Abstract
Background & aims
To assess the impact of pre-admission renin-angiotensin-aldosterone system inhibitor (RAASi) and statin use on mortality following COVID-19 hospitalization in adults with pre-existing diabetes.
Methods
Retrospective cohort study of adults with diabetes admitted to ninety-nine participating hospitals in the United Kingdom, France and Spain during the first wave of the COVID-19 pandemic. Logistic regression models adjusted for demographic factors and comorbidity were used to describe associations with mortality in hospital or within 28 days of admission and individual or combined RAASi and statin therapy prescription followed by a country level meta-analysis.
Results
Complete data were available for 3474 (42.6%) individuals. Prescribing patterns varied by country: 25–50% neither RAASi nor statin therapy, 14–36% both RAASi and statin therapy, 9–24% RAASi therapy alone, 12–36% statin alone. Overall, 20–37% of patients died within 28 days. Meta-analysis found no evidence of an association between mortality and prescription of RAASi therapy (OR 1.09, CI 0.78–1.52 (I2 22.2%)), statin (OR 0.97, CI 0.59–1.61 (I2 72.9%)) or both (OR 1.14, CI 0.67–1.92 (I2 78.3%)) compared to those prescribed neither drug class.
Conclusions
This large multicentre, multinational study found no evidence of an association between mortality from COVID-19 infection in people with diabetes and use of either RAASi, statin or combination therapy. This provides reassurance that clinicians should not change their RAASi and statin therapy prescribing practice in people with diabetes during the COVID-19 pandemic.
1. Introduction
The COVID-19 pandemic has been one of the biggest human catastrophes of the modern age; killing almost 5 million people in 2 years and being one of the leading causes of death in the USA in 2021 [1,2]. The SARS-CoV-2 viral infection, initially identified in China in December 2019, rapidly became a global pandemic, reaching Europe in January 2020 [3]. Almost two years on, the pandemic continues with new variants emerging.
The main intracellular entry point into humans for the SARS-CoV-2 virus is the spike protein that binds to angiotensin-converting enzyme-2 (ACE-2) receptor. ACE-2 shows homology to ACE, a key player in the renin-angiotensin-aldosterone system (RAAS), suggesting hypothetical benefits and harms for people using medications affecting RAAS. Statin use has also been proposed as modulating COVID-19 infectivity through its upregulation of ACE2 and pleiotropic effect on oxidative stress [4].
International guidance for management of both type 1 and type 2 diabetes advocates the use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) as first line agents to manage hypertension and reduce deterioration of diabetic nephropathy [5]. Additionally, the use of statin therapy is advocated for both primary and secondary prevention of cardiovascular mortality and morbidity in these higher risk populations. The COVID-19 pandemic has disproportionately affected people with diabetes, raising questions as to whether certain pharmacological agents have played a contributory role [6]. Recent studies have indicated that glucose-lowering agents are safe to use during COVID-19 but large study data on the use of both RAASi and statins in people with diabetes are lacking [7]. This study aims to address the uncertainty of benefit and harm on the concomitant use of statin and RAASi therapy in adults with pre-existing type 1 and type 2 diabetes hospitalized with COVID-19 infection, using data from a multicentre, multinational study across Europe (France, Spain and United Kingdom (UK)).
2. Research design and methods
2.1. Study design and participants
Retrospective data from hospitalized adults with pre-existing diabetes and concomitant COVID-19 infection were collected in the UK, France and Spain. Adults with hyperglycaemia at admission, but not pre-existing or subsequent diagnosis of diabetes (based on WHO criteria) were excluded from analysis [8]. Further descriptions of each nation's dataset have been published previously [[9], [10], [11], [12]].
2.1.1. United Kingdom: association of British clinical diabetologist (ABCD) COVID-19 audit
ABCD COVID-19 audit collects data retrospectively from hospitals across the UK. Clinicians submit data for adults with pre-existing type 1 and type 2 diabetes infected with COVID-19. The audit is registered with Oxford University Hospitals NHS Foundation Trust (OUH) and a Data Protection Impact Assessment was carried out and approved by the OUH Caldicott Guardian and the Public Benefit and Privacy Panel in Scotland (reference 2021–0111). The NHS supports audit with clear guidance for the contributing centers on the use of routine clinical practice data submitted in anonymized form via the secure NHS network [12].
2.1.2. France: CORONADO (CORONAvirus-SARS-CoV-2 and diabetes outcomes)
The CORONADO study set out to describe the phenotypic characteristics and prognosis of people with diabetes admitted with COVID-19 between March 10 and April 10, 2020 [10,11]. CORONADO is a retrospective study from French hospitals volunteering to share data on hospitalized COVID-19 patients with diabetes. The study was sponsored by Nantes University Hospital and designed in accordance with the Declaration of Helsinki. It obtained all regulatory approvals.
2.1.3. Spain
The six hospitals in the HM Hospitales group collected anonymized observational data for people infected with COVID-19 during the first wave. This dataset is made available to researchers via “Covid Data Save Lives” [9]. The electronic hospital health records were collected for admitted persons including pre-existing disease status, medication use, demographic and outcome. A subset of people with pre-existing diabetes from this cross-sectional database was used in this study. Before access was granted, a formal petition, specific study protocol, and ethics committee approval were obtained. The study was approved by the Ethics Committee of the Primary Health Care University Research Institute (IDIAP) Jordi Gol, Barcelona (approval number: 20/089-PCV).
2.2. Data collection: definitions and outcomes
Demographic data included: age, gender and type of diabetes. UK and France collected ethnicity data (White/Europid, Black/African, Asian/Asian, Other/Middle East and North African (MENA)). Medication use at point of admission was collected with particular focus on those medications associated with diabetes or diabetes-related comorbidities. This study focused on statin, ACEi and ARB use.
Microvascular disease (including retinopathy, neuropathy and nephropathy) was collected for the French and UK cohorts. The Spanish cohort collected presence of chronic kidney disease (CKD) alone based on clinical coding record. CKD was defined by eGFR <60 ml/min or presence of macroalbuminuria. To improve homogeneity of the data and reduce collinearity we reported on CKD only. Macrovascular disease was collected for all datasets and included a diagnosis of cardiovascular, peripheral vascular or cerebrovascular disease.
The primary outcome was death. French data was collected to day 28 of admission, Spanish and UK data included outcome to conclusion of hospital episode.
2.3. Statistical analysis
We conducted complete-case analyses and reported descriptive statistics comparing the complete dataset to those excluded due to missing variables. Clinical characteristics were reported as frequency and percentages for categorical variables, and as mean and standard deviation for continuous variables. Multivariable logistic regression models were used to obtain odds ratios for the outcome variable, death. The main exposure was RAASi use, statin use, or both, and associations were estimated using as a reference category neither RAASi nor Statin. Potential confounders were ethnicity, CKD, macrovascular complications, hypertension, gender and type of diabetes (categorical) and age (continuous). Logistic regressions were performed using R in each contributing country; country-specific odds ratios were then pooled in a random-effects DerSimonian and Laird meta-analysis, with estimates stratified by model adjustment. Heterogeneity across studies was evaluated using the statistic I 2. Results are reported with 95% confidence interval (CI) and P-values of less than 0.05 were considered statistically significant.
3. Results
The UK ABCD COVID-19 audit collected data on 3007 people with diabetes from twenty-five hospitals between March and October 2020. Of these 1849 individuals (61.4%) had complete data required for this study and were included in this analysis. CORONADO investigators collected data on 2843 people from 68 hospitals with 1219 (43%) having complete data set. Spanish investigators collected data on 2310 individuals at six hospital sites, the majority without hyperglycaemia or diabetes. There was complete data for 406 Spanish individuals (18%) with pre-existing diabetes.
Comparison of the complete dataset to that with missing data showed no difference in mean age, gender, ethnicity or type of diabetes across all three countries (Supplementary Material Table 1). Ethnicity data were not available for the Spanish cohort.
The final dataset included 3474 individuals with diabetes and COVID-19 infection. One thousand- and nineteen (29.3%) people died. Characteristics of the study populations including prescribing patterns and mortality by country are shown in Table 1 .
Table 1.
Characteristics of people with diabetes and COVID-19 infection by combinations of medication prescription per country; United Kingdom (UK), France and Spain. Abbreviations used: Chronic kidney disease (CKD), renin-angiotensin-aldosterone-system inhibitors (RAASi).
| UK ABCD N = 1849 |
France CORONADO N = 1219 |
Spain HM Hospitales N = 406 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither RAASi nor Statin (n = 547, 29.6%) | Statin alone (n = 669, 36.2%) | RAASi alone (n = 163, 8.8%) | RAASi and Statin (n = 470, 25.4%) | Neither RAASi nor Statin (n = 310, 25.4%) | Statin alone (n = 194, 15.9%) | RAASi alone (n = 279, 22.9%) | RAASi and Statin (n = 436, 35.8%) | Neither RAASi nor Statin (n = 203, 56.7%) | Statin alone (n = 50, 12.3%) | RAASi alone (n = 96, 23.6%) | RAASi and statin (n = 57, 14%) | |
| Age (mean, SD) years | 72 (16) | 72 (13) | 72 (14) | 71 (12) | 69.2 (15.9) | 73.3 (11.7) | 71.8 (13.3) | 71.3(10.9) | 73.5 (13.0) | 77.1 (10.3) | 74.5 (11.0) | 77.0 (9.74) |
| Men n (%) | 314 (57%) | 428 (64%) | 93 (57%) | 301 (64%) | 176 (56.8%) | 125 (64.4%) | 166 (59.5%) | 278 (63.8%) | 123 (60.6%) | 30 (60.0%) | 61 (63.5%) | 36 (63.2%) |
| Ethnicity: | ||||||||||||
| White | 421 (77%) | 428 (64%) | 117 (72%) | 320 (68%) | 181 (58.4%) | 123 (63.4%) | 164 (58.8%) | 253 (58%) | NA | NA | NA | NA |
| Asian | 57 (10%) | 119 (18%) | 18 (11%) | 78 (17%) | 18 (5.8%) | 6 (3.1%) | 5 (1.8%) | 14 (3.2%) | ||||
| Black | 31 (6%) | 44 (7%) | 5 (3%) | 24 (5%) | 58 (18.7%) | 21 (10.8%) | 55 (19.7%) | 65 (14.9%) | ||||
| Other | 38 (7%) | 78 (11%) | 23 (14%) | 48 (10%) | 53 (17.1%) | 44 (22.7%) | 55 (19.7%) | 104 (23.9%) | ||||
| Type of diabetes | ||||||||||||
| Type 1 | 40 (7%) | 29 (4%) | 9 (6%) | 19 (4%) | 19 (6.1%) | 4 (2.1%) | 6 (2.2%) | 12 (2.8%) | 2 (0.99%) | 2 (4.00%) | 0 (0.00%) | 0 (0.00%) |
| Type 2 | 507 (93%) | 640 (96%) | 154 (94%) | 451 (96%) | 291 (93.9%) | 190 (97.9%) | 273 (97.8%) | 424 (97.2%) | 201 (99.0%) | 48 (96.0%) | 96 (100%) | 57 (100%) |
| Presence of CKD | 130 (24%) | 186 (28%) | 32 (20%) | 114 (24%) | 119 (38.4%) | 100 (51.5%) | 141 (50.5%) | 212 (48.6%) | 15 (7.39%) | 13 (26.0%) | 16 (16.7%) | 7 (12.3%) |
| Presence of macrovascular disease | 184 (34%) | 260 (39%) | 69 (42%) | 218(46%) | 87 (28.1%) | 100 (51.5%) | 101 (36.2%) | 243 (55.7%) | 35 (17.2%) | 23 (46.0%) | 13 (13.5%) | 24 (42.1%) |
| Presence of hypertension | 326 (60%) | 427 (64%) | 142 (87%) | 403 (86%) | 184 (59.4%) | 146 (75.3%) | 266 (95.3%) | 409 (93.8%) | 111 (54.7%) | 33 (66.0%) | 89 (92.7%) | 53 (93.0%) |
| Death | 212 (39%) | 229 (34%) | 70 (43%) | 165 (35%) | 55 (17.7%) | 49 (25.3%) | 49 (17.6%) | 111 (25.5%) | 42 (20.7%) | 9 (18.0%) | 18 (18.8%) | 10 (17.5%) |
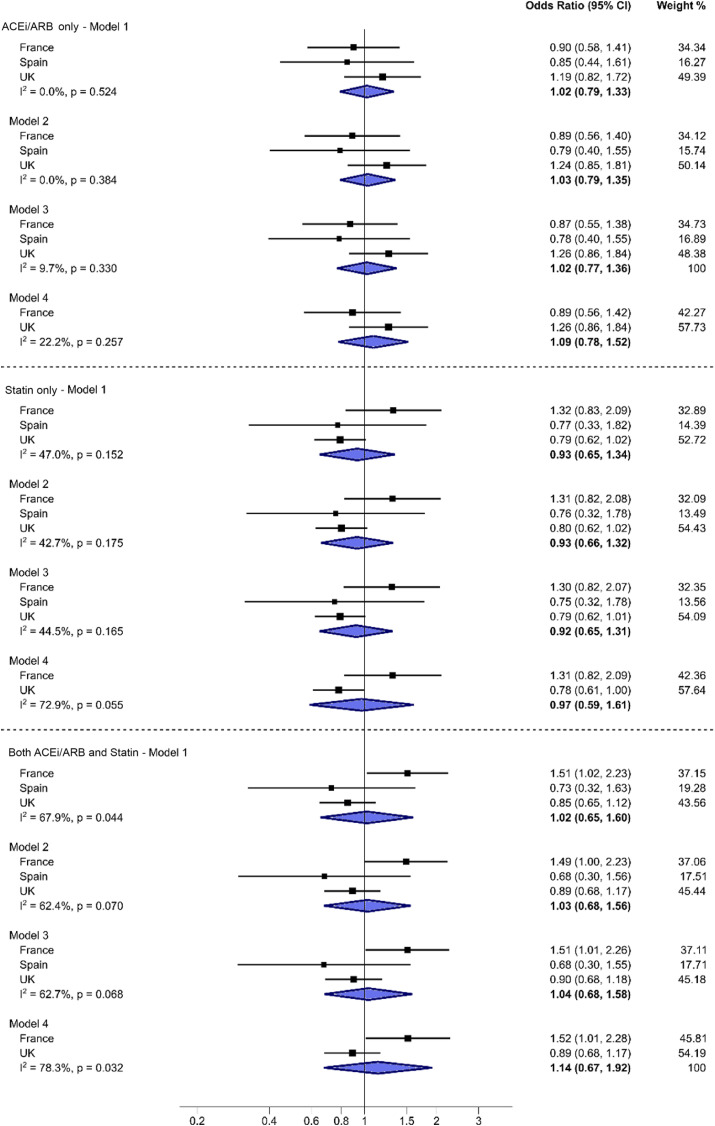
Output from individual logistic regression models for each country were combined in a meta-analysis and showed no significant association between death and use of either RAASi or statin therapy with considerable heterogeneity (Fig. 1 ). The overall odds ratio for RAASi use and mortality was 1.09 after adjusting for age, gender, type of diabetes, presence of macrovascular disease, CKD and hypertension and ethnicity (model 4), however this did not reach significance (CI 0.78–1.52) with little evidence of heterogeneity (I 2 22.2%). Similar non-significant association was seen for statin use (OR 0.97, CI 0.59–1.61) after further adjustment for ethnicity in UK and French cohorts with high heterogeneity (Fig. 1) (I 2 72.9%). Combined use of statin and RAASi therapy had similar non-significant association (OR 1.14, CI 0.67–1.92) with high heterogeneity (Fig. 1) (I 2 78.3%)
Fig. 1.
Country-specific and pooled odds ratios.
Legend:Odds ratio of death associated with use of either renin-angiotensin-aldosterone system inhibitor (RAASi)(indicated as ACEi/ARB), Statin or combined therapy in each country and overall (random-effects meta-analysis). I2indicates heterogeneity in the estimates. The baseline level for odds ratio estimates is the category “neither on ACEi/ARB nor on Statin”.
Odds Ratio estimates adjusted for.
Model 1 – Admission age, gender, Type of diabetes, presence of macrovascular complications.
Model 2 – Model 1 + Hypertension.
Model 3 – Model 2 + CKD.
Model 4 – Model 3 + Ethnicity.
Spain is excluded from Model 4 due to lack of ethnicity data.
4. Discussion
This is the first multinational European retrospective study to specifically investigate the outcomes of hospitalized adults with pre-existing diabetes and COVID-19 in relation to prior use of RAASi and statin therapy. We found no significant association between the prior use of RAASi or statin and the primary outcome of death in the meta-analysis, although the findings for statins were highly heterogeneous between the participating countries. The findings remained non-significant after adjusting for age, gender, diabetes type, hypertension, macrovascular disease and CKD. Further adjustment for ethnicity in two of the nations’ data had no further impact on the findings.
COVID-19 infection is associated with poorer outcome for those with long-term conditions, such as diabetes, and several potential mechanisms have been proposed [6,9]. This paper specifically examined medications used by many people with diabetes (type 1 and 2) for the prevention of diabetic complications and, therefore, the potential detrimental or beneficial effect of these medications on outcomes for this specific population. It uses large heterogenous real world datasets to draw clinically relevant conclusions.
Early use of statin therapy is globally advocated to prevent diabetes complications however the proportions receiving statin prescriptions in our study cohort varied between 26% in Spain and 61% in the UK [8]. Current guidance does not advise stopping statin therapy during intercurrent illness.
Prior to the COVID-19 pandemic, secondary analysis of a randomised, placebo-controlled trial in acute respiratory distress syndrome (ARDS) showed improved survival with simvastatin treatment in a sub-group of participants with hyperinflammatory state [13]. This raises a hypothetical benefit from statin use in populations with high inflammatory burden, such as type 2 diabetes, due to potential suppression of the hyperinflammatory response triggered by SARS-CoV-2 infection. Observational studies of statin therapy and COVID-19 infection support this. A US study showed reduced severity of COVID-19 infection where statins were used within 1 month prior to admission with faster recovery in those without severe disease, whilst another study found 12% reduced risk of in-hospital mortality in a diabetes subset [4,14]. A large Chinese retrospective sub-study found reduced COVID-19 related mortality associated with statin use (5.2% v 9.4% death rate) but no additional effect of additive RAASi therapy [15].
Our analysis revealed high heterogeneity in mortality for those using statin therapy, with differences in the relationship in France compared to both Spain and UK. The CORONADO statin therapy outcomes are surprising and oppose other evidence [16]. Strandberg and Kivimäki suggested that they may be outlier findings [17]. Closer review of this French cohort reveals a higher burden of macrovascular disease, CKD and hypertension [16]. However the mortality was considerably lower in the French than the UK cohort, suggesting unmeasured differences may explain these opposing relationships, such as diabetes stage or severity, diabetes therapies, smoking status, socioeconomic status or national healthcare systems.
Antecedent statin use with an associated lower lipid profile on admission has been associated with lower 30-day mortality in a general USA population [18]. A meta-analysis of adults with COVID-19 infection found almost 50% mortality risk reduction associated with in-hospital use of statin in a mixed patient population, but no benefit from statin use pre-admission [19]. In a population with high diabetes prevalence (44%), similar beneficial results were found with in-hospital statin use [20]. However, a recent meta-analysis of 25 studies, including 33% people with diabetes, found benefit of statin therapy only in chronic use prior to admission [21].
Our study did not examine either inflammatory markers or lipid levels, nor collect data on duration of prior use of statin and/or continuation of therapy on admission, which may have explained national differences.
Mounting evidence suggests beneficial or neutral effects of statin therapy but further investigation is needed in diabetes-specific populations of in-hospital and pre-admission statin use in relation to mortality and ICU admission. At least 34 randomised controlled trials using lipid-lowering medications are currently underway in general populations, including 14 using statins (both high and low dose with patients in and out of intensive care settings) [22].
The potential pleiotropic interactions between statin therapy and COVID-19 include effects on inflammatory response and ACE2 protein. One may anticipate more conclusive evidence from therapies exclusively affecting ACE2 protein, and therefore SARS-CoV-2 transmission and infection, such as RAASi. To date, evidence from cohort studies and a global meta-analysis of over 19,000 people show no increased risk of SARS-CoV-2 infection or COVID-19 disease severity for those taking RAASi [23,24]. In fact, meta-analysis of over 33,000 people found beneficial effect of RAASi use on disease severity [25]. A UK primary care prospective study also observed reduced disease severity but reported ethnic disparities in association between RAASi use and SARS-CoV-2 positivity, with those from Caribbean or Black-African ethnic groups having increased risk of infection compared to White [26]. Within our diabetes-specific population, with 43% RAASi use overall (Supplementary Table 1), there was no significant association with mortality, including after adjusting for ethnicity. These findings are in keeping with a meta-analysis of people with co-existent hypertension and COVID-19, which showed a beneficial effect of RAASi use that was not modulated by diabetes in meta-regression [27].
RAASi treatment may be interrupted to minimise potential nephrotoxic effects [28]. Further research is required to investigate whether stopping RAASi is appropriate in the face of COVID-19 infection. As with statin therapy, this research is in progress, but it will be important to look at the diabetes-specific population where prescribing indications differ [29].
This study has clinical relevance to the general practitioner, diabetologist and their patients. It provides reassurance about continued outpatient use of RAASi and statin therapy during the pandemic, with no significant association with increased mortality or disease severity in those prescribed them. The multinational and demographically diverse datasets allow for wider generalisability of findings.
Our study is limited by the heterogeneity of data collection methods across the nations due to use of databases not initially created in collaboration to answer the study question and, therefore, by some missing data. The meta-analysis combines a larger dataset to provide an overall relationship of association, rather than causality, but masks heterogeneity across nations. Markers of inflammation and lipid levels were not collected, as well as other potential confounding factors such as smoking status, body mass index and diabetes duration. Lastly, drug histories were often collected from health records, rather than being confirmed with individuals, introducing the possibility of error in the context of access to pharmacies being restricted during pandemic lockdowns.
5. Conclusion
This large multinational study of people with diabetes requiring hospitalization for COVID-19 infection demonstrates no significant association with the pre-admission use of statin and/or RAAS inhibitor therapy with mortality. It provides reassurance to clinicians who have continued prescribing RAAS inhibitors throughout the pandemic despite publicity surrounding unadjusted associations between statin therapy and poor outcomes from COVID-19. Further studies, including randomised controlled trials, are required to provide further reassurance. These studies should consider prescribing indications specific to diabetes, such as microalbuminuria, which may produce conflicting results to those seen in the general population.
Funding
This study received the following funding: the Fondation Francophone de Recherche sur le Diabète (FFRD), supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly and FFD (Fédération Française des Diabétiques) – CORONADO initiative emergency grant; Société Francophone du Diabète (SFD) – CORONADO initiative emergency grant; Air Liquide Health Care international. CORONADO initiative emergency grant; Allergan. CORONADO initiative emergency grant; AstraZeneca. CORONADO initiative emergency grant; Elivie. CORONADO initiative emergency grant; Fortil. CORONADO initiative emergency grant; Lifescan. CORONADO initiative emergency grant; CORONADO initiative emergency grant; Nantes Métroplole. NHC. CORONADO initiative emergency grant; Novo Nordisk. CORONADO initiative emergency grant; Sanofi. CORONADO emergency grant; PHRC National COVID-19 Hospitalization and Care Organization Division (DGOS) as part of the Hospital Clinical Research Program (PHRC COVID-19-20-0138). All research facilities are acknowledged for providing research associates and research technicians for clinical investigations pro bono. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
The ABCD nationwide COVID-19 & Diabetes is an independent audit which has received support from Public Health Wales and Novo Nordisk.
Author contributions
MW, PG, SaH and BC designed the CORONADO study.
RRe, EGW, SHW, SoH, BCTF, REJR, PN, KV, YR and KK designed the ABCD COVID-19 National audit. YR conducted the statistical analysis for the ABCD study. FZ conducted the meta-anlysis. SoH, KK and SW drafted the first version of the manuscript. All authors approved the final manuscript. SoH and KK are the guarantors of this work.
Authors relationships
No potential conflicts of interest relevant to this article were reported.
BC reports grants and personal fees from Amgen, personal fees from Astra-Zeneca, personal fees from Akcea, personal fees from Genfit, personal fees from Gilead, personal fees from Eli Lilly, personal fees from Novo Nordisk, personal fees from Merck (MSD), grants and personal fees from Sanofi, grants and personal fees from Regeneron.
PG reports personal fees from Abbott, personal fees from Amgen, personal fees from Astra-Zeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from MSD, personal fees from Mundipharma, grants and personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Servier.
SaH reports personal fees and non-financial support from Astra Zeneca, grants and personal fees from Bayer, personal fees from Boehringer Ingelheim, grants from Dinno Santé, personal fees from Eli Lilly, non-financial support from LVL, personal fees and non-financial support from MSD, personal fees from Novartis, grants from Pierre Fabre Santé, personal fees and non-financial support from Sanofi, personal fees and non-financial support from Servier, personal fees from Valbiotis.
MW reports personal fees from Novo Nordisk.
RRe has acted as a consultant, speaker or received grants from Novo Nordisk, Eli Lilly and Boehringer Ingelheim.
KK has acted as a consultant, speaker or received grants for investigator-initiated studies for Astra Zeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG/Menarini Group, Janssen, and Napp.
EGW has received personal fees from: Abbott Diabetes Care, Dexcom, Diasend, Eli Lilly, Insulet, Medtronic, Novo Nordisk, Sanofi Aventis. Chair of the Ethnicity Subgroup of the UK Scientific Advisory Group for Emergencies (SAGE) and Member of SAGE.
SHW attends meetings of the Scottish Study Group of Diabetes in the Young that receives support from Novo Nordisk.
SoH has received educational funding support from Sanofi Aventis, and consulting fees from Eli Lilly and Oviva
BCTF has acted as a consultant, speaker, or received grants from Abbott Diabetes, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GSK, Janssen, Medtronic, MSD, Napp, Novo Nordisk and Sanofi.
REJR has received speaker fees and/or consultancy fees and/or educational sponsorships from AstraZeneca, BioQuest, GI Dynamics, Janssen, Novo Nordisk, Sanofi-Aventis and Takeda.
DP has acted as a consultant, speaker or received grants from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Napp and Novo Nordisk.
Data availability
Data is available on request from corresponding author and/or national study leads.
Declaration of competing interest
All other authors declare no competing interests.
Acknowledgments
The contributors to national studies are listed in supplementary material.
With regard to the CORONADO initiative, we thank the sponsor (DRCI, Nantes University Hospital), Clinical Project Manager (Maëva Saignes) and assistant (Jeanne Saunier), Clinical Research Associates (Selma El Andaloussi, Joëlle Martin-Gauthier, Emily Rebouilleau) and data manager (Tanguy Roman). We thank the Communication Manager of l’Institut du Thorax (Vimla Mayoura). We acknowledge all medical staff involved in the diagnosis and treatment of patients with COVID-19 in participating centers. We thank all GPs, specialists, pharmacists and biological laboratories in charge of hospitalized patients for providing additional medical information to our investigators. We thank the Société Francophone du Diabète (SFD) and Société Française d’Endocrinologie (SFE) for disseminating study design and organization, the Fédération Française des Diabétiques (FFD) for participating in the organization of the study. KK is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC).
With regard to the ABCD-COVID-19 national Audit, we are grateful to all the people who collected the data for this study, to Ben Maylor and Joanne Miksza for data template development, and to Melissa Cull of the ABCD secretariat for administrative support.
See contributors of the ABCD COVID-19 audit study in supplementary material.
We are grateful to all the people who collected the data for this study, to Ben Maylor and Joanne Miksza for data template development, and to Melissa Cull of the ABCD secretariat for administrative support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2022.102484.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organisation. WHO Coronavirus COVID-19 dashboard.
- 2.Woolf S.H., Chapman D.A., Lee J.H. COVID-19 as the leading cause of death in the United States. JAMA J Am Med Assoc. 2021;325(2):123–124. doi: 10.1001/jama.2020.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiteri G., Fielding J., Diercke M., et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European region, 24 january to 21 february 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels L.B., Sitapati A.M., Zhang J., et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136 doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosentino F., Grant P.J., Aboyans V., et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Euro Heart. J. 2019;41(2) doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 6.Singh A.K., Gillies C.L., Singh R., et al. Prevalence of co-morbidities and their association with mortality in patients with <scp>COVID</scp> -19: a systematic review and meta-analysis. Diabetes Obes Metabol. 2020;22(10) doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khunti K., Knighton P., Zaccardi F., et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. 2021;9(5) doi: 10.1016/S2213-8587(21)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation . 2016. Global report on diabetes. [Google Scholar]
- 9.Ortega E., Corcoy R., Gratacòs M., et al. Risk factors for severe outcomes in people with diabetes hospitalised for COVID-19: a cross-sectional database study. BMJ Open. 2021;(7):11. doi: 10.1136/bmjopen-2021-051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cariou B., Hadjadj S., Wargny M., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8) doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wargny M., Potier L., Gourdy P., et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64(4):778—794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagi D., Ryder R.E., Ruan Y., et al. An audit of people admitted to hospital with diabetes and coronavirus (SARS-CoV-2): data collection methods. The Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Br J Dermatol. 2021;21(1):96–99. doi: 10.15277/bjd.2021.299. [DOI] [Google Scholar]
- 13.Calfee C.S., Delucchi K.L., Sinha P., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9) doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeed O., Castagna F., Agalliu I., et al. Statin use and in-hospital mortality in patients with diabetes mellitus and COVID-19. J Am Heart Assoc. 2020;9(24) doi: 10.1161/JAHA.120.018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X.J., Qin J.J., Cheng X., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metabol. 2020;32(2) doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou B., Goronflot T., Rimbert A., et al. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metabol. 2021;47(2):101202. doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strandberg T.E., Kivimäki M. Increased mortality risk associated with statins in the CORONADO study. Diabetes Metabol. 2021;47(3):101250. doi: 10.1016/j.diabet.2021.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A., Madhavan M.v., Poterucha T.J., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Permana H., Huang I., Purwiga A., et al. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol Rep. 2021;73(3) doi: 10.1007/s43440-021-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohia P., Kapur S., Benjaram S., et al. Statins and clinical outcomes in hospitalized COVID-19 patients with and without Diabetes Mellitus: a retrospective cohort study with propensity score matching. Cardiovasc Diabetol. 2021;20(1):140. doi: 10.1186/s12933-021-01336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Arocutipa C., Melgar-Talavera B., Á Alvarado-Yarasca, et al. Statins reduce mortality in patients with COVID-19: an updated meta-analysis of 147 824 patients. Int J Infect Dis. 2021;110:374–381. doi: 10.1016/j.ijid.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talasaz AH, Sadeghipour P, Aghakouchakzadeh M, et al. Lipid-modulating agents for prevention or treatment of COVID-19 in randomized trials. medRxiv. Published online May 4, 2021. doi:10.1101/2021.05.03.21256468.
- 23.Rezel-Potts E., Douiri A., Chowienczyk P.J., Gulliford M.C. Antihypertensive medications and COVID-19 diagnosis and mortality: population-based case-control analysis in the United Kingdom. Br J Clin Pharmacol. 2021:14873. doi: 10.1111/bcp.14873. Published online May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Yu J., Pan L ya, Jiang H yin. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Long C, Xiong Q, et al. Association of <scp>angiotensin converting enzyme inhibitors and angiotensin II receptor blockers</scp> with risk of <scp>COVID</scp> -19, inflammation level, severity, and death in patients with <scp>COVID</scp> -19: a rapid systematic review and <scp>meta-analysis</scp>. Clin Cardiol. Published online August 5, 2020:clc.23421. doi:10.1002/clc.23421. [DOI] [PMC free article] [PubMed]
- 26.Hippisley-Cox J., Young D., Coupland C., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19) doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Chen B., Li Y., et al. The use of renin–angiotensin–aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2021;93(3) doi: 10.1002/jmv.26625. [DOI] [PubMed] [Google Scholar]
- 28.TREND-UK Type 2 diabetes: what to do when you are ill. https://trend-uk.org/wp-content/uploads/2020/03/A5_T2Illness_TREND_FINAL.pdf
- 29.Gnanenthiran S.R., Borghi C., Burger D., et al. Prospective meta-analysis protocol on randomised trials of renin–angiotensin system inhibitors in patients with COVID-19: an initiative of the International Society of Hypertension. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-043625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available on request from corresponding author and/or national study leads.