Breathing is one of the perpetual rhythms of life that is often taken for granted, its apparent simplicity belying the complex neural machinery involved. This behavior is more complicated than just producing inspiration, as breathing is integrated with many other motor functions such as vocalization, orofacial motor behaviors, emotional expression (laughing and crying), and locomotion (1, 2). In addition, cognition can strongly influence breathing. Conscious breathing during yoga, meditation, or psychotherapy can modulate emotion, arousal state, or stress (3). Therefore, understanding the links between breathing behavior, brain arousal state, and higher-order brain activity is of great interest. On page 1411 of this issue, Yackle et al. (4) identify an apparently specialized, molecularly identifiable, small subset of ~350 neurons
“…the motor act of breathing can influence higher-order brain functions.”
in the mouse brain that forms a circuit for transmitting information about respiratory activity to other central nervous system neurons, specifically with a group of noradrenergic neurons in the locus coeruleus (LC) in the brainstem, that influences arousal state (see the figure). This finding provides new insight into how the motor act of breathing can influence higher-order brain functions.
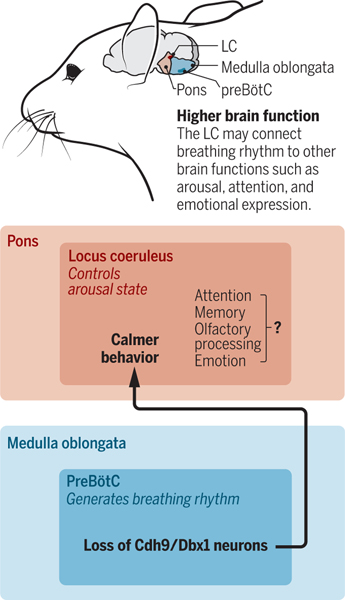
figure. Breathing affects brain activity.
The preBötzinger complex (preBötC) and locus coeruleus (LC) are located in the medulla oblongata and pons regions of the brainstem (mouse shown). A subset of neurons that express Cdh9 and Dbx1 in the preBötC form connections with LC neurons, controlling arousal state.
The innate process of breathing is regulated by neural circuits throughout the brain for automatic and volitional control. The current theory of how the basic rhythm and motor patterns of breathing are generated in mammals is centered around functionally distinct regions located in the medulla oblongata and pons of the brainstem, which drive and modulate rhythmic breathing (1, 5). It is now established that the inspiratory rhythm is generated endogenously by the preBötzinger complex (preBötC) (1, 6), a bilateral, core medullary structure whose activity is modulated either directly or indirectly by convergent inputs from many brain regions, including from structures functioning as chemosensors for carbon dioxide and oxygen (7). PreBötC activity is also broadcasted to the other respiratory control and central nervous system circuits, including the LC (5, 8).
The LC, located in the pons, has been functionally linked to the control of sleep-wakefulness state, vigilance, and emotions (9). LC neurons are spontaneously active, receive inputs from many brain areas, and conversely have extensive projections throughout the brain and spinal cord (10). The activity of a subset of LC neurons is augmented by elevated brain carbon dioxide (the cells exhibit chemosensory activity), and some LC neurons can exhibit respiratory-related activity, indicating a connection to respiratory function (11). The specific neurons that establish circuits with LC neuron subpopulations involved in respiratory behavior had not been delineated before.
Yackle et al. used a combination of techniques, including genetically engineered mice, neuronal circuitry mapping, and behavioral analyses, to establish that a small subpopulation of preBötC neurons expressing two molecular markers—the cell adhesion protein cadherin-9 (Cdh9) and a transcription factor called developing brain homeobox protein 1 (Dbx1)—represent functionally distinct neurons that are directly connected to a subset of LC neurons. Genetic database screening and expression profiling suggested that the Cdh9 gene is selectively expressed in and immediately around the preBötC. Expression of Cdh9 in preBötC neurons that also express Dbx1 is notable because many preBötC Dbx1–derived neurons have a glutamatergic, excitatory neuronal phenotype and are proposed to generate and transmit the inspiratory rhythm (12). Indeed, some of the neurons expressing both Cdh9 and Dbx1 (Cdh9/Dbx1 neurons) exhibited rhythmic inspiratory activity. Yet, remarkably, genetic-based deletion of these neurons did not disrupt normal breathing rhythm, sighs (periodic augmented breaths that also originate in the preBötC) (13), or respiratory responses to hypercapnic (high carbon dioxide) or hypoxic (low oxygen) environmental conditions. However, ablation of these neurons caused mice to spend more time in a calm versus a mild arousal state, as defined by electrocorticographic measurements. Thus, the Cdh9/Dbx1 LC–projecting preBötC neurons seem to be dedicated to transmitting inspiratory activity to LC neurons.
Understanding the mechanistic details of how activation of the LC by the Cdh9/Dbx1 neurons produces arousal remains a challenging problem. Currently, it is unclear if the respiratory-related input to the LC that rhythmically augments LC activity is directly transmitted to higher central nervous system regions involved in arousal state control. In general, how the LC generates behavioral arousal is currently unknown.
Deciphering the functional and structural properties of the mammalian preBötC has been a long-standing problem. This structure, discovered more than 25 years ago in rats (6), has been investigated intensively to uncover the mechanisms of rhythm generation at the molecular, biophysical and neuronal circuit levels—problems that are still not solved (1). Unexpected complexity in structural-functional organization has been revealed. There is greater molecular and functional diversity of preBötC neurons than suspected, and the concept has emerged that there are molecularly distinct subpopulations with functional roles other than generating normal breathing rhythm. Another small population of ~400 preBötC neurons appears to be dedicated to generating sighs (13). The evidence for a subpopulation of presumably excitatory neurons expressing Cdh9 and Dbx1 that regulate the activity of LC noradrenergic neurons and behavioral arousal, without roles in normal breathing rhythm and sigh generation, further advances this important concept.
The preBötC neurons involved in generating normal breathing are also functionally and, likely, molecularly heterogeneous. There are subpopulations of excitatory and inhibitory neurons with electrophysiological phenotypes that subserve distinct functions during normal breathing (14). Transcriptomic analyses (for gene expression) of these rhythmic neurons, detailed studies of local morphology, and brain-wide mapping of their output and input connections are essential to further decipher molecular properties and functional roles. Other cell types, such as astrocytes, also may be important in modulating breathing activities (15), yet much remains to be discovered on the local mechanisms of neuron-glial communication in the preBötC and other respiratory circuits. Ultimately, unraveling molecular, cellular, and circuit mechanisms of rhythm generation and the neural control of breathing should lead to new therapeutic strategies to treat breathing arrhythmias and other disorders of respiratory control.
REFERENCES
- 1.Feldman JL, Del Negro CA, Gray PA, Ann. Rev. Physiol. 75, 423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleinfeld D, Moore JD, Wang F, Deschênes M, Cold Spring Harbor Symp. Quant. Biol. 79, 29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown RP, Gerbarg PL, J. Altern. Compl. Med. 11, 711 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Yackle K. et al. , Science 355, 1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JC et al. , Trends Neurosci. 36, 152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JC et al. , Science 254, 726 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyenet PG, Compr. Physiol. 4, 1511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan W. et al. , J. Comp. Neurol. 518, 1862 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge CW, Waterhouse BD, Brain Res. Rev. 42, 33 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Schwarz LA, Luo L, Curr. Biol. 25, R1051 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Li A, Nattie E, J. Physiol. (London) 570, 385 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray PA et al. ,J. Neurosci. 30, 14883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P. et al. ,Nature 530, 293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter DW, Smith JC, Physiology 29, 58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelova PR et al. ,J. Neurosci. 35, 10460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]