Abstract
Quinupristin-dalfopristin (Q-D) is an injectable streptogramin active against most gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA). In experimental endocarditis, however, Q-D was less efficacious against MRSA isolates constitutively resistant to macrolide-lincosamide-streptogram B (C-MLSB) than against MLSB-susceptible isolates. To circumvent this problem, we used the checkerboard method to screen drug combinations that would increase the efficacy of Q-D against such bacteria. β-Lactams consistently exhibited additive or synergistic activity with Q-D. Glycopeptides, quinolones, and aminoglycosides were indifferent. No drugs were antagonistic. The positive Q-D–β-lactam interaction was independent of MLSB or β-lactam resistance. Moreover, addition of Q-D at one-fourth the MIC to flucloxacillin-containing plates decreased the flucloxacillin MIC for MRSA from 500 to 1,000 mg/liter to 30 to 60 mg/liter. Yet, Q-D–β-lactam combinations were not synergistic in bactericidal tests. Rats with aortic vegetations were infected with two C-MLSB-resistant MRSA isolates (isolates AW7 and P8) and were treated for 3 or 5 days with drug dosages simulating the following treatments in humans: (i) Q-D at 7 mg/kg two times a day (b.i.d.) (a relatively low dosage purposely used to help detect positive drug interactions), (ii) cefamandole at constant levels in serum of 30 mg/liter, (iii) cefepime at 2 g b.i.d., (iv) Q-D combined with either cefamandole or cefepime. Any of the drugs used alone resulted in treatment failure. In contrast, Q-D plus either cefamandole or cefepime significantly decreased valve infection compared to the levels of infection for both untreated controls and those that received monotherapy (P < 0.05). Importantly, Q-D prevented the growth of highly β-lactam-resistant MRSA in vivo. The mechanism of this beneficial drug interaction is unknown. However, Q-D–β-lactam combinations might be useful for the treatment of complicated infections caused by multiple organisms, including MRSA.
Quinupristin-dalfopristin (Q-D) is a new injectable streptogramin potent against most gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant enterococci (4, 14, 15, 19). The two Q-D components bind synergistically to the 23S RNA of the bacterial ribosome and thus confer efficacy against both macrolide-lincosamide-streptogramin B (MLSB)-susceptible and MLSB-resistant bacteria (6, 20). However, although both compounds are intrinsically active against MLSB-susceptible S. aureus, the presence of a second component, i.e., dalfopristin, is absolutely required for efficacy against constitutively MLSB-resistant (C-MLSB) isolates (6). Since most MRSA in the clinical environment are C-MLSB resistant (9, 25), this phenotype may pose a therapeutic challenge.
Potential problems with C-MLSB-resistant staphylococci were first detected in early studies with animals (9, 12). Indeed, while Q-D given two times a day (b.i.d.) was successful treatment for rats with experimental endocarditis due to MLSB-susceptible MRSA, it failed as therapy against C-MLSB-resistant isolates (9). This correlated with the short life span of dalfopristin in the serum, and therapeutic efficacy could be restored by prolonging the presence of dalfopristin in the blood by using a programmable infusion pump (9; J. Vouillamoz, J. M. Entenza, M. P. Glauser, and P. Moreillon, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C91, p. 50, 1996). Moreover, experiments with the rabbit model of endocarditis indicated that dalfopristin did not penetrate cardiac vegetations as well as quinupristin (13). Thus, adequate dalfopristin levels at the infection site were critical for treatment efficacy.
To solve this problem one can either increase the Q-D dosage or seek drug combinations that would decrease the need for Q-D to be effective. First, an increase in the Q-D dosage was achieved in animals either by augmenting the number of daily doses or by delivering the drug as a continuous infusion. The two strategies improved the therapeutic efficacy of Q-D (Vouillamoz et al., 36th ICAAC) and helped define newer recommendations for Q-D administration to humans, which are now 7.5 mg/kg three times a day (t.i.d.) (18; J. Moses, E. Brown, W. Lynn, J. White, L. K. Goldberg and G. H. Talbot, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother. abstr. MN-52, p. 603, 1998) rather than 7 mg/kg b.i.d., as proposed earlier (10).
Second, seeking synergistic drug combinations is a reasonable approach that might help circumvent the risk of toxicity associated with dose escalation. Previous studies indicated that exposure of staphylococci to subinhibitory concentrations of Q-D yielded bacteria with very thick and abnormal cell walls (22, 23). On the basis of this observation, we hypothesized that concomitant treatment of staphylococci with Q-D plus a second antibiotic that specifically interferes with cell wall synthesis might result in a positive interaction. Testing of this possibility was the very purpose of the present experiments. First, the checkerboard method was used to test combinations of Q-D with a variety of antibiotics with unrelated modes of action against both MLSB-susceptible and C-MLSB-resistant S. aureus isolates. Second, selected drugs that interacted positively with Q-D were tested alone or in combination with Q-D in rats with experimental endocarditis.
MATERIALS AND METHODS
Microorganisms and growth conditions.
A panel of 8 clinical isolates of methicillin-susceptible S. aureus (MSSA) (4 MLSB-susceptible isolates and 4 C-MLSB-resistant isolates) and 10 MRSA isolates (5 MLSB-susceptible isolates and 5 C-MLSB-resistant isolates) that differed in their pulsed field gel electrophoresis profiles (3) were tested for their susceptibilities to both Q-D and unrelated antibiotics (see Table 1). Two of these isolates, namely, isolates AW7 and P8, were further used for experiments with animals. They were both C-MLSB resistant and expressed heterogeneous resistance to methicillin (9).
TABLE 1.
Ranges of MICs of several antibiotics for MLSB-susceptible and C-MLSB-resistant MSSA and MRSA isolatesa
| Antibiotic | MIC range (mg/liter)
|
|||
|---|---|---|---|---|
| MSSA MLSB-S | MSSA C-MLSB-R | MRSA MLSB-S | MRSA C-MLSB-R | |
| Q-D | 0.25–0.5 | 0.25–1 | 0.25 | 0.25–0.5 |
| Quinupristin | 1–4 | 32 | 1–4 | 32 |
| Dalfopristin | 1–4 | 1–8 | 1–4 | 1–8 |
| Flucloxacillin | 0.06–0.012 | 0.06–0.012 | 0.12–>64 | 0.12–>64 |
| Amoxicillin | 0.25–>64 | 0.25–>64 | >64 | >64 |
| Cefuroxime | 1 | 1 | >64 | 8–>64 |
| Cefamandole | 0.25–0.5 | 0.25–1 | 4–16 | 2–8 |
| Cefepime | 0.25–1 | 0.25–1 | >64 | >64 |
| Imipenem | 0.01–0.03 | 0.01–0.03 | 4–16 | 4–16 |
| Vancomycin | 0.5–1 | 0.5–1 | 2 | 1–2 |
| Rifampin | 0.004 | 0.004 | 0.004–>1 | 0.004–>1 |
| Tetracycline | 0.25 | 0.25–>64 | >64 | 32–>64 |
| Co-trimoxazole | 0.25–4 | 0.25 | 2–>8 | 2–>8 |
| Ciprofloxacin | 0.125–0.5 | 0.06–0.25 | 0.12–16 | 0.12–0.25 |
| Gentamicin | 0.125–0.5 | 0.06–16 | 6–32 | 8–32 |
The MSSA isolates include four MLSB-susceptible (MLSB-S) and four C-MLSB-resistant (C-MLSB-R) clinical isolates; the MRSA isolates include five MLSB-susceptible and five C-MLSB-resistant clinical isolates.
Unless otherwise stated, the bacteria were grown at 35°C either in tryptic soy broth (Difco Laboratories, Detroit, Mich.), in cation-supplemented Mueller-Hinton broth (Difco), or on tryptic soy agar (Difco). Except for time-kill experiments, all media were supplemented with 2% NaCl to increase the level of expression of β-lactam resistance by MRSA. Stocks were kept at −70°C in tryptic soy broth supplemented with 10% (vol/vol) glycerol.
Antibiotics and chemicals.
Q-D (RP 59500), quinupristin, and dalfopristin were provided by Rhône-Poulenc Rorer (Antony, France); cefepime was provided by Bristol-Myers Squibb AG (Baar, Switzerland). All other drugs and chemicals were commercially available products.
Susceptibility testing and antibiotic interactions.
The antibiotic MICs were determined by a previously described broth macrodilution method (1) with a final inoculum of 105 to 106 CFU/ml. Antibiotic interactions were assessed by the checkerboard method in 96-well microtiter plates (Dynatech Microtiter, Chantilly, Va.) as described previously (7). The wells were inoculated with 105 CFU/ml from a logarithmic-phase culture, and the plates were incubated for 18 h at 35°C before visible bacterial growth was determined. Fractional inhibitory concentration (FIC) indices were interpreted as follows: ≤0.5 for drug synergism, >0.5 but ≤1 for additivity, >1 but ≤4 for indifference, and >4 for antagonism (7).
Population analysis profiles and time-kill curves.
The phenotypic expression of β-lactam resistance was determined by spreading large bacterial inocula (≥109 CFU) as well as appropriate dilutions onto NaCl-supplemented agar plates containing twofold serial dilutions of antibiotics (24). In certain experiments, the plates were supplemented with a constant subinhibitory concentration (one-fourth the MIC) of Q-D to test the effect of this drug on the expression of β-lactam resistance. The numbers of colonies growing on the plates were enumerated after 48 h of incubation at 35°C. The results were expressed by plotting the numbers of colonies growing on the plates against the β-lactam concentrations in the plates.
For time-kill curve studies, series of flasks containing fresh prewarmed medium were inoculated with ca. 106 CFU/ml (final concentration) from an overnight culture of bacteria. Immediately after inoculation, the antibiotics were added to the flasks at final concentrations that approximated the peak and trough antibiotic levels produced in the serum of humans and rats by therapeutic doses of the drugs (see Results section). Viable counts were determined at various times before and after antibiotic addition by plating dilutions of the cultures onto nutrient agar. To avoid antibiotic carryover the drugs were removed from the samples by serial centrifugation as described previously (8). As an additional precaution the plates were supplemented with penicillinase (final concentration, 2,000 U/ml; Bacto-Penase concentrate; Difco) when β-lactams were used. Due to the prolonged postantibiotic effect of Q-D, the plates were incubated for at least 48 h before viable counts were obtained (5, 21).
Production of endocarditis and infusion pump installation.
The production of aortic vegetations and the installation of a central jugular line (Dow Corning Corp., Midland, Mich.) and a programmable pump (Pump 44; Harvard Apparatus, Inc., South Natick, Mass.) to deliver the antibiotics were as described previously (16, 17). In certain experiments, Q-D was injected into the animals in combination with another drug. This necessitated the use of two infusion pumps (one for each drug) which were connected to a two-way swivel (BOC Ohmeda AB, Helsinborg, Sweden) and to two independent jugular lines. No intravenous (i.v.) lines were placed in the control animals.
Bacterial endocarditis was induced 24 h after catheterization by i.v. challenge of the animals with 0.5 ml of saline containing 105 CFU of the test bacteria. This inoculum was 10 times larger than the minimum inoculum that produced endocarditis in 90% of untreated controls.
Therapy for experimental endocarditis.
Treatment was started 12 h after bacterial challenge and lasted for 3 or 5 days. The antibiotics were delivered at changing flow rates to simulate the kinetics of the drugs in humans. The Q-D treatment simulated treatment with 7 mg/kg b.i.d. (every 12 h) (10). This was a lower daily dose than that from the t.i.d. regimen now proposed for this drug (18; Moses et al., 38th ICAAC; S. A. Nachman, A. Phillips, S. L. Gray, and G. H. Talbot, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-167, p. 217, 1998) but helped detect positive drug interactions in the present experimental setting (see Results). Cefamandole was tested as a proof of concept, because it is one of the rare cephalosporins that has some activity against MRSA (24). It was administered as a continuous infusion that produced constant levels in serum of 30 mg/liter, which is one of the highest dosages that was given to a human (24). Cefepime was given to simulate i.v. treatment of humans with 2 g b.i.d. (2). This required a total amount of antibiotic (in milligrams per kilogram of body weight per 12 h) of ca. 38 mg of Q-D, 360 mg of cefamandole, and 142 mg of cefepime.
Determination of serum antibiotic concentrations.
Concentrations of antibiotics in serum were determined in groups of four to nine uninfected or infected rats. Levels in the serum of infected animals came from an internal control for adequate drug delivery in therapeutic experiments. Blood was drawn by puncturing the periorbital sinuses. For Q-D, blood was acidified and processed as described previously (9). Micrococcus luteus ATCC 9341 was used as an indicator organism to measure the total Q-D activity. Individual quinupristin and dalfopristin concentrations were measured occasionally as described previously (9). Bacillus subtilis ATCC 6633 was used to titrate cefamandole and cefepime. Standard curves were constructed with pooled rat serum as the diluent, and the standard samples were acidified for Q-D titration as described above. The limits of detection for the assay were ca. 0.3 mg/liter for Q-D and cefamandole and 3 mg/liter for cefepime. The linearity of the standard curve was assessed by use of a regression coefficient of 0.998, and the coefficient of variation of the assay was consistently less than 10%.
Evaluation of infection.
Control rats were killed at treatment onset (12 h after inoculation) in order to measure both the frequency and the severity of valve infection at the start of therapy. Treated rats were killed 12 h after the trough level after administration of the last antibiotic dose was achieved, a time at which no residual antibiotic could be detected in the blood. The vegetations were dissected, weighed, homogenized in 1 ml of saline, and serially diluted before being plated for colony counts. Quantitative blood and spleen cultures were performed in parallel. Several animals died before the end of treatment due to either complications of the operation (such as possible catheter-induced arrhythmia) or the infective process. The blood and spleens from these animals were not cultured. Only rats that had received at least two-thirds of the treatment were taken into account for vegetation bacterial counts. The numbers of colonies growing on the plates were determined after 48 h of incubation at 35°C. Bacterial densities in the vegetations were expressed as log10 CFU per gram of tissue. The minimum detection level was ≥2 log10 CFU/g of vegetation. For statistical comparisons of differences between the vegetation bacterial densities of various treatment groups, culture-negative vegetations were considered to contain 2 log10 CFU/g.
Selection for antibiotic resistance in vitro an in vivo.
Highly β-lactam- and/or Q-D-resistant MRSA subpopulations were assessed from an in vitro population analysis profile. Colonies growing on drug-containing agar were enumerated after 48 h of incubation at 35°C, and the MICs for these colonies were determined. The emergence of resistance in vivo was evaluated by plating 0.1-ml portions from undiluted vegetation homogenates both on plain agar and on agar supplemented with five times the MICs of the test antibiotics. As described above, the MICs for bacteria growing on antibiotic-containing plates were redetermined in liquid media. The in vivo screening was performed only for Q-D and cefepime.
Statistical analysis.
The median bacterial densities in the vegetations of various treatment groups were compared by the nonparametric Kruskal-Wallis one-way analysis of variance on ranks, with subsequent pairwise multiple comparison procedures done by Dunn's method. The differences in mortality rates were analyzed by the χ2 test with Yates' correction. Overall, differences were considered significant when P was ≤0.05 by use of two-tailed significance levels.
RESULTS
Antibiotic susceptibility and FIC indices.
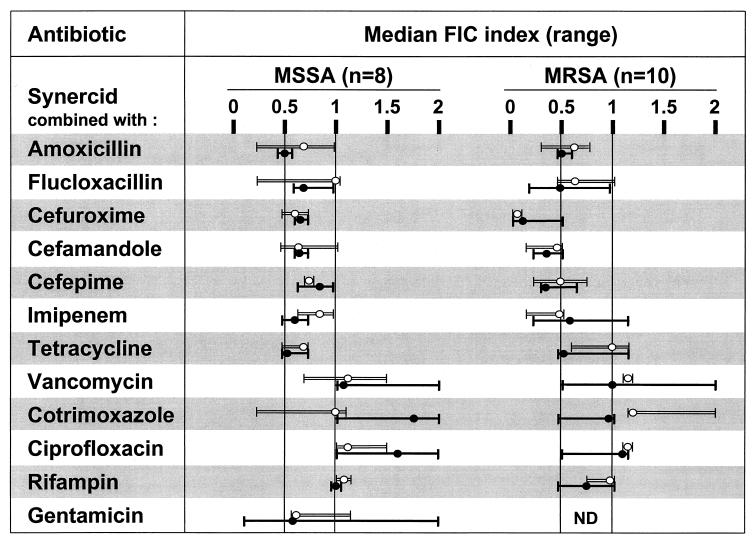
Table 1 presents the MICs of several antibiotics for the 8 MSSA and 10 MRSA isolates used in vitro. Figure 1 presents the FIC indices of Q-D combined with other antibiotics for these bacteria. As can be seen, Q-D demonstrated either additive activity (FIC index, between 0.5 and 1) or synergistic activity (FIC index, ≤0.5) with all the β-lactams and with tetracycline. FIC indices were not affected either by MLSB resistance or by β-lactam resistance. Combinations of Q-D with other antibiotics were indifferent (FIC indices, >1 but ≤4). No antagonism (FIC index, >4) was observed.
FIG. 1.
FIC indices for Q-D (Synercid) combined with a variety of antibiotics as determined by the checkerboard method. Median (range) values are indicated for each combination tested against both MLSB-susceptible (four MSSA and five MRSA isolates; open bars) and C-MLSB-resistant (four MSSA and five MRSA isolates; plain bars) clinical isolates of S. aureus. The vertical line at 0.5 indicates the limit for synergism (FIC index, ≤0.5). The vertical lines between 0.5 and 1 delineate the area of addition (FIC index, >0.5 but ≤1). The area above 1 indicates indifference (FIC index, >1 but ≤4) (7). No antagonism between Q-D and any of the other drug tested was observed. ND, not determined.
Two previously described C-MLSB-resistant MRSA isolates (isolates AW7 and P8) (9, 24) were selected for further studies (see below). For these strains, the MICs of the antibiotics used to treat animals were as follows: (i) for Q-D, 0.5 mg/liter (susceptibility and resistance breakpoints, 1 and 4 mg/liter, respectively [National Committee for Clinical Laboratory Standards Meeting, 8 June 1999, Reston, Va.]); (ii) for cefamandole, 8 mg/liter (susceptibility and resistance breakpoints, 8 and 32 mg/liter, respectively); and (iii) for cefepime, 64 mg/liter (susceptibility and resistance breakpoints, 8 and 32 mg/liter, respectively).
Population analysis profile and time-kill experiments.
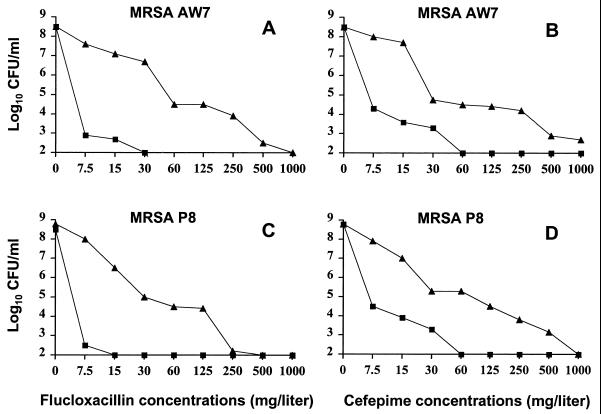
Figure 2 presents the population analysis profiles determined on agar plates that contained either flucloxacillin (Fig. 2A and C) or cefepime (Fig. 2B and D) and that were supplemented or not with one-fourth the MIC of Q-D. In the absence of Q-D, both MRSA grew on plates that contained up to 1,000 mg of the β-lactams per liter. In the presence of subinhibitory Q-D concentrations, on the other hand, neither of the organisms grew on plates containing ≥30 mg of flucloxacillin per liter or ≥60 mg of cefepime per liter. This bacteriostatic synergism was in accordance with the FIC indices (Fig. 1) and was also supported by a recent report that indicated that non-cell wall inhibitors, including Q-D, could affect the expression of β-lactam resistance by MRSA (26).
FIG. 2.
Population analysis profiles of MRSA AW7 (A and B) and MRSA P8 (C and D) grown on agar plates containing increasing concentrations of either flucloxacillin (A and C) or cefepime (B and D). Large numbers of bacteria (ca. 109 CFU) were spread onto plates supplemented with increasing concentrations of either flucloxacillin or cefepime used alone (triangles) or in combination with a constant subinhibitory concentration of Q-D of 0.125 mg/liter (equal to one-fourth the MIC) (squares). The plates were incubated for 48 h at 35°C before the colonies were counted. The number of colonies was plotted as a function of the β-lactam concentrations present in the plates.
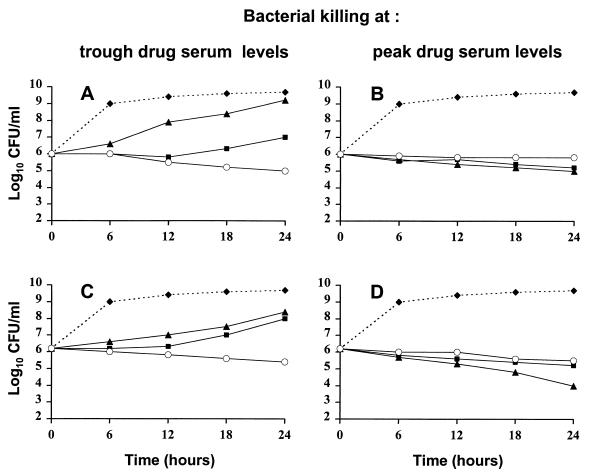
To test whether this enhanced growth inhibition translated into bacterial killing, bactericidal experiments were performed by using the peak and through antibiotic concentrations produced in serum human during standard therapy (Fig. 3). At trough concentrations (Fig. 3A and C), both drugs used alone failed to prevent bacterial growth. In contrast, the Q-D–cefepime combination successfully blocked growth and even inflicted a marginal yet reproducible viability loss of 1 to 2 log10 CFU/ml after 24 h. Similar results were obtained when Q-D was combined with the other β-lactams and staphylococci presented in Fig. 1 (J. Vouillamoz, J. M. Entenza, M. Giddey, M. P. Glauser, and P. Moreillon, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E7, p. 82, 1996).
FIG. 3.
Time-kill experiments with MRSA AW7 (A and B) and MRSA P8 (C and D) exposed to concentrations of Q-D and/or cefepime that mimic either trough antibiotic levels (A and C) or peak antibiotic levels (B and D) obtained during i.v. treatment in humans or rats. Cultures received either no drug (diamonds), Q-D alone (squares), cefepime alone (triangles), or Q-D and cefepime combined (open circles). Trough concentrations (A and C) of Q-D and cefepime were 0.5 mg/liter (1× the MIC) and 5 mg/liter (1/12× the MIC), respectively. Peak concentrations (B and D) of Q-D and cefepime were 5 mg/liter (20× the MIC) and 160 mg/liter (2.5× the MIC), respectively. Antibiotics were added to the cultures at time zero. At various times before and after antibiotic addition, samples were removed from the cultures, diluted, and plated for colony count determinations, with adequate measures taken to avoid antibiotic carryover (see Materials and Methods). Each dot represents the mean of at least three separate experiments.
At peak concentrations in serum (Fig. 3B and D), on the other hand, the combination of the two drugs was not more bactericidal than either drug alone. The two antibiotics even tended to be antagonistic in terms of killing. This paradoxical high-dose, low-dose effect of Q-D–β-lactam combinations was also observed with other β-lactams and other staphylococcal isolates (Vouillamoz et al., 36th ICAAC). To clarify the potential relevance of these effects in vivo, Q-D was used alone or in combination with cefamandole or cefepime to treat rats with experimental MRSA endocarditis.
Concentrations of antibiotics in serum of rats.
Q-D concentrations in serum were 5 mg/liter at 1 h after treatment onset, 2 mg/liter after 2 h, 0.7 mg/liter after 4 h, and below the level of detection after 6 h, as measured by the global bioassay (9). Cefamandole was given continuously to produce constant concentrations of 30 mg/liter, as described previously (24). Cefepime concentrations were 166.2 ± 18.8 mg/liter at 0.5 h after treatment onset, 62.8 ± 10.7 mg/liter after 2 h, 47.3 ± 9.8 mg/liter after 4 h, and 7.1 ± 3.3 mg/liter after 12 h (2). These concentrations encompassed the genuine in vivo dynamic between the peak and trough antibiotic concentrations tested in vitro.
Efficacy of Q-D alone or in combination with cefamandole or cefepime in the treatment of experimental endocarditis.
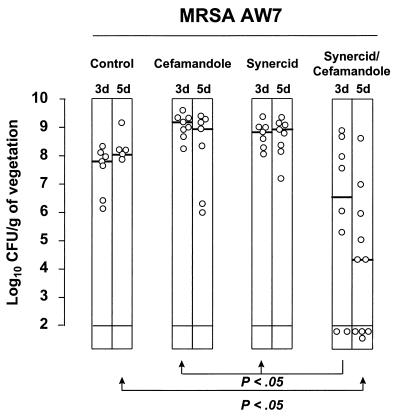
Figure 4 depicts the therapeutic results with Q-D and cefamandole against infection due to MRSA AW7. Both drugs used alone failed to cure the animals. Bacteria even continued to grow in spite of antibiotic treatment. In sharp contrast, Q-D plus cefamandole progressively reduced the bacterial densities in the vegetations. After 3 days, this reduction was statistically significant (P < 0.05) compared to the densities in the vegetations of rats treated with Q-D or cefamandole alone but not compared to those in animals killed at treatment onset (P > 0.05). After 5 days, on the other hand, combination therapy had significantly (P < 0.05) decreased the vegetation bacterial titers compared to those for both of these control groups.
FIG. 4.
Treatment of experimental endocarditis due to MRSA AW7 with Q-D (Synercid) and cefamandole used alone or in combination. Each dot indicates the bacterial density in the vegetations of independent animals. The horizontal bars in the columns indicate the median values for the group. Treatment groups and treatment duration are indicated at the top of the graph. Control animals were killed at treatment onset, i.e., 12 h after inoculation, in order to determine the frequency and severity of valve infection. Treatment duration was either 3 days (3 d) or 5 days (5 d). A P value of <0.05 indicates that the difference between the groups was statistically significant.
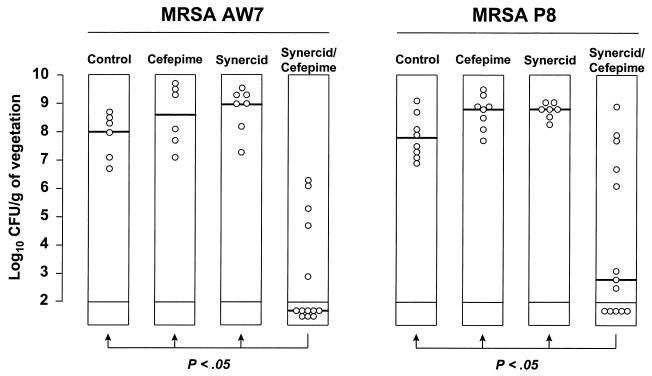
Figure 5 depicts the results of a similar experiment performed with Q-D and cefepime against both MRSA AW7 and MRSA P8. The result resembled those observed with cefamandole; i.e., monotherapy failed, whereas the Q-D–cefepime combination significantly (P < 0.05) decreased the vegetation bacterial titers compared to those both for rats that received single-drug therapy and for untreated control rats.
FIG. 5.
Treatment of experimental endocarditis due to MRSA AW7 or MRSA P8 with Q-D (Synercid) and cefepime used alone or in combination. Each dot indicates the bacterial density in the vegetations of independent animals. The horizontal bars in the columns indicate the median values for the group. Control animals were killed at treatment onset, i.e., 12 h after inoculation, in order to determine the frequency and severity of valve infection. Animals receiving antibiotics were treated for a total of 5 days. A P value of <0.05 indicates that the difference between the groups was statistically significant.
Mortality, blood cultures, and spleen cultures.
The spontaneous mortality in these experiments was high, reaching 30 to 40% at 3 days and 60 to 80% at 5 days. However, mortality did not vary among the various treatments groups and thus was instead due to the complex experimental setting. When pooled together, the specific 5-day mortality rate in the experiments whose results are depicted in Fig. 4 and 5 was 13 of 21 (61%) rats in the cephalosporin monotherapy groups, 20 of 23 (86%) rats in the Q-D monotherapy group, and 25 of 36 (69%) rats in the Q-D–cephalosporin treatment groups (P > 0.05 when the results were compared by the χ2 test with Yates' correction).
Since relatively few eligible rats were left for blood and spleen cultures, analysis of blood and spleens was done with pooled blood and spleens from animals from the two experiments performed with MRSA AW7, the results of which are presented in Fig. 4 and Fig. 5, respectively. At the start of therapy, the blood of 18 of 18 (100%) untreated controls grew bacteria with a median of 500 CFU/ml (range, 7 to >1,000 CFU/ml). At the end of treatment, six of six available animals in the cefamandole and cefepime groups and four of four animals in the Q-D group had positive blood cultures that contained ≥1,000 CFU/ml. In contrast, only 4 of 11 (36%) rats treated with the drug combinations had positive blood cultures, which contained a median of only 12 CFU/ml (range, 1 to 100 CFU/ml).
Similar observations were made with the spleens. At treatment onset, the spleens of 18 of 18 (100%) control animals were positive by culture, growing a median of 1,000 CFU/g of tissue (range, 120 to ≥1,000 CFU/g of tissue). At the end of treatment, all animals that received single-drug therapy had similar bacterial densities in the spleens. In comparison, the spleens of only 4 of 11 rats that received combination therapy were positive by culture, containing a median of 56 CFU/g of tissue (range, 3 to 128 CFU/g of tissue). While these numbers are only indicative, they clearly underline the effect of combination therapy compared to the control.
Selection of antibiotic resistance.
In vitro population analysis profiles determined with cefepime and MRSA AW7 and P8 indicated that the frequency of highly resistant subpopulations (defined by growth on ≥50 mg of cefepime per liter) was ≥10−4 (Fig. 2). In contrast, similar experiments performed with Q-D showed a sharp decrease in bacterial growth with the drug at between 0.15 and 0.3 mg/liter and no residual bacterial growth with the drug at 0.6 mg/liter. This was below the 1-mg/liter susceptibility breakpoint of the drug.
In vivo emergence of Q-D resistance was routinely tested and remained undetectable in any of the experiments whose results are presented in Fig. 4 and 5. In addition, the emergence of cefepime resistance was tested in the experiment with MRSA AW7, depicted in Fig. 5. In this experiment, the valves of all rats that received cefepime alone grew highly resistant derivatives for which the MICs were ≥128 mg/liter. On the other hand, the valve of only one of the five rats (20%) in the Q-D–cefepime group with positive valve cultures grew derivatives for which the cefepime MIC was increased five or more times. Therefore, as observed in vitro, Q-D tended to impede the outgrowth of highly β-lactam-resistant MRSA subpopulations in vivo as well.
DISCUSSION
The present study investigated the potential benefit of combining Q-D with β-lactams in vitro and in rats with experimental endocarditis. The rationale for testing such drug associations was based on previous work by Lorian et al. (21, 22), who observed that staphylococci exposed to subinhibitory concentrations of Q-D produced very thick, abnormal cell walls. Thus, use of a combination of a drug that produces abnormal cell wall accumulation with drugs that inhibit cell wall assembly might result in some kind of cooperative antibacterial effect. Moreover, one in vitro study and one in vivo study suggested that this assumption might be correct (11, 26).
The present results confirmed the beneficial Q-D–β-lactam interaction against several MRSA strains. In contrast, this beneficial interaction was less obvious with other classes of antibiotics, except maybe for tetracycline, which might interact with Q-D at the ribosome level. A striking observation was that subinhibitory Q-D concentrations could prevent the outgrowth of highly β-lactam-resistant MRSA, as determined from population analysis profiles. This phenomenon could have practical implications because Q-D might decrease the MICs of certain β-lactams for MRSA to concentrations that can be achieved during standard therapy in human.
One may only speculate on the mechanism(s) of this interaction. In a recent study, Sieradzki and Tomasz (26) observed that several non-β-lactam antibiotics could affect the expression of methicillin resistance by MRSA. This suggested that exposure of bacteria to certain non-cell wall inhibitors might have deleterious repercussions on the cell wall building machinery. Likewise, we recently observed that exposure of MRSA to subinhibitory concentrations of Q-D affected the compositions of their walls, as determined by high-pressure liquid chromatography (J. Vouillamoz, P. A. Majcherczyk, H. Nadler, M. Giddey, M. P. Glauser, and P. Moreillon, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1260, p. 262, 1999). This phenomenon was also observed to a lesser extent with erythromycin but not with other ribosome inhibitors such as tetracycline and gentamicin (Vouillamoz et al., 39th ICAAC). Hence, it is possible that the dual interference of certain non-cell wall inhibitors and cell wall-acting drugs on peptidoglycan assembly might have potentiating effects.
While the positive bacteriostatic interaction between Q-D and β-lactams was detected at low concentrations, higher drug doses suggested a possible antagonism in time-kill experiments. This was reminiscent of the bactericidal interference between protein inhibitors and cell wall-active antibiotics and raised the question of its relevance in vivo. Most interestingly, however, this antagonism did not prevail in rats with experimental endocarditis. Indeed, Q-D combined with either cefamandole or cefepime significantly decreased vegetation bacterial titers and even resulted in negative valve cultures, even though the β-lactams were given at quasimaximal doses and the Q-D concentration fluctuated between the peak and trough concentrations tested in vitro. This in vitro-in vivo dissociation might be explained by the constant variation in drug concentrations at the infected site in animals. Note that the present experiments did not test definitive cure, as relapses were not evaluated in animals kept for a prolonged duration after the end of treatment. However, the significant decrease in vegetation bacterial titers conferred by the combination treatment clearly indicated its superiority over that of a single-drug therapy.
Another important question was the risk of resistance selection. In the present experiments, Q-D used alone did not select for derivatives for which MICs were increased either in vitro or in vivo. Moreover, Q-D could prevent the growth of highly β-lactam-resistant subpopulations in vitro, and also hindered—but did not entirely prevent—the overgrowth of highly cefepime-resistant subpopulations in vivo. Therefore, resistance selection was not a major issue in this particular experimental setting.
Taken together, the present study underlines the beneficial effect of Q-D–β-lactam combinations against C-MLSB-resistant MRSA. The observation was valid both in vitro and in rats with experimental endocarditis, indicating that the finding held true when testing was done in the complicated context of in vivo therapy. The mechanism of this beneficial interaction has yet to be determined. Nevertheless, the present experiments with MRSA, as well as previous experiments with Q-D–ampicillin against experimental endocarditis due to MLSB-resistant Enterococcus faecium (11), support the potential usefulness of this strategy. Moreover, it is now suggested that Q-D be administered t.i.d. rather than b.i.d. for the treatment of severe infections (18; Moses et al., 38th ICAAC; Nachman et al., 38th ICAAC), thus adding to the therapeutic margin of the compound. In this context, the present observations indicate that Q-D plus the broad-spectrum cefepime could be of use, for instance, for treatment of severely ill patients who require multiple-antibiotic therapy.
ACKNOWLEDGMENTS
We thank Marlyse Giddey and Dominique Blanc for outstanding technical assistance and the Rhône-Poulenc Rorer working group on the Q-D animal models (Harriette Nadler, Michael Dowzicky, Guy Montay, Philippe Picaut, Nadine Berthaud, and Céline Féger) for stimulating discussions.
REFERENCES
- 1.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 52–111. [Google Scholar]
- 2.Barbhaiya R H, Forgue S T, Gleason C R, Knupp C A, Pittman K A, Weidler D J, Movahhed H, Tenney J, Martin R R. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992;36:552–557. doi: 10.1128/aac.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc D S, Petignat C, Moreillon P, Entenza J M, Eisenring M C, Kleiber H, Wenger A, Troillet N, Blanc C H, Francioli P. Unusual spread of a penicillin-susceptible methicillin-resistant Staphylococcus aureus clone in a geographic area of low incidence. Clin Infect Dis. 1999;29:1512–1518. doi: 10.1086/313522. [DOI] [PubMed] [Google Scholar]
- 4.Brumfitt W, Hamilton-Miller J M, Shah S. In-vitro activity of RP 59500, a new semisynthetic streptogramin antibiotic, against gram-positive bacteria. J Antimicrob Chemother. 1992;30(Suppl. A):29–37. doi: 10.1093/jac/30.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- 5.Chin N X, Neu H C. Post-antibiotic effect of the new streptogramin RP 59500. Eur J Clin Microbiol Infect Dis. 1992;11:642–645. doi: 10.1007/BF01961676. [DOI] [PubMed] [Google Scholar]
- 6.Cocito C, Di Giambattista M, Nyssen E, Vannuffel P. Inhibition of protein synthesis by streptogramins and related antibiotics. J Antimicrob Chemother. 1997;39(Suppl. A):7–13. doi: 10.1093/jac/39.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulos G M, Moellering R C., Jr . Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 330–396. [Google Scholar]
- 8.Entenza J, Marchetti O, Glauser M P, Moreillon P. Y-688, a new quinolone active against quinolone-resistant Staphylococcus aureus: lack of in vivo efficacy in experimental endocarditis. Antimicrob Agents Chemother. 1998;42:1889–1894. doi: 10.1128/aac.42.8.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entenza J M, Drugeon H, Glauser M P, Moreillon P. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob Agents Chemother. 1995;39:1419–1424. doi: 10.1128/aac.39.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne S D, Montay G, Le Liboux A, Frydman A, Garaud J J. A phase I, double-blind, placebo-controlled study of the tolerance and pharmacokinetic behaviour of RP 59500. J Antimicrob Chemother. 1992;30(Suppl. A):123–131. doi: 10.1093/jac/30.suppl_a.123. [DOI] [PubMed] [Google Scholar]
- 11.Fantin B, Leclercq R, Garry L, Carbon C. Influence of inducible cross-resistance to macrolides, lincosamides, and streptogramin B-type antibiotics in Enterococcus faecium on activity of quinupristin-dalfopristin in vitro and in rabbits with experimental endocarditis. Antimicrob Agents Chemother. 1997;41:931–935. doi: 10.1128/aac.41.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantin B, Leclercq R, Merle Y, Saint-Julien L, Veyrat C, Duval J, Carbon C. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP 59500 (quinupristin-dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:400–405. doi: 10.1128/aac.39.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fantin B, Leclercq R, Ottaviani M, Vallois J M, Maziere B, Duval J, Pocidalo J J, Carbon C. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob Agents Chemother. 1994;38:432–437. doi: 10.1128/aac.38.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fass R J. In vitro activity of RP 59500, a semisynthetic injectable pristinamycin, against staphylococci, streptococci, and enterococci. Antimicrob Agents Chemother. 1991;35:553–559. doi: 10.1128/aac.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finch R G. Antibacterial activity of quinupristin/dalfopristin. Rationale for clinical use. Drugs. 1996;51(Suppl. 1):31–37. doi: 10.2165/00003495-199600511-00007. [DOI] [PubMed] [Google Scholar]
- 16.Fluckiger U, Francioli P, Blaser J, Glauser M P, Moreillon P. Role of amoxicillin serum levels for successful prophylaxis of experimental endocarditis due to tolerant streptococci. J Infect Dis. 1994;169:1397–1400. doi: 10.1093/infdis/169.6.1397. [DOI] [PubMed] [Google Scholar]
- 17.Heraief E, Glauser M P, Freedman L R. Natural history of aortic valve endocarditis in rats. Infect Immun. 1982;37:127–131. doi: 10.1128/iai.37.1.127-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson C A, Taylor III C A, Zimmerman S W, Bridson W E, Chevalier P, Pasquier O, Baybutt R I. Pharmacokinetics of quinupristin-dalfopristin in continuous ambulatory peritoneal dialysis patients. Antimicrob Agents Chemother. 1999;43:152–156. doi: 10.1128/aac.43.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones R N, Ballow C H, Biedenbach D J, Deinhart J A, Schentag J J. Antimicrobial activity of quinupristin-dalfopristin (RP 59500, Synercid) tested against over 28,000 recent clinical isolates from 200 medical centers in the United States and Canada. Diag Microbiol Infect Dis. 1998;31:437–451. doi: 10.1016/s0732-8893(98)80002-3. [DOI] [PubMed] [Google Scholar]
- 20.Leclercq R, Nantas L, Soussy C J, Duval J. Activity of RP 59500, a new parenteral semisynthetic streptogramin, against staphylococci with various mechanisms of resistance to macrolide-lincosamide-streptogramin antibiotics. J Antimicrob Chemother. 1992;30(Suppl. A):67–75. doi: 10.1093/jac/30.suppl_a.67. [DOI] [PubMed] [Google Scholar]
- 21.Lorian V, Amaral L, Fernandes F. RP 59500 postantibiotic effect defined by bacterial ultrastructure. Drugs Exp Clin Res. 1995;21:125–128. [PubMed] [Google Scholar]
- 22.Lorian V, Esanu Y, Amaral L. Ultrastructure alterations of Staphylococcus aureus exposed to RP59500. J Antimicrob Chemother. 1994;33:625–628. doi: 10.1093/jac/33.3.625. [DOI] [PubMed] [Google Scholar]
- 23.Lorian V, Fernandes F. Electron microscopy studies of the bactericidal effects of quinupristin/dalfopristin on Staphylococcus aureus. J Antimicrob Chemother. 1999;43:845–848. doi: 10.1093/jac/43.6.845. [DOI] [PubMed] [Google Scholar]
- 24.Que Y A, Entenza J M, Francioli P, Moreillon P. The impact of penicillinase on cefamandole treatment and prophylaxis of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 1998;177:146–154. doi: 10.1086/513805. [DOI] [PubMed] [Google Scholar]
- 25.Scheel O, Lyon D J, Rosdahl V T, Adeyemi-Doro F A, Ling T K, Cheng A F. In-vitro susceptibility of isolates of methicillin-resistant Staphylococcus aureus 1988–1993. J Antimicrob Chemother. 1996;37:243–251. doi: 10.1093/jac/37.2.243. [DOI] [PubMed] [Google Scholar]
- 26.Sieradzki K, Tomasz A. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J Antimicrob Chemother. 1997;39(Suppl. A):47–51. doi: 10.1093/jac/39.suppl_1.47. [DOI] [PubMed] [Google Scholar]