Abstract
Background
Minimal change disease (MCD) is a common cause of the nephrotic syndrome. Several studies have shown an increased incidence of cancer in patients with MCD. However, there are no reports on the association between MCD and gastrointestinal stromal tumor (GIST).
Case presentation
We report a case of a 66-year-old female with severe nephrotic syndrome and concomitant duodenal GIST. Immunoglobulin test showed a significant increase of IgE levels. The diagnosis of renal histopathology was MCD with subacute tubulointerstitial injury. The combination of preoperative Imatinib mesylate chemotherapy and tumor excision was accompanied by significant remission of proteinuria, and IgE level decreasing, without immunosuppressivetherapy.
Conclusions
It is the first case report that MCD was associated with GIST and elevated IgE level. Clinically, in patients with elevated IgE level associated with nephrotic syndrome, the possibility of tumor must be taken into account when allergic factors are excluded.
Keywords: Minimal change disease, gastrointestinal stromal tumor, Immunoglobulin E
Background
Glomerular disease in malignant tumors has been recognized for decades. The most frequent types of malignant tumors are pulmonary, renal, and gastrointestinal solid tumors. Membranous nephropathy (MN) is the most common glomerulopathy associated with malignant tumors. However, other types have been reported, especially IgA nephropathy, minimal change disease (MCD), and anti-glomerular basement membrane (GBM) disease [1]. MCD is relatively uncommon.
In adults, MCD represents approximately 10-15% of patients with idiopathic nephrotic syndrome. Secondary MCD is involved in neoplasia, drugs (e.g., non-steroidal anti-inflammatory drugs), infections, and atopy. Frequently reported solid tumor associated with MCD are lymphoma, lung cancer, colorectal carcinoma, renal cell carcinoma, and thymoma. Pancreatic, breast, bladder, ovarian, and esophageal cancers have little correlation with MCD [2]. Here, we report a rare case of MCD associated with GIST and elevated serum IgE.
Case presentation
A 66-year-old female was admitted to the hospital on October 26th, 2019, with edema of the eyelid and lower limbs for 1 month. 2 weeks ago, edema was aggravated, accompanied by increased foam in urine, nausea, abdominal distension, and poor appetite. Previous history: More than 10 years of type 2 diabetes, blood glucose was controlled satisfactorily. Hypertension has a history of more than 10 years, with poorly controlled blood pressure. A history of chronic bronchitis for many years. Physical examination disclosed an anemic appearance, moderate pitting edema of eyelids and lower limbs. Blood pressure was 176/92 mmHg, and serum glucose level was 5.4 mmol/L. Proteinuria was 22.224 g/24 h, serum albumin was 19.2 g/L, serum creatinine was 233.2 μmol/L, accompanied by hyperlipidemia (triglyceride: 10.10 mmol/L, Cholesterol: 13.65 mmol/L) and significantly increased erythrocyte sedimentation rate (ESR, 103 mm/h). Serum complement 3 (C3) level was in normal range, and C4 level was slightly high (41.2 mg/dL). Serum protein electrophoresis showed no M-spike. Coagulation function and thyroid function were normal. Antinuclear antibodies (ANA), anti-double-stranded DNA antibody, anti-neutrophil cytoplasmic antibody (ANCA) and anti-phospholipase A2 receptor (PLA2R) antibody were negative. Tests of hepatitis B surface antigen, antibodies against hepatitis C virus and human immunodeficiency virus were negative. Immunoglobulin examination revealed markedly elevated IgE level (7080 IU/mL, normal range 0-165 IU/mL), slightly decreased IgG level (505 mg/dL). IgA and IgM levels were normal. Mild abnormalities were found in serum light chain tests (κ chain: 114.69 mg/dL, λ chain: 64.41 mg/dL), but the ratio of κ to λ was normal (κ/λ:1.78). Both level of Ca125 and Ca199 were elevated (188.92 U/mL, and 48.65 U/mL, individually). Abdominal ultrasound indicated that the size and shape of the kidney was normal. There was a hypoechoic mass (5.0 cm × 3.0 cm × 4.1 cm) near the upper part of the right kidney. Computerized tomography (CT) scan for the abdomen revealed a space occupying lesion between the gastric antrum and the duodenum. Gastroscopy (November 13rd, 2019) showed that the proximal end of the posterior duodenal bulb had a raised lesion about 2.0 cm in size. The pathological result of gastroscope biopsy was inflammation. Kidney biopsy was performed. Of 10 glomeruli, 3 were globally ischemic sclerosed. Glomerular mesangium was normal. No nodular glomerulosclerosis, microaneurysm, hyaline insudation, crescent and capsule adhesion was seen. There was mild tubular atrophy, mild interstitial edema, inflammation and fibrosis in tubulointerstium. Tubular epithelial necrosis and brush border loss was occasionally observed. Arteries and arterioles exhibited mild intimal fibrosis and hyalinosis. Immunofluorescence microscopy for immunoglobulins G, A, and M, C3, C4, C1q, and fibrinogen was negative. It was observed by electron microscopy that there was no electron-dense deposits, the thickness of GBM was normal (343 nm ~ 405 nm, average 382 nm), and foot process of podocyte was widespreadly effaced. MCD and subacute tubulointerstitial injury was diagnosed (Fig. 1). The patient was discharged after remission under treatments including diuresis, antihypertensive treatment, blood glucose control, and anemia correction. We observed quickly decline of proteinuria (2.514 g/24 h), with serum albumin level elevated to 31.5 g/L, serum IgE level decreased to 6540 IU/mL and serum creatinine decreased to normal.
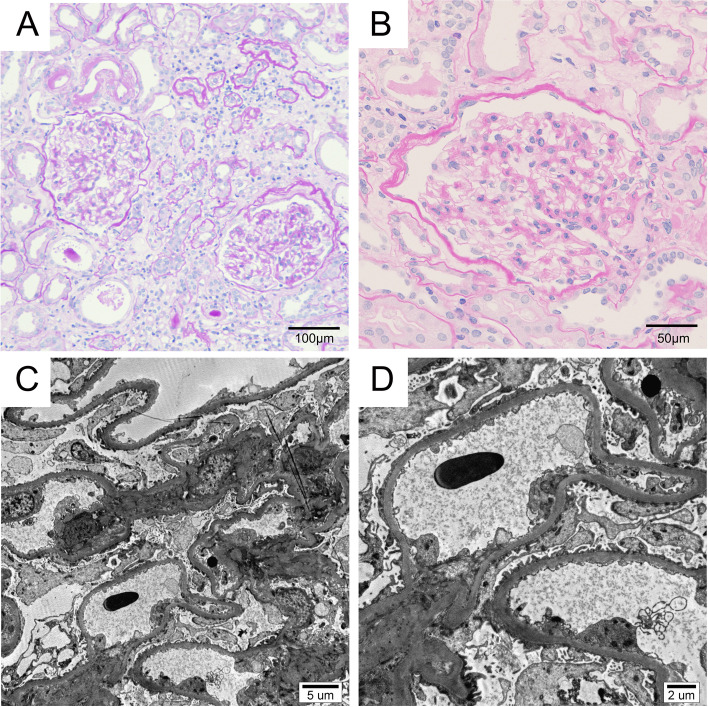
Fig. 1.
Kidney biopsy findings. A Appearance of glomeruli was essentially normal. Mild tubular atrophy, mild interstitial edema, inflammation and fibrosis in interstium, arteriole hyalinosis was observed (PAS stain, × 200). B No mesangial expansion and hypercelluarity was observed (PAS stain, × 400). C No mesangial matrix accumulation was observed and width of mesangium was normal (Electron microscopy, magnification × 3000). D No electron-dense deposit was found, the thickness of glomerular basement membrane (GBM) was normal (343 nm ~ 405 nm, average 382 nm), and footprocess of podocyte was widespreadly effaced (Electron microscopy, magnification × 6000)
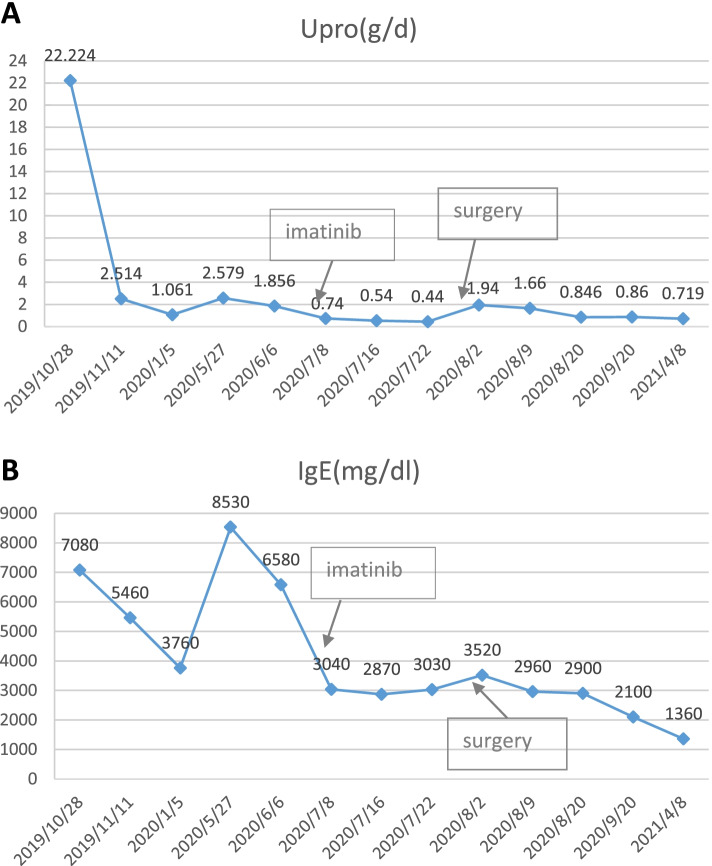
On May 10th, 2020, the patient was readmitted for review. Proteinuria was 2.579 g/24 h and serum albumin was 34.1 g/L. But the serum IgE level went up to 8530 IU/mL. Abdominal enhanced magnetic resonance imaging (June 2nd, 2020) disclosed that the intestinal wall of the descending junction area of the duodenum was thickened, with a range of about 47 mm × 61 mm, lymphoma was not excluded. Electronic ultrasound endoscopy was performed again on June 8th, 2020. A 1.0 cm × 1.5 cm deep ulcer was found in the upper part of the descending duodenum. The tumor was located in the posterior lateral wall of the descending duodenum, with a range of about 4.5 cm × 3 cm × 3 cm. The pathologic diagnosis of the tumor was duodenalgastrointestinal stromal tumor (GIST), spindle cell type, low-risk type. Only one mitose was observed per 50 HPF (Fig. 2A). Immunoperoxidase staining for CD117 (c-kit), Dog-1, and Vimentin were positive in the tumor cells (Fig. 2B), while staining for CD34, smooth muscle actin (SMA), desmin and S-100 were negative. The positive ratio of Ki-67 staining was lower than 10%. On June 24st, 2020, the patient was administrated with imatinib mesylate (400 mg/day), and the surgical excision of the tumor was performed after 1 month. Imatinib mesylate has been administered subsequently. The histopathological result of the tumor was also duodenal GIST (spindle cell type, and low-risk type). The patient had never received any specific immunosuppressive therapy for proteinuria. Proteinuria slowly declined to 0.846 g/d 1 month after surgery, with serum albumin increased to 36.4 g/L and IgE decreased to 2900 IU/mL. 2 months after surgery, proteinuria was 0.86 g/d, serum albumin was 36.3 g/L and serum IgE was 2100 IU/mL. Eight months after surgery, proteinuria was 0.719 g/d, serum albumin was 45.3 g/L (Fig. 3A) and serum IgE was 1360 IU/mL (Fig. 3B).
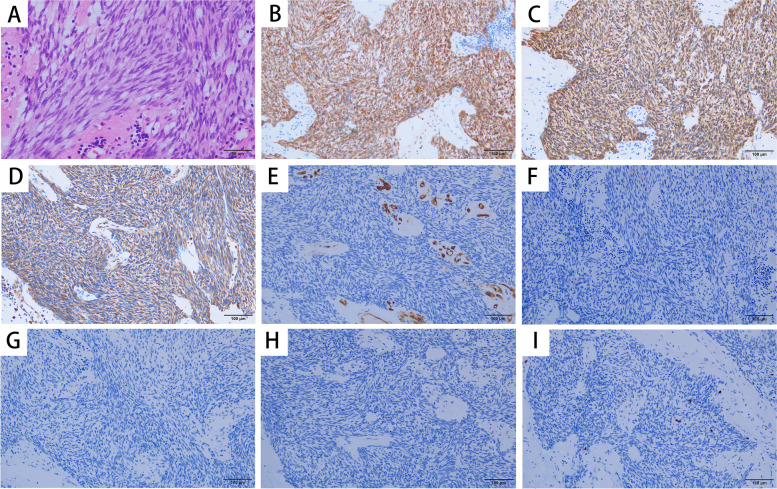
Fig. 2.
Tumor cells were spindle shaped cells with rod like hyperchromatic nuclei (A). Only one mitose was observed per 50 HPF (HE stain, × 400). The tumor cells were positive for CD117 (× 400) (B), Dog-1 (C) and Vimentin (D), while stains for CD34 (E), smooth muscle actin (SMA) (F), desmin (G) and s-100 (H) were negative. The positive ratio of Ki-67 staining was less than 10% (I)
Fig. 3.
Proteinuria (A) and serum IgE level (B) declined with imatinib treatment and surgical removal of the tumor
Discussion and conclusion
The clinical manifestation of this patient was nephrotic syndrome with a history of diabetes, and the possibility of diabetic nephropathy was considered at admission. However, the kidney biopsy confirmed MCD, which can be caused by a variety of factors, such as allergy, tumor, drugs, and etc [2].
Imaging examination of the patient indicated that a space occupying lesion was in the duodenum. GIST was confirmed by pathological examination after surgical resection. Previously, two cases of nephrotic syndrome caused by GIST have been reported, one of which was confirmed to be MN by renal biopsy [3] and the other was not subjected to renal biopsy [4]. MCD in this case may be caused by GIST, and it is speculated that some tumor factors secreted by GIST may cause the increased permeability of the glomerular basement membrane (GBM), then lead to proteinuria [5, 6].
Another finding in this case was a significant increase in serum IgE level, and a significant decrease in serum IgE level and remission of the nephrotic syndrome after surgical resection of the tumor. It was suggested there is a correlation among elevated IgE level, GIST and MCD. With the deepening of IgE related studies, not only IgE is the relevant indicator of anaphylaxis, but also its correlation with tumors has increasingly become a research hotspot [7]. In the human body, tumor pathogenic factors and tumor cells act as a foreign antigen or allergen, constantly stimulate the body to generate antibodies, including IgE antibodies and forming a symptom similar to allergic reaction [8]. In recent decades, evidences have emerged relating allergies with cancer development [9]. However, most of the results of epidemiology studies have been controversial. One indicate that allergies can reduce the risk of cancer [10, 11], another indicate that they may increase this risk [12, 13]. In any cases, IgE production involves activation of helper T cell subsets 2 (Th2) to generate interleukin-4 (IL-4) and interleukin-13 (IL-13), which stimulate B lymphocytes to synthesize IgE [14, 15]. IgE acts on mast cells and basophil cells to release histamine and leuktriene [16, 17], and acts on monocyte and macrophage to release tumor necrosis factor α (TNF-α) [18], which might enhance permeability of the GBM. In addition, IL-13 can also cause the disorder of podocyte skeleton protein and affect the function of foot process, which might be involved in proteinuria [19, 20].
In conclusion, for patients with elevated IgE level associated with nephrotic syndrome, the possibility of tumor must be taken into account when allergic factors are excluded.
Acknowledgments
We thank our patient for allowing us to publish her case.
Abbreviations
- MCD
Minimal change disease
- GIST
Gastrointestinal stromal tumor
- MN
Membranous nephropathy
- ANA
Antinuclear antibodies
- ANCA
Antineutrophil cytoplasmic antibody
- PLA2R
Phospholipase A2 receptor
- CT
Computerized tomography
- SMA
Smooth muscle actin
- GBM
Glomerular basement membrane
- TNF-α
Tumor necrosis factor α
Authors’ contributions
CYY, JL and XGZ were involved in the clinical management of the patient. CYY collected the data and wrote the first version of the manuscript. XGZ and CHQ completed the renal histologic study. ZYW performed the surgical excision of the tumor. CHQ realized the histologic study of the tumor. CYY, YFX and XGZ approved the final version of the manuscript. The authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor of this journal.
Competing interests
The authors have no conflict of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yue-Fei Xiao, Email: xyf01_2012@163.com.
Xue-Guang Zhang, Email: xg301301@163.com.
References
- 1.Mazanowska O, Klinger M. Glomerulonephritis in neoplastic disease. Pol Merkur Lekarski. 2005;19(110):211–214. [PubMed] [Google Scholar]
- 2.Bacchetta J, Juillard L, Cochat P, Droz JP. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70(1):39–58. doi: 10.1016/j.critrevonc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Cimic A, Pastan SO, Bijol V. Membranous nephropathy associated with gastrointestinal stromal tumor: a case report. NDT Plus. 2009;2(4):306–308. doi: 10.1093/ndtplus/sfp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takane K, Midorikawa Y, Yamazaki S, Kajiwara T, Yoshida N, Kusumi Y, et al. Gastrointestinal stromal tumor with nephritic syndrome as a paraneoplastic syndrome: a case report. J Med Case Rep. 2014;27(8):108. doi: 10.1186/1752-1947-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi K, Fujioka H, Torashima Y, Yamaguchi J, Izawa K, Kanematsu T. Rectal cancer with paraneoplastic nephropathy: association of vascular endothelial growth factor. Dig Surg. 2004;21(5-6):455–457. doi: 10.1159/000083474. [DOI] [PubMed] [Google Scholar]
- 6.Li JY, Yong TY, Kuss BJ, Klebe S, Kotasek D, Barbara JA. Malignant pleural mesothelioma with associated minimal change disease and acute renal failure. Ren Fail. 2010;32(8):1012–1015. doi: 10.3109/0886022X.2010.502275. [DOI] [PubMed] [Google Scholar]
- 7.Josephs DH, Spicer JF, Karagiannis P, Gould HJ, Karagiannis SN. IgE immunotherapy: A novel concept with promise for the treatment of cancer. MAbs. 2014;6(1):54–72. doi: 10.4161/mabs.27029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zennaro D, Capalbo C, Scala E, Liso M, Spillner E, Braren I, et al. IgE, IgG4 and IgG response to tissue-specific and environmental antigens in patients affected by cancer. Istanbul: European Academy of Allergy and Clinical Immunology (EAACI); 2011. [Google Scholar]
- 9.Kozłowska R, Bożek A, Jarząb J. Association between cancerand allergies. Allergy, Asthma Clin Immunol. 2016;12:39. doi: 10.1186/s13223-016-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KinetJP. The high-affinity IgE receptor (FceRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 11.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu RevImmunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 12.Tirado-Rodríguez B, Huerta-Yépez S. Allergies:diseases closely related to cancer. Bol Med Hosp Infant Mex. 2016;73(6):432–445. doi: 10.1016/j.bmhimx.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Hayes MD, Ward S, Crawford G, Seoane RC, Jackson WD, Kipling D, et al. Inflammation-induced IgE promotes epithelial hyperplasia and tumor growth. Elife. 2020;9:e51862. doi: 10.7554/eLife.51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Hafez M, Shimada M, Lee PY, Johnson RJ, Garin EH. Idiopathic nephrotic syndrome and atopy: is there a common link? Am J Kidney Dis. 2009;54(5):945–953. doi: 10.1053/j.ajkd.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105(2 Pt 2):S547–S558. doi: 10.1016/S0091-6749(00)90059-9. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie BM. Reagin-like antibodies in animals immune to helminth parasites. Nature. 1964;204:91–92. doi: 10.1038/204091a0. [DOI] [PubMed] [Google Scholar]
- 18.Karagiannis SN, Josephs DH, Bax HJ, Spicer JF. Therapeutic IgE antibodies: harnessing a macrophage-mediated immune surveillance mechanism against cancer. Cancer Res. 2017;77(11):2779–2783. doi: 10.1158/0008-5472.CAN-17-0428. [DOI] [PubMed] [Google Scholar]
- 19.Lai KW, Wei CL, Tan LK, Tan PH, Chiang GS, Lee CG, et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J Am Soc Nephrol. 2007;18(5):1476–1485. doi: 10.1681/ASN.2006070710. [DOI] [PubMed] [Google Scholar]
- 20.Berghea EC, Balgradean M, Popa IL. Correlation between idiopathic nephrotic syndrome and atopy in children-short review. Maedica (Bucur) 2017;12(1):55–58. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.