Abstract
Background
Patients with inborn errors of immunity (IEI) are at increased risk of severe coronavirus disease-2019 (COVID-19). Effective vaccination against COVID-19 is therefore of great importance in this group, but little is known about the immunogenicity of COVID-19 vaccines in these patients.
Objectives
We sought to study humoral and cellular immune responses after mRNA-1273 COVID-19 vaccination in adult patients with IEI.
Methods
In a prospective, controlled, multicenter study, 505 patients with IEI (common variable immunodeficiency [CVID], isolated or undefined antibody deficiencies, X-linked agammaglobulinemia, combined B- and T-cell immunodeficiency, phagocyte defects) and 192 controls were included. All participants received 2 doses of the mRNA-1273 COVID-19 vaccine. Levels of severe acute respiratory syndrome coronavirus-2–specific binding antibodies, neutralizing antibodies, and T-cell responses were assessed at baseline, 28 days after first vaccination, and 28 days after second vaccination.
Results
Seroconversion rates in patients with clinically mild antibody deficiencies and phagocyte defects were similar to those in healthy controls, but seroconversion rates in patients with more severe IEI, such as CVID and combined B- and T-cell immunodeficiency, were lower. Binding antibody titers correlated well to the presence of neutralizing antibodies. T-cell responses were comparable to those in controls in all IEI cohorts, with the exception of patients with CVID. The presence of noninfectious complications and the use of immunosuppressive drugs in patients with CVID were negatively correlated with the antibody response.
Conclusions
COVID-19 vaccination with mRNA-1273 was immunogenic in mild antibody deficiencies and phagocyte defects and in most patients with combined B- and T-cell immunodeficiency and CVID. Lowest response was detected in patients with X-linked agammaglobulinemia and in patients with CVID with noninfectious complications. The assessment of longevity of immune responses in these vulnerable patient groups will guide decision making for additional vaccinations.
Key words: Inborn errors of immunity, primary immunodeficiency disorders, SARS-CoV-2, mRNA-1273 COVID-19 vaccine, immunogenicity, antibody response, T-cell response, CVID, CID, XLA
Abbreviations used: CID, Combined B- and T-cell immunodeficiency; COVID-19, Coronavirus disease-2019; CVID, Common variable immunodeficiency; GMT, Geometric mean titer; IEI, Inborn errors of immunity; RBD, Receptor-binding domain; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; SPAD, Specific polysaccharide antibody deficiency; S, Spike; XLA, X-linked agammaglobulinemia
Inborn errors of immunity (IEI), also referred to as primary immunodeficiencies, are a heterogeneous group of inborn disorders affecting a single or multiple component(s) of the immune system. Clinically, IEI are characterized by an increased susceptibility to infections, autoimmune complications, autoinflammatory diseases, allergies, and malignancies. Variants in more than 450 genes that give rise to IEI have been identified.1 , 2
Reports on the severity of coronavirus disease-2019 (COVID-19) in patients with IEI are conflicting. An early report in 94 patients demonstrated that IEI was not an independent risk factor for severe COVID-19, but more recent studies showed increased morbidity and mortality.3, 4, 5 Importantly, common complications in IEI include chronic lung diseases, such as bronchiectasis, asthma, or interstitial lung disease, which are additional risk factors for severe COVID-19.4, 5, 6 Prevention of COVID-19 in patients with IEI is therefore important. It has been demonstrated that messenger RNA (mRNA)- and adenovirus-based COVID-19 vaccines are effective in preventing severe disease in the general population.7, 8, 9, 10 However, in IEI absent or disturbed response to vaccination is a common finding,11 which may hamper effective protection by immunization in this vulnerable group. Until now, few studies in mainly patients with common variable immune deficiency (CVID) were conducted,12, 13, 14, 15, 16 showing that vaccination in IEI was safe, and most patients developed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-specific immune responses.
In this study, we measured SARS-CoV-2–specific immune responses following vaccination with the mRNA-1273 COVID-19 vaccine (Moderna) in 505 adult patients with IEI, and compared these with responses in 192 adult controls. The patients with IEI were stratified into cohorts of patients with CVID, isolated antibody deficiencies (IgG subclass deficiency ± IgA deficiency, specific polysaccharide antibody deficiency [SPAD]), undefined antibody deficiencies (patients with primary hypogammaglobulinemia and intact cellular immunity who do not fulfill diagnostic criteria of any of the primary antibody deficiencies), X-linked agammaglobulinemia (XLA), combined B- and T-cell immunodeficiency (CID), and phagocyte defects (see this article’s Methods section in the Online Repository at www.jacionline.org). In XLA, an absent antibody response, with conserved T-cell responses, would be expected, whereas variable, but diminished, antibody responses could be found in patients with CVID, isolated antibody deficiencies, undefined antibody deficiencies, and CID. Moreover, T-cell responses in patients with CID and patients with CVID with immunologic or clinical features of T-cell dysfunction could be diminished. We performed a complete immunologic assessment, including the measurement of SARS-CoV-2–specific binding and neutralizing antibodies and T-cell responses at baseline, 28 days after the first vaccination, and 28 days after the second vaccination.
Methods
Ethical statement
The Vaccination Against COvid in Primary Immune Deficiencies study is a prospective, controlled, multicenter study performed among patients with IEI from 7 academic hospitals in the Netherlands. The study adheres to the principles of the Declaration of Helsinki and was approved by the Dutch Central Committee on Research Involving Human Subjects (CCMO, NL7647.078.21, EudraCT number 2021-000515-24), the Medical Research Ethics Committee from Erasmus University Medical Center (MEC-2021-0050), and the local review boards of all other participating centers. All participants provided written informed consent before enrollment.
Study participants and design
Patients with a clinical and/or genetic diagnosis of IEI, older than 18 years, and who were treated in the outpatient clinic at one of the study sites were eligible for participation. Pregnant patients or patients with an active malignancy were excluded from participation. An extensive description of the IEI diagnoses and all inclusion and exclusion criteria can be found in this article’s Online Repository at www.jacionline.org. The study was initiated in collaboration with the Dutch patient organization for primary immune deficiencies (Stichting voor Afweerstoornissen), and patients were informed on start of this study by the patient organization. Consequently, a high number of interested patients were eligible for participation. Therefore, participants were selected by draw. In total, 505 adult patients with IEI were included and stratified into the following cohorts: CVID (n = 212), isolated antibody deficiencies (IgG subclass deficiency ± IgA deficiency; n = 133), SPAD (n = 64), CID (n = 25), undefined antibody deficiencies (n = 23), XLA (n = 21), phagocyte defects (n = 17), and “other” (n = 10). Detailed information on the phenotype of patients with CID is summarized in Table E1 in this article’s Online Repository at www.jacionline.org. The isolated IgG subclass deficiency ± IgA deficiency and the SPAD cohorts, which are clinically comparable, were analyzed as 1 group. A total of 192 adult controls, defined as not diagnosed with IEI, were included. This control group consisted of partners, siblings, or other family/household members from included patients with IEI. Baseline characteristics, including medical history and medication use, were recorded.
Study participants received COVID-19 vaccination according to the Dutch vaccination program against COVID-19 as implemented by the Dutch Ministry of Health, Welfare and Sport. Vaccinations were prepared and administered at study sites according to the manufacturer’s instructions. Patients with IEI and controls received a first vaccination with the mRNA-1273 (100 μg) COVID-19 vaccine in the deltoid muscle at study visit 1 (day 0). A nasopharyngeal swab was taken to exclude (asymptomatic) natural SARS-CoV-2 infection. The second mRNA-1273 vaccination was administered at study visit 2 (interval between vaccinations was 28 days). Study visit 3 was scheduled 28 days after visit 2. Blood samples were collected at all visits. Severe adverse events were recorded during follow-up.
Immunogenicity
The analysis of humoral and cellular immune responses is extensively described in this article’s Online Repository at www.jacionline.org. Briefly, total immunoglobulin antibodies against the receptor-binding domain (RBD) of SARS-CoV-2 were measured in serum by qualitative ELISA (Wantai).17 Full spike (S) protein–specific binding antibodies were measured by a quantitative anti-S IgG Luminex assay.18 , 19 Neutralizing antibodies against infectious SARS-CoV-2 D614G (Global Initiative on Sharing All Influenza Data: hCov-19/Netherlands/ZH-EMC-2498) in a selection of sera were assessed in an infectious plaque reduction neutralization test (PRNT) on Vero-E6 cells.20 , 21 A pseudovirus neutralization assay was performed in the same sample selection using a pseudovirus system based on SARS-CoV-2-S and HIV-1-NL43 ΔEnv-NanoLuc reporter virus and HEK293T-ACE2 cells.22 , 23 All binding and neutralization titers were normalized to the WHO International Standard for anti–SARS-CoV-2 immunoglobulin (NIBSC 20/136). SARS-CoV-2–specific T-cell responses were assessed by IFN-γ release assay (QuantiFERON, QIAGEN or EuroImmun) at study visits 1, 2, and 3 as previously described.21 , 24
Statistical analysis
Sample size
A sample size calculation was performed for patients with IgG subclass deficiencies ± IgA deficiency and SPAD. A total of 175 participants were required, assuming immunogenicity of 75% in this cohort with alpha 0.05 and beta 0.2 (compared with an assumed immunogenicity of 90% in the controls). A total of 200 participants were assigned to the control group. For the other cohorts with IEI, no power calculation was made because of the heterogeneous character of these cohorts.
Statistical analysis plan
Baseline characteristics were described for the patients with IEI and controls. Categorical variables were displayed as numbers and percentages and analyzed with Pearson chi-square test or with Fisher exact test when the (expected) cell count was less than 5. Continuous baseline variables were presented as mean ± SD and analyzed using independent t test. P values less than .05 were considered statistically significant.
The primary end point was defined as seroconversion rates 28 days after vaccination of patients with IEI compared with controls. Participants were classified as responders or nonresponders on the basis of a qualitative RBD-specific total immunoglobulin ELISA and compared by Fisher exact test.
Levels of binding antibodies, neutralizing antibodies, and SARS-CoV-2–specific T-cell responses were defined as secondary end points. Differences in response rates were calculated by Fisher exact test. Log-transformed results were displayed in figures and text as geometric mean titer (GMT) ± 95% CI and were analyzed by Mann-Whitney U test. Multivariate logistic regression was performed in the cohort with CVID, to associate patient characteristics with seroconversion. To avoid overfitting, 3 variables were chosen on the basis of univariate analysis and clinical relevance. Spearman ρ test was used to perform correlation analysis.
Software
Study data were collected and stored in an online database (Castor, Amsterdam, the Netherlands), which is compliant with the General Data Protection Regulation. SPSS (SPSS Statistics 25, IBM, New York, NY), R studio, and GraphPad PRISM, version 9.1.2 (San Diego, Calif) were used for statistical analyses. Graphs were made with GraphPad PRISM.
Results
Baseline characteristics
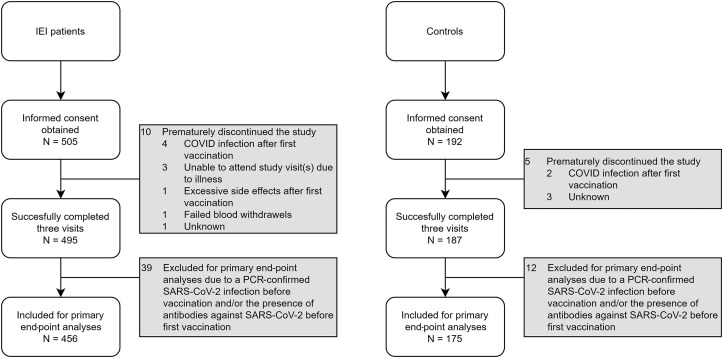
A total of 505 adult patients with IEI and 192 adult controls without IEI were screened for eligibility; 682 participants completed all 3 study visits. The most frequent reason for drop-out of 15 study participants was contracting COVID-19 after the first vaccination (Fig 1 ). Of the 682 participants, 51 were found to have a history of a proven COVID-19 infection. These participants were excluded for primary end-point analysis (but were analyzed separately), leaving 631 participants eligible for evaluation of the primary end point of this study (Table I ).
Fig 1.
Subject enrollment and outcome after 3 visits. In total, 697 patients signed informed consent (505 patients with IEI, 192 controls). Fifteen participants did not complete the 3 visits (10 patients with IEI, 5 controls). A total of 51 participants (39 patients with IEI, 12 controls) were considered as COVID-19 recovered patients and are discussed separately. The 631 remaining participants (456 patients with IEI, 175 controls) are described in detail in Table I.
Table I.
Baseline characteristics of the 631 participants eligible for primary end-point evaluation
| Characteristic | Patients with IEI (N = 456) | Controls (N = 175) | P value |
|---|---|---|---|
| Sex: male, n (%) | 184 (40.4) | 100 (57.1) | .00015∗ |
| Age (y), mean ± SD | 49.0 ± 14.9 | 51.6 ± 13.8 | .052† |
| IEI diagnosis, n (%) | |||
| Antibody deficiency | |||
| CVID | 196 (43.0) | ||
| Isolated IgG subclass deficiency ± IgA deficiency | 121 (26.5) | ||
| SPAD | 58 (12.7) | ||
| Undefined antibody deficiency‡ | 16 (3.5) | ||
| Absent B cells§ | |||
| XLA | 19 (4.2) | ||
| Autosomal-dominant agammaglobulinemia | 1 (0.2) | ||
| CID | 22 (4.8) | ||
| Phagocyte defects | 15 (3.3) | ||
| Other‖ | 8 (1.7) | ||
| Immunoglobulin replacement therapy, n (%) | 319 (70.0) | 0 | |
| Noninfectious complications, n (%) | |||
| Autoimmune cytopenias | 26 (5.7) | 0 | |
| Other autoimmune diseases | 71 (15.6) | 1 (0.6) | |
| Enteropathies | 42 (9.2) | 2 (1.1) | |
| Lymphoproliferative diseases | 33 (7.2) | 0 | |
| Granulomatous-lymphocytic interstitial lung disease | 30 (6.6) | 0 | |
| Granulomatous complications affecting other organs | 11 (2.4) | 0 | |
| Malignancies | 21 (4.6) | 2 (1.1) | |
| Most frequent other comorbidities, n (%) | |||
| Asthma | 8 (1.7) | 3 (1.7) | |
| Bronchiectasis | 10 (2.2) | 0 | |
| Cardiac diseases¶ | 5 (1.1) | 5 (2.9) | |
| Diabetes | 2 (0.4) | 2 (1.1) | |
| Hypertension | 6 (1.3) | 17 (9.7) | |
| Immunosuppressive medication in past 2 y, n (%) | 96 (21.1) | 5 (2.9) | <.0001∗ |
Pearson’s χ2 test.
Independent t test.
Patients with primary hypogammaglobulinemia and intact cellular immunity who do not fulfill diagnostic criteria of any of the other primary antibody deficiencies.
In the text, this cohort is referred to as XLA, although it also includes 1 participant with autosomal-dominant agammaglobulinemia (TCF3 mutation).
Patients with an unknown classification of their IEI, high B-cell numbers, or hyper-IgM syndrome.
Including myocardial infarction, chemotherapy-induced cardiomyopathy, coronary artery bypass grafting, arrhythmias, and heart valve diseases.
Seventy percent of the patients with IEI received immunoglobulin replacement therapy (Table I). Most frequent noninfectious complications in patients with IEI were autoimmune complications (n = 71), enteropathies (n = 42), lymphoproliferative complications (n = 33), and granulomatous-lymphocytic interstitial lung disease (n = 30). In the past 2 years, any immunosuppressive medication was used by 21.0% of the patients with IEI and in 2.9% of controls (P < .0001). The mean interval between the first and second vaccinations was 28.4 ± 1.78 days, and between the second vaccination and the third visit was 28.7 ± 3.78 days.
Seroconversion based on RBD-specific binding antibodies
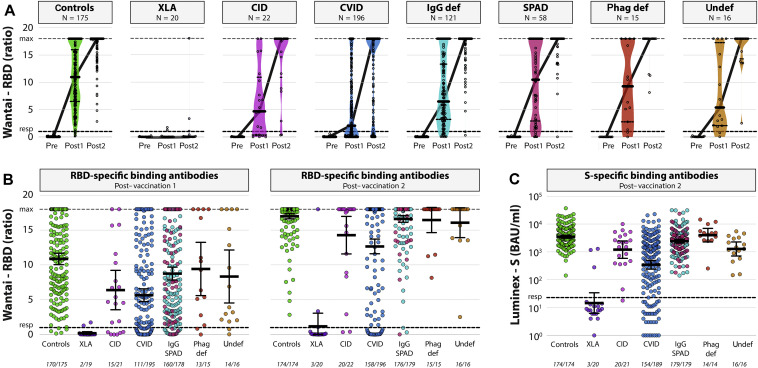
Seroconversion, defined as the primary end point, was based on the detection of total immunoglobulins (ratio >1) against RBD in a qualitative ELISA. After the first vaccination, the seroconversion rate of the controls was 97%. This was significantly higher than the seroconversion rates found in the XLA cohort (11%; P < .0001), the CID cohort (71%; P = .00024), the CVID cohort (57%; P < .0001), and the IgG/SPAD cohort (90%; P = .0084) (Fig 2 ). Patients with phagocyte defects (87%; P = .10) and patients with undefined antibody deficiency (88%; P = .11) showed comparable seroconversion rates after the first vaccination compared with controls. Clinical details of the seroconverted patients with XLA are summarized in Table E2 in this article’s Online Repository at www.jacionline.org.
Fig 2.
SARS-CoV-2–specific binding antibodies. A, Levels (GMT ± 95% CI) of total RBD-specific immunoglobulins at baseline (pre), after vaccination 1 (post 1), and after vaccination 2 (post 2) in all different patients with PID. Symbols show individual data points, violin plots reflect data distribution, and lines connect the GMT. B, Comparison of total RBD-specific immunoglobulins (GMT ± 95% CI) after vaccination 1 (left panel) and after vaccination 2 (right panel) between groups. Lower level of detection is a ratio of 0, and responder (resp) cutoff is a ratio of 1 (black dotted line). A ratio of 18 is the maximum dynamic range of the assay. Number of participants above responder cutoff is indicated beneath the x-axis. C, Comparison of S-specific antibodies (GMT ± 95% CI) after vaccination 2 between groups. LLoD is 1 BAU/mL, and responder (resp) cutoff is set at 22.87 BAU/mL (black dotted line). Number of participants above responder cutoff is indicated beneath the x-axis. Patients with IgG deficiencies and patients with SPAD are combined in panels B and C, but the original color coding (A) is maintained. Color coding is the same in all (Online Repository) figures. def, Deficiency; LLoD, lower level of detection; phag, phagocyte; PID, primary immunodeficiency; undef, undefined.
Seroconversion rates, measured 28 days after the second vaccination, were comparable in patients with selective IgG subclass deficiency ± IgA deficiency/SPAD (IgG/SPAD) (98.3%), undefined antibody deficiencies (100%), and phagocyte defects (100%), when compared with controls (100%). Significantly lower seroconversion rates were found in the XLA cohort (15%; P < .0001), the CID cohort (91%; P = .012), and the CVID cohort (81%; P = .0001) (Fig 2).
Fifty-one of 682 participants (12 of 192 [6.3%] controls and 39 of 505 [7.7%] patients with IEI) were considered as COVID-19 recovered patients on the basis of detection of S-specific binding antibodies at baseline, or positive PCR result before study inclusion. Forty-six experienced mild disease with spontaneous recovery, not necessitating hospital admission. Five patients had been admitted to a hospital, of which 1 was admitted to an intensive care unit. Baseline characteristics of this recovered cohort are reported in Table E3 in this article’s Online Repository at www.jacionline.org. At baseline, antibodies (ratio >1) were found in 36 of 39 patients with IEI (92.3%) and in all 12 controls. One of the 3 nonresponders (IgG/SPAD cohort) seroconverted after first vaccination. The other 2 patients (XLA and CVID cohorts) remained seronegative after 2 vaccinations (see Fig E1 in this article’s Online Repository at www.jacionline.org).
Quantitative evaluation of S-specific antibodies
The GMT of SARS-CoV-2 S-specific IgG antibodies of the control cohort was 3503 BAU/mL (95% CI, 3098-3961). Significantly lower GMTs were found in the XLA cohort (14.51 BAU/mL; 95% CI, 6.186-34.02; P < .0001), the CID cohort (1204 BAU/mL; 95% CI, 584.7-2479; P = .0019), the CVID cohort (345.4 BAU/mL; 95% CI, 240.4-496.1; P < .0001), the IgG/SPAD cohort (2414 BAU/mL; 95% CI, 2073-2811; P = .00081), and the undefined antibody deficiency cohort (1274 BAU/mL; 95% CI, 711.5-2282; P < .00049). Patients with a phagocyte defect showed similar GMTs compared with controls (3991 BAU/mL; 95% CI, 2285-6969; P = .27) (Fig 2).
Virus neutralization determined by 2 different neutralization tests
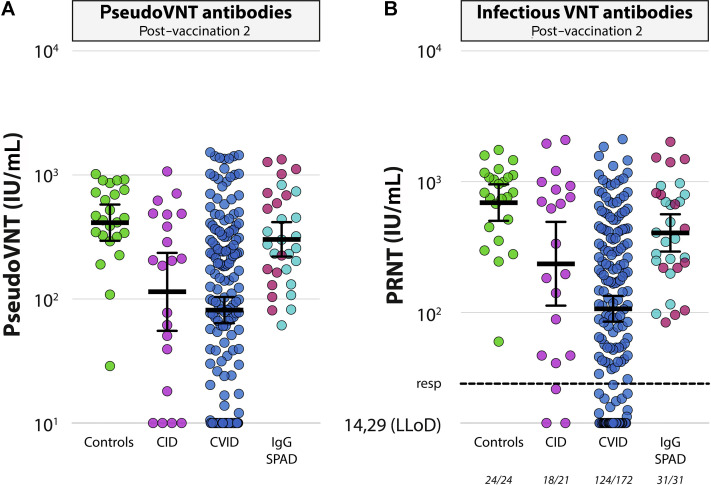
Additional functional serological tests were performed with samples from the CID and CVID cohorts, and compared with a random selection of sera from controls (pseudovirus neutralization and infectious virus neutralization assays, Fig 3 ). In addition, a selection of sera from patients with IgG deficiency/SPAD was included. Based on the infectious neutralization test, neutralizing antibodies (>28.57 IU/mL, corresponding to a titer of 40) were detected in 100% of sera obtained from both controls (n = 24) and patients with IgG deficiency/SPAD (n = 31). Comparable to controls, 86% of the patients with CID developed neutralizing antibodies (P = .094), but a significantly lower response rate was observed in patients with CVID (response rate of 72%; P = .0015). GMT of the control cohort in the infectious neutralization test was 694 IU/mL (95% CI, 504-955), significantly higher than the titers/dilutions found in the other cohorts (CID: 236 IU/mL, 95% CI, 113-494, P = .019; CVID: 106 IU/mL, 95% CI, 84-133, P < .0001; IgG deficiency/SPAD: 407 IU/mL, 95% CI, 294-566, P = .011). Neutralization results in the 2 different neutralization tests correlated excellently (r = 0.93). S-specific binding antibodies were significantly correlated to pseudovirus neutralization as well (r = 0.83) (see Fig E2 in this article’s Online Repository at www.jacionline.org).
Fig 3.
SARS-CoV-2–specific neutralizing antibodies. A, Comparison of neutralizing antibodies (GMT ± 95% CI) determined by pseudovirus neutralization test after vaccination 2 between groups. LLoD is a 10 IU/mL. B, Comparison of neutralizing antibodies (GMT ± 95% CI) determined by PRNT after vaccination 2 between groups. LLoD is 14.29 IU/mL, and responder (resp) cutoff is 28.57 IU/mL (black dotted line). Number of participants above responder cutoff is indicated beneath the x-axis. Patients with IgG deficiencies and patients with SPAD are combined, but the original color coding (Fig 2, A) is maintained. LLoD, Lower level of detection; PRNT, plaque reduction neutralization test; VNT, virus neutralisation test.
T-cell responses
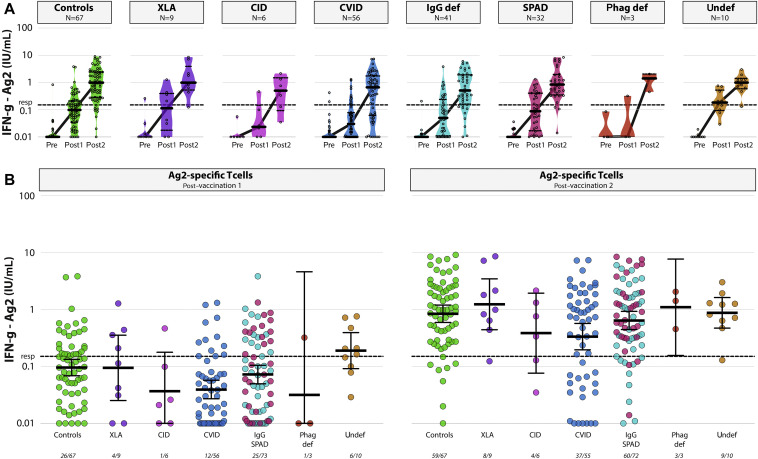
Levels of SARS-CoV-2–specific T cells were determined in samples obtained from 4 of 7 study sites, with 2 different IFN-γ release assays (QIAGEN, Fig 4 , and EuroImmun [see Fig E3 in this article’s Online Repository at www.jacionline.org]). In the QIAGEN assay using a peptide pool covering the S protein (Ag2), SARS-CoV-2–specific T cells were detectable (IFN-γ levels > 0.15) in 59 of 67 controls (88%). When comparing the different IEI cohorts, only in the CVID cohort significantly lower responder rates were found when compared with controls (67% vs 88%; P = .010) (Fig 4). In addition, when comparing geometric mean IFN-γ levels in serum after stimulation, all cohorts were comparable to controls with the exception of the CVID cohort (0.33 IU/mL, 95% CI, 0.20-0.57, compared with 0.84 IU/mL, 95% CI, 0.59-1.12, P = .019). Results obtained with an RBD peptide pool (Ag1; see Fig E4 in this article’s Online Repository at www.jacionline.org) and the EuroImmun assay (Fig E3) were comparable to the results described above.
Fig 4.
SARS-CoV-2-T-cell responses. A, Levels of S-specific T cells (GMT ± 95% CI) determined by QuantiFERON assay (Ag2) at baseline (pre), after vaccination 1 (post 1), and after vaccination 2 (post 2) in patients with IEI at 2 inclusion sites (Erasmus MC and LUMC). LLoD is 0.01 IU/mL, and responder (resp) cutoff is set at 0.15 IU/mL (black dotted line). Symbols show individual data points, violin plots reflect data distribution, and lines connect the GMT. B, Comparison of S-specific T-cell responses (GMT ± 95% CI) determined by QuantiFERON assay (Ag2) after vaccination 1 (left panel) and after vaccination 2 (right panel) between groups. LLoD is 0.01 IU/mL, and responder (resp) cutoff is 0.15 IU/mL (black dotted line). Number of participants above responder cutoff is indicated beneath the x-axis. def, Deficiency; LLoD, lower level of detection; phag, phagocyte; LLoD, Lower level of detection; undef, undefined.
Limited correlation was observed between S-specific binding antibodies and T-cell responses (Fig E2; R = 0.30). However, most patients with IEI and controls with detectable binding antibody responses also developed T-cell responses with the exception of the patients with XLA, who exclusively developed T-cell responses.
Clinical characteristics of responders and nonresponders in the CVID cohort
In the CVID cohort, we observed a large variation in antibody and T-cell responses, spreading from nonresponders to responders comparable to controls. This cohort was also consistently lower in responder rates, levels of binding and neutralizing antibodies, and T-cell responses when compared with controls (Fig 2, Fig 3, Fig 4). Clinically, patients with CVID are known to experience recurrent (bacterial) infections with varying severity. Some patients with CVID have additional autoimmune, granulomatous, lymphoproliferative, and/or oncological complication.25 In our cohort, noninfectious complications were more frequent in nonresponders (29 of 35), compared with responders (83 of 154) (P < .0001) (Table II ). Thereby, the GMT of patients with CVID with multiple complications was significantly lower compared with that of patients with CVID without noninfectious complications or a single noninfectious complication (see Fig E6 in this article’s Online Repository at www.jacionline.org). Higher age (P = .017), autoimmune cytopenia (P < .0001), lymphoproliferative diseases (P < .0001), granulomatous-lymphocytic interstitial lung disease (P < .0001), and the concomitant use of immunosuppressive medication (P = .019) were significantly more present in nonresponders (Table II). The presence of a noninfectious complication in combination with or without the use of immunosuppressive medication was negatively associated with being a responder in a multivariable logistic regression model (adjusted odds ratios of 0.099, 95% CI, 0.025-0.32, P = .00028, and 0.19, 95% CI, 0.053-0.55, respectively) (see Table E4 in this article’s Online Repository at www.jacionline.org). Noninfectious complications were also more present in patients with CVID with a lower T-cell response (see Table E5 in this article’s Online Repository at www.jacionline.org). The presence of a monogenic defect was not associated with a poorer antibody response (Table II).
Table II.
Differences in patient characteristics between responders and nonresponders in patients with CVID
| Characteristic | Responder (Luminex-S > 22.87 BAU/mL) (N = 154) |
Nonresponder (Luminex-S ≤ 22.87 BAU/mL) (N = 35) |
P value |
|---|---|---|---|
| Sex: male, n (%) | 62 (40.3) | 14 (40.0) | .997 |
| Age (y), mean ± SD | 46.3 ± 16.4 | 51.5 ± 10.0 | .017∗ |
| Genetic defect known, n (%) | 23 (14.9) | 6 (17.1) | .744 |
| Noninfectious complications present, n (%) | 83 (53.9) | 29 (82.9) | .002 |
| Autoimmune cytopenia | 8 (5.2) | 12 (34.3) | <.0001 |
| Other autoimmune diseases | 28 (18.2) | 11 (31.4) | .080 |
| Enteropathy | 22 (14.3) | 3 (8.6) | .580 |
| Malignancy | 9 (5.8) | 5 (14.3) | .143 |
| Lymphoproliferative diseases | 12 (7.8) | 16 (45.7) | <.0001 |
| Granulomatous-lymphocytic interstitial lung disease | 13 (8.4) | 15 (42.9) | <.0001 |
| Other granulomatous diseases | 3 (1.9) | 4 (11.4) | .023 |
| Immunosuppressive medicine(s) used in last 2 y,∗ n (%) | 37 (24.0) | 16 (45.7) | .010 |
| Immunosuppressive medicine(s) used during vaccination period, n (%) | 29 (18.8) | 13 (37.1) | .019 |
| Steroids | 16 (10.4) | 6 (17.1) | .254 |
| Anti–TNF-α | 4 (2.6) | 3 (8.6) | .120 |
| Azathioprine | 3 (1.9) | 3 (8.6) | .078 |
| Rituximab (year of treatment) | 2 (1.3) (2017) | 2 (5.7) (2014, 2017, 2020) | .157 |
Independent t test. All other P values are calculated using Pearson χ2 test, except for parameters with total (expected) cell counts <5 (Fischer exact test).
Serious adverse events in patients with IEI after mRNA-1273 COVID-19 vaccine
During follow-up, 9 severe adverse events were reported. Three patients experienced shortness of breath following the first vaccination. On evaluation no abnormalities were found and complaints diminished spontaneously. The second vaccination followed without problems in these patients. One patient was admitted 2 months after vaccination because of cerebral hemorrhage and thrombosis, 1 patient because of diverticulitis. One patient was admitted because of COVID-19 infection after the first vaccination and 1 patient because of dyspnea and low oxygen levels, probably related to underlying pulmonary condition. In the control group, 1 study participant experienced bradyarrhythmia following vaccination, with spontaneous recovery, and 1 participant suffered from ongoing tinnitus after full vaccination.
Discussion
This study investigated the immunogenicity of mRNA-1273 COVID-19 vaccination in a large cohort of adult patients with IEI.
In patients with mild antibody deficiencies, seroconversion rates were comparable to those in controls The mRNA-1273 vaccine seemed to mount a robust antibody response, whereas these patients are known for diminished or absent response to polysaccharide vaccines.11 Although seroconversion rates were comparable to those found in controls, levels of (neutralizing) antibodies were lower. Because neutralizing antibodies are considered an important correlate of protection, the lower levels of neutralizing antibodies potentially warrant booster vaccination.26 This holds especially true with the recent emergence of SARS-CoV-2 variants of concern, which can (partially) escape vaccine-induced antibodies. Presently, long-term immunogenicity data are lacking and waning of antibody response will be assessed in future studies. In addition to antibody response, the assessment of memory B- and T-cell responses is important to understand vaccine-induced immunity in patients with IEI. In this study, T-cell responses after vaccination in these patients were comparable to those in controls, implicating that the induction of T-cell responses by mRNA-1273 vaccination is not disturbed.
As expected, antibody responses were not present in most patients with XLA. XLA is caused by a genetic defect in Bruton tyrosine kinase gene, which results in agammaglobulinemia and (near) absence of CD19+ B cells.25 Three patients with XLA developed an antibody response. The positive antibody responses in these patients could be explained by residual B-cell function due to hypomorphic gene variant defects, and/or as a result of incomplete penetrance.27 Because anti–SARS-CoV-2 antibodies were not present in immunoglobulin preparations at time of evaluation of the response in our study, the increase in titers reflects a true immune response in these patients with XLA. Interestingly, all patients with XLA developed a robust SARS-CoV-2–specific T-cell response, indicating that patients who lack B cells could still benefit from mRNA-1273 vaccination.28 , 29
Most patients with CVID developed SARS-CoV-2–specific antibody and/or T-cell responses. Several clinical characteristics, such as age and noninfectious complications, differed between responders and nonresponders. The association of inadequate responses with age has been previously described.12 However, the negative association of antibody responses with noninfectious complications in IEI is a novel finding. The occurrence of autoimmune, autoinflammatory, and lymphoproliferative complications points toward more severe immune dysregulation in these patients, which could explain the lower responses. Moreover, we showed that the number of noninfectious complications is correlated with poorer antibody response, which supports this hypothesis. We could not find an association between the presence of a monogenic defect in CVID and poor response. The numbers of included patients with CVID using specific immunosuppressive medication in this study was too low to link specific treatment to reduced vaccination responses and should be evaluated in future registration studies. Previous studies did show a negative impact of B-cell–depleting therapies (rituximab) and mycophenolate on COVID-19 vaccination responses.24 , 30 Altogether, our results suggest a need for personalized vaccination regimens and follow-up in specific subgroups of patients with CVID.
We included a low number of patients with CID (22 in total) in our study, with heterogeneous clinical phenotypes, which makes it difficult to draw definite conclusions on the immunogenicity of the mRNA1273 COVID-19 vaccine. However, our findings of antibody and T-cell response rates being comparable to those in controls should encourage vaccination of patients with CID.
Finally, in patients with phagocyte defects, no disturbed antibody or T-cell responses were found, which is in line with the underlying defects in these patients, in whom disease is characterized by a defect in phagocyte number and/or function, while antibody and T-cell functions are not affected.
In our study, the assessment of the initial immune response following COVID-19 vaccination in patients with IEI was limited to the mRNA-1273 COVID-19 vaccine. In the Netherlands, mRNA-based vaccines have been the vaccines of choice for immunocompromised patients. Previous studies in small cohorts of patients with IEI vaccinated with BNT162b2 mRNA COVID-19 vaccine showed comparable results to our study.12 , 13 Long-term follow-up of patients with IEI on vaccination is essential to better understand COVID-19 vaccine immunogenicity, and the additional evaluation of booster vaccinations will be important in the near future. This is specifically important as novel variants of concern are continuously emerging, such as the Omicron BA.1 and BA.2, which can efficiently evade antibody responses.31 However, vaccine-induced T-cell responses are cross-reactive to circulating variants.32 , 33
In conclusion, we show distinct differences in responses between cohorts or subgroups of patients with IEI, based on underlying disease and immune defects. Ninety percent of included patients suffered from a predominantly antibody deficiency. In more than 80% of these patients, seroconversion and T-cell response were demonstrated. In specific subgroups of patients with IEI, diminished responses were found.
On the basis of our data, all patients with IEI should receive full COVID-19 vaccination, because this population is at risk of severe clinical course after infection. In addition, we show that patients with XLA could benefit from COVID-19 vaccination despite the absence of functional antibody responses by mounting a cellular response. Additional vaccinations should be considered for patients with IEI who are likely to have suboptimal antibody response, although not all patients may benefit from these.34 It will therefore still be important to maintain precautionary measures for patients with IEI after additional vaccination. In specific subgroups of patients with IEI, personalized vaccination regimens or temporary reduction of immunosuppressive medication before vaccination could be considered, when clinical condition allows.35
Clinical implications.
Most patients with IEI mount an immune response after a standard primary vaccination series with mRNA-1273 COVID-19 vaccine, but variable efficacy may necessitate disease-specific or personalized booster regimens.
Footnotes
This study was funded by ZonMw (grant no. 10430072010006), EudraCT number 2021-000515-24.
Disclosure of potential conflict of interest: M. J. van Gils received funding from the Amsterdam UMC Fellowship to support her research activities. J. Potjewijd received a grant from GlaxoSmithKline (GSK) for an improvement of clinical care project, received support from Prothva Biosolutions for attending meetings and cover of travel expenses, and participates in an Advisory Board for Janssen. A. A. J. M. van de Ven received a grant of the Stichting voor Afweerstoornissen (SAS, Dutch Patients Organization for inborn errors of immunity) and lecture honoraria from Takeda Pharmaceutical Company. S. F. J. de Kruijf-Bazen reported honoraria for lectures and support for attending meetings and/or travel from Takeda Pharmaceutical Company and participated in an advisory board for Takeda Pharmaceutical Company. F. van de Veerdonk received a grant from ZonMW for a study on lanadelumab in COVID-19, and consulting fees from GSK made to his department. H. L. Leavis received a Takeda Pharmaceutical Company research grant and consulting fees from Takeda Pharmaceutical Company made to her institution. P. M. van Hagen is cochair of the medical board of the International Patients Organisation for Primary Immunodeficiencies. V. A. S. H. Dalm received consulting fees from GSK for Advisory board meetings and honoraria for lectures from Takeda Pharmaceutical Company. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Tangye S.G., Al-Herz W., Bousfiha A., Chatila T., Cunningham-Rundles C., Etzioni A., et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40:24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangye S.G., Al-Herz W., Bousfiha A., Cunningham-Rundles C., Franco J.L., Holland S.M., et al. The ever-increasing array of novel inborn errors of immunity: an Interim Update by the IUIS Committee. J Clin Immunol. 2021;41:666–679. doi: 10.1007/s10875-021-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields A.M., Burns S.O., Savic S., Richter A.G., UK PIN COVID-19 Consortium COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147:870–875.e1. doi: 10.1016/j.jaci.2020.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goudouris E.S., Pinto-Mariz F., Mendonça L.O., Aranda C.S., Guimarães R.R., Kokron C., et al. Outcome of SARS-CoV-2 infection in 121 patients with inborn errors of immunity: a cross-sectional study. J Clin Immunol. 2021;41:1479–1489. doi: 10.1007/s10875-021-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milito C., Soccodato V., Auria S., Pulvirenti F., Quinti I. COVID-19 in complex common variable immunodeficiency patients affected by lung diseases. Curr Opin Allergy Clin Immunol. 2021;21:535–544. doi: 10.1097/ACI.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orange J.S., Ballow M., Stiehm E.R., Ballas Z.K., Chinen J., De La Morena M., et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130:S1–S24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amodio D., Ruggiero A., Sgrulletti M., Pighi C., Cotugno N., Medri C., et al. Humoral and cellular response following vaccination with the BNT162b2 mRNA COVID-19 vaccine in patients affected by primary immunodeficiencies. Front Immunol. 2021;12:727850. doi: 10.3389/fimmu.2021.727850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delmonte O.M., Bergerson J.R.E., Burbelo P.D., Durkee-Shock J.R., Dobbs K., Bosticardo M., et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148:1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulvirenti F., Fernandez Salinas A., Milito C., Terreri S., Piano Mortari E., Quintarelli C., et al. B cell response induced by SARS-CoV-2 infection is boosted by the BNT162b2 vaccine in primary antibody deficiencies. Cells. 2021;10:2915. doi: 10.3390/cells10112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arroyo-Sánchez D., Cabrera-Marante O., Laguna-Goya R., Almendro-Vázquez P., Carretero O., Gil-Etayo F.J., et al. Immunogenicity of anti-SARS-CoV-2 vaccines in common variable immunodeficiency. J Clin Immunol. 2022;42:240–252. doi: 10.1007/s10875-021-01174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., Bogers S., Embregts C.W.E., Laksono B.M., et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11:3436. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobben M., van der Straten K., Brouwer P.J., Brinkkemper M., Maisonnasse P., Dereuddre-Bosquet N., et al. Cross-reactive antibodies after SARS-CoV-2 infection and vaccination. Elife. 2021;10 doi: 10.7554/eLife.70330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keuning M.W., Grobben M., de Groen A.C., Berman-de Jong E.P., Bijlsma M.W., Cohen S., et al. Saliva SARS-CoV-2 antibody prevalence in children. Microbiol Spectr. 2021;9 doi: 10.1128/Spectrum.00731-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sablerolles R.S.G., Rietdijk W.J.R., Goorhuis A., Postma D.F., Visser L.G., Geers D., et al. Immunogenicity and reactogenicity of booster vaccinations after Ad26.COV2.S priming. N Engl J Med. 2022;386:951–963. doi: 10.1056/NEJMoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C., et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217 doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer P.J.M., Brinkkemper M., Maisonnasse P., Dereuddre-Bosquet N., Grobben M., Claireaux M., et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell. 2021;184:1188–1200.e19. doi: 10.1016/j.cell.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders J.F., Bemelman F.J., Messchendorp A.L., Baan C.C., van Baarle D., van Binnendijk R., et al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2022;106:821–834. doi: 10.1097/TP.0000000000003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picard C., Bobby Gaspar H., Al-Herz W., Bousfiha A., Casanova J.L., Chatila T., et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. 2018;38:96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 27.Stewart D.M., Lian L., Nelson D.L. The clinical spectrum of Bruton’s agammaglobulinemia. Curr Allergy Asthma Rep. 2001;1:558–565. doi: 10.1007/s11882-001-0065-8. [DOI] [PubMed] [Google Scholar]
- 28.Gadani S.P., Reyes-Mantilla M., Jank L., Harris S., Douglas M., Smith M.D., et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73:103636. doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moderbacher C.R., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;83:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moor M.B., Suter-Riniker F., Horn M.P., Aeberli D., Amsler J., Möller B., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mykytyn A.Z., Rissmann M., Kok A., Rosu M.E., Schipper D., Breugem T.I., et al. Omicron BA.1 and BA.2 are antigenically distinct SARS-CoV-2 variants. bioRxiv. 2022 doi: 10.1126/sciimmunol.abq4450. 2022.02.23.481644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GeurtsvanKessel C.H., Geers D., Schmitz K.S., Mykytyn A.Z., Lamers M.M., Bogers S., et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022 doi: 10.1126/sciimmunol.abo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Bello A., Abravanel F., Marion O., Couat C., Esposito L., Lavayssière L., et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 2022;22:322–323. doi: 10.1111/ajt.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly C.M., Chiang T.P., Boyarsky B.J., Ruddy J.A., Teles M., Alejo J.L., et al. Temporary hold of mycophenolate augments humoral response to SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Rheum Dis. 2022;81:293–295. doi: 10.1136/annrheumdis-2021-221252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.