Abstract
Rationale
Nontuberculous mycobacteria (NTM) are ubiquitous environmental bacteria, and some pathogenic species cause lung disease. Environmental factors contribute to increased NTM abundance, with higher potential for exposure and infection.
Objectives
To identify water-quality constituents that influence the risk of NTM infection in Oregon.
Methods
We conducted a population-based cohort study using patient incidence data from the Oregon statewide NTM laboratory data collected as part of a public health surveillance project from 2007 through 2012. To estimate the risk of NTM pulmonary infection (PI) from exposure to water constituents, we extracted water-quality data from the Water Quality Portal and associated these data with corresponding patient county of residence. Using generalized linear models, we modeled two outcomes: Mycobacterium avium complex species PI and Mycobacterium abscessus group species PI.
Results
For every 1-unit increase in the log concentration of vanadium in surface water, infection risk increased by 49% among persons with Mycobacterium avium complex PI. Among those with Mycobacterium abscessus PI, we observed that for every 1-unit increase in the log concentration of molybdenum in surface water, infection risk increased by 41%. The highest risk of infection due to Mycobacterium abscessus group infection was concentrated in counties within the Northwestern region of Oregon. High infection risk associated with Mycobacterium avium complex species did not show any geographic pattern.
Conclusions
Concentrations of the trace metals molybdenum and vanadium in surface water sources were associated with NTM infection in Oregon. These findings may help identify regions at higher risk of NTM infection to guide risk reduction strategies.
Keywords: nontuberculous mycobacteria, molybdenum, vanadium, surface water, environmental epidemiology
The incidence and prevalence of nontuberculous mycobacterial pulmonary infection (NTM PI) have increased over the past decades (1), with an increasing burden of lung disease in the United States (2–4). Nontuberculous mycobacteria are widespread in natural and engineered environments, such as soil, natural water, water distribution systems, and biofilms in municipal water supplies. However, the distribution of NTM disease across the United States varies by geographic region for both the general and high-risk populations (2, 5). Certain environmental conditions likely contribute to increased NTM abundance, leading to increased exposure with higher potential for NTM infection and disease. One study (6) found that NTM abundance in showerheads, as measured by 16S rRNA gene sequencing, was significantly correlated with higher NTM disease prevalence in those same areas.
Analysis of environmental risks within a single geographic area such as a state allow for more precise data regarding environmental risk. In prior studies, we identified high-risk regions for NTM infection in Colorado (7) and found that molybdenum in surface water was significantly associated with a higher likelihood of NTM infection in both a hospital-based cohort (8) and in a cystic fibrosis patient population (9). Here, we extend our inquiries into the state of Oregon to identify water-quality constituents associated with NTM infection in a different geographic region. We associated water-quality data from the Water Quality Portal (WQP) (10), with Oregon statewide NTM microbiology data from a public health surveillance project.
Methods
Population-based NTM patient data for January 1, 2007, through December 31, 2012 were provided by the Oregon Health Authority and the Oregon Health and Science University (OHSU) (11). NTM PI cases were defined using the ATS microbiologic definition of NTM PI (12). This study was approved by the National Jewish Health Institutional Review Board (HS-3410). We used 2010 U.S. Census data from Oregon (13) for total population per county as well as age, sex, and racial/ethnic categories, and population density.
Oregon surface water-quality data were extracted from the WQP (10) for the period January 1, 1997, through December 31, 2012. Surface water describes all the water on the Earth’s surface, such as in a stream, river, lake, or reservoir (14). The cleaned dataset included 33 water-quality constituents from 78,141 total samples collected from 1,373 unique sampling sites. Following data curation steps (see online supplement), 20 water-quality constituents remained for analysis (see Table E1 in the online supplement). We obtained precipitation data from the National Centers for Environmental Information at the National Oceanic and Atmospheric Administration (15) and calculated the median value for each county in Oregon in 2010. Data were analyzed using R packages (see Methods in the online supplement). We calculated the median value of each water-quality constituent for each county (see Methods in the online supplement). Water-quality constituents were eliminated if data were not available for more than 50% of counties, leaving 20 constituents for analysis (Tables E1 and E2), which were then natural log transformed and standardized; missing values were imputed (see Methods in the online supplement). Principal component analysis (PCA) was performed on the county-level dataset using the PCA function. We retained the top four principal components, which explained 64.9% of the variability in the water-quality dataset. We identified the most important variables in explaining variability of the top four principal components (see online supplement). Any constituent with a contribution above the reference line (see red dashed line in Figure E1) was considered important in contributing to the principal components (16, 17).
We used negative binomial regression models to model infection risk as a function of water-quality constituents. Infection risk was modeled in R through negative binomial regression models with the observed number of cases in a given county during the study period as the numerator, and the annual county population as the denominator (modeled using the log of the population as the offset). County-level median values of each water-quality constituent as well as county-level mean age, race, sex, and precipitation were included as predictors. We estimated NTM PI incidence given exposure to water-quality constituents in surface water sources, with significance assessed at P < 0.05. We modeled two separate outcomes: NTM PI incidence associated with Mycobacterium avium complex (MAC) species, and NTM PI incidence associated with Mycobacterium abscessus species, as a function of water-quality constituents and other covariates (Table E3). We calculated the variance inflation factor (VIF) for each water-quality constituent in each model, and included only those water-quality constituents with VIFs less than 10 (Table 1; Model 1). We constructed separate single-constituent regression models (Models 2 and 3) for the metals that demonstrated statistical significance from Model 1 (P < 0.05). Lastly, to create Figures 1 and 2, we used the best-fit estimates of the county-specific risks from the negative binomial models.
Table 1.
Model 1. Negative binomial regression model examining water-quality constituents (with VIF values less than 10) associated with NTM infection risk in Oregon
| MAC Species Variable Relative Risk (95% CI) P Value |
M. Abcessus Complex Species Variable Relative Risk (95% CI) P Value |
||
|---|---|---|---|
| Age: (1 year) |
0.99 (0.91–1.07) 0.841 |
Age: (1 year) |
0.99 (0.82–1.17) 0.943 |
| Sex: Female |
1.10 (0.83–1.46) 0.503 |
Sex: Female |
1.81 (0.94–3.91) 0.100 |
| Race: Non-White* |
0.96 (0.89–1.02) 0.159 |
Race: Non-White* |
1.04 (0.92–1.17) 0.503 |
| Precipitation (inches) |
1.01 (0.99–1.03) 0.135 |
Precipitation (inches) |
1.01 (0.97–1.04) 0.777 |
| Aluminum (1-log unit) |
0.84 (0.59–1.21) 0.354 |
Arsenic (1-log unit) |
1.11 (0.55–2.24) 0.758 |
| Arsenic (1-log unit) |
1.10 (0.84–1.44) 0.492 |
Boron (1-log unit) |
1.19 (0.67–2.21) 0.563 |
| Boron (1-log unit) |
1.10 (0.79–1.52) 0.568 |
Calcium (1-log unit) |
1.36 (0.48–3.80) 0.553 |
| Calcium (1-log unit) |
1.34 (0.80–2.23) 0.248 |
Iron (1-log unit) |
1.13 (0.66–1.88) 0.641 |
| Iron (1-log unit) |
0.87 (0.61–1.24) 0.430 |
Manganese (1-log unit) |
1.27 (0.77–2.10) 0.356 |
| Manganese (1-log unit) |
1.02 (0.76–1.38) 0.892 |
Molybdenum (1-log unit) |
2.08 (1.02–4.49) 0.047 |
| Molybdenum (1-log unit) |
0.93 (0.63–1.37) 0.721 |
Nickel (1-log unit) |
0.61 (0.37–1.03) 0.054 |
| Nickel (1-log unit) |
1.23 (0.89–1.71) 0.232 |
Potassium (1-log unit) |
0.73 (0.32–1.71) 0.459 |
| Potassium (1-log unit) |
0.92 (0.60–1.409) 0.679 |
Vanadium (1-log unit) |
1.16 (0.64–2.06) 0.616 |
| Vanadium (1-log unit) |
1.66 (1.12–2.50) 0.005 |
||
Definition of abbreviations: CI = confidence interval; MAC = Mycobacterium avium complex species; NTM = Nontuberculous mycobacteria; VIF = variance inflation factor.
Bolded estimates are statistically significant (P < 0.05).
Reference group is White Alone.
Figure 1.
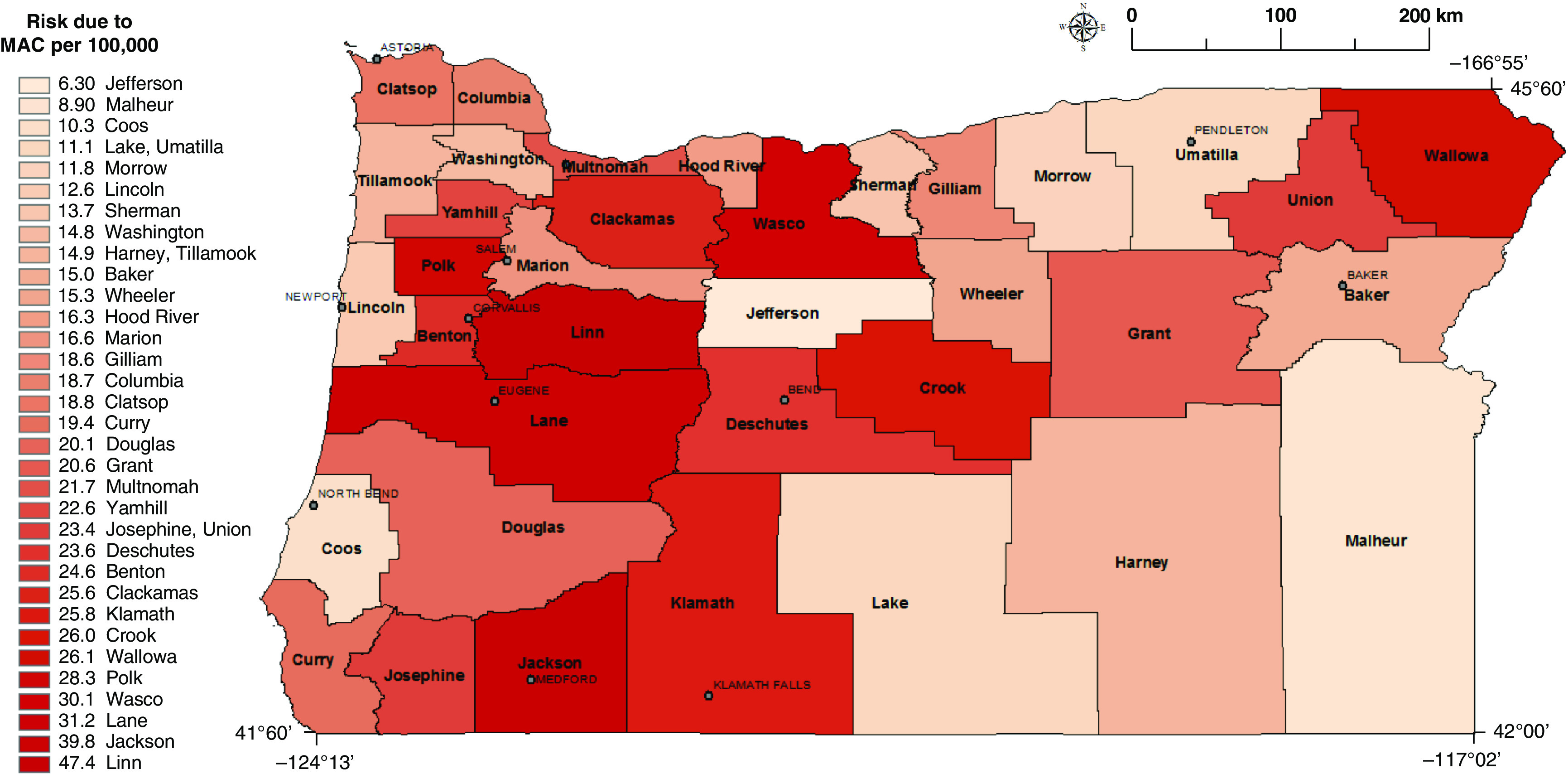
Risk of MAC infection for counties where NTM patients resided based on a vanadium regression model (Model 2; Table 2). Gray lines represent county line boundaries in Oregon. The county names are printed in boldface type. The city names are printed in capital font. MAC = Mycobacterium avium complex species; NTM = nontuberculous mycobacteria.
Figure 2.
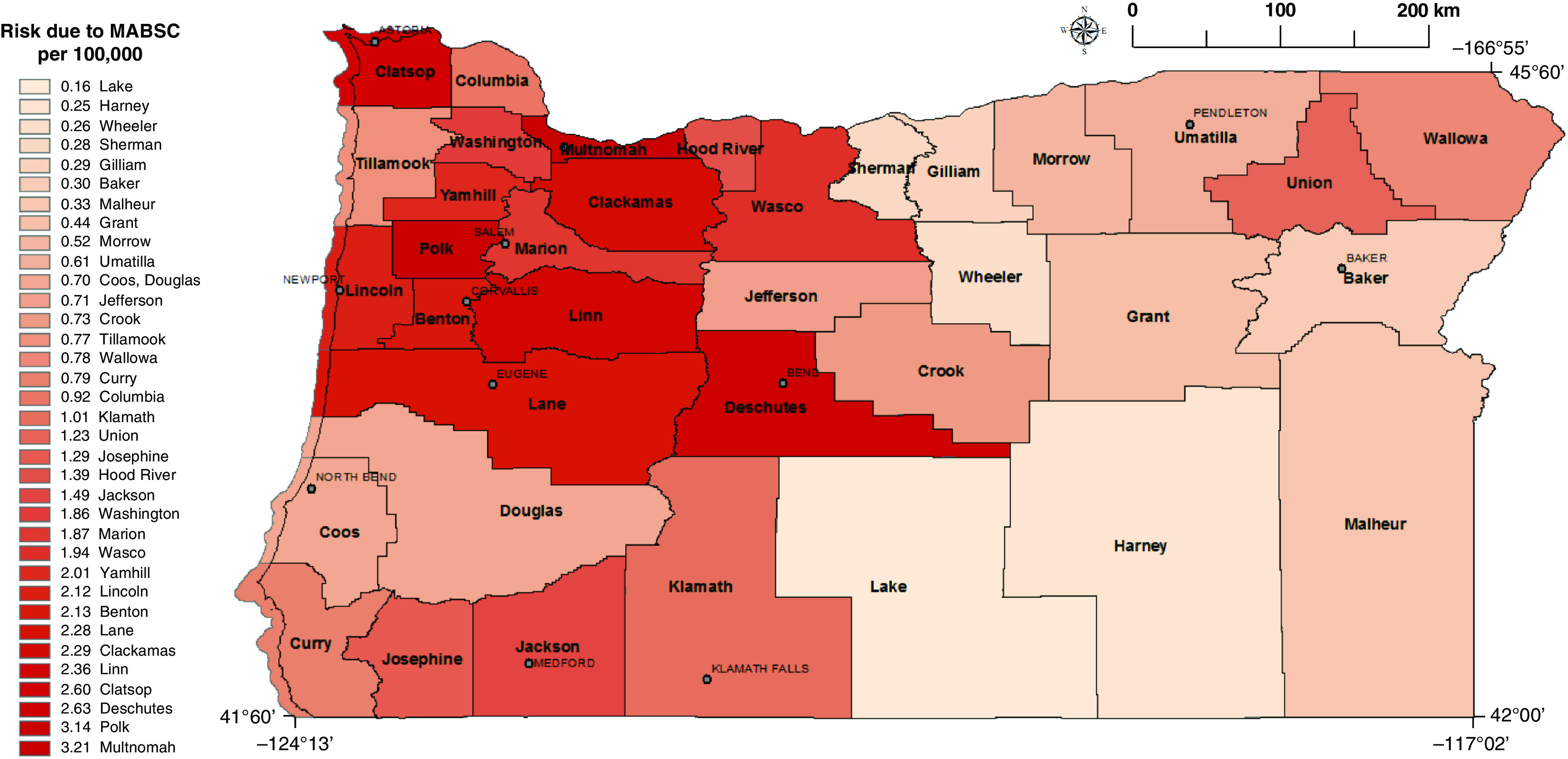
Risk of Mycobacterium abscessus infection for counties where NTM patients resided based on a molybdenum regression model (Model 3; Table 3). Gray lines represent county line boundaries in Oregon. The county names are printed in boldface type. The city names are printed in capital font. MABSC = Mycobacterium abscessus complex; NTM = nontuberculous mycobacteria.
Results
Study Population Characteristics
Our study population comprised 1,138 persons with incident microbiologically confirmed NTM PI at any point during 2007 through 2012 and resident in Oregon. The mean age of pulmonary NTM patients was 66.5 years (range, 0.9 to 97 yr). The proportion female was 55.9%. Of the 1,138 NTM PI patients, 1,015 (89.2%) had slowly growing species, including 980 (86.1%) with MAC species. An additional 93 (8.2%) had rapidly growing species, including 80 (7.0%) with M. abscessus. Because MAC and M. abscessus comprised the majority of the slowly growing and rapidly growing species isolated from patients, respectively, we focused our analysis on two main outcomes: NTM PI associated with MAC species, and NTM PI associated with M. abscessus species.
Regression Models with Individual Metals from Top 4 Principal Components
Based on a predefined threshold (see Methods in the online supplement), we identified 13 out of 20 constituents that were important contributors to the top four principal components (Figure E1): aluminum, arsenic, boron, calcium, copper, iron, magnesium, manganese, molybdenum, nickel, potassium, sodium, and vanadium. We modeled the risk of NTM PI as a function of these 13 water-quality constituents (Table E3). To build Model 1 (Table 1), we selected water-quality constituents (Table E3) whose VIFs were below 10 to mitigate the potential impact of collinear covariate constituents. In each model, we sequentially removed the constituent with the highest VIF and reran the model until all constituents had VIFs less than 10. In Table 1, sodium, magnesium, and copper were omitted from each final model. When modeling NTM PI associated with M. abscessus complex, aluminum was also omitted. The correlation matrix for water-quality constituents is available in Table E4.
In Model 1 (Table 1) for NTM PI associated with MAC, vanadium was positively associated with infection. For NTM PI associated with M. abscessus complex, molybdenum was significantly positively associated with infection and nickel had borderline significance. We then modeled the risk of NTM PI as a function of each significant metal from Model 1 in separate single-constituent models (Models 2 and 3) (P < 0.05). When we modeled the risk of MAC infection (Table 2; Model 2) as a function of each significant metal from Model 1, vanadium remained statistically significant. For every 1-log unit increase in vanadium concentrations in surface water at the county level, the risk of infection associated with MAC increased by 49%. When we modeled the risk of M. abscessus infection as a function of each significant metal in Model 1, molybdenum remained significant, while nickel did not (Table 3; Model 3). For every 1-log unit increase in molybdenum concentrations in surface water at the county level, the risk of infection increased by 41%. In all models, we controlled for age, sex, race, and precipitation. Precipitation was included as a covariate because it has been shown to be related to infection risk (18) as well as being significantly associated with many individual water-quality constituents in our dataset. When adjusting for multiple comparisons using the Bonferroni method (5 models, new P value = 0.01), vanadium remained significant in Model 1 (P = 0.005), but did not exceed significance in Model 2 (P = 0.015). Molybdenum did not retain significance in either Model 1 (P = 0.047) or Model 3 (P = 0.027).
Table 2.
Model 2. Negative binomial regression model examining significant metals from Model 1 and other covariates associated with NTM infection risk for MAC species in Oregon
| Characteristic | Relative Risk 95% CI P Value |
|---|---|
| Age: (1 year) |
0.99 (0.92–1.08) 0.953 |
| Sex: Female |
1.18 (0.92–1.53) 0.207 |
| Race: Non-White* |
0.97 (0.92–1.02) 0.239 |
| Precipitation (inches) |
1.01 (0.99–1.02) 0.273 |
| Vanadium (1-log unit) |
1.49 (1.06–2.10) 0.015 |
Definition of abbreviations: CI = confidence interval; MAC = Mycobacterium avium complex species; NTM = Nontuberculous mycobacteria.
Bolded estimates are statistically significant (P < 0.05).
Reference group is White Alone.
Table 3.
Model 3. Negative binomial regression model examining significant metals from Model 1 and other covariates associated with NTM infection risk for M. abscessus species in Oregon
| Characteristic | Relative Risk 95% CI P Value |
Characteristic | Relative Risk 95% CI P Value |
|---|---|---|---|
| Age: (1 year) |
0.92 (0.80–1.03) 0.189 |
Age: (1 year) |
0.91 (0.79–1.02) 0.123 |
| Sex: Female |
1.60 (0.98–2.95) 0.092 |
Sex: Female |
1.30 (0.84–2.21) 0.293 |
| Race: Non-White* |
1.03 (0.96–1.11) 0.420 |
Race: Non-White* |
0.98 (0.93–1.05) 0.624 |
| Precipitation (inches) |
1.01 (0.99–1.03) 0.181 |
Precipitation (inches) |
1.01 (0.99–1.02) 0.372 |
| Molybdenum (1-log unit) |
1.41 (1.05–1.93) 0.027 |
Nickel (1-log unit) |
1.04 (0.74–1.45) 0.812 |
Definition of abbreviations: CI = confidence interval; NTM = Nontuberculous mycobacteria.
Bolded estimates are statistically significant (P < 0.05).
Reference group is White Alone.
When examining model fit for M. abscessus, we noticed that counties with small population size often exhibited evidence of poor fit by the model. To further study this effect, we performed a sensitivity analysis by including only counties with populations of ⩾30,000. The effect of molybdenum on NTM risk for M. abscessus infection remained significant (Table E5). We also included population density as a variable in our final single-constituent models to determine whether this variable contributed to NTM infection risk. When we included population density (Table E6), the association between vanadium and risk of MAC infection remained significant; however, population density was also statistically significant. When we included population density (Table E7), the association observed between molybdenum and risk of M. abscessus infection remained significant, and population density was not statistically significant.
We estimated NTM PI incidence by county for MAC and M. abscessus infections using models adjusted for demographic covariates. Figure E1 shows the adjusted model for MAC infection with vanadium included as an independent predictor (Model 2; Table 2). We observed the highest risk counties dispersed throughout the state (Crook, Wallowa, Polk, Wasco, Lane, Jackson, and Linn counties). Figure E2 is based on the adjusted regression model for those with M. abscessus infection and included molybdenum as an independent predictor (Model 3; Table 3). We observed the highest risk counties for M. abscessus infection in the Northwestern region of the state, while the remaining counties were at lower risk.
Discussion
We found that for MAC and M. abscessus infection, increasing concentrations of vanadium and molybdenum in surface water, respectively, were associated with an increased risk of NTM PI. Counties with a high risk of M. abscessus were concentrated in the Northwestern region of the state and in Deschutes county in the center of the state, whereas counties with high risk of MAC showed no discernable pattern. Interestingly, while Multnomah county demonstrated the highest risk of M. abscessus infection, the risk estimate for MAC infection represented the average compared with all counties. This finding from Oregon residents with pulmonary NTM infections confirms our previous findings from the Colorado residents with NTM infections, that molybdenum was significantly associated with an increased risk of NTM infection (8). In addition, in a separate case-control study with a population-based sample of CF patients in Colorado, molybdenum in the surface waters of the county of residence significantly increased the odds of having an M. abscessus infection. We found that among CF patients, for every 1-unit increase in the molybdenum concentration in surface water, the odds of M. abscessus infection increased by 79% compared with those who were NTM-negative (9). This finding from an independent patient population in a separate geographic area of the United States lends validity to our current results.
Our study is the first to report that increased risk of MAC infection is associated with vanadium concentrations in surface water. Experimental evidence demonstrating the relationship between vanadium concentrations and environmental MAC abundance is not currently available. Several mechanisms are plausible. Vanadium could stimulate or inhibit growth of MAC depending on the concentration. Dose-dependent inhibition has been observed with other metals. For example, zinc (Zn) concentrations were correlated with NTM numbers in acidic, brown water coastal swamps of the Southeastern United States (19), yet high zinc concentrations are toxic. Vanadate (VO4) substitutes for phosphate (PO4) and has been shown to be an inhibitor of membrane-bound ATPase in vesicles of Mycobacterium phlei (20), suggesting that mycobacteria might be sensitive to high vanadium concentrations. However, the ATPase inhibition was measured using membrane vesicles of M. phlei, not whole cells where the ATPase would likely be protected by the thick, lipid-rich outer membrane of mycobacteria (21). As MAC are resistant to heavy metals (22), high vanadium concentrations might not inhibit energy generation in MAC as much as it would in other microbes. That would provide a competitive advantage to MAC in natural soils and waters. Other demonstrated activities of vanadium as cofactors for nitrogenases and haloperoxidases (23) might be operative in MAC to provide them with reduced nitrogen (NH4) or protect them from toxic halides (respectively). As molybdenum (Mo) is a known cofactor of nitrogen and nitrate-reduction (24), the presence of vanadium in Oregon soils and waters might provide an alternative source of reduced nitrogen for mycobacterial growth. The growth of mycobacteria in natural, low nutrient waters (25) suggests that the mycobacteria can obtain nitrogen via N2 fixation. Current data from U.S. Geological Surveys (26), demonstrate that Oregon has elevated concentrations of vanadium in soil throughout the state (Figure E2). Although our reported association is based on vanadium concentrations in surface water, vanadium soil content may be a proxy for vanadium concentrations in surface water (direct communication with U.S. Geological Survey scientist, Dr. Katherine Walton-Day). Further studies in other geographic regions are necessary to confirm or refute this association.
The risk of M. abscessus infection from environmental molybdenum in water sources could be related to either the mycobacteria or humans independently. Molybdenum enzymes in mycobacteria exert important physiological functions, and other research suggests a physiological connection linking molybdenum and essential metabolism of Mycobacterium tuberculosis, potentially affecting survival, pathogenesis, and persistence. Mycobacteria tuberculosis as well as NTM contain proteins for the importation and utilization of molybdenum, including the molybdate ATP-binding cassette (ABC) importer genes modA, modB, and modC (27–30). Several studies on M. tuberculosis have shown that ABC importers are associated with physiology and pathogenicity (31–33), implying that this pathogen cannot grow in the host without specific nutrients. It has been reported for M. tuberculosis that a mutant of molybdate transport protein, pModA, contributed to decreased survival in mice lungs, suggesting that the uptake of molybdenum was required for the survival of this pathogen (34). In a recent study (35) that explored the role of ABC importers for potential drug and vaccine targets in M. tuberculosis, the authors indicated that a lack of molybdenum importation may affect the biosynthesis of molybdenum cofactor (MoCo), which has been suggested to be associated with pathogenesis (29) and persistence (28) of M. tuberculosis. Because M. tuberculosis and NTM are phylogenetically related organisms, this connection offers biological plausibility that molybdenum in water sources influences growth and persistence of NTM as well.
Our study has some limitations. First, we are estimating infections and not disease, because detailed patient clinical and radiographic data for patients with these infections were not available. However, the presence of these infections is a marker for NTM in the environment. In addition, the positive predictive value (PPV) of 2 cultures for predictive disease is high. As stated in the current NTM diagnostic guidelines, “Clinically significant MAC pulmonary disease is unlikely in patients who have a single positive sputum culture during the initial evaluation [5–7], but can be as high as 98% in those with at least 2 positive cultures [5]” (12). In addition, the ATS microbiologic criteria have been found to have a predictive value for true disease of 86% (36). Second, because this is an observational study, we cannot infer causation from these findings alone. However, the association between molybdenum and risk of M. abscessus infection has now been upheld in three studies using different study designs, patient populations and geographic locations (8, 9). These findings strengthen the possible causal relationship between the presence of molybdenum in surface water and the risk of NTM infection. In our study, we used infection incidence as a proxy for NTM abundance in the environment. Again, the effects of molybdenum could be influencing the bacteria, the humans, or both. For example, molybdenum could increase M. abscessus numbers in waters, increasing the probability of infection. Alternatively, or in conjunction, molybdenum could be influencing human susceptibility of M. abscessus infection. In a Korean study, Oh and colleagues (37) reported that pulmonary NTM patients had significantly higher molybdenum concentrations in their blood serum (1.70 μg/L) compared with healthy controls (0.96 μg/L) and patients with pulmonary tuberculosis (0.67 μg/L). Our study used an ecologic design, which is both a strength and a limitation: by definition, an ecologic study measures exposure at the group level, in this case county, and not the individual. Thus, water-quality constituent exposure may vary for each patient even within the same county. However, measurement of exposure to water quality constituents at the population level may capture exposures in a population more fully than is feasible at the individual level. In addition, both host and environmental factors each contribute to infection risk, with host susceptibility playing an important role. For these reasons, we should not be extrapolating population risk to individual probability of infection (38).
Our approach assumes that areas with high infection incidence correlate with regions of high NTM abundance (6), where regional environmental factors create a favorable environment for NTM to persist, thereby increasing the risk of infection. Our causal inferences were strengthened by obtaining water quality data for a period prior to the incidence of NTM infections. Although we did not have information on the duration of residence in a given county, one study from Oregon showed a relatively long residence duration, mean 13.6 years, in Multnomah County, Oregon, the most highly populated county in the state (39). While the incubation period for NTM infection has not been defined, our findings are further strengthened by the fact that the trace metals analyzed did not show much fluctuation over the 15-year period (1997–2012) (data not shown), such that measured concentrations of these surface metals likely represent an average exposure of the population in that area.
We hypothesize that some environmental water sources present a higher abundance or risk of exposure to NTM due to the presence of certain trace metals in surface water sources, particularly molybdenum or vanadium, that may alter the metabolism and pathogenicity of these organisms. Ongoing in vitro studies as well as population-based studies conducted in other geographic regions will help to confirm this hypothesis and further assess the evidence for causality. Whether molybdenum or vanadium in the human host alters the ability to respond to or contain infection is also the subject of future research.
Acknowledgments
Acknowledgment
The authors thank Dr. Daniel Wise (USGS Oregon) and Dr. Katherine Walton-Day (USGS Colorado) for their expertise and consultation on our water-quality constituent dataset.
Footnotes
Supported by the Cystic Fibrosis Foundation, Clinical Pilot and Feasibility Award (E.M.L.), the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (D.R.P.), National Science Foundation awards 1463642 and 1915277 (J.P.F.), NSF award 1743597 (J.L.C.), and NSF award DGE-1545433 (R.A.M.).
Author Contributions: E.M.L. conceived of the study. E.M.L., J.L.C., and D.R.P. designed the study. E.M.L., R.A.M., and E.H. acquired the data. E.M.L. and J.P.F. analyzed the data. E.M.L., J.O.F., and D.R.P. interpreted the data and drafted the manuscript. E.M.L., J.P.F., J.O.F., J.L.C., R.A.M., E.H., and D.R.P. provided intellectual input into the manuscript revisions.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med . 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med . 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc . 2015;12:1458–1464. doi: 10.1513/AnnalsATS.201503-173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc . 2020;17:178–185. doi: 10.1513/AnnalsATS.201804-236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adjemian J, Olivier KN, Prevots DR. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: screening practices and environmental risk. Am J Respir Crit Care Med . 2014;190:581–586. doi: 10.1164/rccm.201405-0884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebert MJ, Delgado-Baquerizo M, Oliverio AM, Webster TM, Nichols LM, Honda JR, et al. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. MBio . 2018;9:e01614-18. doi: 10.1128/mBio.01614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipner EM, Knox D, French J, Rudman J, Strong M, Crooks JL. A geospatial epidemiologic analysis of nontuberculous mycobacterial infection: an ecological study in Colorado. Ann Am Thorac Soc . 2017;14:1523–1532. doi: 10.1513/AnnalsATS.201701-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipner EM, French J, Bern CR, Walton-Day K, Knox D, Strong M, et al. Nontuberculous mycobacterial disease and molybdenum in Colorado watersheds. Int J Environ Res Public Health . 2020;17:E3854. doi: 10.3390/ijerph17113854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipner EM, Crooks JL, French J, Strong M, Nick JA, Prevots DR.Nontuberculous mycobacterial infection and environmental molybdenum in persons with cystic fibrosis: a case-control study in Colorado J Expo Sci Environ Epidemiol 10.1038/s41370-021-00360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Geological Survey NWQMC. 2012. https://www.waterqualitydata.us/portal/
- 11. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc . 2015;12:642–647. doi: 10.1513/AnnalsATS.201412-559OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis . 2020;71:e1–e36. doi: 10.1093/cid/ciaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bureau USC.2016.
- 14.Survey USG.Dictionary of Water Terms. In: Interior Dot, editor. [Google Scholar]
- 15.National Centers for Environmental Information NOAA. 2021.
- 16.Kassambara A.http://www.sthda.com/english/wiki/practical-guide-to-principal-component-methods-in-r
- 17.Kassambara A.http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/113-ca-correspondence-analysis-in-r-essentials/
- 18. Winthrop KL, Varley CD, Ory J, Cassidy PM, Hedberg K. Pulmonary disease associated with nontuberculous mycobacteria, Oregon, USA. Emerg Infect Dis . 2011;17:1760–1761. doi: 10.3201/eid1709.101929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirschner RA, Jr, Parker BC, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am Rev Respir Dis . 1992;145:271–275. doi: 10.1164/ajrccm/145.2_Pt_1.271. [DOI] [PubMed] [Google Scholar]
- 20. Yoshimura F, Brodie AF. Interaction of vanadate with membrane-bound ATPase from Mycobacterium phlei. J Biol Chem . 1981;256:12239–12242. [PubMed] [Google Scholar]
- 21. Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem . 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 22. Falkinham JO, III, George KL, Parker BC, Gruft H. In vitro susceptibility of human and environmental isolates of Mycobacterium avium, M. intracellulare, and M. scrofulaceum to heavy-metal salts and oxyanions. Antimicrob Agents Chemother . 1984;25:137–139. doi: 10.1128/aac.25.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rehder D. The role of vanadium in biology. Metallomics . 2015;7:730–742. doi: 10.1039/c4mt00304g. [DOI] [PubMed] [Google Scholar]
- 24. Glass JB, Axler RP, Chandra S, Goldman CR. Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front Microbiol . 2012;3:331. doi: 10.3389/fmicb.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. George KL, Parker BC, Gruft H, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am Rev Respir Dis . 1980;122:89–94. doi: 10.1164/arrd.1980.122.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Smith DB, Solano F, Woodruff L.G., Cannon W.F., Ellefsen K.J.U.S. Department of the Interior, U.S. Geological Survey. Denver, CO: 2019. https://pubs.usgs.gov/sir/2017/5118/sir20175118_element.php?el=23 [Google Scholar]
- 27.Levillain F, Poquet Y, Mallet L, Mazères S, Marceau M, Brosch R, et al. Horizontal acquisition of a hypoxia-responsive molybdenum cofactor biosynthesis pathway contributed to Mycobacterium tuberculosis pathoadaptation. PLoS Pathog. 2017;13:e1006752. doi: 10.1371/journal.ppat.1006752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams MJ, Kana BD, Mizrahi V. Functional analysis of molybdopterin biosynthesis in mycobacteria identifies a fused molybdopterin synthase in Mycobacterium tuberculosis. J Bacteriol . 2011;193:98–106. doi: 10.1128/JB.00774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGuire AM, Weiner B, Park ST, Wapinski I, Raman S, Dolganov G, et al. Comparative analysis of Mycobacterium and related Actinomycetes yields insight into the evolution of Mycobacterium tuberculosis pathogenesis. BMC Genomics . 2012;13:120. doi: 10.1186/1471-2164-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braibant M, Gilot P, Content J. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol Rev . 2000;24:449–467. doi: 10.1111/j.1574-6976.2000.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 31. Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci USA . 2001;98:12712–12717. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA . 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Price CT, Bukka A, Cynamon M, Graham JE. Glycine betaine uptake by the ProXVWZ ABC transporter contributes to the ability of Mycobacterium tuberculosis to initiate growth in human macrophages. J Bacteriol . 2008;190:3955–3961. doi: 10.1128/JB.01476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol . 1999;34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 35. Soni DK, Dubey SK, Bhatnagar R. ATP-binding cassette (ABC) import systems of Mycobacterium tuberculosis: target for drug and vaccine development. Emerg Microbes Infect . 2020;9:207–220. doi: 10.1080/22221751.2020.1714488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med . 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 37.Oh J, Shin SH, Choi R, Kim S, Park HD, Kim SY, et al. Assessment of 7 trace elements in serum of patients with nontuberculous mycobacterial lung disease. J Trace Elem Med Biol. 2019;53:84–90. doi: 10.1016/j.jtemb.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Szklo M, Nieto FJ. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers; 2007. Epidemiology Beyond the Basics. [Google Scholar]
- 39. Sedman R, Funk LM, Fountain R. Distribution of residence duration in owner occupied housing. J Expo Anal Environ Epidemiol . 1998;8:51–58. [PubMed] [Google Scholar]


