy Evaluable: ‐‐ Vs. ‐‐ Hormone Therapy: Total androgen suppression consisted of Luteinizing hormone‐releasing hormone agonist plus an antiandrogen. Radiotherapy: · Pelvic Region dose:64 Gy Patients received treatment over a period of 6.5 weeks to the relevant part of the body. Number of fractions: 32 |
|
| Outcomes | i. Progression‐free survival | |
| Notes | · We are only interested in study 1, because study 2 considers a combination of neo‐adjuvant, concomitant, and adjuvant treatment. · Poor study as few details are given for each arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McLeod 2005.
| Methods | Casodex Early Prostate Cancer Trialists' Group. Randomised study of adjuvant Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. Quality score: 1/1/1 | |
| Participants | Participants, inclusion and exclusion and endpoints as Tyrrell 2005 study. | |
| Interventions | Overall, the early prostate cancer programme (an amalgamation of 3 trials powered for combined analysis) has enrolled 8113 patients of whom 28% ie 2285 patiets underwent watchful waiting and these patients are not considered in this review as the review is only considering active therapy such as surgery or radiotherapy 1370 patients (from trials 23,24 and 25) who received radiotherapy in the early prostate cancer programme who either received bicalutamide or placebo were analysed. Treatment duration was between 2 and 5 years. 4454 patients who underwent prostatectomy were also analysed. Median follow‐up was 7.4 years. | |
| Outcomes | Primary endpoint was progression free survival and survival. Side effects were evaluated. | |
| Notes | : Patients with localize disease do not appear to derive clinical benefit from added bicalutamide. However adding bicalutamide 150 mg to standard care provides significant clinical benefits in patients with locally advanced prostate cancer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Messing 1999.
| Methods | Randomised study examining the use of immediate androgen therapy compared with observation after radical prostatectomy in the treatment of prostate cancer (stage T2‐T3) Quality Score (1/0/1) | |
| Participants | Patients entered: 100
Patients evaluable: 98
Age (Median): 65.6
Range: 45 ‐ 78 Eligibility Criteria: · Undergone radical prostatectomy and bilateral pelvic lymphadenectomy for clinically localised disease (to more than stage T2), but who were found on review of the histological studies to have nodal metastases · No evidence of metastases on radionuclide bone scans or chest x‐ray films obtained before prostatectomy · No previous hormonal therapy · Gave informed consent Ineligibility Criteria: · None stated Exclusion Criteria: · 1 patient did not undergo prostatectomy · 1 patient did not undergo lymphadenectomy |
|
| Interventions | Radical Prostatectomy and Immediate hormone therapy Vs. Radical Prostatectomy alone
Evaluable: 40 Vs. 38 Hormone Therapy: · Received immediate anti‐androgen therapy, with either goserelin at a dose of 3.6 mg subcutaneously every 28 days or bilaterial orchiectomy, or to be followed until there was signs of progression other than newly detectable or rising levels of serum prostate specific antigen (PSA). · Radical Prostatectomy group received no hormone treatment until the onset of disease progression |
|
| Outcomes | i. Disease‐specific Survival ii. Overall Survival iii. Progression‐Free Survival iv. Side Effects of Treatment | |
| Notes | · An intention‐to‐treat analysis was conducted that included all eligible patients as originally randomised. · NB. 37 men in the observation group received hormonal therapy ‐ 3 received hormonal therapy relatively early ‐ 2 requested and received it immediately after randomisation ‐ 1 received it along with radiotherapy of the prostatic bed on the basis of rising serum PSA level alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pilepich 2001.
| Methods | Randomised study comparing neo‐adjuvant androgen suppression prior to and during radiotherapy with radiotherapy alone in the treatment of prostate cancer (stage T2b ‐ T4). Quality Score (1/0/1) | |
| Participants | Patients entered: 471
Patients evaluable: 456 Eligibility Criteria: · Evidence of histologically confirmed adenocarcinoma of the prostate either confined to the prostate or extending beyond the capsule · No evidence of dissemination beyond the lymph nodes · Tumour size required to be = 25 cm's as measured by the surface area palpable by digital rectal examination · Karnofsky performance score (KPS) = 60 · Patients with positive lymph nodes were eligible if the involved nodes remained below the common iliac level Ineligibility Criteria: · Patients with involved common iliac or peri‐aortic lymph node involvement were not eligible Exclusion Criteria: 15 patients excluded for the following reasons: · 4 ‐ had no follow‐up · 5 ‐ had tumour too small · 3 ‐ refused all treatment and follow‐up · 1 ‐ lung primary · 1 ‐ bone metastasis · 1 ‐ benign disease |
|
| Interventions | Androgen suppression prior to and during Radiotherapy Vs. Radiotherapy alone Entered: 226 Vs. 230 Evaluable: 226 Vs. 230 Hormone Therapy: 3.6 mg of Goserelin acetate (Zoladex) was administered subcutaneously every 4 weeks starting 2 months prior to initiation of radiotherapy. It was continued during radiation therapy for a total of 4 injections. The overall treatment time being 112 days. Flutamide (Eulexin) was administered by mouth (250 mg) 3 times per day over the same period (112 days). Radiotherapy: · Pelvic Region dose: 44 ‐ 46 Gy · Prostate dose: 65 ‐ 70 Gy Patients received treatment over a period of 6.5 weeks to the relevant part of the body. Number of fractions: 33 |
|
| Outcomes | i. Local Failure ii. Distant metastasis iii. Biochemical Disease‐Free Survival iv. Overall Survial v. Cause‐Specific Mortality | |
| Notes | Hormone therapy was given for a period of 16 weeks (approx. 4 months). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pilepich 2005.
| Methods | Randomised study (Zelen Method) examining use of Gorselin adjuvant to radiotherapy in the treatment of prostate cancer (stages T1‐T3) Quality Score (1/0/1) | |
| Participants | Patients Entered: 977
Patients evaluable: 945 Eligibility Criteria: · Histologically confirmed adenocarcinoma of the prostate who either had grossly palpable tumour beyond the confines of the prostate (clinical stage T3) or documented involvement of the regional lymphatics · Patients with primary tumour confined to the prostate (clinical stage T1 or T2) were eligible if there was evidence of spread to the regional lymph nodes (external or internal iliac, common iliac, and/ or paraaortic) either radiographically or histologically. · Patients who have undergone prostatectomy were eligible if there was penetration through the prostatic capsule to the margin of resection and/ or seminal vesicle involvement was histologically documented. · Karnofsky performance status had to be > 60% · Informed written consent. Ineligibility Criteria: · Patients with bulky primary lesions, defined as those with a product of palpable tumour dimension of ³ 25cm. Exceptions were those with evidence of spread to the lymphatics outside the pelvis (common iliac and/ or peri‐aortic lymph nodes) we eligible regardless of size of the primary tumour. Exclusion Criteria: · 32 patients were classified as ineligible. The most common reason was a T2 primary tumour with negative lymph nodes. |
|
| Interventions | Radiotherapy and Goserelin adjuvant treatment Vs. Radiotherapy alone
Entered: 488 Vs. 489
Eligible: 488 Vs. 489
Evaluable: 477 Vs. 468 Hormone Therapy: · Goserelin (Zoladex) was started during the last week of treatment and continued indefinitely or until sign of disease progression. A dose of 3.6 mg of Zoladex was administered subcutaneously in the anterior abdomen wall · None until relapse, where Goserelin was administered as in intervention 1 above. Radiotherapy: · Pelvic Region dose:44 ‐ 46 Gy · Prostate dose:70 Gy Patients received a dose of 2 Gy over a period of 6.5 weeks to the relevant part of the body. Number of fractions: 33 |
|
| Outcomes | i. Disease free survival ii. Metastatic Disease iii. Overall survival | |
| Notes | Hormone treatment was supplied at the time of relapse on the radiotherapy only arm and this seems to be in compassed into the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Prezioso 2004.
| Methods | Randomised study examining the use of neo‐adjuvant hormone treatment prior to prostatectomy in patients with T1a ‐T2b adenocarcinoma of the prostate. Quality Score (1/0/1) | |
| Participants | Patients entered: 183
Patients evaluable: 167
Age (Median): 64.9 in group A
Range: 45 ‐ 78 and 64.5 in group B. Eligibility Criteria: · Undergone radical prostatectomy and bilateral pelvic lymphadenectomy for clinically localised disease (to more than stage T2), but who were found on review of the histological studies to have nodal metastases · No evidence of metastases on radionuclide bone scans or chest x‐ray films obtained before prostatectomy · No previous hormonal therapy · Gave informed consent Ineligibility Criteria: · None stated Exclusion Criteria: · 1 patient did not undergo prostatectomy · 1 patient did not undergo lymphadenectomy |
|
| Interventions | Radical Prostatectomy and Immediate hormone therapy Vs. Radical Prostatectomy alone
Evaluable: 40 Vs. 38 Hormone Therapy: · Received immediate anti‐androgen therapy, with either goserelin at a dose of 3.6 mg subcutaneously every 28 days or bilateral orchiectomy, or to be followed until there was signs of progression other than newly detectable or rising levels of serum prostate specific antigen (PSA). · Radical Prostatectomy group received no hormone treatment until the onset of disease progression |
|
| Outcomes | i. Adverse effects ii. Biochemical Relapse/ Disease Recurrence iii. Pathological Staging iv. Positive surgical margin status v. PSA Levels | |
| Notes | · An intention‐to‐treat analysis was conducted that included all eligible patients as originally randomised. · NB. 37 men in the observation group received hormonal therapy ‐ 3 received hormonal therapy relatively early ‐ 2 requested and received it immediately after randomisation ‐ 1 received it along with radiotherapy of the prostatic bed on the basis of rising serum PSA level alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schulman 2000.
| Methods | Randomised study examining the use of hormone therapy neo‐adjuvant to prostatectomy in the treatment of prostate cancer (tumour stages T2‐3, Nx, M0) Quality Score (1/0/1) | |
| Participants | Patients entered: 487
Patients eligible: 402
Patients evaluable: 398 Eligibility Criteria: · Newly diagnosed T2‐3, Nx, M0 prostate carcinoma confirmed by histopathologic analysis · PSA concentration < 100 ng/ ml · Patients clinically downstaged if after 3 months of neo‐adjuvant treatment, the clinical stage of the tumour was lower than the clinical stage at baseline. · Patients pathologically 'down staged' if the pathological T stage was lower than the clinical T stage at baseline Ineligibility Criteria: · Postsurgical treatment was not allowed inpatients with a postoperative PSA concentration < 1ng/ml at 4 weeks except in patients with pathologically confirmed pT4, pN2 (more than one lymph node involved) or cM + prostatic carcinoma. NB. Patients with a postoperative PSA concentration > 1ng/ ml on two subsequent occasions, and in patients with pT4, pN2 or cM+ tumours, treatment was started at the discretion of the urologist, and the patient was followed for the assessment of time to local recurrence and distant metastases. Exclusion Criteria: · 21 patients excluded because they were not eligible · Hormone Group ‐ 12 excluded because: · 3 had metastatic prostate cancer ‐ 2 had grade T1 prostate cancer ‐ 1 had lymphocytic lymphoma ‐ 1 bladder cancer ‐ 2 surgery postponed (angina) ‐ 1 pleuritis ‐ 1 Cirrhosis ‐ 1 hip fracture. · Prostatectomy group ‐ 9 excluded because: ‐ 5 Metastatic cancer; 3 T1 cancer; ‐ 1 patient PSA >100 ng/ ml · 64 patients because no data available ‐ 30 [17 hormone, 13 Prostatectomy] refused surgery ‐ 34 [24 hormone, 10 Prostatectomy] lost to follow up before surgery could take place · 5 randomised to hormone treatment received a Prostatectomy · 2 randomised to have a prostatectomy received hormone therapy. |
|
| Interventions | Radical Prostactectomy (RP) Vs. Luteinizing hormone‐releasing hormone (LHRH) and RP
Entered: 242 Vs. 245
Evaluable: 209 Vs. 189 Hormone Therapy: Luteinizing hormone‐releasing hormone (LHRH) analogue goserelin (3.6 mg subcutaneously depot injection each month) plus flutamide (250 mg TID) for a period of 3 months. |
|
| Outcomes | i. Disease recurrence ii. Distant Metatstases iii. Pathological Staging iv. Positive surgical margin status v. PSA Progression | |
| Notes | · Study is analysed to treatment actually received · Poor study as few base patient characteristics are provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Selli 2002.
| Methods | Randomised study comparing short‐term (12 weeks) neo‐adjuvant hormone therapy with long‐term (24 weeks) before radical prostatectomy in the treatment of prostate cancer (stage T2‐T3, N0, M0) Quality Score (1/0/1) | |
| Participants | Patients entered: 431
Patients evaluable: 393 Eligibility Criteria: · Not stated in paper Ineligibility Criteria: · Not stated in paper Exclusion Criteria: · 24 did not undergo surgery · 14 did not comply with the protocol and therefore excluded |
|
| Interventions | Short‐term androgen therapy plus surgery Vs. Long‐term androgen therapy plus surgery Evaluable: 128 Vs. 143 Hormone Therapy: Short‐term (12 weeks) or Long‐term (24 weeks) of the following: 'Zoladex' depot 3.5 mg subcutaneously every 28 days plus 'Casodex' 50 mg/ day orally |
|
| Outcomes | i. Disease‐specific Survival ii. Overall Survival iii. Positive surgical margin status iv. PSA progression v. Time to clinical Progression | |
| Notes | · It is 'Open labels' study, and therefore subjects to bias! · Pathological staging of the prostate cancer was defined according to the Whitmore Jewitt system. The protocol for the trial was drawn up when this system was popular among urologists and pathologists, and the TNM system was not considered the international standard. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Soloway 2002.
| Methods | Results update of a randomised study comparing radical prostatectomy alone with 3 months neo‐adjuvant hormone therapy before prostatectomy in the treatment of prostate cancer (Stage T2b) Quality Score (1/0/1) | |
| Participants | Patients entered: 303 Eligibility Criteria: · Patients had not received previous hormonal therapy or chemotherapy · Patients age < 75 years · Serum Prostate Specific Antigen (PSA) < 50 mg/ ml · Normal bone scan Ineligibility Criteria: · Not stated Exclusion Criteria: · 20 patients lost to follow‐up (8 hormone group and 12 prostatectomy only) · Data missing on the following patients: ‐ 6 surgical margins ‐ 7 Seminal vesicle involvement ‐ 11 biopsy grade ‐ 16 surgical specimen gleason score · 11 from the hormone group: ‐ 2 non‐compliant with medication ‐ 2 adverse reaction to medication ‐ 3 refused participation ‐ 1 lost to follow‐up ‐ 3 cardiovascular or pulmonary events precluding surgery · 10 from the Surgery only group: ‐ 7 refused participation ‐ 1 non‐compliant with visits ‐ 2 cardiovascular or pulmonary events precluding surgery Also, 1 patient in the hormone group with positive lymph nodes and 6 patients in the surgery group with lymph node involvement did not have their prostate removed during radical prostatectomy. |
|
| Interventions | Surgery (Radical prostatectomy) only Vs. 3 months hormone therapy plus surgery Entered: 154 Vs. 149 Eligible: 144 Vs. 138 Evaluable: 138 Vs. 137 Hormone Therapy: 3 months of combined androgen blockade with 7.5 mg leuprolide injections monthly and 250 mg flutamide 3 times daily. |
|
| Outcomes | i. Disease‐free survival ii. Positive surgical margin status iii. Seminal vesicle invasion Rate | |
| Notes | The study included patients with more aggressive cancer than that operated on today. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tyrrell 2005.
| Methods | Bicalutamide ('Casodex') 150 mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: results from the randomised Early Prostate Cancer Programme. Quality score: 1/1/1 | |
| Participants | T1‐4 MO any N prostate cancer clinically or pathologically confirmed. Inclusion criteria: Trial 23 only included patients who had undergone surgery or radiotherapy but trials 24 and 25 also included patients who were considered suitable for watchful waiting. Lymph node involvement was accepted in trial 23 but not in the other two. Exclusion criteria: No distant metastasis on bone scan and lymph node involvement in trials 24 and 25. | |
| Interventions | T1‐4 MO any N prostate cancer clinically or pathologically confirmed. Inclusion criteria: Trial 23 only included patients who had undergone surgery or radiotherapy but trials 24 and 25 also included patients who were considered suitable for watchful waiting. Lymph node involvement was accepted in trial 23 but not in the other two. Exclusion criteria: No distant metastasis on bone scan and lymph node involvement in trials 24 and 25. Methods: Overall, the early prostate cancer programme (an amalgamation of 3 trials powered for combined analysis) has enrolled 8113 patients of whom 28% ie 2285 patients underwent watchful waiting and these patients are not considered in this review as the review is only considering active therapy such as surgery or radiotherapy. 1370 patients (from trials 23,24 and 25) who received radiotherapy in the early prostate cancer programme who either received bicalutamide or placebo were analysed. Treatment duration was between 2 and 5 years. Median follow up was 5.3 years. | |
| Outcomes | Primary endpoint was progression free survival and survival. Side effects were evaluated. | |
| Notes | Bicalutamide 150 mg/day given as adjuvant to radiotherapy significantly improved progression‐free survival in patients with locally advanced prostate cancer. Hormonal therapy does not appear to be appropriate for localized disease, from the results so far. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Van Der Kwast 1999.
| Methods | Randomised study comparing 3 months of neo‐adjuvant hormone therapy with 6 months before radical prostatectomy in the treatment of prostate cancer (stage T1‐T3) Quality Score (1/0/1) | |
| Participants | Patients entered: 47
Patients evaluable: 40 Eligibility Criteria: · Not Stated Ineligibility Criteria: · Not Stated Exclusion Criteria: · 5 patients withdrew from the study before radical prostatectomy · 1 patient had surgery in another institution · 1 patinet was excluded from the study because of prior transurethral resection |
|
| Interventions | 3 mths hormone therapy plus prostatectomy Vs. 6 mths hormone therapy plus prostatectomy Evaluable: 18 Vs. 22 Hormone Therapy: Patients received either daily injections of LHRH agonist or monthly injections of Lupron. In addition, patients were given oral flutamide (Eulaxin, 250 mg 3 time daily). |
|
| Outcomes | i. Positive surgical margin status ii. Seminal vesicle invasion Rate | |
| Notes | Poor study as no details provided on patient eligibility. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wirth 2004a.
| Methods | Randomised study examining the use of adjuvant flutamide after radical surgery compared with surgery alone in the treatment of prostate cancer (stage T3‐T4, N0) Quality Score (1/0/1) | |
| Participants | Patients entered: 352
Patients evaluable: 309 Eligibility Criteria: · < 75 years old · have locally advanced, lymph node‐negative prostate cancer (pT3‐4pN0) Ineligibility Criteria: · Secondary Primary Cancer Exclusion Criteria: · 43 patients were excluded for the following reasons: 10 ‐ No study entry documents available 5 ‐ Tumour stage less than pT3 1 ‐ Secondary primary cancer 27 ‐ No or inadequately documented treatment |
|
| Interventions | Surgery and Adjuvant Flutamide Vs. Surgery Alone
Evaluable: 152 Vs. 157 Hormone Therapy: Patients were randomised to receive flutamide 250 mg three time daily (total 750 mg a day) indefinitely. |
|
| Outcomes | i. Overall Survival ii. Progression‐Free Survival | |
| Notes | · 12% of the initially enrolled patients were excluded. · Incompleteness of the toxicity and treatment withdrawal data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zagars 1988.
| Methods | Randomised study examining the use of Adjuvant estrogen (Diethylstilbestrol) with radiotherapy in the treatment of prostate cancer (clinical stage C) Quality Score (1/0/1) | |
| Participants | Patients entered: 82
Patients evaluable: 78
Age (Mean/Median): 64
Range: 51‐73 Eligibility Criteria: · No Prior treatment for prostatic carcinoma except where transurethral resection of the prostate was done Ineligibility Criteria: · Not stated Exclusion Criteria: · 3 patients from radiation therapy arm: ‐ 1 did not have carcinoma on slide review ‐ 1 refused to complete radiotherapy ‐ 1 lost to follow‐up · 1 patient from radiation and estrogen arm: ‐ 1 unequivocal bone metastases demonstrated on his pre‐treatment x rays and bone scan |
|
| Interventions | Radiation Therapy Vs. Radiation Therapy and adjuvant estrogen treatment Entered: 43 Vs. 39 Evaluable: 40 Vs. 38 Hormone Therapy: Hormone therapy consisted of Diethylstilbestrol (DES) in a daily dose of 5 gm, which began immediately after completion of radiotherapy, and was continued indefinitely. However, after concerns from other studies over the use of DES, this was reduced to 2 mg DES daily. · 38 patients were randomised to receive estrogen, but 4 actually never received treatment. Of the remaining 34 patients: ‐ 20 patients had 5mg DES daily ‐ 12 patients had 2mg DES daily ‐ 2 patients had chlorotrianisene (Trace) 12 mg daily · For relapse (27 patients) in the radiotherapy arm, 26 patients received hormonal treatment. Radiotherapy Therapy: · Pelvic Region dose:50 Gy · Prostate dose:70 Gy Patients received a dose of 2 Gy over a period of 7 weeks to the relevant part of the body. Number of fractions: 35 |
|
| Outcomes | i. Disease‐free survival ii. Overall Survival | |
| Notes | · Badly designed study providing little data on the base characteristics of patients or with the basic details of the study. · Hormone treatment is given on relapse in intervention 1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlgren 1999 | Earlier study of Ahlgren 2000a |
| Ahlgren 2000a | Analysis of samples from earlier trial |
| Ahlgren 2000b | Earlier Study of Ahlgren 2000a |
| Akakura 1999 | Not a randomised controlled trial |
| Akaza 2003 | Does not fit our protocol ‐ Evaluates hormonal treatment as primary therapy |
| Akaza 2004 | Review |
| Anderson 1997 | Not a randomised controlled trial |
| Andriole 1998 | Does not fit our protocol ‐ Evaluates PSA and Finasteride in the detection of prostate cancer |
| Armstrong 2004 | Preliminary results of trial |
| Asbell 1996 | Does not fit our protocol ‐ Documents anaemia in prostate cancer treated patients |
| Aus 1994 | Not a randomised controlled trial |
| Bolla 1997 | Earlier study of Bolla 2002 |
| Bolla 1999 | Review of RTOG trials |
| Bonney 1998 | Review |
| Bono 2001 | Earlier study of Selli 2002 |
| Bullock 2002 | Earlier study of Klotz 2003 |
| Chang 2000 | Update of Cookson 1997 |
| Cookson 1997 | Not a randomised Study |
| D'Amico 2004 | Examines hormone treatment before, during, and after primary treatment when compared to primary treatment alone |
| De Leval 2002 | Does not fit our protocol ‐ Evaluates continuous versus intermittent hormone therapy for patients with prostate cancer |
| Debruyne 1994 | Preliminary results of trial |
| Debruyne 2000 | Earlier study of Schulman 2000 |
| Fellows 1992 | This study utilised neo‐adjuvant orchidectomy as the method of hormone manipulation. This implies that androgen deprivation was permanent and therefore could not be included in the neo‐adjuvant comparisons. |
| Galalae 2004 | Not a randomised controlled trial |
| Goldenberg 1996 | Earlier study of Klotz 2003 |
| Granfors 1998 | This study utilised neo‐adjuvant orchidectomy as the method of hormone manipulation. This implies that androgen deprivation was permanent and therefore could not be included in the neo‐adjuvant comparisons. |
| Hanks 2003 | Does not fit our protocol ‐ Compares neo‐adjuvant and concurrent hormone treatment with neo‐adjuvant, concurrent and adjuvant treatment after radiotherapy. |
| Homma 1999 | Earlier study of Homma 2004 |
| Homma 2004 | Does not fit our protocol ‐ Evaluates hormonal therapy before and after surgery with surgery followed by hormonal treatment |
| Horwitz 2001 | Review of RTOG trials |
| Hugosson 1996 | Earlier report of the Aus 2002 study |
| Isaka 1994 | Does not fit our protocol ‐ Evaluates neo‐adjuvant hormonal therapy with different primary treatments in prostate cancer patients |
| Iversen 2002 | Unable to separate results into individual primary treatments |
| Iversen 2004a | Not able to separate results |
| Iversen 2004b | Review |
| Jani 2003 | Review |
| Klotz 1999 | Earlier study of Klotz 2003 |
| Klotz 2000 | Earlier study of Klotz 2003 |
| Labrie 1993a | Review |
| Labrie 1993b | Earlier study of Labrie 1997 |
| Labrie 1994 | Earlier study of Labrie 1997 |
| Labrie 1995 | Earlier study of Labrie 1997 |
| Lawton 1997 | Earlier study of Lawton 2005 |
| Lawton 2001 | Earlier study of Lawton 2005 |
| Lawton 2005 | Subset analysis of Pilepich 2005 |
| Lee 2002 | Not a randomised controlled trial |
| Matveev 1993 | Comparison of various treatment methods that does not fit our protocol |
| Mazzucchelli 2001 | Not a randomised controlled trial |
| Montironi 1999 | Earlier study of Selli 2002 |
| Pilepich 1989 | Earlier study of Pilepich 1995a |
| Pilepich 1993 | Preliminary results of Pilepich 2001 study |
| Pilepich 1995a | Does not fit our protocol ‐ RTOG 83‐07: Comparison of efficacy and toxicity of different hormone neo‐adjuvant to radiotherapy |
| Pilepich 1995b | Earlier study of Pilepich 2001 |
| Pilepich 1997 | Earlier study of Pilepich 2005 |
| Prezioso 1999 | Earlier study of Mazzucchelli 2002 |
| Rabbani 1998a | Analysis based on Goldenberg 1996 study |
| Rabbani 1998b | Review |
| Schmidt 1993 | Earlier study of Schmidt 1996b |
| Schmidt 1996a | Earlier study of Schmidt 1996b |
| Schmidt 1996b | Examines adjuvant chemotherapy treatment an not hormone treatment as specified in our protocol. |
| See 2002 | Earlier study of Wirth 2004b |
| See 2003 | Earlier report of Tyrell 2005 and McLeod 2005 |
| Shipley 2002 | Earlier study of Pilepich 1993 |
| Solomon 1993 | Not a randomised controlled trial |
| Soloway 1995 | Earlier study of Soloway 2002 |
| Vaillancourt 1996 | Review of results given by Labrie 1993 |
| Van Poppel 1992 | Not a randomised controlled trial |
| Van Poppel 1995 | Interim results of trial |
| Van Poppel 2001 | Review |
| Wirth 2004b | Pooled study paper where individual study paper was unavailable |
| Witjes 1997 | Earlier study of Schulman 2000 |
| Yamanaka 2005 | Does not fit our protocol ‐ Evaluates continuous versus intermittent hormone therapy after radiotherapy |
| Zagars 1999 | Not a randomised controlled trial |
| Zurlo 2002 | Review |
Contributions of authors
Satish Kumar‐Concept, literature search, screen databases for selection of papers, data extraction, data analysis, quality assessment, primary manuscript preparation.
Mike Shelley‐Concept, literature search, screen databases for selection of papers, data extraction, analysis, manuscript preparation.
Craig Harrison‐literature search, screen databases for selection of papers, data extraction, data analysis, quality assessment and manuscript review.
Timothy J Wilt‐review data analysis, review manuscript.
Malcolm D Mason‐provide clinical data and review data analysis and manuscript.
Sources of support
Internal sources
Velindre NHS Trust, Cardiff, UK.
Minneapolis VA Centre for Chronic Disease Outcomes Research, USA.
Cardiff University, Cardiff, Wales,, UK.
External sources
National Collaborating Centre For Cancer, Cardiff, UK.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Aus 2002 {published data only}
- Hugosson J, Abrahamsson PA, Ahlgren G, Aus G, Lundberg S, Schelin S, Schain M, Pedersen K. The risk of malignancy in the surgical margin at radical prostatectomy reduced almost three‐fold in patients given neo‐adjuvant hormone treatment. European Urology 1996;29(4):413‐9. [DOI] [PubMed] [Google Scholar]
Bolla 2002 {published data only}
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Mattelaer J, Lopez Torecilla J, Pfeffer JR, Lino Cutajar C, Zurlo A, Pierart M. Long‐term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360(9327):103‐6. [DOI] [PubMed] [Google Scholar]
Crook 2004 {published data only}
- Crook J, Ludgate C, Malone S, Lim J, Perry G, Eapen L, Bowen J, Robertson S, Lockwood G. Report of a multicenter Canadian phase III randomized trial of 3 months vs. 8 months neoadjuvant androgen deprivation before standard‐dose radiotherapy for clinically localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2004;60(1):15‐23. [DOI] [PubMed] [Google Scholar]
Dalkin 1996 {published data only}
- Dalkin BL, Ahmann FR, Nagle R, Johnson CS. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. Journal of Urology 1996;155(4):1357‐60. [PubMed] [Google Scholar]
Denham 2005 {published data only}
- Denham JW, Steigler A, Lamb DS, Mameghan H, Turner S, Matthews J, Franklin I, Atkinson C, North J, Paulsen M, Christie D, Spry NA, Tai K‐H, Wynne C, Duchesne G, Kovacev O, D'Este C. Short‐term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans‐Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncology 2005;6:841‐50. [DOI] [PubMed] [Google Scholar]
Gleave 2001 {published data only}
- Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, Jewett M, Kassabian V, Chetner M, Dupont C, Rensselaer S. Randomized comparative study of 3 versus 8‐month neoadjuvant hormonal therapy before radical prostatectomy: Biochemical and pathological effects. Journal of Urology 2001;166(2):500‐6. [PubMed] [Google Scholar]
Klotz 2003 {published data only}
- Klotz LH, Goldenberg SL, Jewett MAS, Fradet Y, Nam R, Barkin J, Chin J, Chatterjee S. Long‐term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. Journal of Urology 2003;170(3):791‐4. [DOI] [PubMed] [Google Scholar]
Labrie 1997 {published data only}
- Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay M, Tetu B, Fradet Y, Belanger A, Candas B. Neoadjuvant hormonal therapy: The Canadian experience. Urology 1997;49(3 Suppl):56‐64. [DOI] [PubMed] [Google Scholar]
Lamb 2003 {published data only}
- Lamb DS, Denham JW, Mameghan H, Joseph D, Turner S, Matthews J, Franklin I, Atkinson C, North J, Poulsen M, Kovacev O, Robertson R, Francis L, Christie D, Spry NA, Tai KH, Wynne C, Duchesne G. Acceptability of short term neo‐adjuvant androgen deprivation in patients with locally advanced prostate cancer. Radiotherapy and Oncology 2003;68(3):255‐67. [DOI] [PubMed] [Google Scholar]
Laverdiere 2004 {published data only}
- Laverdiere J, Nabid A, Bedoya LD, Ebacher A, Fortin A, Wang CS, Harel F. The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2‐T3 prostate cancer. Journal of Urology 2004;171(3):1137‐40. [DOI] [PubMed] [Google Scholar]
McLeod 2005 {published data only}
- McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP. Bicalutamide 150mg plus standard care vs standard care alone for early prostate cancer. British Journal Urology International 2005;97:247‐54. [DOI] [PubMed] [Google Scholar]
Messing 1999 {published data only}
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node‐positive prostate cancer.[see comment]. New England Journal of Medicine 1999;341(24):1781‐8. [DOI] [PubMed] [Google Scholar]
Pilepich 2001 {published data only}
- Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, Lawton C, Machtay M, Grignon D. Phase III radiation therapy oncology group (RTOG) trial 86‐10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 2001;50(5):1243‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pilepich 2005 {published data only}
- Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma ‐ Long‐term results of phase III RTOG 85‐31. International Journal of Radiation Oncology, Biology, Physics 2005;61(5):1285‐90. [0360‐3016] [DOI] [PubMed] [Google Scholar]
Prezioso 2004 {published data only}
- Prezioso D, Lotti T, Polito M, Montironi R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and cyproterone acetate, before radical prostatectomy: A randomized study. Urologia Internationalis 2004;72(3):189‐95. [DOI] [PubMed] [Google Scholar]
Schulman 2000 {published data only}
- Schulman CC, Debruyne FMJ, Forster G, Selvaggi FP, Zlotta AR, Witjes WPJ. 4‐Year follow‐up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2‐3N0M0 prostate cancer. European Urology 2000;38(6):706‐13. [DOI] [PubMed] [Google Scholar]
Selli 2002 {published data only}
- Selli C, Montironi R, Bono A, Pagano F, Zattoni F, Manganelli A, Selvaggi FP, Comeri G, Fiaccavento G, Guazzieri S, Lembo A, Cosciani‐Cunico S, Potenzoni D, Muto G, Mazzucchelli R, Santinelli A. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. Journal of Clinical Pathology 2002;55(7):508‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soloway 2002 {published data only}
- Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP, Jr, Puras‐Baez A, Lupron Depot Neoadjuvant Prostate Cancer Study G. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5‐year results. Journal of Urology 2002;167(1):112‐6. [PubMed] [Google Scholar]
Tyrrell 2005 {published data only}
- Tyrrel CJ, Payne H, See W, McLeod DG, Wirth M, Iversen P, Armstrong J, Morris C, on behalf of the 'Casodex' early Prostate Trialist Group. Bicalutamide ('Casodex') 150mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: Results from the randomised Early Prostate Cancer Programme. Radiotherapy and Oncology 2005;76:4‐10. [DOI] [PubMed] [Google Scholar]
Van Der Kwast 1999 {published data only}
- Kwast TH, Tetu B, Candas B, Gomez JL, Cusan L, Labrie F. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: Three versus six months of endocrine therapy. Urology 1999;53(3):523‐9. [DOI] [PubMed] [Google Scholar]
Wirth 2004a {published data only}
- Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, Riedmiller H, Noack B, Hinke A, Froehner M. Prospective Randomized Trial Comparing Flutamide as Adjuvant Treatment versus Observation after Radical Prostatectomy for Locally Advanced, Lymph Node‐Negative Prostate Cancer. European Urology 2004;45(3):267‐70. [DOI] [PubMed] [Google Scholar]
Zagars 1988 {published data only}
- Zagars GK, Johnson DE, Eschenbach AC, Hussey DH. Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: Long‐term results of a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 1988;14(6):1085‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahlgren 1999 {published data only}
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson PA. Tumor cell proliferation in prostate cancer after 3 months of neoadjuvant LHRH analogue treatment is a prognostic marker of recurrence after radical prostatectomy. Urology 1999;54(2):329‐34. [DOI] [PubMed] [Google Scholar]
Ahlgren 2000a {published data only}
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson P. Neuroendocrine differentiation is not prognostic of failure after radical prostatectomy but correlates with tumor volume. Urology 2000;56(6):1011‐5. [DOI] [PubMed] [Google Scholar]
Ahlgren 2000b {published data only}
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson PA. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate 2000;42(4):274‐9. [DOI] [PubMed] [Google Scholar]
Akakura 1999 {published data only}
- Akakura K, Isaka S, Akimoto S, Ito H, Okada K, Hachiya T, Yoshida O, Arai Y, Usami M, Kotake T, Tobisu K, Ohashi Y, Sumiyoshi Y, Kakizoe T, Shimazaki J. Long‐term results of a randomized trial for the treatment of Stages B2 and C prostate cancer: radical prostatectomy versus external beam radiation therapy with a common endocrine therapy in both modalities. Urology 1999;54(2):313‐8. [DOI] [PubMed] [Google Scholar]
Akaza 2003 {published data only}
- Akaza H, Homma Y, Okada K, Yokoyama M, Usami M, Hirao Y, Tsushima T, Ohashi Y, Aso Y. A prospective and randomized study of primary hormonal therapy for patients with localized or locally advanced prostate cancer unsuitable for radical prostatectomy: results of the 5‐year follow‐up. BJU International 2003;91(1):33‐6. [1464‐4096] [DOI] [PubMed] [Google Scholar]
Akaza 2004 {published data only}
- Akaza H, Yamaguchi A, Matsuda T, Igawa M, Kumon H, Soeda A, Arai Y, Usami M, Naito S, Kanetake H, Ohashi Y. Superior anti‐tumor efficacy of bicalutamide 80 mg in combination with a luteinizing hormone‐releasing hormone (LHRH) agonist versus LHRH agonist monotherapy as first‐line treatment for advanced prostate cancer: interim results of a randomized study in Japanese patients. Japanese Journal of Clinical Oncology 2004;34(1):20‐8. [DOI] [PubMed] [Google Scholar]
Anderson 1997 {published data only}
- Anderson PR, Hanlon AL, Movsas B, Hanks GE. Prostate cancer patient subsets showing improved bNED control with adjuvant androgen deprivation. International Journal of Radiation Oncology, Biology, Physics 1997;39(5):1025‐30. [DOI] [PubMed] [Google Scholar]
Andriole 1998 {published data only}
- Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, Hudson P, Jackson CL, Romas NA, Patterson L, Cook TJ, Waldstreicher J. Treatment with finasteride preserves usefulness of prostate‐specific antigen in the detection of prostate cancer: results of a randomized, double‐blind, placebo‐controlled clinical trial. PLESS Study Group. Proscar Long‐term Efficacy and Safety Study. Urology 1998;52(2):195‐201. [DOI] [PubMed] [Google Scholar]
Armstrong 2004 {published data only}
- Armstrong J, Taylor J, Thirion P, Henry P, Hollywood D, O' Neill L, Cosgrove S, Horan C, Stevenson M, Butler M. Preliminary results of a randomised trial comparing short vs. protracted neoadjuvant hormonal therapy (NHT) prior to radiation therapy (RT) of localized prostate cancer. (Irish Clinical Oncology Research Group Protocol # 97‐01). Radiotherapy and Oncology 2004;73:S78. [Google Scholar]
Asbell 1996 {published data only}
- Asbell SO, Leon SA, Tester WJ, Brereton HD, Ago CT, Rotman M. Development of anemia and recovery in prostate cancer patients treated with combined androgen blockade and radiotherapy. Prostate 1996;29(4):243‐8. [DOI] [PubMed] [Google Scholar]
Aus 1994 {published data only}
- Aus G, Brandstedt S, Haggman M, Pedersen K, Torre M, Hugosson J. Effects of 3 months' neoadjuvant hormonal treatment with a GnRH analogue (triptorelin) prior to radical retropubic prostatectomy on prostate‐specific antigen and tumour volume in prostate cancer. European Urology 1994;26(1):22‐8. [0302‐2838] [DOI] [PubMed] [Google Scholar]
Bolla 1997 {published data only}
- Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. New England Journal of Medicine 1997;337(5):295‐300. [DOI] [PubMed] [Google Scholar]
Bolla 1999 {published data only}
- Bolla M. Adjuvant hormonal treatment with radiotherapy for locally advanced prostate cancer. European Urology 1999;35 Suppl 1:23‐5; discussion 26. [MEDLINE: ] [PubMed] [Google Scholar]
Bonney 1998 {published data only}
- Bonney WW, Schned AR, Timberlake DS. Neoadjuvant androgen ablation for localized prostatic cancer: Pathology methods, surgical end points and meta‐analysis of randomized trials. Journal of Urology 1998;160(5):1754‐60. [DOI] [PubMed] [Google Scholar]
Bono 2001 {published data only}
- Bono AV, Pagano F, Montironi R, Zattoni F, Manganelli A, Selvaggi FP, Comeri G, Fiaccavento G, Guazzieri S, Selli C, Lembo A, Cosciani‐Cunico S, Potenzoni D, Muto G, Diamanti L, Santinelli A, Mazzucchelli R, Prayer‐Galletti T. Effect of complete androgen blockade on pathologic stage and resection margin status of prostate cancer: Progress pathology report of the Italian PROSIT study. Urology 2001;57(1):117‐21. [DOI] [PubMed] [Google Scholar]
Bullock 2002 {published data only}
- Bullock MJ, Srigley JR, Klotz LH, Larry Goldenberg S. Pathologic effects of neoadjuvant cyproterone acetate on nonneoplastic prostate, prostatic intraepithelial neoplasia, and adenocarcinoma: A detailed analysis of radical prostatectomy specimens from a randomized trial. American Journal of Surgical Pathology 2002;26(11):1400‐13. [DOI] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- Chang SS, Reuter VE, Heston WDW, Hutchinson B, Grauer LS, Gaudin PB. Short term neoadjuvant androgen deprivation therapy does not affect prostate specific membrane antigen expression in prostate tissues. Cancer 2000;88(2):407‐15. [PubMed] [Google Scholar]
Cookson 1997 {published data only}
- Cookson MS, Sogani PC, Russo P, Sheinfeld J, Herr H, Dalbagni G, Reuter VE, Begg CB, Fair WR. Pathological staging and biochemical recurrence after neoadjuvant androgen deprivation therapy in combination with radical prostatectomy in clinically localized prostate cancer: results of a phase II study. British Journal of Urology 1997;79(3):432‐8. [0007‐1331] [DOI] [PubMed] [Google Scholar]
D'Amico 2004 {published data only}
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6‐Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. Journal of the American Medical Association 2004;292(7):821‐7. [DOI] [PubMed] [Google Scholar]
De Leval 2002 {published data only}
- Leval J, Boca P, Yousef E, Nicolas H, Jeukenne M, Seidel L, Bouffioux C, Coppens L, Bonnet P, Andrianne R, Wlatregny D. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone‐naive prostate cancer: results of a prospective randomized multicenter trial. Clinical Prostate Cancer 2002;1(3):163‐71. [DOI] [PubMed] [Google Scholar]
Debruyne 1994 {published data only}
- Debruyne FMJ, Witjes WPJ, Schulman CC, Cangh PJ, Oosterhof GON. A multicentue trial of combined neoadjuvant androgen blockade with Zoladex and flutamide prior to radical prostatectomyin prostate cancer. European Urology 1994;26(Suppl 1):4. [DOI] [PubMed] [Google Scholar]
Debruyne 2000 {published data only}
- Debruyne FMJ, Witjes WPJ, Zietman A, Bruchovsky N, Klotz L, Fair W. Neoadjuvant hormonal therapy prior to radical prostatectomy: The European experience. Molecular Urology 2000;4(3):251‐7. [PubMed] [Google Scholar]
Fellows 1992 {published data only}
- Fellows GJ, Clark PB, Beynon LL, Boreham J, Keen C, Parkinson MC, Peto R, Webb JN. Treatment of advanced localised prostatic cancer by orchiectomy, radiotherapy, or combined treatment. A Medical Research Council Study. Urological Cancer Working Party‐‐Subgroup on Prostatic Cancer. British Journal of Urology 1992;70(3):304‐9. [DOI] [PubMed] [Google Scholar]
Galalae 2004 {published data only}
- Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N, Eulau S, Gustafson G, Gribble M, Kovacs G. Long‐term outcome by risk factors using conformal high‐dose‐rate brachytherapy (HDR‐BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2004;58(4):1048‐55. [0360‐3016] [DOI] [PubMed] [Google Scholar]
Goldenberg 1996 {published data only}
- Goldenberg SL, Klotz LH, Srigley J, Jewett MA, Mador D, Fradet Y, Barkin J, Chin J, Paquin JM, Bullock MJ, Laplante S. Randomized, prospective, controlled study comparing radical prostatectomy alone and neoadjuvant androgen withdrawal in the treatment of localized prostate cancer. Canadian Urologic Oncology Group. [see comment]. Journal of Urology 1996;156(3):873‐7. [PubMed] [Google Scholar]
Granfors 1998 {published data only}
- Granfors T, Modig H, Damber JE, Tomic R. Combined orchiectomy and external radiotherapy versus radiotherapy alone for nonmetastatic prostate cancer with or without pelvic lymph node involvement: a prospective randomized study. Journal of Urology 1998;159(6):2030‐4. [DOI] [PubMed] [Google Scholar]
Hanks 2003 {published data only}
- Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA, Sandler HM, Shipley WU. Phase III trial of long‐term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92‐02. Journal of Clinical Oncology 2003;21(21):3972‐8. [DOI] [PubMed] [Google Scholar]
Homma 1999 {published data only}
- Homma Y, Akaza H, Okada K, Yokoyama M, Moriyama N, Usami M, Hirao Y, Tsushima T, Sakamoto A, Ohashi Y, Aso Y. Early results of radical prostatectomy and adjuvant endocrine therapy for prostate cancer with or without preoperative androgen deprivation. The Prostate Cancer Study Group. International Journal of Urology 1999;6(5):229‐37; discussion 238‐9. [DOI] [PubMed] [Google Scholar]
Homma 2004 {published data only}
- Homma Y, Akaza H, Okada K, Yokoyama M, Usami M, Hirao Y, Tsushima T, Sakamoto A, Ohashi Y, Aso Y, Prostate Cancer Study G. Endocrine therapy with or without radical prostatectomy for T1b‐T3N0M0 prostate cancer. International Journal of Urology 2004;11(4):218‐24. [0919‐8172] [DOI] [PubMed] [Google Scholar]
Horwitz 2001 {published data only}
- Horwitz EM, Winter K, Hanks GE, Lawton CA, Russell AH, Machtay M. Subset analysis of RTOG 85‐31 and 86‐10 indicates an advantage for long‐term vs. short‐term adjuvant hormones for patients with locally advanced nonmetastatic prostate cancer treated with radiation therapy. International Journal of Radiation Oncology, Biology, Physics 2001;49(4):947‐56. [DOI] [PubMed] [Google Scholar]
Hugosson 1996 {published data only}
- Hugosson J, Abrahamsson PA, Ahlgren G, Aus G, Lundberg S, Schelin S, Schain M, Pedersen K. The risk of malignancy in the surgical margin at radical prostatectomy reduced almost three‐fold in patients given neo‐adjuvant hormone treatment. European Urology 1996;29:413‐9. [DOI] [PubMed] [Google Scholar]
Isaka 1994 {published data only}
- Isaka S, Shimazaki J, Akimoto S, Okada K, Yoshida O, Arai Y, Usami M, Kotake T, Tobisu K, Kakizoe T, et al. A prospective randomized trial for treating stages B2 and C prostate cancer: radical surgery or irradiation with neoadjuvant endocrine therapy. Japanese Journal of Clinical Oncology 1994;24(4):218‐23. [PubMed] [Google Scholar]
Iversen 2002 {published data only}
- Iversen P, Tammela TLJ, Vaage S, Lukkarinen O, Lodding P, Bull‐Njaa T, Viitanen J, Hoisaeter P, Lundmo P, Rasmussen F, Johansson JE, Persson BE, Carroll K. A randomised comparison of bicalutamide ('Casodex') 150 mg versus placebo as immediate therapy either alone or as adjuvant to standard care for early non‐metastatic prostate cancer first report from the Scandinavian Prostatic Cancer Group Study no. 6. European Urology 2002;42(3):204‐11. [DOI] [PubMed] [Google Scholar]
Iversen 2004a {published data only}
- Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, Tammela TLJ, Tasdemir I, Morris T, Carroll K. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3‐Year median followup from the Scandinavian Prostate Cancer Group Study Number 6. Journal of Urology 2004;172(5 pt 1):1871‐6. [DOI] [PubMed] [Google Scholar]
Iversen 2004b {published data only}
- Iversen P, Wirth MP, See WA, McLeod DG, Klimberg I, Gleason D, Chodak G, Montie J, Tyrrell C, Wallace DM, Delaere KP, Lundmo P, Tammela TL, Johansson JE, Morris T, Carroll K. Is the efficacy of hormonal therapy affected by lymph node status? data from the bicalutamide (Casodex) Early Prostate Cancer program. Urology 2004;63(5):928‐33. [1527‐9995] [DOI] [PubMed] [Google Scholar]
Jani 2003 {published data only}
- Jani AB, Kao J, Hellman S. Hormone therapy adjuvant to external beam radiotherapy for locally advanced prostate carcinoma: a complication‐adjusted number‐needed‐to‐treat analysis. Cancer 2003;98(11):2351‐61. [DOI] [PubMed] [Google Scholar]
Klotz 1999 {published data only}
- Klotz LH, Goldenberg SL, Jewett M, et al. CUOG randomised trial of neo‐adjuvant androgen ablation before radical prostatectomy: 36 month post treatment PSA results. Urology 1999;53:757. [DOI] [PubMed] [Google Scholar]
Klotz 2000 {published data only}
- Klotz L, Gleave M, Goldenberg SL, Garnick M, Moul J, Sylvester J, Stone N. Neoadjuvant hormone therapy: The Canadian trials. Molecular Urology 2000;4(3):233‐7. [PubMed] [Google Scholar]
Labrie 1993a {published data only}
- Labrie F, Dupont A, Cusan L, Gomez J, Diamond P, Koutsilieris M, Suburu R, Fradet Y, Lemay M, Tetu B, Emond J, Candas B. Downstaging of localized prostate cancer by neoadjuvant therapy with flutamide and lupron: The first controlled and randomized trial. Clinical and Investigative Medicine. Medecine Clinique et Experimentale 1993;16(6):499‐509. [PubMed] [Google Scholar]
Labrie 1993b {published data only}
- Labrie F, Belanger A, Simard J, Labrie C, Dupont A. Combination therapy for prostate cancer. Endocrine and biologic basis of its choice as new standard first‐line therapy. Cancer 1993;71(3 Suppl):1059‐67. [0008‐543X] [DOI] [PubMed] [Google Scholar]
Labrie 1994 {published data only}
- Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay M, Tetu B, Fradet Y, Candas B. Down‐staging of early stage prostate cancer before radical prostatectomy: The first randomized trial of neoadjuvant combination therapy with flutamide and a luteinizing hormone‐releasing hormone agonist. Urology 1994;44(6 Suppl):29‐37. [Google Scholar]
Labrie 1995 {published data only}
- Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay MT, tu B, Fradet Y, Candas B. Downstaging by combination therapy with flutamide and an LHRH agonist before radical prostatectomy. Cancer Surveys 1995;23:149‐56. [MEDLINE: ] [PubMed] [Google Scholar]
Lawton 1997 {published data only}
- Lawton CA, Winter K, Byhardt R, Sause WT, Hanks GE, Russell AH, Rotman M, Porter A, McGowan DG, DelRowe JD, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with D1 (pN+) adenocarcinoma of the prostate (results based on a national prospective randomized trial, RTOG 85‐31). International Journal of Radiation Oncology, Biology, Physics 1997;38(5):931‐9. [DOI] [PubMed] [Google Scholar]
Lawton 2001 {published data only}
- Lawton CA, Winter K, Murray K, Machtay M, Mesic JB, Hanks GE, Coughlin CT, Pilepich MV. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85‐31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. International Journal of Radiation, Oncology, Biology, Physics 2001;49(4):937‐46. [DOI] [PubMed] [Google Scholar]
Lawton 2005 {published data only}
- Lawton CA, Winter K, Grignon D, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node‐positive adenocarcinoma of the prostate: updated results based on national prospective randomized trial Radiation Therapy Oncology Group 85‐31. Journal of Clinical Oncology 2005;23(4):800‐7. [0732‐183X] [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee LN, Stock RG, Stone NN. Role of hormonal therapy in the management of intermediate‐ to high‐risk prostate cancer treated with permanent radioactive seed implantation. International Journal of Radiation Oncology, Biology, Physics 2002;52(2):444‐52. [DOI] [PubMed] [Google Scholar]
Matveev 1993 {published data only}
- Matveev BP, Liazaliev KT. The combined treatment of patients with locally disseminated prostatic cancer. Urologiia i Nefrologiia 1993;4:21‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Mazzucchelli 2001 {published data only}
- Mazzucchelli R, Montironi R, Prezioso D, Bono AV, Ferrari P, Manganelli A, Morelli P, Vito ML, Santinelli A, Diamanti L, Lotti T, Polito M, Takeda Italian TAPSG. Surgical pathology examination of radical prostatectomy specimens. Updated protocol based on the Italian TAP study. Anticancer Research 2001;21(5):3599‐607. [PubMed] [Google Scholar]
Montironi 1999 {published data only}
- Montironi R, Diamanti L, Santinelli A, Galetti‐Prayer T, Zattoni F, Selvaggi FP, Pagano F, Bono AV. Effect of total androgen ablation on pathologic stage and resection limit status of prostate cancer. Initial results of the Italian PROSIT study. Pathology, Research and Practice 1999;195(4):201‐8. [DOI] [PubMed] [Google Scholar]
Pilepich 1989 {published data only}
- Pilepich MV, Krall JM, John MJ, Rubin P, Porter AT, Marcial VA, Martz KL. Hormonal cytoreduction in locally advanced carcinoma of the prostate treated with definitive radiotherapy: Preliminary results of RTOG 83‐07. International Journal of Radiation Oncology, Biology, Physics 1989;16(3):813‐7. [DOI] [PubMed] [Google Scholar]
Pilepich 1993 {published data only}
- Pilepich MV. Phase III trial of hormone cytoreduction in conjunction with definative radiotherapy in loacally advanced prostate carcinoma: the emerging role of PSA in the assessment of outcome. abstract no: 193. International Journal of Radiation Oncology, Biology, Physics 1993;27(S1):246. [Google Scholar]
Pilepich 1995a {published data only}
- Pilepich MV, Buzydlowski JW, John MJ, Rubin P, McGowan DG, Marcial VA. Phase II trial of hormonal cytoreduction with megestrol and diethylstilbestrol in conjunction with radiotherapy for carcinoma of the prostate: Outcome results of RTOG 83‐07. International Journal of Radiation Oncology, Biology, Physics 1995;32(1):175‐80. [DOI] [PubMed] [Google Scholar]
Pilepich 1995b {published data only}
- Pilepich MV, Krall JM, al‐Sarraf M, John MJ, Doggett RL, Sause WT, Lawton CA, Abrams RA, Rotman M, Rubin P, et al. Androgen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomized comparative trial of the Radiation Therapy Oncology Group. Urology 1995;45(4):616‐23. [DOI] [PubMed] [Google Scholar]
Pilepich 1997 {published data only}
- Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavourable prognosis carcinoma of the prostate treated with definitive radiotherapy: report of RTOG protocol 85 ‐31. Journal of Clinical Oncology 1997;15:1013‐21. [DOI] [PubMed] [Google Scholar]
Prezioso 1999 {published data only}
- Prezioso D, Lotti T, Montironi R, Polito M. Role of neoadjuvant treatment in clinically confined prostate cancer. Takeda NHT Italian Group. European Urology 1999;35(Suppl 1):17‐21; discussion 22. [DOI] [PubMed] [Google Scholar]
Rabbani 1998a {published data only}
- Rabbani F, Goldenberg SL, Klotz LH. Predictors of pathological stage before neoadjuvant androgen withdrawal therapy and radical prostatectomy. The Canadian Urologic Oncology Group. [see comment]. Journal of Urology 1998;159(3):925‐8. [PubMed] [Google Scholar]
Rabbani 1998b {published data only}
- Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false‐negative sextant prostate biopsies. Journal of Urology 1998;159(4):1247‐50. [PubMed] [Google Scholar]
Schmidt 1993 {published data only}
- Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Adjuvant therapy for localized prostate cancer. Cancer 1993;71(3 Suppl):1005‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmidt 1996a {published data only}
- Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Adjuvant therapy for clinical localized prostate cancer treated with surgery or irradiation. European Urology 1996;29(4):425‐33. [DOI] [PubMed] [Google Scholar]
Schmidt 1996b {published data only}
- Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Evaluation of adjuvant estramustine phosphate, cyclophosphamide, and observation only for node‐positive patients following radical prostatectomy and definitive irradiation. Investigators of the National Prostate Cancer Project. Prostate 1996;28(1):51‐7. [DOI] [PubMed] [Google Scholar]
See 2002 {published data only}
- See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, Chodak G, Montie J, Tyrrell C, Wallace DMA, Delaere KPJ, Vaage S, Tammela TLJ, Lukkarinen O, Persson BE, Carroll K, Kolvenbag G. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: First analysis of the early prostate cancer program. Journal of Urology 2002;168(2):429‐35. [PubMed] [Google Scholar]
See 2003 {published data only}
- See W, Iveresen P, Wirth M, McLeod D, Garside L, Morris T. Immediate treatment with bicalutamide 150mg as adjuvant therapy significantly reduces the risk of PSA progression in early prostate cancer. European Urology 2003;44:512‐8. [DOI] [PubMed] [Google Scholar]
Shipley 2002 {published data only}
- Shipley WU, Lu JD, Pilepich MV, Heydon K, Roach IM, Wolkov HB, Sause WT, Rubin P, Lawton CA, Machtay M. Effect of a short course of neoadjuvant hormonal therapy on the response to subsequent androgen suppression in prostate cancer patients with relapse after radiotherapy: A secondary analysis of the randomized protocol RTOG 86‐10. International Journal of Radiation Oncology, Biology, Physics 2002;54(5):1302‐10. [DOI] [PubMed] [Google Scholar]
Solomon 1993 {published data only}
- Solomon MH, McHugh TA, Dorr RP, Lee F, Siders DB. Hormone ablation therapy as neoadjuvant treatment to radical prostatectomy. Clinical and Investigative Medicine. Medecine Clinique et Experimentale 1993;16(6):532‐8. [PubMed] [Google Scholar]
Soloway 1995 {published data only}
- Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood Jr DP, Puras‐Baez A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. Journal of Urology 1995;154(2 I):424‐428. [PubMed] [Google Scholar]
Vaillancourt 1996 {published data only}
- Vaillancourt L, Tetu B, Fradet Y, Dupont A, Gomez J, Cusan L, Suburu ER, Diamond P, Candas B, Labrie F. Effect of neoadjuvant endocrine therapy (combined androgen blockade) on normal prostate and prostatic carcinoma: A randomized study. American Journal of Surgical Pathology 1996;20(1):86‐93. [DOI] [PubMed] [Google Scholar]
Van Poppel 1992 {published data only}
- Poppel H, Ameye F, Oyen R, Voorde W, Baert L. Neo‐adjuvant hormonotherapy does not facilitate radical prostatectomy. Acta Urologica Belgica 1992;60(3):73‐82. [PubMed] [Google Scholar]
Van Poppel 1995 {published data only}
- Poppel H, Ridder D, Elgamal AA, Voorde W, Werbrouck P, Ackaert K, Oyen R, Pittomvils G, Baert L. Neoadjuvant hormonal therapy before radical prostatectomy decreases the number of positive surgical margins in stage T2 prostate cancer: interim results of a prospective randomized trial. The Belgian Uro‐Oncological Study Group. Journal of Urology 1995;154(2 Pt 1):429‐34. [DOI] [PubMed] [Google Scholar]
Van Poppel 2001 {published data only}
- Poppel H. Neoadjuvant hormone therapy and radical prostatectomy: The jury is still out. European Urology 2001;39(Suppl 1):10‐14. [DOI] [PubMed] [Google Scholar]
Wirth 2004b {published data only}
- Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K, Casodex Early Prostate Cancer Trialists G. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. Journal of Urology 2004;172(5 Pt 1):1865‐70. [0022‐5347] [DOI] [PubMed] [Google Scholar]
Witjes 1997 {published data only}
- Witjes WP, Schulman CC, Debruyne FM. Preliminary results of a prospective randomised study comparing radical prostatectomy versus radical prostatectomy associated with neo‐adjuvant hormonal combination therapy in T2‐3N0M0 prostatic carcinoma. Urology 1997;49(Suppl 3A):65. [DOI] [PubMed] [Google Scholar]
Yamanaka 2005 {published data only}
- Yamanaka H, Ito K, Naito S, Tsukamoto T, Usami M, Fujimoto H, Matsuoka N, Fukui I, Harada M, Ohashi Y, Kotake T, Kakizoe T. Effectiveness of adjuvant intermittent endocrine therapy following neoadjuvant endocrine therapy and external beam radiation therapy in men with locally advanced prostate cancer. Prostate 2005;63(1):56‐64. [0270‐4137] [DOI] [PubMed] [Google Scholar]
Zagars 1999 {published data only}
- Zagars GK, Pollack A, Smith LG. Conventional external‐beam radiation therapy alone or with androgen ablation for clinical stage III (T3, NX/N0, M0) adenocarcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 1999;44(4):809‐19. [DOI] [PubMed] [Google Scholar]
Zurlo 2002 {published data only}
- Zurlo A, Collette L, Tienhoven G, Blank LE, Warde P, Dubois JB, Jeanneret W, Storme G, Bernier J, Kuten A, Pierart M, Bolla M, Sternberg C, Kate FWJ, Mattelaer J, Lopez Torrecilla J, Pfeffer JR, Cutajar CL, Roberts T, Fossa S, Horiot JC, Hamers H, Bossett JF, Kovner F, Poppel H, Jager J, Svoboda V, Zambon JJ, Wijndaele JJ, Koriakine O, Boekenkruger C. Acute toxicity of conventional radiation therapy for high‐risk prostate cancer in EORTC trial 22863. European Urology 2002;42(2):125‐32. [DOI] [PubMed] [Google Scholar]
Additional references
Aus 1997
- Aus G, Hugosson J, Abrahamsson PA, et al. Pretreatment with triptorelin before radical prostatectomy: a 3 year follow up. Journal of Urology 1997;158(Suppl):393. [Google Scholar]
Baert 1998
- Baert LV, Goethuys HJ, Ridder DJ, et al. Neo‐adjuvant treatment before radical prostatectomy decreases the number of positive margins in CT2‐T3 but has no impact on PSA progression or survival in CT2‐T3. Journal of Urology 1998;159(Suppl):61. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Hanks 2000
- Hanks GE, Lu JD, Machtay, et al. RTOG protocol 9202: a phase 111 trial of the use of long term androgen suppression following neo adjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate. International Journal of Radiation Oncology Biology and Physics 2000;48:112. [Google Scholar]
Huggins 1941
- Huggins C, Hodges CV. Studies on prostate cancer 1: the effect of castration, of oestrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research 1941;1:293‐7. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [0197‐2456] [DOI] [PubMed] [Google Scholar]
Konski 2005
- Konski A, Sherman E, Krahn M, Bremner K, Beck JR, Watkins‐Bruner D, Pilepich M. Ecomomic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86‐10). International Journal Radiation Oncology Biology and Physics 2005;63(3):788‐94. [DOI] [PubMed] [Google Scholar]
Konski 2006
- Konski A, Watkins‐Bruner D, Brereton H, Feigenberg S, Hanks G. Long‐term hormone therapy and radiation is cost‐effective for patients with locally advanced prostate cancer. Cancer 2006;106:51‐7. [DOI] [PubMed] [Google Scholar]
Laverdiere 1997
- Laverdiere J, Gomez JL, Cusan L, et al. Beneficial effect of combination hormonal therapy administered prior and following external beam irradiation in localised prostate cancer. International Journal of Radiation Oncology Biology and Physics 1997;37:247‐52. [DOI] [PubMed] [Google Scholar]
Meng 2002
- Meng MV, Grosfeld GD, Carrol PR, Small EJ. Neoadjuvant Strategies for Prostate Cancer Prior Radical Prostatectomy. Seminars in Urological Oncology 2002;20(3, suppl 1):10‐18. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Jadad AR, et al. Assessing the quality of reports of randomised trials: implications for the conduct of meta‐analyses.. Health Technology Assessment 1999;3(12):1‐98. [PubMed] [Google Scholar]
Pilepich 2000
- Pilepich MV, Winter K, Byhardt RW, et al. Androgen ablation adjuvant to definitive radiotherapy in carcinoma of the prostate: year 2000 update of RTOG phase 111 studies 86‐10 and 85‐31. International Journal Radiation Oncology Biology Physics 2000;48(3 Suppl):169. [Google Scholar]
Samant 2003
- Samant RS, Dunscombe PB, Roberts GH. A cost‐outcome analysis of long‐term adjuvant gorserelin in addition to radiotherapy for locally advanced prostate cancer. Urological Oncology 2003;21(May ‐ June (3)):171‐7. [DOI] [PubMed] [Google Scholar]
Stone 1999
- Stone N, Stock RG. Prostate brachytherapy: treatment strategies. Journal of Urology 1999;162:421‐6. [DOI] [PubMed] [Google Scholar]


