Abstract
Rationale
Data on longitudinal recovery after hospitalization for coronavirus disease (COVID-19) currently remain scarce, just as outcomes beyond 3 months of follow-up do.
Objectives
To evaluate the sequelae up to 6 months after hospitalization for COVID-19 by considering 1) recovery as it relates to pulmonary function, radiological abnormalities, physical and mental health status, and health-related quality of life (HR-QoL) and 2) the predictors of the most clinically relevant sequelae.
Methods
Patients were evaluated at 6 weeks, 3 months, and 6 months after hospitalization by using pulmonary function testing, radiological evaluation, and online questionnaires on the physical and mental health status and HR-QoL. Outcomes were analyzed using repeated-measurement analyses.
Results
Ninety-two patients were included (mean age, 58.2 ± 12.3 yr; 58 [63.0%] men). The estimated percentage of patients with impaired forced vital capacity improved from 25% at 6 weeks to 11% at 6 months; for impaired diffusion capacity, this percentage improved from 63% to 46%. Radiologically, ground-glass opacity decreased but fibrosis persisted. The majority of patients (89.1%) still reported one or more symptoms 6 months after discharge. Fatigue decreased significantly over time (P = 0.006). Nonetheless, fatigue remained in 51% of the patients at 6 months. HR-QoL (nearly) normalized in most domains at 6 months, except for physical role functioning, with persistent fatigue and the length of hospitalization being the most important predictors.
Conclusions
During the first 6 months after hospitalization for COVID-19, most patients demonstrated continuing recovery across all health domains, but persistent sequelae were frequent. Fatigue was the most frequent residual and persistent symptom up to 6 months after hospitalization, importantly impacting HR-QoL.
Keywords: coronavirus disease, pulmonary function, trajectories of recovery, patient-reported outcomes, fatigue
The coronavirus disease (COVID-19) pandemic overwhelmed the world in 2020, and the pandemic is still ongoing, affecting millions of people. Infection with COVID-19 in humans is associated with respiratory symptoms that range from very mild symptoms to severe bilateral pneumonia. In 5–14% of patients, the infection is severe, requiring oxygen supplementation or even prolonged ventilatory support (1, 2). Progressive respiratory failure is the primary cause of death after COVID-19 infection, with overall hospital mortality being approximately 15–20% (2).
Knowledge on the sequelae of the COVID-19 infection is still scarce. However, it becomes increasingly clear that up to 10% of patients experience persistent symptoms 3 months after infection or even longer (3). These numbers are even higher (approaching 50%) in patients who were more severely affected, such as those who survived hospitalization, with dyspnea and fatigue being the most prevalent symptoms (4). In addition, impaired mental health (including anxiety, depression, post-traumatic stress, and cognitive dysfunction) and health-related quality of life (HR-QoL) have been reported (5, 6).
Because persistent symptoms and impairment of pulmonary function are known to last for months or even years in survivors from other coronavirus pneumoniae (severe acute respiratory syndrome [SARS] and Middle East respiratory syndrome) (7, 8), we hypothesize that there are long-lasting sequelae from COVID-19 as well and that there is a relation between the severity of COVID-19 and outcomes.
However, longitudinal data on recovery after hospitalization for COVID-19 remain scarce, just as outcomes beyond 3 months of follow-up (FU) do (9–11). Moreover, it is unclear to what extent patients suffer from persistent pulmonary sequelae, especially in the mid to long term, and how hospitalization for COVID-19 affects the physical and mental health status and HR-QoL. Early reports indicate that at discharge after hospitalization for COVID-19, a considerable proportion of patients had impaired pulmonary function, especially reduced diffusion capacity (47%) (12). Similarly, at 3 months of FU, restrictive defects (7–13%), diffusion impairment (24–34%), and development of pulmonary fibrosis (19–26%) have been reported and are more frequent after critical COVID-19 (6, 11, 13, 14). Recently, similar numbers of persistent pulmonary function impairment and residual radiological abnormalities at 6 and 12 months after hospitalization were reported, but whether recovery over time occurs and to what extent remains unclear (9–11).
Given the lack of long-term longitudinal data on recovery after hospitalization for COVID-19, we aimed to evaluate 1) recovery as it relates to pulmonary function, radiological abnormalities, physical and mental health status, and HR-QoL over time up to 6 months after hospitalization for COVID-19 and 2) the predictors of the most clinically relevant sequelae in these domains.
Methods
Study Design and Participants
This was a prospective cohort study of patients with COVID-19 who were discharged from the Erasmus Medical Center (MC) (University MC, Rotterdam, the Netherlands) in the first phase of the pandemic. We collected data as part of CO-FUS (COVID-19 FU Study). Within the Erasmus MC, there was universal opt-out informed consent established regarding all clinical data of patients who had been hospitalized for COVID-19 (Erasmus MC COVID-19 Observational Research). The medical ethics committee of our center reviewed this study and found it not to be subject to the Medical Research Involving Human Subjects Act (MEC-2020-0511). Nonetheless, participants could use opt-out online consent before filling out the questionnaires, and in addition, written informed consent was given at the outpatient clinic during FU.
All consecutive patients who were discharged between February 28 and July 31, 2020, were potential candidates for the study, and FU was completed on January 31, 2021. Patients were eligible for inclusion if they visited the outpatient clinic for FU after hospitalization for COVID-19, were over 18 years old, and had an established diagnosis of COVID-19. This diagnosis either was based on a positive reverse transcription–polymerase chain reaction result or detection of nucleic acid from COVID-19 before or during the initial hospitalization or was based on a multidisciplinary team decision regarding symptoms and chest computed tomography (CT) scan findings during hospitalization, which were later confirmed with positive serology results.
Study Procedures
FU visits were scheduled around 6 weeks and 3 and 6 months after hospitalization. Patients without signs of residual radiological or pulmonary function abnormalities at one of the FU visits were not scheduled for continuing visits. Before all the outpatient visits, patients were asked to fill out several online questionnaires. Patients who were discharged from clinical FU continued completing the online questionnaires.
Pulmonary function tests were performed at all outpatient visits and consisted of spirometry measuring forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and the diffusing capacity of the lung for carbon monoxide adjusted for hemoglobin (DlCOc). Radiographic FU consisted of chest radiography at 6 weeks, which was followed by thin-section noncontrast chest CT scans at 3 and 6 months.
Measurements
Pulmonary sequelae
Pulmonary function tests were performed to assess the FVC and FEV1 in liters and the DlCOc in mmol/(min ⋅ kPa).We performed measurements according to the current guidelines from the American Thoracic Society and the European Respiratory Society (15). The Global Lung Function Initiative Network reference values were used to express the percentages of predicted values of FVC, FEV, and DLCOc% (FVC%, FEV1%, DLCOc%), the z-scores, and the lower limit of normal (LLN) (16, 17). A z-score below −1.64 indicated a measurement under the LLN.
Experienced chest radiologists interpreted the CT scans during FU by using a standardized assessment. Chest radiographs were classified as normal or as demonstrating moderate or severe abnormalities on the basis of their report. CT scans were classified as normal; as demonstrating the presence of ground-glass opacities (GGO) (moderate/severe) only or GGO with other abnormalities, including bronchiectasis or bronchiolectasis (moderate/severe), consolidations, reticulation/fibrosis, and subpleural lines and bands; or as demonstrating other abnormalities only.
To assess the antibody kinetics over time, sera were collected at every FU visit. Qualitative enzyme-linked immunosorbent assays were performed for the detection of total antibodies and immunoglobulin M (IgM) class antibodies to COVID-19 (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.). Optical density ratios above 1 were interpreted as positive results, as indicated by the manufacturer. In this assay, total IgM class antibodies above 10 indicate the presence of neutralizing antibodies (18).
Physical and mental health status
Together with patients, a new Corona Symptom Checklist was developed on “novel symptoms since the onset of COVID-19” during the first 3 months, which was added to the online questionnaires with the answer options of “yes” or “no” (see the online supplement for the complete questionnaires).
Fatigue was assessed by using the Fatigue Assessment Scale (FAS), which is a 10-item self-report questionnaire and has been validated in patients with chronic lung disease (19, 20). The items are scored on a Likert scale ranging from 1 to 5, and the total score ranges from 10 to 50, increasing with more frequent fatigue problems. Total scores of ⩾22 are considered to represent substantial fatigue. A change in the FAS score of 4 points or more indicates a clinically significant change in fatigue (21).
Anxiety and depression were measured by using the Hospital Anxiety and Depression Scale (HADS) (22). The HADS is a 14-item self-report measure and is commonly used to determine the degree of anxiety (HADS anxiety subscale) and depression (HADS depression subscale) (each including 7 items, which are scored on a Likert scale ranging from 0 to 3). A sum score ⩾8 on either the HADS anxiety subscale or the HADS depression subscale is classified as clinically relevant anxiety or depression, respectively (22). The HADS has demonstrated its validity in many settings, including during the aftermath of a critical illness (23).
Symptoms of post-traumatic stress disorder (PTSD) were assessed by using the Impact of Event Scale–Revised (IES-R) (24). The IES-R is a self-report measure consisting of 22 items, which are scored on a Likert scale ranging from 0 to 4, assessing subjective distress caused by a traumatic event, and has previously been validated in intensive care unit (ICU) survivors (25). The IES-R consists of three subscales, indicating symptoms of intrusion, avoidance, and hyperarousal. Total scores range from 0 to 88, with higher scores indicating more severe symptoms. An IES-R total score ⩾24 is considered clinically meaningful PTSD, with mild (score of 24–32), moderate (score of 33–38), or severe (score of 39–88) complaints.
HR-QoL
HR-QoL was assessed by using the 36-item Short Form Health Survey (SF-36) (26, 27). The SF-36 is a 36-item patient-reported survey and consists of eight domains: physical functioning, social functioning, physical role functioning, emotional role functioning, mental health, vitality, bodily pain, and general health perception (27). Each domain score is directly transformed to a scale score ranging from 0 (worst score) to 100 (best score). The SF-36 has been extensively validated in the Dutch population (28).
Data Collection
Patient characteristics (age at admission, sex, and body mass index [BMI]) and clinical data (medical history, laboratory values and chest radiography findings at admission, occurrence of thrombosis and delirium during hospitalization, ICU admission, invasive ventilation, prone positioning, ICU length of stay [LOS], hospital LOS, in-hospital COVID-19–directed treatment, and pulmonary function and radiology at FU) were collected in Castor EDC software. Questionnaires on physical and mental health status and HR-QoL were completed online and directly collected in Castor EDC. All data were pseudonymized before storing and were not directly retraceable to the individual patient.
Statistical Analysis
Normally distributed variables were reported as means with standard deviations or were otherwise reported as medians with interquartile ranges, and categorical variables were reported as numbers with percentages. For longitudinal analyses, we used linear mixed model (LMM) analyses and generalized LMM (GLMM) analyses to take into account the correlation within and between repeated measurements and to use all available measurements despite missing data. The LMM and GLMM can handle missing data, under the missing-at-random (MAR) assumption, by estimating the within- and between-subject covariance matrix on the basis of the observed data that are all included in these repeated-measurement analyses. Missing data were assumed to be MAR, as the missing data at 6 months depend on the observations at 3 months and 6 weeks of FU.
We performed LMM analyses to assess recovery over time for variables on an interval scale (pulmonary function, fatigue, anxiety/depression, PTSD, and HR-QoL), and these results are presented as estimated means with standard errors. We used a GLMM for dichotomous outcomes to model the probability of abnormal outcomes over time, and these results are presented as estimated proportions with standard errors. The dependent variables in these models were the observed outcomes at 6 weeks, 3 months, and 6 months. The measurement time point was entered as a factor into each model to evaluate changes over the total FU time and to make pairwise comparisons between the time points in post hoc analyses. Fixed baseline risk factors (sex, age, BMI, length of hospital stay, length of ICU stay) and time-varying covariates (FVC%, DlCOc%, FAS, HADS, IES-R, chest CT abnormalities) were added to these models one by one to assess their predictive value. Subsequently, all significant predictors were entered into multivariable models for each outcome of interest to assess their independent predictive value. Goodness of fit was checked by using the Akaike information criterion. For all analyses, a P value of <0.05 was considered to indicate statistical significance. All analyses were performed by using SPSS version 25 software (IBM SPSS Statistics).
Results
Study Sample
Between February 28 and July 31, 2020, 171 patients were discharged from the Erasmus MC after admission for COVID-19. Our center served as a referral center for mostly ICU patients, and because of many transfers from other regions during the first phase of the pandemic, a portion of these patients did not undergo FU in our hospital. Figure E1 in the online supplement presents the numbers of patients during each FU visit at the outpatient clinic. At 6 weeks after hospitalization, 92 patients were included in this study and had a mean age of 58.2 ± 12.3 years, 58 (63.0%) were male, 74 (80.4%) had one or more comorbidities, 36 (39.1%) had two or more comorbidities, and the median LOS in the hospital was 21.5 (13.0–40.8) days (Table 1). Of the included patients, 61 (66.3%) had been admitted to the ICU and had a median LOS of 20 (11.0–33.0) days. At admission, radiographic abnormalities were seen in 80 (87.0%) patients, and 30 (32.6%) had a thrombotic event during admission. In total, 25 (27.2%) patients received one or more COVID-19–directed drug therapies, with therapies consisting of (hydroxy)chloroquine in 3 (3.3%) patients, antivirals in 3 (3.3%) patients (remdesivir in 1, lopinavir/ritonavir in 2), steroids in 17 (18.5%) patients (dexamethasone in 1, prednisone in 6, and high-dose methylprednisolone in 10), and other antiinflammatory agents in 4 (4.3%) patients (anti-IL6 in 1 and anti-IL1 in 3).
Table 1.
Baseline characteristics of patients hospitalized for COVID-19
| n * | All (N = 92) | |
|---|---|---|
| Patient characteristic | ||
| Age, yr | — | 58.2 ± 12.3 |
| Sex, male | — | 58 (63.0) |
| BMI | — | 29.9 (6.6) |
| Comorbidities | ||
| None | — | 18 (19.6) |
| Comorbidities, >1 | — | 36 (39.1) |
| Obesity, BMI > 30 kg/m2 | — | 37 (40.2) |
| Diabetes | — | 15 (16.7) |
| Cardiovascular disease and/or hypertension | — | 28 (31.1) |
| Pulmonary disease | — | 14 (15.6) |
| Renal disease | — | 5 (5.4) |
| Gastrointestinal disease | — | 1 (1.1) |
| Neuromuscular disease | — | 3 (3.3) |
| Malignancy | — | 4 (4.3) |
| In-hospital characteristics | ||
| PCR-confirmed COVID-19 | — | 86 (93.5) |
| Serology-confirmed COVID-19 | — | 6 (6.5) |
| Laboratory values | ||
| Creatinine, μmol/L | 87 | 83.0 (68.0–102.0) |
| CKD-EPI equation–derived eGFR, ml/min | 87 | 85.0 (64.0–92.0) |
| CRP, mg/L | 82 | 108.5 (53.8–195.3) |
| Ferritin, μg/L | 63 | 839.0 (454.0–1,565.0) |
| ALAT, U/L | 87 | 30.0 (22.0–51.0) |
| Hemoglobin, mmol/L | 86 | 8.0 (7.5–8.9) |
| MCV, fl | 83 | 89.0 (86.0–91.0) |
| Thrombocyte count, 109/L | 84 | 224.5 (171.0–294.8) |
| Lymphocyte absolute count, 109/L | 66 | 0.90 (0.68–1.2) |
| D-dimer, mg/L | 24 | 0.72 (0.52–1.5) |
| NT-pro-BNP, pmol/ml | 18 | 14.5 (7.3–41.3) |
| IL-6, pmol/ml | 25 | 58.0 (29.0–153.5) |
| Chest X-ray abnormalities | 86 | — |
| Normal | — | 6 (7.0) |
| Moderate | — | 15 (17.4) |
| Severe | — | 65 (75.6) |
| Thrombosis | — | 30 (32.6) |
| Deep vein thrombosis | — | 4 (4.3) |
| Pulmonary embolism | — | 25 (27.2) |
| Subsegmental pulmonary embolism | — | 18 (19.6) |
| Segmental pulmonary embolism | — | 10 (10.9) |
| Saddle pulmonary embolism | — | 3 (3.3) |
| Delirium | — | 48 (52.2) |
| Requiring oxygen supplementation | — | 85 (92.4) |
| Requiring high flow nasal cannula | — | 20 (22.5) |
| ICU admission | — | 61 (66.3) |
| Invasive mechanical ventilation | — | 55 (90.2) |
| Prone positioning | — | 52 (85.2) |
| Length of intubation, d | — | 15.0 (10.0–31.5) |
| Tracheostomy | — | 33 (54.1) |
| Length of ICU stay, d | — | 20.0 (11.0–33.0) |
| Length of hospital stay, d | — | 21.5 (13.0–40.8) |
| In-hospital treatment | ||
| (Hydroxy)chloroquine | — | 3 (3.3) |
| Antivirals | — | 3 (3.3) |
| Steroids | — | 3 (3.3) |
| Antiinflammatory therapy | — | 3 (3.3) |
| Time interval between discharge and follow-up visit | ||
| 6-wk visit, d | 92 | 48.0 ± 10.0 |
| 3-mo visit, d | 89 | 92.3 ± 11.7 |
| 6-mo visit, d | 46 | 171.2 ± 17.0 |
Definition of abbreviations: ALAT = alanine aminotransferase; BMI = body mass index; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; CRP = C-reactive protein; COVID-19 = coronavirus disease; eGFR = estimated glomerular filtration rate; ICU = intensive care unit; IL-6 = interleukin 6; MCV = mean corpuscular volume; NT-pro-BNP = N-terminal-pro hormone B-type natriuretic peptide; PCR = polymerase chain reaction.
Data are presented as the n (%), mean ± standard deviation, or, for non–normally distributed variables, median (interquartile range).
The adjusted n is presented for variables with a total number of patients less than 92.
Pulmonary Function
Spirometry was available for 77 (83.7% of patients with an FU visit), 89 (100%), and 43 (89.5%) patients at 6 weeks, 3 months, and 6 months of FU, respectively. The estimated results from the LLMs over time are presented in Table 2. At 6 weeks, the FVC was below the LLN in 25% (±4.7%) of the patients, decreasing to 21% (±4.3%) and 11% (±5.3%) after 3 and 6 months, respectively. The estimated mean FVC increased significantly over time from 3.4 L (±0.11 L) to 3.7 L (±0.12 L) at 6 months (P < 0.001), and the estimated mean FVC% predicted increased from 86.3% (±1.64%) at 6 weeks to 93.8% (±1.98%) (P < 0.001) at 6 months (Figure 1A). Likewise, the mean estimated FEV1% predicted improved significantly from 6 weeks to 6 months (P = 0.001) (Figure 1A). Spirometry results improved similarly across the whole range of pulmonary function.
Table 2.
Pulmonary function at 6-week and 3- and 6-month follow-up in patients hospitalized for COVID-19
| 6 Weeks | 3 Months | 6 Months | P Value | |
|---|---|---|---|---|
| Spirometry | ||||
| FVC, L | 3.4 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.1 | <0.001 |
| FVC% predicted | 86.3 ± 1.6 | 90.0 ± 1.6 | 93.8 ± 2.0 | <0.001 |
| FVC z-score | −1.0 ± 0.1 | −0.69 ± 0.1 | −0.44 ± 0.1 | <0.001 |
| FVC% < LLN | 25 ± 4.7 | 21 ± 4.3 | 11 ± 5.3 | 0.127 |
| FEV1, L | 2.7 ± 0.08 | 2.8 ± 0.08 | 2.8 ± 0.1 | 0.319 |
| FEV1% predicted | 88.4 ± 1.7 | 89.6 ± 1.6 | 92.6 ± 1.9 | 0.001 |
| FEV1 z-score | −0.80 ± 0.1 | −0.70 ± 0.1 | −1.4 ± 0.9 | 0.061 |
| FEV1% < LLN | 18 ± 4.6 | 16 ± 3.9 | 12 ± 4.8 | 0.663 |
| Gas exchange | ||||
| DlCOc, mmol/(min*kPa) | 5.9 ± 0.2 | 6.2 ± 0.2 | 6.8 ± 0.2 | <0.001 |
| DlCOc% predicted | 69.5 ± 1.8 | 73.0 ± 1.8 | 80.0 ± 1.8 | <0.001 |
| DlCOc z-score | −2.3 ± 0.2 | −2.0 ± 0.1 | −1.4 ± 0.1 | <0.001 |
| DlCOc% < LLN | 63 ± 5.5 | 51 ± 5.4 | 46 ± 7.0 | 0.021 |
Definition of abbreviations: COVID-19 = coronavirus disease; DlCOc = diffusing capacity of the lung for carbon monoxide adjusted for hemoglobin; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; LLN = lower limit of normal.
Data are presented as the mean ± standard error or proportion ± standard error. Estimates and P values were obtained by using linear mixed model analyses in cases of continuous outcomes and by using generalized linear mixed model analyses in cases of binary outcomes.
Figure 1.

Pulmonary function at 6 weeks, 3 months, and 6 months. (A) FVC and FEV1 values in liters (scale on the left side, dashed lines) and the percent-predicted values (scale on the right side, solid lines) are shown. (B) DlCOc values in mmol/min ⋅ kPa/L (scale on the left side, dashed line) and the percent-predicted values (scale on the right side, solid line) are shown. DlCOc = diffusing capacity of the lung for carbon monoxide adjusted for hemoglobin; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Diffusion capacity at 6 weeks was below the LLN in 63.0% (±5.5%) of the patients, decreasing to 51% (±5.4%) and 46% (±7.0%) at 3 and 6 months, respectively. The mean value estimate increased significantly over time from 5.9 mmol/(min ⋅ kPa) [±0.20 mmol/(min ⋅ kPa)] at 6 weeks to 6.8 mmol/(min ⋅ kPa) [±0.22 mmol/(min ⋅ kPa)] (P < 0.001) at 6 months, and the estimated DlCOc% predicted increased from 69.5% (±1.78%) at 6 weeks to 79.9% (±1.81%) at 6 months (P < 0.001) (Figure 1B).
Radiology
Six weeks after hospitalization, chest radiography findings had normalized in 17 (21.3%) patients. Chest CT scans were available in 87 (94.6%) and 46 (95.8%) patients at 3 and 6 months, respectively (Table 3). After 3 months, chest CT scans were normal in 12 (13.8%) patients, and persistent GGO were seen in 50 (57.5%) patients, of whom 12 (24.0%) had severe GGO. Bronchiectasis or bronchiolectasis was present in 31 (35.6%) patients, consolidations were present in 13 (14.9%) patients, reticulation and fibrotic changes were present in 29 (33.3%) patients, and subpleural lines and bands were present in 27 (31.0%) patients. Patients with minimal residual abnormalities and normalized pulmonary function did not undergo a repeat chest CT scan. Among patients with chest CT scans (n = 45) at both 3 and 6 months, 32 (71.0% [±6.8%]) patients had GGO at 3 months, which decreased to 27 patients (60.0% [±7.3%]) at 6 months (P = 0.126), and the presence of consolidation decreased from 27% (±6.6%) to 13.0% (±5.1%) (P = 0.030). Fibrotic changes did not decrease over time (P = 0.763).
Table 3.
Radiographic characteristics at 6-week and 3- and 6-month follow-up in patients hospitalized for COVID-19
| 6 Weeks | 3 Months | 6 Months | |
|---|---|---|---|
| Chest X-ray abnormalities, n | 80 | — | — |
| Normal | 17 (21.3) | — | — |
| Moderate | 37 (46.3) | — | — |
| Severe | 26 (32.5) | — | — |
| Chest CT scan abnormalities, n* | — | 87 | 46 |
| Normal | — | 12 (13.8) | 1 (1.2)* |
| GGO | |||
| Moderate | — | 38 (43.7) | 19 (21.8) |
| Severe | — | 12 (13.8) | 8 (9.2) |
| Bronchiectasis or bronchiolectasis | |||
| Moderate | — | 20 (23.0) | 14 (16.1) |
| Severe | — | 11 (12.6) | 7 (8.0) |
| Consolidation | — | 13 (14.9) | 6 (6.9) |
| Reticulation/fibrosis | — | 29 (33.3) | 22 (25.3) |
| Subpleural lines and bands | — | 27 (31.0) | 19 (21.8) |
| Chest CT scan abnormalities, n† | — | 45 | 45 |
| Normal | — | 1 (2.2) | 1 (2.2) |
| GGO | |||
| Moderate | — | 22 (48.9) | 19 (42.2) |
| Severe | — | 10 (22.2) | 8 (17.8) |
| Bronchiectasis or bronchiolectasis | |||
| Moderate | — | 14 (31.1) | 14 (31.1) |
| Severe | — | 11 (24.4) | 7 (15.6) |
| Consolidation | — | 12 (26.7) | 6 (13.3) |
| Reticulation/fibrosis | — | 21 (46.7) | 22 (48.9) |
| Subpleural lines and bands | — | 20 (44.4) | 18 (40.0) |
Definition of abbreviations: COVID-19 = coronavirus disease; CT = computed tomography; GGO = ground-glass opacities.
Data are presented as n (%), unless otherwise noted.
Patients without signs of radiological abnormalities at the 3-month follow-up were not scheduled for continuing visits; the percentages of abnormalities at the 6-month follow-up are based on the total number of patients at the 3-month follow-up.
For 45 patients, chest CT scans were available at both 3 and 6 months.
Antibody Kinetics
Sera were tested for 76 (83%), 75 (84%), and 47 (98%) patients at 6 weeks, 3 months, and 6 months, respectively. At 6 months after discharge, all tested patients had detectable total antibodies to COVID-19, and 96% showed optical density ratios above 10, which correlated with the presence of neutralizing antibodies (18). IgM class antibodies to COVID-19 showed gradual waning, with positivity decreasing from 78% at 6 weeks to 36% at 6 months (see Figure E2 in the online supplement).
Physical and Mental Health Symptoms
Through the newly developed Corona Symptom Checklist, 89.1% of the patients at 6 months still reported one or more physical or mental symptoms since COVID-19 infection (Table 4). Patients most frequently experienced reduced fitness (71.9%), followed by muscle weakness (54.7%), concentration and/or memory problems (53.1%), and joint complaints (46.9%). Reduced taste and/or smell was still reported by 17.2% of patients at 6 months. Several patients were discharged with supplemental oxygen during rest or exercise. This could be weaned in all patients during the first 6 weeks after discharge.
Table 4.
Prevalence of symptoms at 6-month follow-up in patients hospitalized for COVID-19
| 6 Months | |
|---|---|
| New symptoms since COVID-19 infection | n = 64 |
| One or more symptoms | 57 (89.1) |
| Reduced fitness | 46 (71.9) |
| Muscle weakness | 35 (54.7) |
| Concentration and/or memory problems | 34 (53.1) |
| Joint complaints | 30 (46.9) |
| Sleeping problems | 25 (39.1) |
| Dizziness | 22 (34.4) |
| Skin rash | 22 (34.4) |
| Tingling and/or pain in extremities | 22 (34.4) |
| Hoarseness | 16 (25.0) |
| Reduced vision | 16 (25.0) |
| Hair loss | 14 (21.9) |
| Reduced taste and/or smell | 11 (17.2) |
| Reduced hearing | 9 (14.1) |
Definition of abbreviation: COVID-19 = coronavirus disease.
Data are presented as n (%), indicating the number of patients with symptoms.
The mean FAS score was higher than the cutoff score at all FU visits, indicating the presence of overt fatigue (Figure 2A). Fatigue measured by using the FAS did not decrease significantly between 6 weeks and 3 months (P = 0.863), with a decrease only being shown from 3 months to 6 months (P = 0.002). At 6 months, a large proportion of the patients (50.8%) still experienced fatigue, as indicated by FAS score ⩾22 (Table E1).
Figure 2.
Fatigue, post-traumatic stress, anxiety, and depression at 6 weeks, 3 months, and 6 months. For each domain the cutoff score is shown. FAS = Fatigue Assessment Scale; HADS = Hospital Anxiety and Depression Scale; IES-R = Impact of Event Scale–Revised.
Symptoms of PTSD, as measured by using the IES-R, decreased significantly over time (P < 0.001), as presented in Figure 2B. Of all patients, 28.6% had signs and symptoms of PTSD at 6 weeks (which decreased to 16.4% and 3.6% at 3 and 6 mo, respectively) (Table E1).
Both mean anxiety (P = 0.003) and depression (P < 0.001) scores decreased significantly over time (Figures 2C and 2D; Table E1). The prevalence rates of anxiety were 20.6%, 19.6%, and 14% at 6 weeks, 3 months, and 6 months, respectively, and the prevalence rates of depression were 23.5%, 16.1%, and 16.1% at 6 weeks, 3 months, and 6 months, respectively.
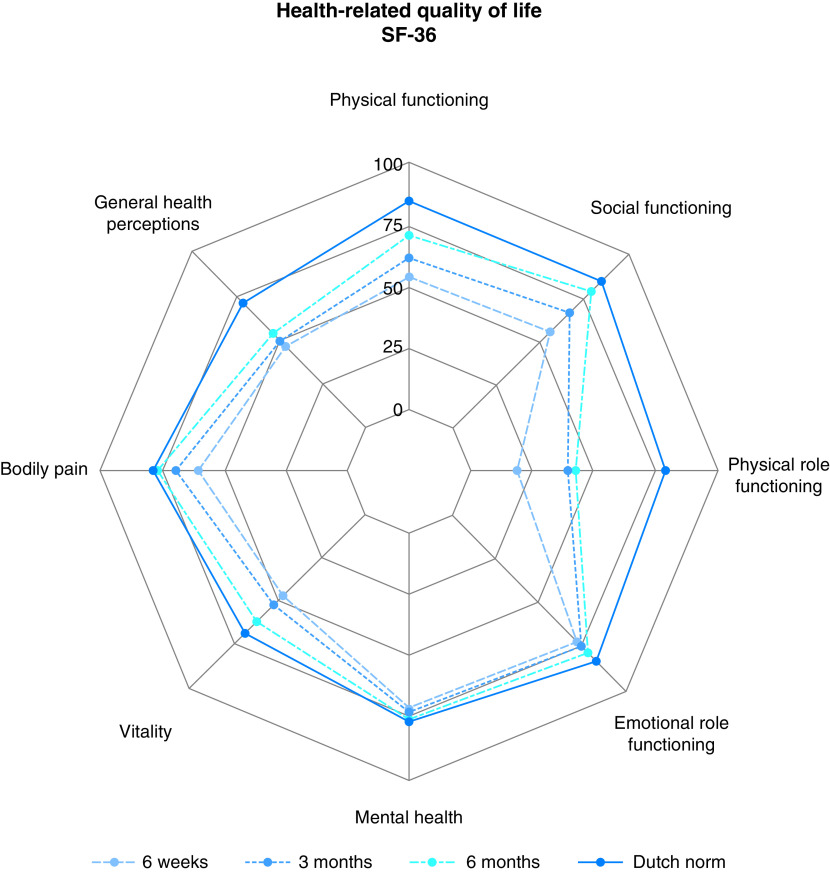
HR-QoL
Figure 3 shows the profiles of the domain scores of the SF-36 for each time point compared with the Dutch norm. Most domains improved significantly over time (P < 0.001), except for emotional role functioning (P = 0.292) and mental health (P = 0.103) (Table E1). The degrees of impairment for both emotional role functioning and mental health were limited and were similar to the degrees of impairment among the norm population at all time points. In addition, most domains (nearly) normalized at 6 months of FU, except for physical role functioning. Although gradual improvement over time was evident, a gap was still seen between its 6-month outcome and the Dutch norm outcome.
Figure 3.
Scores for the SF-36 health-related quality of life domains at 6 weeks, 3 months, and 6 months compared with the Dutch norm population. SF-36 = 36-item Short Form Health Survey.
Predictors
We analyzed the predictors of the most clinically relevant sequelae after hospitalization for COVID-19: namely DlCOc, fatigue measured by using the FAS, and physical role functioning as measured by using the SF-36. Table 5 shows the results of the LMM analyses.
Table 5.
Multivariable analyses for DlCOc%, fatigue, and physical role function up to 6 months
| DlCOc% (N Obs = 122) |
FAS (N Obs = 176) |
SF-36 RP (N Obs = 132) |
||||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | |
| Time | ||||||
| 6 wk | — | — | Ref | — | Ref | — |
| 3 mo | Ref* | — | 0.18 | 0.863 | 15.70 | 0.019 |
| 6 mo | 7.13 | <0.001 | −2.11 | 0.046 | 17.83 | 0.017 |
| Sex, female | — | — | 4.05 | 0.027 | — | — |
| Hospital LOS, d | −0.23 | 0.007 | — | — | −0.42 | 0.007 |
| Chest CT scan abnormalities | — | 0.145 | — | — | — | — |
| Normal | Ref | — | — | — | — | — |
| GGO | 2.24 | 0.632 | — | — | — | — |
| GGO + others | −3.44 | 0.361 | — | — | — | — |
| Others | −5.21 | 0.169 | — | — | — | — |
| FAS score | — | — | — | — | −2.88 | <0.001 |
| HADS-A score | — | — | — | — | ||
| HADS-D score | — | — | — | — | −1.58 | 0.122 |
| IES-R score | — | — | — | — | 0.53 | 0.089 |
Definition of abbreviations: CT = computed tomography; DlCOc = diffusing capacity of the lung for carbon monoxide adjusted for hemoglobin; FAS = Fatigue Assessment Scale; GGO = ground-glass opacities; HADS-A = Hospital Anxiety and Depression Scale anxiety subscale; HADS-D = Hospital Anxiety and Depression Scale depression subscale; IES-R = Impact of Event Scale–Revised; LOS = length of stay; N Obs = number of observations; Ref = reference category; RP = physical role functioning; SF-36 = 36-item Short Form Health Survey.
Models are estimated with linear mixed models, using time as a factor. Predictive value of fixed baseline risk factors and time-varying covariates was assessed.
The 3-month time point is the reference category as chest CT scan was added to the model, only having data at 3 and 6 months.
DlCOc was the most frequently and significantly affected pulmonary function parameter. Bivariate predictors for a lower DlCOc% were a longer time since hospitalization (P < 0.001), a longer hospital LOS (P < 0.001), and more chest CT scan abnormalities (P = 0.048). In the multivariable analysis, a longer time since hospitalization (P < 0.001) and a longer hospital LOS (P = 0.007) remained independent predictors for a lower DlCOc%.
Fatigue was the most frequent symptom during FU. Female sex was the only significant predictor of fatigue over time (mean difference of 4.05 between women and men on a FAS scale; P = 0.027). Decreased pulmonary function and chest CT scan abnormalities were not predictors of fatigue.
Physical role functioning was the most affected domain of the SF-36 after hospitalization for COVID-19 at all time points. The hospital LOS (P = 0.015), the FAS score (P < 0.001), depression (P < 0.001), and PTSD (P = 0.003) over time were bivariate predictors for physical role functioning, whereas ICU admission and reduced pulmonary function were not. In the multivariable analyses, a longer LOS in the hospital (P = 0.007) and a higher FAS score (P < 0.001) persisted as independent predictors of a lower physical role functioning score over time.
Discussion
We assessed recovery in relation to pulmonary function, radiological abnormalities, the physical and mental health status, and HR-QoL during the first 6 months after hospitalization for COVID-19 in a prospective cohort study and demonstrated recovery across all health domains in most patients.
Our data on persistent pulmonary injury are in line with earlier reports, with restrictive defects, diffusion impairment, and pulmonary fibrosis being reported in a proportion of patients depending on the cohort and occurring more frequently after critical COVID-19 (6, 11, 13, 14). This is also very much in line with outcomes after severe acute respiratory syndrome and Middle East respiratory syndrome infection (7, 8). Given the large numbers of persistent physiological and radiological signs of pulmonary fibrosis, a large cohort of patients with pulmonary fibrosis after COVID-19 with persistent and potentially progressive impairment was feared by experts (29, 30). In our cohort, reassuringly, no progressive impairment was noted. On the contrary, despite the presence of some fibrosis in almost a quarter of the patients, pulmonary function and other residual radiological abnormalities improved significantly over time.
This leads to speculation of the underlying pathophysiology of the pulmonary injury, as purely fibrotic changes do not improve over time. In a series of autoptic lungs from patients with severe endothelial injury caused by COVID-19 (associated with the presence of intracellular virus), disrupted cell membranes as well as widespread vascular thrombosis with microangiopathy and occlusion of alveolar capillaries were demonstrated (1). At the same time, the lungs from patients with COVID-19 had significant new vessel growth. Decreased diffusion and ground-glass abnormalities are possibly due to vascular damage and microangiopathy, with angiogenesis explaining partial recovery in many. Other changes, such as the parenchymal bands and reticulation, are more likely purely fibrotic in origin and remain stable over time, causing permanent physiological impairment. This impairment is mild in most patients, even after critical COVID-19, and was not related to symptoms such as dyspnea in rest or during exercise or HR-QoL, indicating limited clinical significance.
A substantial number of patients reported impaired physical and mental health up to 6 months after hospitalization. Although this may be expected as a general fitness degree after a severe illness, the number of patients reporting symptoms (including, but not limited to reduced fitness, muscle weakness, joint complaints, dizziness, and concentration and/or memory problems) was remarkable. Our findings are in line with those of various studies reporting on mid-term/long-term symptoms. We demonstrate evident recovery over time among most evaluated symptoms, such as anxiety and depression.
Notably, this was not as much the case for fatigue, which was the most frequent and persistent symptom. There was significant improvement after 3 months, but at 6 months, 51% of the patients still had fatigue on the basis of the FAS score. The phenomenon that female sex is predictive for fatigue has been described before, and further investigation of the differences in outcomes between sexes is needed (31, 32). More research into the underlying biological mechanisms is warranted, especially because of its significant impact and the distressing finding that the high prevalence did not substantially decrease over time.
Many patients after ICU treatment suffer from post–intensive care syndrome. Although it is not possible to discriminate between common sequelae of post–intensive care syndrome and effects specific to the post–COVID-19 period in these patients, we observed long-lasting physical, cognitive, and psychological consequences from COVID-19 in patients who survived ICU treatment as well as in those who did not undergo ICU treatment. It is as yet unclear how longer-term recovery will develop and whether the reduced health status will persist chronically. Earlier studies demonstrated that survivors of acute respiratory distress syndrome continued to have persistent exercise limitations and a reduced physical quality of life 5 years after their discharge from the ICU, but pulmonary function usually normalized over time (33, 34). Early mobility and rehabilitation are promising interventions for ameliorating such impairments, but it remains unclear how rehabilitation programs should be tailored to meet the needs of these patients. Given that the virus may affect multiple body systems beyond the pulmonary system, such as the cardiac, neurological, and renal systems, a one-size-fits-all approach probably will not suffice. Rather, rehabilitation programs should be individualized and integrated into care pathways aimed at early discharge from the hospital with a focus beyond restoring physical and respiratory function alone, thereby addressing fatigue, anxiety, depression, and the return to participation in society (35). Many questions remain regarding the clinical effectiveness of multimodal post–COVID-19 rehabilitation and the optimal timing of rehabilitation interventions, which will hopefully be addressed in the near future (36). This question is deemed a research priority by many societies (36, 37).
Lastly, our findings confirm that the majority of the hospitalized patients with COVID-19 develop a sustained humoral response, with detectable antibodies being found in all tested patients at 6 months after discharge. This is in agreement with a recent report that describes the evolution of COVID-19 immunity up to 6.2 months after symptom onset (38). IgM antibodies gradually wane after clinical recovery. In this population, IgM antibodies were still detectable in 36% of cases at 6 months. This illustrates that the utility of COVID-19 IgM as a marker of acute infection is limited. Because of the assay used in this study, no quantitative data are reported on (neutralizing) antibody titers.
Strengths and Limitations
We believe this study adds important knowledge about recovery after hospitalization for COVID-19. A strength of the current study are the repeated measurements over time in patients with a broad range of COVID-19 severity degrees. At the same time, compared with all patients admitted for COVID-19 in the Netherlands, the number of patients who were admitted to the ICU were overrepresented in this cohort (39). Our center mostly served as a referral center for ICU patients, hence explaining the large numbers of patients who received ICU treatment in this cohort. Furthermore, patients who had normalized pulmonary outcomes were no longer followed up in the clinic, leading to missing FU measurements. Under the MAR assumption, we used all observed data of this cohort in repeated-measurement analyses. These analyses can handle this kind of missing data by using the within- and between-subject covariance under the assumption that subjects with (nearly) normalized pulmonary outcomes at 3 months will still have good outcomes at 6 months. However, other unobserved reasons for dropout cannot be completely ruled out, and the results should therefore be interpreted with caution. Likewise, given the relatively small numbers of patients, the results of the multivariable models should be considered as explorative and hypothesis-generating, and they need further in-depth evaluation and confirmation. We will continue to prospectively follow up with a large cohort of (>500) patients within 10 hospitals, rehabilitation centers, and nursing homes in our region up to 2 years after hospitalization in the CO-FLOW (COVID-19 FU care Paths and Long-term Outcomes within the Dutch Healthcare System) study (Netherlands trial registration number NL74252.078.20), assessing long-term sequelae after COVID-19 infection, care paths, and the effects of various rehabilitation interventions.
Conclusions
In conclusion, in this prospectively followed Dutch cohort, we longitudinally describe recovery as it relates to pulmonary function, radiological abnormalities, physical and mental health status, and HR-QoL after hospitalization for COVID-19 and its main predictors. Persistent pulmonary impairment can be found for up to 6 months, but gradual improvement is seen over time. Similarly, the majority of patients reported persistent symptoms and reduced HR-QoL related to COVID-19 infection. Most of these improved over time, but fatigue was not only the most frequent but also the most persistent symptom, also seriously affecting HR-QoL. Fatigue could not be explained by the severity of COVID-19 or pulmonary function at FU. The underlying cause and optimal treatment need to be established and will be the topic of further investigations.
Footnotes
Supported by ZonMw grant 10430022010026 and Rijndam Rehabilitation, Laurens, and Erasmus Medical Center.
Author Contributions: M.E.H., S.H., and J.G.J.V.A. devised the project the main conceptual ideas and proof outline. M.E.H. and S.H. enrolled the patients. L.M.B., J.C.B., G.N., P.C., and M.C.S. contributed to data collection and aggregation. M.E.H. and J.H.V. designed the figures. L.M.B., J.C.B., S.J.B., and M.H.H.-K. performed the statistical analyses. M.E.H., S.H., L.M.B., J.C.B., G.N., C.A.M.S., J.H.V., M.E.v.G., J.v.B., D.G., A.O., P.C., M.C.S., C.G.v.K., S.J.B., G.M.R., R.J.G.v.d.B.-E., M.H.H.-K., and J.G.J.V.A. wrote the manuscript. All authors discussed the results, commented on the manuscript, and approved the final version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med . 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3. Yelin D, Margalit I, Yahav D, Runold M, Bruchfeld J. Long COVID-19: it’s not over until? Clin Microbiol Infect . 2021;27:506–508. doi: 10.1016/j.cmi.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect . 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. COVID-19 BioB Outpatient Clinic Study group Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun . 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis . 2021;73:e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest . 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park WB, Jun KI, Kim G, Choi J-P, Rhee J-Y, Cheon S, et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med Sci . 2018;33:e169. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet . 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology . 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-Month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med . 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J . 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marvisi M, Ferrozzi F, Balzarini L, Mancini C, Ramponi S, Uccelli M. First report on clinical and radiological features of COVID-19 pneumonitis in a Caucasian population: factors predicting fibrotic evolution. Int J Infect Dis . 2020;99:485–488. doi: 10.1016/j.ijid.2020.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J . 2021;57:2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J . 2017;49:1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 16. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, et al. Global Lung Function Initiative TLCO working group Global Lung Function Initiative (GLI) TLCO. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J . 2017;50:1700010. doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 18. GeurtsvanKessel CH, Okba NMA, Igloi Z, Bogers S, Embregts CWE, Laksono BM, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun . 2020;11:3436. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J Psychosom Res . 2003;54:345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 20. Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J . 2012;40:255–263. doi: 10.1183/09031936.00002512. [DOI] [PubMed] [Google Scholar]
- 21. de Kleijn WPE, De Vries J, Wijnen PAHM, Drent M. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med . 2011;105:1388–1395. doi: 10.1016/j.rmed.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 22. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand . 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23. Scragg P, Jones A, Fauvel N. Psychological problems following ICU treatment. Anaesthesia . 2001;56:9–14. doi: 10.1046/j.1365-2044.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 24. Zilberg NJ, Weiss DS, Horowitz MJ. Impact of Event Scale: a cross-validation study and some empirical evidence supporting a conceptual model of stress response syndromes. J Consult Clin Psychol . 1982;50:407–414. doi: 10.1037//0022-006x.50.3.407. [DOI] [PubMed] [Google Scholar]
- 25. Brück E, Schandl A, Bottai M, Sackey P. The impact of sepsis, delirium, and psychological distress on self-rated cognitive function in ICU survivors: a prospective cohort study. J Intensive Care . 2018;6:2. doi: 10.1186/s40560-017-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res . 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care . 1992;30:473–483. [PubMed] [Google Scholar]
- 28. Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol . 1998;51:1055–1068. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 29. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med . 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raghu G, Wilson KC. COVID-19 interstitial pneumonia: monitoring the clinical course in survivors. Lancet Respir Med . 2020;8:839–842. doi: 10.1016/S2213-2600(20)30349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshida Y, Gillet SA, Brown MI, Zu Y, Wilson SM, Ahmed SJ, et al. Clinical characteristics and outcomes in women and men hospitalized for coronavirus disease 2019 in New Orleans. Biol Sex Differ . 2021;12:20. doi: 10.1186/s13293-021-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol . 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 33. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med . 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 34. Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med . 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 35. Gorna R, MacDermott N, Rayner C, O’Hara M, Evans S, Agyen L, et al. Long COVID guidelines need to reflect lived experience. Lancet . 2021;397:455–457. doi: 10.1016/S0140-6736(20)32705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of COVID-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ . 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 37.Kennisagenda COVID-19. 2021. https://www.demedischspecialist.nl/sites/default/files/Kennisagenda%20COVID-19.pdf
- 38.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2 Nature 2021591639–644.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ontwikkeling COVID-19 in grafieken. 2021. https://www.rivm.nl/coronavirus-covid-19/grafieken