Abstract
Although the microflora associated with oral mucositis initiated by cytotoxic therapy is not well characterized, several studies suggest that reduction of the microbial load in the oral cavity has some clinical benefit. The MICs of IB-367, a synthetic protegrin analog, ranged from 0.13 to 64 μg/ml for gram-positive bacteria (Streptococcus mitis, Streptococcus sanguis, Streptococcus salivarius, and Staphylococcus aureus) and from 0.06 to 8 μg/ml for gram-negative species (Klebsiella, Escherichia, and Pseudomonas). IB-367 exhibited rapid, microbicidal activity against both log- and stationary-phase cultures of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. At concentrations near the MICs for these two organisms (4 and 2 μg/ml, respectively), IB-367 reduced viability by more than 3 logs in less than 16 min. Similarly, IB-367 effected a 4-log reduction of the endogenous microflora in pooled human saliva within 2 min at 250 μg/ml, a concentration readily attained by local delivery. After nine serial transfers at 0.5× the MIC, the MIC of IB-367 for MRSA and P. aeruginosa increased only two to four times. In a phase I clinical study with healthy volunteers, IB-367 was well tolerated, with no detectable systemic absorption. One hour after treatment with 9 mg of IB-367, the prevalence of gram-negative bacteria and yeast was reduced, and the density of the predominant gram-positive oral flora was decreased 1,000 times. IB-367's properties (speed of killing, breadth of spectrum, and lack of resistance) make the compound a strong candidate for the prophylaxis of oral mucositis. Phase II clinical trials with IB-367 are under way for this indication in immunocompromised subjects.
The microflora of the mouth has a complex and diverse ecology. Although more than 200 species of microorganisms have been isolated from the oropharynx, individual surfaces of the oral cavity are dominated by specific subgroups (12). For example, alpha and nonhemolytic viridans group streptococci predominate on the surface of the buccal mucosa (13). The development of illnesses such as cancer has been associated with significant shifts in the numbers of gram-negative bacteria detectable in oral samples (18, 21). An increase in the levels of Candida albicans can also occur; this species is the cause of most oral fungal infections in cancer patients.
The cytotoxic effects of radiation therapy or chemotherapy on rapidly dividing epithelial cells of the oropharyngeal mucosa often lead to a very painful condition known as oral mucositis (5, 26). Lesions associated with oral mucositis are usually found on the buccal and sublingual mucosae (17). Infections with endogenous microflora or opportunistic pathogens are thought to exacerbate this condition, leading to ulceration, inflammation, and accumulation of microorganisms in the necrotic tissue (3, 4, 11, 25). Although the microflora associated with oral mucositis is not well characterized, a reduction in the microbial load of the oral cavity appears to have some benefit in the treatment of oral mucositis in cancer patients (2, 6, 22, 24). Currently, no effective U.S. Food and Drug Administration-approved therapy exists for prevention or treatment of oral mucositis (14, 18).
We have tested the antimicrobial activity of a synthetic protegrin analog, IB-367 (RGGLCYCRGRFCVCVGRCONH2), against representatives of the most prevalent groups of aerobic oral flora using a modified version of the broth microdilution method recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (15, 23). We have also determined the MICs for multiple strains of aerobic gram-negative species most commonly associated with oral mucositis (Klebsiella, Serratia, Escherichia, and Pseudomonas) and gram-positive species associated with accompanying complications such as bacteremia (Streptococcus mitis and Streptococcus sanguis) or systemic shock (Staphylococcus aureus). In addition, the ability of IB-367 to reduce the level of the oral microflora in vivo was determined in a phase I clinical trial performed with healthy volunteers.
We demonstrate here that IB-367 exhibits properties that may be vital for effective treatment of oral mucositis: (i) broad-spectrum antimicrobial activity, (ii) rapid killing, and (iii) a relative lack of resistance development.
MATERIALS AND METHODS
Bacterial strains.
Strains of gram-positive bacteria obtained from the American Type Culture Collection (ATCC; Rockville, Md.) included methicillin-sensitive S. aureus (MSSA; Smith type; ATCC 19636 and ATCC 29213), methicillin-resistant S. aureus (MRSA; ATCC 33591), vancomycin-sensitive Enterococcus faecalis (ATCC 29212) and Enterococcus faecium (ATCC 19434), Streptococcus salivarius (ATCC 7073, ATCC 31067), Streptococcus mitis (ATCC 9811, ATCC 15914), and Streptococcus pneumoniae (ATCC 49619). Gram-negative bacteria included Acinetobacter calcoaceticus (ATCC 17905, ATCC 23055), Escherichia coli (ATCC 25922, ATCC 23579), Haemophilus influenzae (ATCC 49247), Klebsiella pneumoniae (ATCC 10031, ATCC 9997), Neisseria meningitidis (ATCC 13093), Pseudomonas aeruginosa (ATCC 9027, ATCC 27853, ATCC 39324), and Serratia marcescens (ATCC 13880). Two strains of C. albicans (ATCC 10231 and ATCC 90029) were also tested. Additional clinical isolates were obtained from Patricia Mickelson, Clinical Microbiology Laboratory, Stanford University, Calif. API test strips (BioMerieux, Hazelwood, Mo.) were used to confirm organism identities, and strains were stored frozen in 10% glycerol at −80°C.
Media for in vitro assays.
Mueller-Hinton broth (MHB), cation-adjusted Mueller-Hinton broth II, Trypticase soy broth (TSB), and Trypticase soy agar (TSA) were purchased in powder form from Becton Dickinson, Cockeysville, Md., and were prepared in distilled, deionized water. Haemophilus test medium (HTM) and RPMI medium without NaHCO2 but with 20 mM HEPES and l-glutamine (0.3 g/liter) were purchased premade from Becton Dickinson and Sigma Chemical Co. (St. Louis, Mo.), respectively. Blood agar plates (BAPs) containing TSA with 5% sheep blood added, MacConkey agar plates, Sabouraud dextrose agar plates, mannitol salt agar plates, and horse blood were purchased from Hardy Diagnostics, Santa Maria, Calif. Lysed horse blood (LHB) was prepared by mixing a thawed sampled of frozen blood 1:1 with sterile water, centrifuging, and adding the supernatant to MHB at 2% as needed for adequate growth. Liquid Testing Medium (LTM) contained the following: 10 mM phosphate buffer (pH 6.5), 1% TSB, and 100 mM NaCl. Phosphate-buffered saline (PBS) contained 10 mM phosphate (pH 7.4) and 100 mM NaCl.
Reagents.
Norfloxacin (95% pure by high-performance liquid chromatography [HPLC]), vancomycin (1,118 μg/mg), polymyxin B (7,760 U/mg), and gentamicin (647 μg/mg) were obtained from Sigma Chemical Co., and ciprofloxacin (867 μg/mg) was obtained from Bayer Corporation (Kankakee, Ill.). Human saliva was obtained from healthy volunteers after brushing. IB-367 was synthesized in-house with Rink-amide resins, by 9-fluorenylmethoxy carbonyl solid-phase chemistry, and with an automated peptide synthesizer (model 431A; Applied Biosystems, Foster City, Calif.). The peptide was cleaved from the solid support with trifluoroacetic acid and was subsequently folded by using air oxidation. The peptide was purified by reverse-phase HPLC (Vydac C18 column; 2.2 by 25 cm; solvent A, 0.1% trifluoroacetic acid in water; solvent B, 0.08% trifluoroacetic acid in acetonitrile; linear gradient, 21 to 49% solvent B in 30 min; flow rate, 8 ml/min; detection at 214 nm).
In vitro susceptibility testing.
MICs were determined by a slightly modified version of the NCCLS broth microdilution method as described previously (23). Briefly, antimicrobial agents were prepared as 10× concentrates in the most appropriate solvent. For IB-367, 0.01% acetic acid containing 0.2% bovine serum albumin as a carrier protein was used. Vancomycin, polymyxin B, ciprofloxacin, and gentamicin were dissolved in water, whereas norfloxacin was dissolved in 100% dimethyl sulfoxide and was then serially diluted in water. Inocula were prepared by resuspending colonies from a BAP in medium and adjusting the suspension to match that of a 0.5 McFarland standard. The suspension was diluted into fresh medium (as recommended by NCCLS for the organism) to give 2 × 105 to 7 × 105 CFU/ml for bacteria or 2 × 103 to 7 × 103 CFU/ml for Candida. After dispensing 100-μl aliquots of the microbial suspension into each well of a 96-well polypropylene microtiter plate, 11 μl of test compound was added. The MIC was defined as the lowest concentration of drug which prevented visible turbidity after 16 to 20 h (bacteria) or 46 to 50 h (Candida) at 35°C. Minimum bactericidal concentrations (MBCs) or minimum fungicidal concentrations were determined by transferring 10 μl from each clear well (greater than or equal to the MIC) onto a BAP. After incubation for 20 h, the MBC was identified as the lowest concentration that did not permit any visible growth on the surface of the agar.
Resistance studies.
MRSA and P. aeruginosa were harvested from the well with an IB-367 concentration equal to 0.5× the MIC, diluted to 1 × 105 to 5 × 105 CFU/ml in fresh MHB, and dispensed into microtiter plates as 100-μl aliquots. Compounds were added as described above, and MICs were determined daily for up to 18 serial passages. MICs were also determined for cultures incubated 5 days before serial passage.
Microbicidal assays.
Bacteria were grown overnight in TSB (10 ml in a 50-ml Erlenmeyer flask) at 200 rpm and 37°C to the stationary phase. Stationary-phase cultures in TSB were centrifuged, resuspended in PBS (MRSA) or LTM (P. aeruginosa) at 4 × 105 CFU/ml, and then dispensed into polypropylene microcentrifuge tubes. Exponential-phase cultures were prepared by diluting an overnight culture in MHB into fresh MHB (1:1,000) and incubating the culture at 200 rpm and 37°C until an absorbance (at 600 nm) of 0.2 was reached. The culture was then diluted to 1 × 105 to 4 × 105 CFU/ml in prewarmed MHB, reincubated to allow two cell doublings, and then dispensed into polypropylene microcentrifuge tubes. After addition of the test compounds at a 1/10 volume, the tubes were incubated without aeration at the appropriate temperature. Fresh human saliva was mixed 1:1 with peptide (10 mM sodium acetate buffer [pH 5]) or conventional antimicrobial agents (sterile water), and the mixture was then incubated at 37°C without aeration. The numbers of viable CFU were determined by one of two methods. By the pour plate method, 20-μl aliquots of serial dilutions in 0.87% saline were mixed with approximately 20 ml of tempered (50°C) TSA. By the spread plate method, 20-μl aliquots of serial dilutions in 0.87% saline were spread onto the surface of the desired agar plate. Both methods allowed rapid sampling, minimized drug carryover (i.e., ≤0.01× the MIC), and precluded the need for washing of the cells to remove the drug. The plates were incubated for 18 to 24 h at the appropriate temperature, and the colonies were enumerated to determine the microbicidal effect of the drug.
Phase I clinical trial.
The study population consisted of healthy men and women (ages, 18 to 65 years) who were within 20% of their ideal weight range for age, height, and frame and who were nonsmokers. Use of antibiotics within 28 days of study entry was not allowed, nor was the use of over-the-counter medications and commercially available mouthwashes for at least 14 days prior to the start of the study. In addition, subjects had to be willing and able to refrain from consuming alcohol- or caffeine-containing foods and beverages during the study. Subjects were excluded from the study if they had a history of anaphylaxis or xerostomia or if lesions of the oral mucous membranes were present.
The phase I trial was a multiple-ascending-dose study. The subjects received 3 g of IB-367 gel formulation four times daily for 4 consecutive days. Groups of six subjects each received a gel formulation containing either 0.3, 1, or 3 mg of IB-367 per g of gel. The subjects were asked to hold the gel in their mouth without spitting or swallowing for 5 min. In addition, they were asked not to eat or drink for 1 h after receiving the study medication. Samples of oral microflora were taken prior to administration of the first dose and 1 h after administration of the first dose on days 1, 2, and 4 of treatment. Samples were obtained and the microbial content was determined by a modified version of an oral washing method described previously (19). Briefly, the oral cavity was washed with 20 ml of sterile saline from which 10-fold serial dilutions were made in sterile saline containing 0.1% Tween 80. The total number of aerobic bacteria present was determined by plating each dilution onto BAP. The presence of gram-negative bacteria, staphylococcal species, and yeast was determined by plating 100 μl of the undiluted sample onto MacConkey agar, mannitol salt agar, and Sabouraud agar plates, respectively. All agar plates were incubated overnight at 35°C, and the numbers of viable organisms were determined as the numbers of CFU per milliliter.
RESULTS
In vitro susceptibility.
The MICs of IB-367 and conventional antimicrobial agents for MRSA and P. aeruginosa in MHB were determined (Table 1). When a strain of MRSA was tested in MHB, growth was acceptable and the MIC of IB-367 was 4 μg/ml. When the same strain was tested in MHB plus 2% LHB or HTM, the MIC of IB-367 increased to 16 μg/ml.
TABLE 1.
Comparison of in vitro activity of IB-367 with those of other antimicrobial agents
| Compound | MIC (μg/ml)
|
|
|---|---|---|
| MRSA (ATCC 33591) | P. aeruginosa (ATCC 9027) | |
| IB-367 | 4 | 2 |
| Norfloxacin | 0.5 | 0.25 |
| Gentamicin | 1.0 | 0.5 |
| Vancomycin | 1.0 | NAa |
| Polymyxin B | NA | 0.5 |
NA, not applicable.
Clinical isolates representing aerobic organisms prevalent in the oral cavity were tested for their susceptibilities to IB-367 (Table 2). The MICs of IB-367 ranged from 0.13 to 64 μg/ml for gram-positive bacteria and from 0.06 to 8 μg/ml for gram-negative bacteria. One exception was S. marcescens, for which MICs were in the range of 16 to 256 μg/ml. In general, the MBCs of IB-367 for all organisms were within 1 dilution of the MICs (data not shown).
TABLE 2.
Broad-spectrum antimicrobial activity of IB-367 against oral flora by the modified NCCLS broth microdilution methoda
| Group and organism (no. of strains) | Prevalence range (%)b | MIC range (μg/ml) |
|---|---|---|
| Gram-positive bacteria | ||
| Viridans group streptococcic | 93–99 | |
| S. salivarius (12) | 50–75 | 0.2–5.0 |
| S. sanguis (14) | 25–75 | 4–64 |
| S. mitis (15) | 25–75 | 2–43 |
| S. mutans (3) | 25–75 | 0.7–1.3 |
| Streptococcus spp., group D (6) | 90–100 | 0.25–4 |
| Streptococcus spp. (16)c | 1–50 | 1.3–16 |
| Corynebacterium spp. (5)c | 15–90 | 0.13–0.25 |
| Propionibacterium | 11–12 | NTd |
| Staphylococcus spp. (35) | 3–70 | 0.13–4 |
| Lactobacillus | 1–37 | NT |
| Gram-negative bacteria | ||
| Moraxella spp. (12) | 81–97 | 0.2–0.8 |
| Neisseria sp. (1)c | 5–97 | 8 |
| Haemophilus spp. (15)c | 5–35 | 1–8 |
| Acinetobacter calcoaceticus (4) | 5–30 | 0.06–2 |
| Klebsiella pneumoniae (4) | 5 | 1–5 |
| Pseudomonas aeruginosa (18) | 5 | 1–8 |
| Escherichia coli (5) | NAe | 0.25–1 |
| Serratia marcescens (16) | NA | 16–>256 |
| Yeast, Candida albicans (6) | 3–6 | 4–16 |
Bactericidal activity.
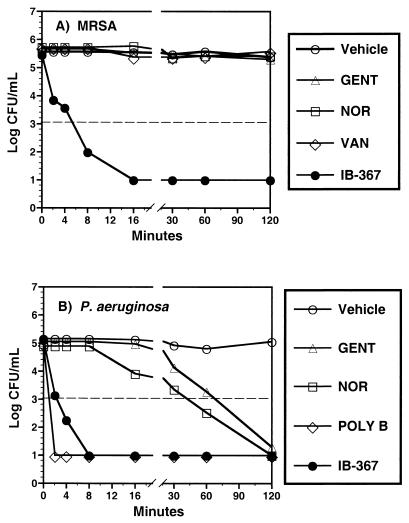
The bactericidal activity of IB-367 was compared to those of conventional antimicrobial agents at concentrations near their respective MICs. After the addition of IB-367 to stationary-phase cultures of MRSA (Fig. 1A) or P. aeruginosa (Fig. 1B), the numbers of viable CFU of both organisms decreased by 3 log units within 8 min. Polymyxin B produced a similar, rapid reduction in the numbers of P. aeruginosa CFU, whereas vancomycin was not bactericidal against MRSA in this time period. Gentamicin and norfloxacin required ≥2 h to effect the same decrease in the numbers of P. aeruginosa CFU and were completely ineffective against MRSA at this concentration.
FIG. 1.
Bactericidal activity of IB-367 against stationary-phase cultures. After overnight growth in TSB, stationary-phase cultures of MRSA or P. aeruginosa were resuspended in either PBS (pH 7.4) (A) or LTM (B). Except for IB-367 (4 μg/ml), all compounds were added at 1 μg/ml, and survivors were enumerated at the indicated times by the pour plate method. The dashed line represents the minimum number of CFU per milliliter which could be accurately determined. GENT, gentamicin; NOR, norfloxacin; VAN, vancomycin; POLY B, polymyxin B; Vehicle, buffer or medium plus the test organism.
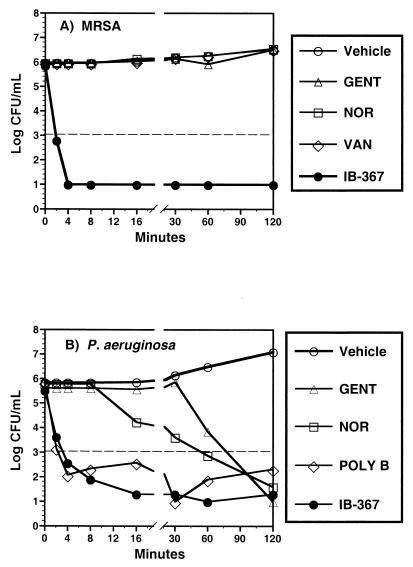
When tested against log-phase cultures of MRSA growing in MHB, IB-367 reduced the numbers of CFU by 4 log units within 4 min, whereas gentamicin, norfloxacin, and vancomycin were completely ineffective, even after 2 h (Fig. 2A). Similar results were observed for exponentially growing cultures of P. aeruginosa (Fig. 2B). IB-367 reduced the numbers of CFU more rapidly than either norfloxacin or gentamicin did, and the reduction achieved with IB-367 was similar to the reduction achieved with polymyxin B.
FIG. 2.
Bactericidal activity of IB-367 against log-phase cultures. Exponentially growing MRSA (A) or P. aeruginosa (B) was treated with IB-367 (4 μg/ml) or other antimicrobial agents at 1 μg/ml. Survivors were enumerated at the indicated times by the pour plate method. The dashed line represents the minimum number of CFU per milliliter which could be accurately determined. GENT, gentamicin; NOR, norfloxacin; VAN, vancomycin; POLY B, polymyxin B; Vehicle, buffer or medium plus test organism.
Although increasing the concentrations of conventional antimicrobial agents to 16 μg/ml improved the reduction in the numbers of CFU in both log- and stationary-phase cultures of P. aeruginosa, the reductions did not equal that achieved with IB-367 (data not shown). In contrast, the higher concentration did not affect the reduction in the numbers of CFU when the conventional antimicrobial agents were tested against MRSA.
Microbicidal activity against heterogeneous flora in saliva.
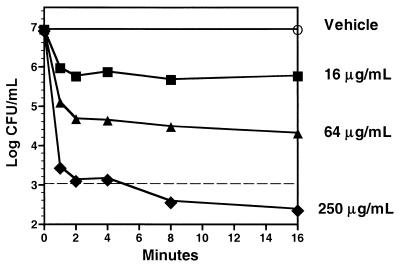
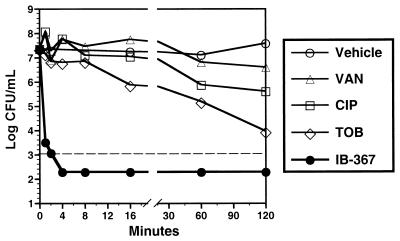
IB-367 exhibited concentration-dependent microbicidal activity against the oral microflora when it was mixed 1:1 with pooled human saliva from healthy volunteers (Fig. 3). At 250 μg/ml, IB-367 effected a 4-log reduction in the levels of the endogenous oral microflora within 2 min, whereas vehicle alone did not exhibit any antimicrobial effect. When compared with conventional antimicrobial agents at 1,000 μg/ml, IB-367 reduced the numbers of CFU by >4 log units in 1 min (Fig. 4). In contrast, tobramycin and ciprofloxacin reduced the numbers of CFU by 1 log unit after 16 and 60 min, respectively, whereas vancomycin was essentially ineffective even after 2 h.
FIG. 3.
Microbicidal activity of IB-367 against polymicrobial flora in saliva from healthy human volunteers. IB-367 (10 mM sodium acetate [pH 5]) was mixed 1:1 with saliva to give the indicated final concentrations. Vehicle contained 10 mM sodium acetate (pH 5) mixed 1:1 with saliva. At the indicated intervals, aliquots were spread onto the surfaces of TSA plates containing 10% fetal bovine serum, and the plates were incubated overnight at 37°C for 48 h prior to enumeration of the number of survivors. The dashed line represents the minimum number of CFU per milliliter which could be accurately determined.
FIG. 4.
Comparison of microbicidal activity of IB-367 and conventional antimicrobial agents against polymicrobial flora in saliva from healthy human volunteers. Saliva was mixed 1:1 with test compound (IB-367; 10 mM sodium acetate [pH 5]; vancomycin [VAN], ciprofloxacin [CIP], or tobramycin [TOB] in sterile deionized water) to a final concentration of 1,000 μg/ml. Vehicle contained 10 mM sodium acetate (pH 5) mixed 1:1 with saliva. At the indicated intervals, aliquots were plated onto TSA containing 5% sheep's blood, and the plates were incubated overnight at 37°C for 24 h prior to enumeration of the number of survivors. The dashed line represents the minimum number of CFU per milliliter which could be accurately determined.
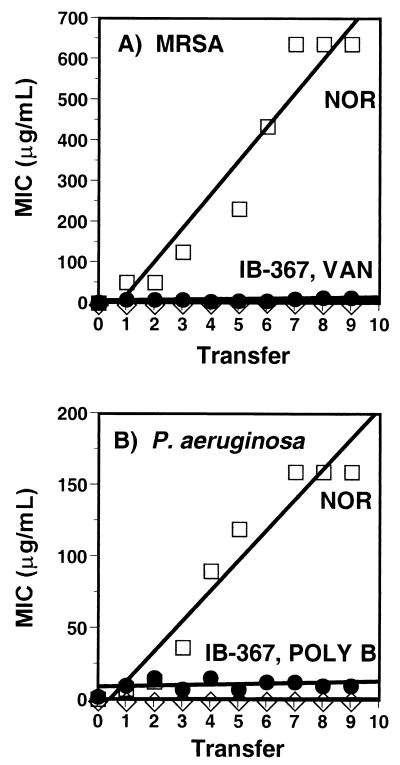
Evaluation of resistance.
When cultures of MRSA or P. aeruginosa were serially transferred daily, the MICs of norfloxacin increased as much as 32 times, whereas little change was observed in the MICs of vancomycin, polymyxin B, or IB-367 (data not shown). In a second study, the incubation period before subculture was extended from 18 to 20 h to 5 days to enhance the detection of low-frequency mutations that might engender resistance. Under these conditions, the initial MICs of norfloxacin for P. aeruginosa and MRSA were slightly higher (1 and 2 μg/ml, respectively) compared with the MICs determined after the standard incubation of 18 to 20 h (0.25 and 0.5 μg/ml, respectively). After nine serial transfers, the MICs of norfloxacin increased 160 times for P. aeruginosa and 320 times for MRSA (Fig. 5). In contrast, the MIC of IB-367 for each strain increased only four times. As before, the MICs of polymyxin B and vancomycin were relatively unaffected.
FIG. 5.
Effect of serial transfer on MICs. Cultures of MRSA (A) or P. aeruginosa (B) were exposed to various concentrations of drugs. After 5 days of incubation, wells containing compounds at concentrations equal to one-half the MIC were subcultured into fresh medium containing the same drugs. NOR, norfloxacin; VAN, vancomycin; POLY B, polymyxin B.
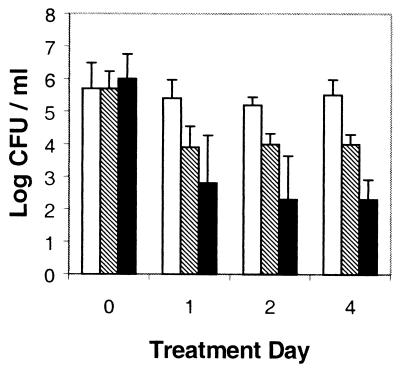
Efficacy of IB-367 in reducing oral microflora.
IB-367 reduced the density of the oral microflora in human volunteers in a concentration-dependent manner by as much as 1,000 times (Fig. 6). The 3-mg/g dose reduced the level of gram-negative bacteria, staphylococcal species, and yeast to undetectable (<10 CFU/ml) levels in all subjects (Table 3). No serious adverse effects, no evidence of allergic or anaphylactoid reactions, and no clinically significant changes in vital signs were observed during the study. In addition, IB-367 remained effective against mixed oral microflora after repeated exposures (Fig. 6).
FIG. 6.
Reduction of oral microflora in human volunteers by IB-367. IB-367 was administered at three doses: 0.3 mg/g (unfilled bars), 1.0 mg/g (shaded bars), and 3.0 mg/g (filled bars). The buccal mucosa was sampled on day 1 prior to treatment (time 0) and 1 h after treatment on days 1, 2, and 4.
TABLE 3.
Prevalence of oral microflora before treatment and 1 h after treatment with IB-367
| Dose (mg/g) | Prevalence (%)
|
|||||
|---|---|---|---|---|---|---|
| Gram-negative bacteria
|
Staphylococcal species
|
Yeast
|
||||
| Before | After | Before | After | Before | After | |
| 0.3 | 33 | 0 | 66 | 0 | 67 | 33 |
| 1.0 | 33 | 0 | 50 | 0 | 50 | 33 |
| 3.0 | 16 | 0 | 100 | 0 | 84 | 0 |
DISCUSSION
In this study, we describe the in vitro and in vivo properties of IB-367, a synthetic protegrin analog that prompted its development as a preventive treatment for oral mucositis. The MICs of IB-367 for MRSA (ATCC 33591) and P. aeruginosa (ATCC 9027) were slightly higher than those of conventional antimicrobial agents. However, IB-367 exhibited good to excellent antimicrobial activity against many of the microorganisms associated with the oral cavity, including C. albicans. In some cases, the elevated MICs may actually represent interference from the addition of blood products to the growth medium. Although such supplements are required for adequate growth of several fastidious organisms such as S. mitis and S. sanguis (2 to 43 and 4 to 64 μg/ml, respectively), they affect the biological activity of IB-367. Since the MICs increased approximately four times in MHB containing LHB or HTM, one might consider that the true MIC of IB-367 for organisms that require these medium supplements may be as much as four times lower than those reported in Table 1.
Extension of the incubation period from 18 to 24 h to 5 days prior to subculture of MRSA or P. aeruginosa produced a substantial increase in the MICs of norfloxacin. However, only a small increase in the MICs of IB-367 was observed. Such a low level of resistance is unlikely to be of clinical significance as the increases in the MICs were negligible compared to the concentration of IB-367 administered to the oral cavity in clinical trials. These data demonstrate that the development of significant resistance of bacteria to IB-367 is highly unlikely, regardless of the incubation conditions. In contrast, resistance to conventional antibiotics such as the fluoroquinolones develops quite easily. Overall, these data imply that the rapid generation of high-level resistance to IB-367 in clinical use is unlikely.
The properties of an agent required for successful treatment of oral mucositis include a broad spectrum of activity, activity that is not compromised by saliva and that can be achieved despite a short contact time with the pathogen, and demonstrated product safety. As described here, IB-367 conforms to all of these criteria. In addition, we have demonstrated that IB-367 can significantly reduce the number of bacteria and yeast that colonize the oral mucosa, a phenomenon previously demonstrated to reduce the severity of oral mucositis and its sequelae (24).
Various antimicrobial agents have been evaluated for their potential to reduce the level of oral mucositis. The narrow-spectrum antimicrobial agents evaluated in clinical studies include clindamycin (6) and nystatin (7), both of which failed to change the local course or modify the systemic sequelae of oral mucositis. Chlorhexidine, a broad-spectrum microbicide, was initially shown to reduce the severity of mucositis as a complication of bone marrow transplantation in a single-center study (8). The efficacy of chlorhexidine was not, however, confirmed in a subsequent study in patients undergoing bone marrow transplant (7, 27) or in patients experiencing oral mucositis as a complication of radiation therapy (20). One possible explanation for these negative results is inactivation of chlorhexidine by saliva, resulting in a failure to achieve a reduction in the microbial burden in the mouth (20).
Higher concentrations of IB-367 were required for effective reduction of the heterogeneous oral flora in pooled normal human saliva. The presence of many negatively charged glycoproteins such as mucin in saliva may bind to the positively charged peptide, rendering it less bioavailable (1, 16). In addition, the increased microbial density in saliva (ca. 5 × 107 CFU/ml) has a substantial inoculum effect on the MIC. However, the levels of IB-367 required for effective reduction of the numbers of CFU (250 to 1,000 μg/ml) are readily achieved in topical formulations of the peptide, as demonstrated by the significant reduction in the oral microflora of human volunteers.
In a multicenter study of patients receiving radiation therapy, administration of chlorhexidine was found to increase oral discomfort compared to administration of a placebo (10). That study was stopped prematurely after interim analysis demonstrated increased oral toxicity. In contrast, the current phase I study of IB-367 demonstrated that the 3-mg/g dose was well tolerated and showed no serious adverse effects. It should also be noted that plasma IB-367 concentrations were below the limit of detection by HPLC-mass spectrometric analysis (<16 ng/ml), indicating little or no systemic absorption of the compound (data not shown).
Conventional antibiotics such as tobramycin or vancomycin have also been used in topical applications for the treatment of oral mucositis with limited success (2, 22). Even at concentrations 1,000 times higher than their MICs, tobramycin, vancomycin, and ciprofloxacin were incapable of exhibiting the rapid reduction in the numbers of CFU in saliva demonstrated by IB-367. These data suggest that IB-367 may offer substantial advantages over conventional antimicrobial agents that require bacterial growth or a longer period of exposure.
IB-367 is a potent, broad-spectrum, rapidly microbicidal agent with superior performance in saliva compared with those of conventional antimicrobial agents and is capable of reducing the prevalence of the oral microflora in humans. IB-367 is currently in phase II clinical trials for the prophylaxis of oral mucositis in bone marrow transplant patients undergoing ablative chemotherapy.
REFERENCES
- 1.Bansil R, Stanley E, LaMont J T. Mucin biophysics. Annu Rev Physiol. 1995;57:635–657. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 2.Barker G J, Call S K, Gamis A S. Oral care with vancomycin paste for reduction in incidence of hemolytic streptococcal sepsis. J Pediatr Hematol Oncol. 1995;17:151–155. doi: 10.1097/00043426-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O J. Oral infections and septicemia in immunocompromised patients with hematologic malignancies. J Clin Microbiol. 1988;26:2105–2109. doi: 10.1128/jcm.26.10.2105-2109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochud P-Y, Eggiman P, Calandra T, Van Melle G, Saghafi L, Francioli P. Bacteremia due to viridans streptococcus in neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis. 1993;18:25–31. doi: 10.1093/clinids/18.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Carl W. Oral complications of local and systemic cancer treatment. Curr Opin Oncol. 1995;7:320–324. doi: 10.1097/00001622-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly J P, Muus P, Horrevorts A M, Sauerwein R W, De Pauw B E. Failure of clindamycin to influence the course of severe oromucositis with streptococcal bacteraemia in allogeneic bone marrow transplant recipients. Scand J Infect Dis. 1993;25:43–50. doi: 10.1080/00365549309169668. [DOI] [PubMed] [Google Scholar]
- 7.Epstein J B, Vickars L, Spinelli J, Reece D. Efficacy of chlorohexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Med Oral Pathol. 1992;73:682–689. doi: 10.1016/0030-4220(92)90009-f. [DOI] [PubMed] [Google Scholar]
- 8.Ferretti G A, Ash R C, Brown A T, Parr M D, Romond E H, Lillich T T. Control of oral mucositis and candidiasis in marrow transplantation: a prospective, double-blind trial of chlorohexidine digluconate oral rinse. Bone Marrow Transplant. 1988;3:483–493. [PubMed] [Google Scholar]
- 9.Ferretti G A, Brown A T, Raybould T P, Lillich T T. NCI monograph 9. Oral complications of cancer. Bethesda, Md: National Cancer Institute; 1990. Oral antimicrobial agents—chlorhexidine; pp. 51–55. [PubMed] [Google Scholar]
- 10.Foote R L, Loprinzi C L, Frank A R, O'Fallon J R, Gulavita S, Tewfik H H, Ryan M A, Earle J M, Novotny P. Randomized trial of a chlorohexidine mouthwash for alleviation of radiation-induced mucositis. J Clin Oncol. 1994;12:2630–2633. doi: 10.1200/JCO.1994.12.12.2630. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg M S, Cohen S G, McKitrick J C, Cassileth P A. The oral flora as a source of septicemia in patients with acute leukemia. Oral Surg. 1982;53:32–36. doi: 10.1016/0030-4220(82)90483-2. [DOI] [PubMed] [Google Scholar]
- 12.Liljemark W F, Bloomquist C G. Normal microbial flora of the human body. In: Nisengard R J, Newman M G, editors. Oral microbiology and immunology. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 120–128. [Google Scholar]
- 13.Loesche W J. Ecology of the oral flora. In: Nisengard R J, Newman M G, editors. Oral microbiology and immunology. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 307–315. [Google Scholar]
- 14.Mueller B A, Millheim E T, Farrington E A, Brusko C, Wiser T H. Mucositis management practices for hospitalized patients: national survey results. J Pain Symptom Manage. 1995;10:510–520. doi: 10.1016/0885-3924(95)00064-6. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. 3rd ed. 1993. Approved standard M7-A3. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 16.Schenkels L C P M, Veerman E C I, Amerongen A V N. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med. 1995;6:161–175. doi: 10.1177/10454411950060020501. [DOI] [PubMed] [Google Scholar]
- 17.Schubert M M, Williams B E, Lloid M E, Donaldson G, Chapko M K. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Cancer. 1991;69:469–477. doi: 10.1002/1097-0142(19920515)69:10<2469::aid-cncr2820691015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Sonis S T. Oral complications of cancer therapy. In: DeVita V, et al., editors. Principles and practice of oncology. J. B. Philadelphia, Pa: Lippincott; 1993. pp. 2385–2394. [Google Scholar]
- 19.Spijkervet F K L, van Saene H K F, Panders A K, Vermey A. Colonisation index of the oral cavity: a novel technique for monitoring colonisation defence. Microb Ecol Health Dis. 1989;2:145–151. [Google Scholar]
- 20.Spijkervet F K L, Saene J J, Saene H K, Panders A K, Vermey A, Fidler V. Chlorohexidine inactivation by saliva. Oral Surg Oral Med Oral Pathol. 1990;69:437–443. doi: 10.1016/0030-4220(90)90377-5. [DOI] [PubMed] [Google Scholar]
- 21.Spijkervet F K L. Irradiation mucositis. Copenhagen, Denmark: Munksgaard Press; 1991b. Oral flora changes in head and neck cancer patients; pp. 43–50. [Google Scholar]
- 22.Spijkervet F K L, van Saene H K F, van Saene J J M, Panders A K, Vermey A, Mehta D M, Fidler V. Effect of selective elimination of the oral flora on mucositis in irradiated head and neck cancer patients. J Surg Oncol. 1991;46:167–173. doi: 10.1002/jso.2930460309. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg D A, Hurst M A, Fujii C A, Kung A H, Ho J F, Cheng F-C, Loury D J, Fiddes J C. Protegrin-1: a broad spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symonds R P, McIlroy P, Khorrami J, Paul J, Pyper E, Alcock S R, McCallum I, Speekenbrink A B J, McMurray A, Lindemann E, Thomas M. The reduction of radiation mucositis by selective decontamination antibiotic pastilles: a placebo-controlled double-blind trial. Br J Cancer. 1996;74:312–317. doi: 10.1038/bjc.1996.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Saene H K, Martin M V. Do microorganisms play a role in irradiation mucositis? Eur J Clin Microbiol Infect Dis. 1990;9:861–863. doi: 10.1007/BF01967499. [DOI] [PubMed] [Google Scholar]
- 26.Verdi C J. Cancer therapy and oral mucositis; an appraisal of drug prophylaxis. Drug Safety. 1993;9:185–195. doi: 10.2165/00002018-199309030-00004. [DOI] [PubMed] [Google Scholar]
- 27.Weisdorf D J, Bostrom B, Raether D, Mattingly M, Walker P, Pihlstrom B, Ferrieri P, Haake R, Goldman A, Woods W, et al. Oropharyngeal mucositis complicating bone marrow transplantation: prognostic factors and the effect of chlorohexidine mouth rinse. Bone Marrow Transplant. 1989;4:89–95. [PubMed] [Google Scholar]