Abstract
Immune checkpoint inhibitors (ICIs) frequently result in cutaneous immune-related adverse events (IrAEs). Whereas the majority of these events are mild-to-moderate in severity, up to 5% are severe, which may lead to morbidity and dose interruption or discontinuation of ICI therapy. In addition, up to 25% of dermatologic IrAEs are corticosteroid-refractory or corticosteroid-dependent. These 2020 MASCC recommendations cover the diagnosis and management of cutaneous IrAEs with a focus on moderate-to-severe and corticosteroid-resistant events. Although the usage of immune-suppressive therapy has been advocated in this setting, there is a lack of randomized clinical trial data to provide a compelling level of evidence of its therapeutic benefit.
Keywords: bullous dermatoses, corticosteroids, cutaneous IrAEs, Inflammatory dermatitis, pruritus, skin rash, vitiligo
Introduction
Dermatologic toxicities secondary to anti-CTLA-4 and anti-PD-1/anti-PD-L1 inhibitors are the most common immune-related adverse events (IrAEs) among all organ systems. Rash, pruritus, and vitiligo are most frequently observed, while immunobullous reactions and severe cutaneous adverse events are less common, but are important to recognize and treat promptly. Rare adverse events (AEs) have been reported and continue to emerge daily. Cutaneous AEs may be a surrogate for clinical benefit; therefore, it is important to recognize these events and appropriately manage them, while trying to avoid discontinuation of immunotherapy if at all possible. With early diagnosis and prompt management, patients may be able to continue treatment with immune checkpoint inhibitors (ICIs), which may ultimately be crucial for overall treatment outcomes. However, it is also important to recognize potentially severe AEs and to know when to hold or discontinue immunotherapy permanently.
The majority of dermatologic reactions secondary to immune checkpoint inhibitors (ICIs) are of grade 1-2, with grade 3-4 reactions occurring in <3% during monotherapy with ipilimumab or anti-PD-1 and <30% with the combination [1]. However, intolerable grade 2 and grade 3-4 reactions need prompt recognition and appropriate management in order to prevent potentially life-threatening outcomes and/or unnecessary permanent discontinuation of life-saving immunotherapy. The pathophysiologic mechanisms of cutaneous IrAEs have not been fully elucidated. Yet they appear to be clearly related to enhanced T or B-cell activation against dermal or epidermal antigens mediated by blockade of PD-1 and CTLA-4 receptors.
Broadly, the skin reactions observed with ICI therapy can be categorized as follows:
Inflammatory dermatitis, which can be of various clinical presentations and histologic patterns, including eczematous/spongiotic, lichenoid (collective term for conditions that are flat-topped, often scaly in appearance that are associated with infiltration/inflammation between the dermis and epidermis), psoriasiform, and urticarial;
Immunobullous reactions, including bullous pemphigoid, pemphigus vulgaris, and lichen planus pemphigoides;
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN);
Pruritus, either in association with, or separate from, visible skin reactions;
Immune-mediated alteration of melanocytes, including regression of nevi and vitiligo.
Clinical examples of these skin reactions are shown in Figure 1.
Figure 1:
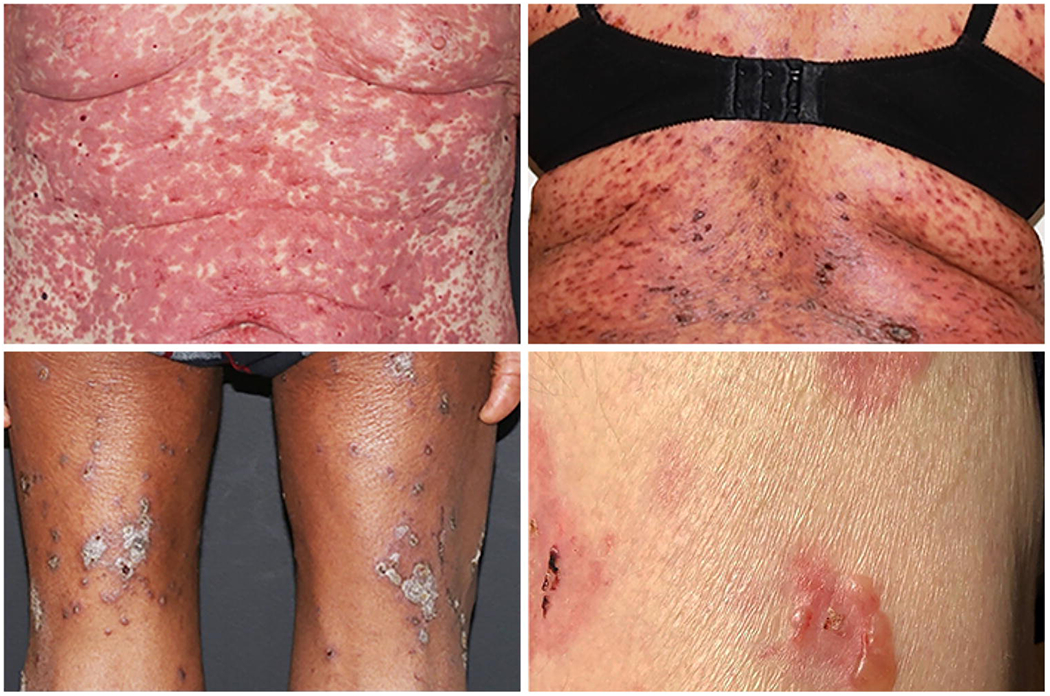
Clinical phenotype of dermatologic Immune-Related Adverse Events (IrAEs): Maculopapular rash (left upper); Lichenoid rash (right upper); Eczematous (left lower); Bullous Pemphigoid (right lower).
Aside from these categories, there are case reports of other more rare types of cutaneous reactions, including autoimmune skin diseases such as dermatomyositis, scleroderma, subacute cutaneous lupus erythematosus, granulomatous disease, and alopecia areata, which will not be discussed at length in this manuscript.
Literature Review and Recommendations
A systematic literature search was conducted for papers from 1960 to May 2019. The Medline (via PubMed) and Thomson-Reuters’ Web of Science databases were searched using the following generic drug names and synonyms as search terms: ipilimumab (Yervoy®), pembrolizumab (MK-3475, SCH900475, lambrolizumab Keytruda®), nivolumab (BMS-936558, MDX-1106, ONO-4538, Opdivo®), atezolizumab (MPDL-3280A, RG-7446, R05541267, Tecentriq®), avelumab (MSB-0010718C, Bavencio®), durvalumab (MEDI-4736, Imfinzi®), and cemiplumab (REGN2810, Libtayo®). Primary case reports, case series, and data from clinical trials were included. All published peer-reviewed literature from the search was reviewed (limited to the English language only, with inclusion of selected non-English reports with abstracts in English). The evidence used for these guidelines consists mainly of systematic reviews of observational data, consensus guidelines [2–6], case series, and case reports guided by dermatologist authors (J.C. and M.E.L.).
Rash/Inflammatory Dermatitis
An inflammatory dermatitis described to be of several different clinical and histologic variants, including non-specific maculopapular or morbilliform rash, eczematous/spongiotic, lichenoid, and psoriasiform [7–10]. The non-specific morbilliform eruption typically occurs in the first 3-6 weeks or sooner primarily with anti-CTLA-4 therapy [11, 12], though delayed onset of months has been reported [13]. Other reactions are more common with PD(L)1 inhibitors as single agents or when combined with anti-CTLA-4; eczematous eruptions clinically present with pruritic, ill-defined, erythematous, scaly papules and plaques, most commonly on the trunk and extremities. Lichenoid eruptions are seen particularly with anti-PD-1 and anti-PD-L1 agents [10, 14–16] that are characterized by an infiltrate of inflammatory cells in the dermis and epidermis, with the lesions having polygonal shapes and a violaceous color, reminiscent of naturally occurring lichen. Onset of lichenoid cutaneous reactions tends to be delayed, with a mean of 4 months, but may occurr up to over a year later [10, 17]. Psoriasiform eruptions secondary to anti-PD-1/anti-PD-L1 therapy have been well-established, either as new onset or as exacerbations of pre-existing psoriasis [18, 19]. Plaque psoriasis is the most common clinical presentation, while guttate, pustular, or inverse variants of psoriasis are possible, albeit less common. [19]. Concomitant psoriatic arthritis can also develop, which may require systemic therapy for control [11].
Bullous dermatoses
Though several types of cutaneous eruptions in the setting of immune checkpoint blockade can present as bullous, including lichenoid reactions, autoimmune blistering disease-like reactions are distinct mucocutaneous manifestations. More specifically, bullous pemphigoid is an autoimmune antibody-mediated disorder that can develop de novo, or as an exacerbation of pre-existing bullous pemphigoid secondary to PD-1 and PD-L1 inhibitor-targeted therapy [20–23]. These are rare with CTLA-4 inhibition. Pruritus is a predominant feature that can sometimes precede onset of visible bullae by months. Bullae develop approximately six to eight months after initiation of immune checkpoint blockade as part of bullous pemphigoid, pemphigus vulgaris, or lichen planus pemphigoides, though a subset of reported cases demonstrate delayed onset up of to one and a half years. Oral mucosa can be involved in approximately 30% of patients [24, 25]. In approximately 75% of patients who develop autoimmune blistering disorders, discontinuation of immunotherapy may be necessary, though cases of successful treatment while continuing immunotherapy have been reported.
Severe Cutaneous Adverse Reactions
Rare cases of SCARs , including SJS, TEN, acute generalized exanthematous pustulosis (AGEP), and drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported with both CTLA-4 and PD-1/PD-L1 inhibitors, some resulting in death [26–32]. It is notable that these reactions may occur without the classic clinical morphology or time course, which is typically within a few days to 4 weeks after initiation of the offending drug. These reactions can occur in a delayed or progressive fashion after several cycles of treatment [26, 32]. Althought two cases of pembrolizumab-induced SJS have been successfully treated with cyclosporine, additional studies are required to improve the level of evidence [32].
Pruritus
Grade 3 incidence secondary to PD-1 inhibitor therapy ranges from 5% to 10% [13, 33]. Pruritus can occur either with or without rash, most commonly on the trunk and extremities, and can be associated with a significantly decreased quality of life [11].
In the case of severe or unremitting pruritus, an occult diagnosis of bullous pemphigoid should be considered, as pruritus can be the presenting symptom in the absence of bullae for many weeks to months before typical changes of bullous pemphigoid present.
I. Rash/inflammatory dermatitis
Diagnostic Work-up:
For any inflammatory dermatitis, each patient should undergo the following evaluations:
Pertinent history and physical examination;
Rule out any other cause for the skin condition, including infection, other drug side-effect, or unrelated primary skin disorder;
Blood laboratory investigations, including complete blood cell count, as well as liver and kidney function tests;
Specific serologic studies if an autoimmune condition is suspected, such as dermatomyositis or lupus: screening for antinuclear (ANA), SS-A/anti-Ro, SS-B/anti-La, autoantibodies and/or other serologic tests;
Skin biopsy.
Grade 3: Macules/papules covering >30% body surfice area (BSA) with or without associated symptoms; limiting self-care ADLs; or grade 2 not responding to therapy.
Management:
-Withhold ICI therapy;
-Dermatology referral for guidance in management;
-Consider skin biopsy and clinical photography to aid in monitoring;
-Initiate oral prednisone or intravenous methylprednisolone (or equivalent) 1 mg/kg daily, tapering over 4 weeks or longer if needed;
-If there is a quick response to steroid treatment, can consider shorter taper over 2 weeks;
-If there is no improvement in rash at 1 mg/kg daily dosing within 5 days, then consider increasing steroid dosing to 2 mg/kg daily (or 1 mg/kg twice daily);
-May consider resuming ICIs if rash reverts to grade 1 or mild grade 2 with close monitoring;
-If rash recurs with steroid taper or discontinuation, consider either maintenance low-dose oral prednisone (e.g. 10 mg daily), while resuming ICI therapy or adjunctive dermatologic treatments with co-management by dermatology (e.g. narrowband ultraviolet B phototherapy 2-3 times a week for several weeks to months and discontinue phototherapy after rash has resolved);
-For psoriasiform eruptions, consider steroid-sparing therapies, such as phototherapy, acitretin (e.g. 10-25 mg daily), apremilast (e.g. 30 mg twice daily), or methotrexate (e.g. 15 mg weekly), in order to avoid long-term treatment with systemic corticosteroids [19, 34–37]. However, the level of evidence for such recommendations is very low, largely based on case reports. TNF-alpha inhibitors, such as etanercept, adalimumab, or infliximab, and interleukin inhibitors, such as ustekinumab (IL-12/23 inhibitor), secukinumab (IL-17 inhibitor), ixekizumab (IL-17 inhibitor), brodalumab (IL-17RA inhibitor), tildrakizumab (IL-23 inhibitor), risankizumab (IL-23 inhibitor), and guselkumab (IL-23 inhibitor), can be considered in severe cases, but must be used with caution and careful monitoring as potential impact on tumor control by immune checkpoint blockade is not yet known. It should be emphasized however, that these recommendations are based largely on case series or case reports and compelling supporting evidence is awaited [38–43].
Grade 4: Macules/papules covering >30% body surface BSA with associated symptoms, unresponsive to prior interventions, rapidly progressing, and/or intolerable.
Management:
-Discontinue ICI
-Urgent dermatology referral for skin biopsy and guidance in management
-Initiate high-dose intravenous methylprednisolone (or equivalent) 1-2 mg/kg daily until toxicity improves, tapering over at least 4 weeks or longer if needed
-Monitor closely for possible progression to SCAR
-Admit patient to hospital with co-management between oncology and dermatology
-Determine appropriateness with dermatology of resuming ICI once skin toxicity resolves and once corticosteroids are reduced to prednisone (or equivalent) ≤ 10 mg daily
-If rash does not resolve to grade 1 or less, discontinue ICI permanently and consider alternative antineoplastic therapy.
II. Bullous dermatoses
Diagnostic Work-up:
For any bullous dermatosis, consider the following evaluations:
Pertinent history and physical examination;
Rule out any other cause for the skin condition, including infection, other drug side-effect, or unrelated primary skin disorder;
Consult dermatology to rule out other causes for blisters of the skin, including herpes simplex, herpes zoster, bullous impetigo;
Blood laboratory investigations, including complete blood cell count, as well as liver and kidney function tests, may be performed if needed;
Specific serologic studies if bullous pemphigoid is suspected, including anti-BP180/BP230, anti-desmogleins 1/3 IgG antibody levels, or under the guidance of dermatology, other serologic studies and sending patient serum for indirect immunofluorescence antibody testing to rule out other autoimmune blistering conditions;
Skin biopsy if the diagnosis of bullous pemphigoid is suspected (lesional biopsy for hematoxylin and eosin evaluation and peri-lesional biopsy for direct immunofluorescence evaluation).
Grade 3: Blisters >30% BSA with skin sloughing with associated pain or pruritus and limiting self-care ADLs
-Withhold ICI therapy;
-Urgent dermatology referral for work-up and guidance in management;
-Consider admission to hospital for initial management and supportive wound care;
-Initiate IV methylprednisolone (or equivalent) 1-2 mg/kg daily until improvement is achieved, then transition to prednisone (or equivalent) 0.5-1 mg/kg daily, tapering over at least 4 weeks;
-For diagnosis of bullous pemphigoid, after initial treatment with high-dose corticosteroids, consider treatment with steroid-sparing therapies, such as rituximab (e.g. 500 mg IV weekly for 4 weeks) or omalizumab (e.g. 300 mg every 4 weeks) in order to avoid long-term treatment with systemic corticosteroids [39–41];
-Treat skin with ointment emollient, such as petrolatum, with non-stick bandages over any open erosions, changed once to twice daily; use of oral antibiotic such as doxycycline at a dose of 50mg to 100mg twice daily may be useful if any signs of bacterial superinfection are evident [44];
-If any concern for overlying infection (e.g. purulent drainage, significant edema or induration, fever), obtain cultures of skin and/or blood and consult infectious diseases specialist for guidance in management.
III. Severe Cutaneous Adverse Reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, and DRESS/DIHS (drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome)
Diagnostic Work-up:
For any potential severe cutaneous adverse reaction, each patient should undergo the following evaluations:
Pertinent history, complete review of systems, and physical examination;
Skin examination should include examination of all areas, including all mucous membranes (eyes, nares, oropharynx, genitals, perianal area);
Rule out any other cause for the skin condition, including infection, other drug side-effect, or unrelated primary skin disorder;
Blood laboratory investigations, including complete blood cell count with differential, liver, and kidney function tests, including urinalysis; if patient is febrile, include blood cultures and urine cultures;
Skin biopsy is indicated to assess for full-thickness epidermal necrosis, as seen in SJS/TEN;
For significant involvement of mucous membranes or blistering of skin, consider admission to the hospital for monitoring and management, and to the intensive care unit or burn unit if needed;
Pay special attention to the following symptoms on review of symptoms as concerning signs: skin pain, fevers, ocular pain or photophobia, sores of discomfort in the nares, sores or discomfort in the oropharynx, pain with swallowing, hoarseness, sores or discomfort in vaginal area for women or meatus of penis in men, sores or discomfort in the peri-anal area, pain with bowel movements, dysuria, abdominal pain, myalgias, arthralgias, malaise;
Physical examination should include assessment for lymphadenopathy, facial swelling, or distal extremity swelling (can be signs of DIHS/DRESS); pustules, vesicles, bullae, or erosions; targetoid lesions with central dusky erythema (as can be seen in SJS/TEN).
Grade 3: Skin sloughing covering <10% BSA with associated signs (e.g. erythema, purpura, epidermal detachment, mucous membrane detachment)
-Withhold ICI therapy;
-Dermatology referral for work-up and guidance in management;
-Consider admission to hospital for initial management and supportive wound care
-Initiate IV methylprednisolone (or equivalent) 1-2 mg/kg daily until improvement is achieved, then transition to prednisone (or equivalent) 0.5-1 mg/kg daily, tapering over at least 4 weeks when toxicity resolves;
-Treat skin with ointment emollient, such as petrolatum, with non-stick bandages over any open erosions, changed once to twice daily; use of topical antibiotic ointment may be useful if any signs of bacterial superinfection;
-For mucous membrane involvement of SJS or TEN, offer appropriate consulting services to help prevent sequelae from scarring (e.g. ophthalmology, ear, nose, and throat, gynecology, urology).
Grade 4: Skin sloughing covering 10-30% BSA with associated signs (e.g. erythema, purpura, epidermal detachment, mucous membrane detachment) or skin erythema ≥30% BSA with systemic symptoms (e.g. fever) and concerning laboratory work-up abnormalities suggestive of DRESS/DIHS (e.g. elevated liver function tests, elevated creatinine, atypical lymphocytes); skin sloughing covering ≥30% BSA with associated symptoms (e.g. erythema, purpura, epidermal detachment) defines toxic epidermal necrolysis.
-Permanently discontinue ICI therapy;
-Urgent dermatology referral for work-up and guidance in management;
-Admit patient immediately to intensive care unit or burn unit for supportive care in wound care and fluid/electrolyte management;
-For mucous membrane involvement of SJS or TEN, offer appropriate consulting services to help prevent sequelae from scarring (e.g. ophthalmology, ear, nose, and throat, gynecology, urology);
-Initiate IV methylprednisolone (or equivalent) 1-2 mg/kg daily until improvement is achieved, then transition to prednisone (or equivalent) 0.5-1 mg/kg daily, tapering over at least 4 weeks when toxicity resolves;
-If no response or resolution with systemic corticosteroids, consider intravenous immunoglobulin (IVIG) (e.g.. 1 gm/kg/day x 3 days) or cyclosporine (e.g. 3-6 mg/kg/day) with slow taper over 4-6 weeks after toxicity has resolved;
-Treat skin with ointment emollient, such as petrolatum, with non-stick bandages over any open erosions, changed once to twice daily; use of topical antibiotic ointment may be useful if any signs of bacterial superinfection;
-If any concern of overlying infection (e.g. purulent drainage, significant edema or induration, fever), obtain cultures of skin and/or blood and consult infectious diseases specialist for guidance in management;
-In a patient who has experienced a SCAR, such as SJS/TEN or DIHS/DRESS, it is generally recommended to avoid re-challenge with the offending drug since re-challenge can result in a SCAR of even higher severity or death. For patients who develop DIHS/DRESS, long-term or later side-effects involving other organs, such as the kidney, thyroid, and heart, can occur; therefore, continued monitoring for at least 6 months should be considered.
IV. Pruritus
Diagnostic Work-up:
For patients with pruritus, consider other causes of pruritus with the following evaluations:
Pertinent history and physical examination;
Rule out any other cause for the skin condition, including infestation (such as scabies), other drug side-effect, or unrelated primary skin disorder;
Blood laboratory values, including complete blood cell count, as well as differential, liver, kidney, and thyroid function tests, and iron levels;
Skin biopsy (lesion biopsy for hematoxylin and eosin evaluation and peri-lesion biopsy for direct immunofluorescence evaluation) can be considered as cases of occult bullous pemphigoid presenting as pruritus without specific skin changes;
Specific serologic studies if bullous pemphigoid is suspected, including anti-BP180 and anti-BP230 IgG antibody levels, IgE levels, or under the guidance of dermatology, other serologic studies and sending patient serum for indirect immunofluorescence testing to rule out other autoimmune blistering conditions.
Grade 3: Widespread and constant, limiting self-care ADLs or sleep for which systemic corticosteroid or immunosuppressive therapy indicated.
-Dermatology referral;
-Initiate oral corticosteroids such as prednisone (or equivalent) 0.5-1 mg/kg/day tapered over 2-4 weeks;
-Initiate addition of an alternative non-steroid agent, such as gabapentin (100-300 mg TID), pregabalin, naltrexone, aprepitant (80 mg daily for 3-5 days or 3-day course of 125 mg, 80 mg and 80 mg) [45], or oral or topical (5% cream) doxepin.
A summary of the management of the dermatological IrAEs is shown in Figure 2.
Figure 2: Management of Dermatologic Immune-Related Adverse Events.
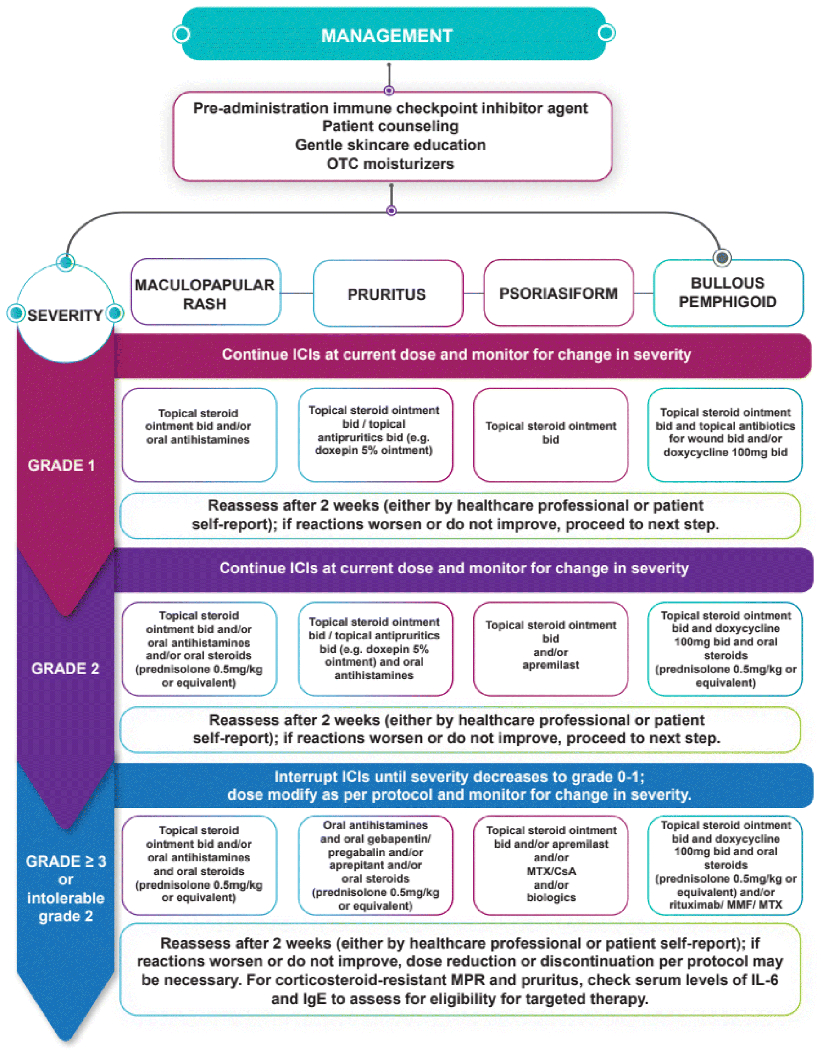
MTX, methotrexate; CsA, cyclosporine A; MMF, Mycophenolate Mofetil; MPR, maculopapular rash; IL-6, interleukin-6; IgE immunoglobulin E.
Conclusions
Given the various clinical presentations of dermatologic IrAEs, accurate diagnosis is critical in order to enable implementation of effective treatment strategies. In addition, since dermatologic events occur early during immunotherapy, recognition at this stage would allow continuation of anti-cancer therapy, while retaining quality of life. The most common clinical presentations include pruritus, lichenoid rash, psoriasis, atopic dermatitis, and vitiligo. Laboratory investigations are also important in aiding diagnosis and treatment, particularly measurement of autoantibodies, both systemically and in skin biopsies. Targeted biologic therapies should be considered in patients with corticosteroid-resistant/refractory dermatologic IrAEs. However, the level of evidence for the usage of these agents remains largely unconfirmed.
Acknowledgments
This work was funded in part by NIH/NIAMS grant U01AR077511 and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Professor BL Rapoport is supported by supported by the Cancer Association of South Africa (CANSA) and the National Research Foundation (NRF) of South Africa.
Dr. I. Glezerman is supported by the NIH/NCI (Cancer Center Support Grant P30 CA008748)
Conflict of interest
AB, RA, JC, TC, PG, DG and VRS have no conflict of interest to declare. MD reports grants from Novartis, other fees from Neoleukin Therapeutics, personal fees from Partner Therapeutics, personal fees from Tillotts Pharma, grants from Genentech, outside the submitted work. MG reports other fees from Bristol Myers Squibb (BMS) and from AstraZeneca, outside the submitted work. IG reports stock ownership from Pfizer Inc., personal fees from CytomX Inc, outside the submitted work. DBJ reports other fees from Array Biopharma, grants and other from BMS, other from Jansen, grants from Incyte, other from Merck, other from Novartis, outside the submitted work. In addition, DBJ has a patent co-inventor on use of CTLA-4 agonist for IrAEs pending. ML reports other (royalties) from Legacy Healthcare Services, from Apricity Health, LLC, from Azitra, Inc., from Deciphera, from Galderma Research and Development, from Johnson and Johnson, from NCODA, from Novocure Inc., from Kyowa Kirin, Inc., from Loxo, from Merck Sharp and Dohme Corporation, from Janssen Research & Development, LLC, from Menlo Therapeutics, from Novartis Pharmaceuticals Corporation, from QED Therapeutics, from F. Hoffmann-La Roche AG, from Amgen Inc., from AstraZeneca Pharmceuticals LP, from Genentech Inc., from Seattle Genetics, from Lutris, from Paxman Coolers, from OnQuality Pharmaceuticals Ltd, and from Takeda Millenium, outside the submitted work. BLR reports personal fees and other from Merck and Co, grants, personal fees and other from BMS, grants, personal fees and other from Roche South Africa, personal fees and other fees from AstraZeneca, during the conduct of the study. MSA reports personal fees from Gilead, grants from Pfizer, personal fees from Abbvie, outside the submitted work. All work with these entities has ended.
References
- 1.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C (2016) Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13(8):473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 2.Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB (2017) Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol 8:49. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassel JC, Heinzerling L, Aberle J, Bähr O, Eigentler TK, Grimm MO, Grünwald V, Leipe J, Reinmuth N, Tietze JK, Trojan J, Zimmer L, Gutzmer R (2017) Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev 57:36–49. doi: 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network (2018) Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS (2016) Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S (2015) Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol 151(11):1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, Carlino MS, Kefford R, Fernandez-Penas P (2016) Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol 74(3):455–461 e1. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, Bosenberg M, Choi JN (2016) Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol 152(10):1128–1136. doi: 10.1001/jamadermatol.2016.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibaud V (2018) Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am J Clin Dermatol 19(3):345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 12.Collins LK, Chapman MS, Carter JB, Samie FH (2017) Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer 41(2):125–128. doi: 10.1016/j.currproblcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD, Lacouture ME (2016) Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP (2016) Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol 28(4):254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 15.Chou S, Hwang SJ, Carlos G, Wakade D, Fernandez-Penas P (2017) Histologic Assessment of Lichenoid Dermatitis Observed in Patients With Advanced Malignancies on Antiprogramed Cell Death-1 (anti-PD-1) Therapy With or Without Ipilimumab. Am J Dermatopathol 39(1):23–27. doi: 10.1097/DAD.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 16.Schaberg KB, Novoa RA, Wakelee HA, Kim J, Cheung C, Srinivas S, Kwong BY (2016) Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J Cutan Pathol 43(4):339–346. doi: 10.1111/cup.12666. [DOI] [PubMed] [Google Scholar]
- 17.Joseph RW, Cappel M, Goedjen B, Gordon M, Kirsch B, Gilstrap C, Bagaria S, Jambusaria-Pahlajani A (2015) Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res 3(1):18–22. doi: 10.1158/2326-6066.CIR-14-0134. [DOI] [PubMed] [Google Scholar]
- 18.Chia PL, John T (2016) Severe Psoriasis Flare After Anti-Programmed Death Ligand 1 (PD-L1) Therapy for Metastatic Non-Small Cell Lung Cancer (NSCLC). J Immunother 39(5):202–204. doi: 10.1097/CJI.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 19.Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, Breton AL, Hubiche T, Bedane C, Legoupil D, Pham-Ledard A, Charles J, Pérol M, Gérard E, Combemale P, Bonnet D, Sigal ML, Mahé E; Groupe de Recherche sur le Psoriasis and the Groupe Cancérologie Cutanée of the Société Française de Dermatologie the GEM Resopso, Apsoderm and the Groupe Français de Pneumo-Cancérologie (2017) Anti-PD1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol 31(5): e254–e257. doi: 10.1111/jdv.14011 [DOI] [PubMed] [Google Scholar]
- 20.Kwon CW, Land AS, Smoller BR, Scott G, Beck LA, Mercurio MG (2017) Bullous pemphigoid associated with nivolumab, a programmed cell death 1 protein inhibitor. J Eur Acad Dermatol Venereol 31(8): e349–e350. doi: 10.1111/jdv.14143. [DOI] [PubMed] [Google Scholar]
- 21.Rofe O, Bar-Sela G, Keidar Z, Sezin T, Sadik CD, Bergman R (2017) Severe bullous pemphigoid associated with pembrolizumab therapy for metastatic melanoma with complete regression. Clin Exp Dermatol 42(3):309–312. doi: 10.1111/ced.13042 [DOI] [PubMed] [Google Scholar]
- 22.Russo I, Sacco G, Frega S, Polo V, Pasello G, Alaibac M (2017) Immunotherapy-related skin toxicity: bullous pemphigoid in a lung adenocarcinoma patient treated with the anti-PDL1 antibody atezolizumab. Eur J Dermatol 27(2):205–208. doi: 10.1684/ejd.2016.2959. [DOI] [PubMed] [Google Scholar]
- 23.Lopez AT, Geskin L (2018) A Case of Nivolumab-Induced Bullous Pemphigoid: Review of Dermatologic Toxicity Associated with Programmed Cell Death Protein-1/Programmed Death Ligand-1 Inhibitors and Recommendations for Diagnosis and Management. Oncologist 23(10):1119–1126. doi: 10.1634/theoncologist.2018-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L (2018) A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol 57(6):664–669. doi: 10.1111/ijd.13984 [DOI] [PubMed] [Google Scholar]
- 25.Naidoo J, Schindler K, Querfeld C, Busam K, Cunningham J, Page DB, Postow MA, Weinstein A, Lucas AS, Ciccolini KT, Quigley EA, Lesokhin AM, Paik PK, Chaft JE, Segal NH, D’Angelo SP, Dickson MA, Wolchok JD, Lacouture ME (2016) Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res 4(5):383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivar KL, Deschaine M, Messina J, Divine JM, Rabionet A, Patel N, Harrington MA, Seminario-Vidal L (2017) Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol 44(4):381–384. doi: 10.1111/cup.12876. [DOI] [PubMed] [Google Scholar]
- 27.Griffin LL, Cove-Smith L, Alachkar H, Radford JA, Brooke R, Linton KM (2018) Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep 4(3):229–231. doi: 10.1016/j.jdcr.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM (2016) Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 60:190–209. doi: 10.1016/j.ejca.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 30.Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, Dummer R (2016) Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res 22(16):4023–4029. doi: 10.1158/1078-0432.CCR-15-2872. [DOI] [PubMed] [Google Scholar]
- 31.Curry JL, Tetzlaff MT, Nagarajan P, Drucker C, Diab A, Hymes SR, Duvic M, Hwu WJ, Wargo JA, Torres-Cabala CA, Rapini RP, Prieto VG (2017) Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol 44(2):158–176. doi: 10.1111/cup.12858. [DOI] [PubMed] [Google Scholar]
- 32.Saw S, Lee HY, Ng QS (2017) Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer 81:237–239. doi: 10.1016/j.ejca.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Ransohoff JD, Kwong BY (2017) Cutaneous Adverse Events of Targeted Therapies for Hematolymphoid Malignancies. Clin Lymphoma Myeloma Leuk 17(12):834–851. doi: 10.1016/j.clml.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Ma VT, Katzman CS, Palmbos PL, Patel RM, Gudjonsson JE, Alva AS (2020) NB-UVB phototherapy in the treatment of anti-PD-1 inhibitor induced psoriasis: A case report. Current Problems in Cancer: Case Reports. doi: 10.1016/j.cpccr.2020.100004. [In Press] [DOI] [Google Scholar]
- 35.Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS (2019) Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol 80(4):990–997. doi: 10.1016/j.jaad.2018.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fattore D, Annunziata MC, Panariello L, Marasca C, Fabbrocini G (2019) Successful treatment of psoriasis induced by immune checkpoint inhibitors with apremilast. Eur J Cancer 110:107–109. doi: 10.1016/j.ejca.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Politi A, Angelos D, Mauri D, Zarkavelis G, Pentheroudakis G (2020) A case report of psoriasis flare following immunotherapy: Report of an important entity and literature review. SAGE Open Med Case Rep 8:2050313X19897707. doi: 10.1177/2050313X19897707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM, Tetzlaff MT, Hwu P, Curry JL, Diab A (2019) IL17A blockade successfully treated psoriasiform dermatologic toxicity from immunotherapy. Cancer Immunol Res 7(6):860–865. doi: 10.1158/2326-6066.CIR-18-0682. [DOI] [PubMed] [Google Scholar]
- 39.Singer S, Nelson CA, Lian CG, Dewan AK, LeBoeuf NR (2019) Nonbullous pemphigoid secondary to PD-1 inhibition. JAAD Case Rep 5(10):898–903. doi: 10.1016/j.jdcr.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damsky W, Kole L, Tomayko MM (2016) Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep 2(6):442–444. doi: 10.1016/j.jdcr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sowerby L, Dewan AK, Granter S, Gandhi L, LeBoeuf NR (2017) Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatol 153(6):603–605. doi: 10.1001/jamadermatol.2017.0091. [DOI] [PubMed] [Google Scholar]
- 42.Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, Miller WH Jr, Calabrese L (2020) Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol 17(8):504–515. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]
- 43.Monsour EP, Pothen J, Balaraman R (2019) A novel approach to the treatment of pembrolizumab-induced psoriasis exacerbation: A case report. Cureus 11(10):e5824. doi: 10.7759/cureus.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, Chalmers JR, Childs M, Walton S, Harman K, Chapman A, Whitham D, Nunn AJ; UK Dermatology Clinical Trials Network BLISTER Study Group (2017) Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet 389(10079):1630–1638. doi: 10.1016/S0140-6736(17)30560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito J, Fujimoto D, Nakamura A, Nagano T, Uehara K, Imai Y, Tomii K (2017) Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer 109:58–61.doi: 10.1016/j.lungcan.2017.04.020. [DOI] [PubMed] [Google Scholar]


