Abstract
Background
Circular RNA (circRNA), as a new members of noncoding RNA family, have vital functions in many biological processes by as microRNA sponges or competing endogenous RNAs (ceRNAs). However, little has been reported about the genetic mechanism of circRNAs regulation of feed efficiency in sheep.
Results
This study aimed to explore the expression of circRNAs in the liver of Hu sheep with High-RFI (High residual feed intake) and Low-RFI (Low residual feed intake) using transcriptome sequencing. A total of 20,729 circRNAs were identified in two groups, in which 219 circRNAs were found as significantly differentially expressed. Several circRNAs were validated by using RT-PCR, sanger sequencing and RT-qPCR methods. These results demonstrated that the RNA-seq result and expression level of circRNAs identified are reliable. Subsequently, GO and KEGG enrichment analysis of the parental genes of the differentially expressed (DE) circRNAs were mainly involved in immunity response and metabolic process. Finally, the ceRNA regulatory networks analysis showed that the target binding sites for miRNA such as novel_41, novel_115, novel_171 and oar-miR-485-3p in the identified DE cirRNAs. Importantly, two metabolic (SHISA3 and PLEKHH2) and four (RTP4, CD274, OAS1, and RFC3) immune-related target mRNAs were identified from 4 miRNAs. Association analysis showed that the polymorphism (RTP4 c.399 A > G) in the target gene RTP4 were significantly associated with RFI (P < 0.05).
Conclusions
Analysis of sequencing data showed some candidate ceRNAs that may play key roles in the feed efficiency in sheep by regulating animal immune and metabolic. These results provide the basis data for further study of the biological functions of circRNAs in regulating sheep feed efficiency.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-022-08517-5.
Keywords: Hu sheep, circRNA, Residual feed intakes, Liver, RTP4
Background
The cost of feed is a major expend in the indoor sheep production industry, accounting for about 65–70% of total expenditures [1]. Due to this cost, the selective breeding of sheep with high feed efficiency has become an important objective of breeding programs. Feed conversion rate (FCR) and residual feed intake (RFI) are two the most commonly used phenotypes for quantifying feed efficiency in livestock species [2]. FCR is the ratio between the total of feed intake and the individual weight gain over a specific time, RFI index was first proposed by Koch et al. (1963) and has successfully applied to the artificial selection of feed efficiency in livestock, which is defined as the difference between actual feed intake and predicted feed intake of each individual requirement for maintaining growth and production [3, 4]. The RFI is an accurate and sensitive index compared with the FCR, because the FCR’s strong negative correlation with body weight gain, and is a ratio traits, it has certain limitations on genetic selection for evaluating FE [5, 6], while the RFI is a trait independent of other production traits. Thus, RFI has gained popularity in recent years, particularly among geneticists, and has been considered as a desirable criterion for the genetic improvement of feed efficiency in livestock breeding.
RFI is a complex quantitative trait, influenced by many biological factors [7]. The digestibility and metabolism processes as well as animal health status are important factor affecting RFI. The liver plays an important role in the metabolic and animal innate immunity, which have vital effects on sheep digestion and metabolism of nutrients. RNA sequencing (RNA-seq) is acknowledged as a mature technology and widely applied to understand the regulatory molecular mechanisms underlying of feed efficiency, and the genetic and breeding approaches are effective to improve feed efficiency. Many studies on the genetic variations affecting feed efficiency of livestock at the mRNA level [8, 9]. CircRNA was first discovered in plant viruses [10], and plays an important role in the regulate expression genes associated with several biological processes as a special of noncoding RNA. To date, many literature showed that circRNAs are mainly related to biological processes such as reproduction [11, 12], growth development [13], milk production [14] and wool follicle development [15] in sheep. However, it is not known about how circRNA regulates feed efficiency in sheep.
Hence, to explore the underlying genetic mechanisms of regulation of feed efficiency in sheep. In the present study, RNA-seq was performed to study the circRNAs of liver samples from Low-RFI and High-RFI lambs. We also describe the genomic features chromosome and length distribution of circRNAs in the liver tissue in sheep. Simultaneously, reverse transcriptase PCR amplification and DNA sequencing technologies were used to validate the presence of circular RNA. Subsequent bioinformatics analyses were performed of the parent genes of the differentially expressed circRNAs, and further constructed a circRNA-miRNA-mRNA interaction network. These findings help us better understand the genetic mechanisms that regulate RFI at the circRNA levels.
Results
Characterization of circRNAs in the ovine liver tissue
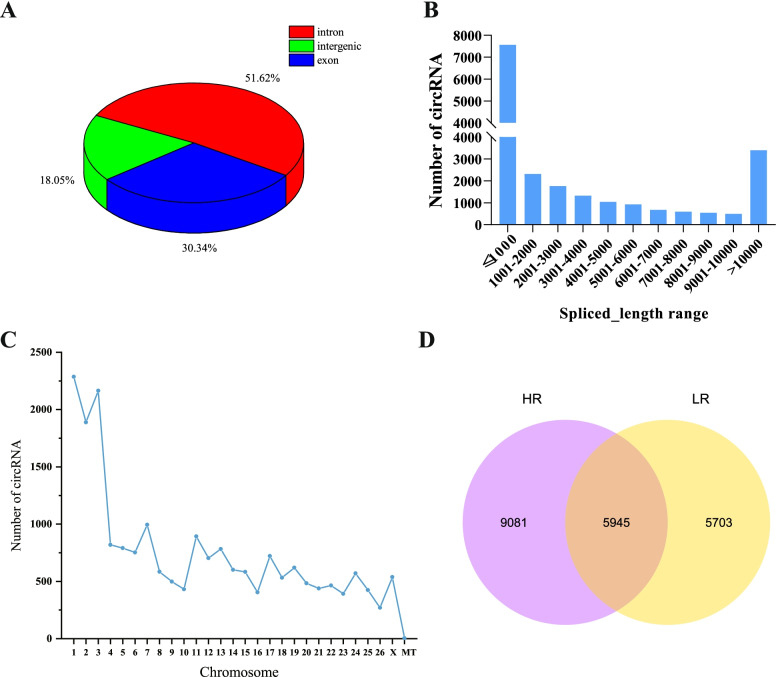
To understand the circRNA expression patterns of liver tissue from the male Hu lambs with different residual feed intakes. We carried out RNA-seq on liver samples from three Low-RFI and three High-RFI lambs. A total of 243 million raw reads were obtained from RNA-seq data. Approximately 227 million clean data obtained by removing adapter and low quality sequences were mapped to the ovine reference genome (Oar_v4.0). Table 1 summarizes the total number of reads generated from six samples, and each sample yielded more than 30 million raw reads data. The average GC content was 62.72% (Table 1). After removing the linear RNA and ribosomal RNA, we detected a total of 20,729 circRNAs from the high quality of the RNA-seq data by find_circ, and the majority of circRNAs consisted mainly of introns and exons, whereas a small fraction are composed of intergenic sequences (Fig. 1A). The length of most candidate circRNAs were mainly focused below 1000 nt (Fig. 1B). The circRNAs in the present study were extensively distributed on 26 autosomes and sex chromosomes, of which most circRNAs were concentrated on chromosomes 1 to 3 (Fig. 1C).
Table 1.
Summary statistics of the RNA-Seq data
| Sample | Raw Reads | Clean Reads | Error rate (%) | Q20 (%) | Q30 (%) | GC content (%) |
|---|---|---|---|---|---|---|
| LR1 | 38,414,647 | 36,495,858 | 0.02 | 98.34 | 94.99 | 62.51 |
| LR2 | 32,883,576 | 30,287,731 | 0.02 | 98.36 | 95.03 | 62.82 |
| LR3 | 47,310,528 | 43,710,001 | 0.02 | 98.33 | 94.98 | 62.85 |
| HR1 | 41,475,895 | 38,357,412 | 0.02 | 98.27 | 94.84 | 63.4 |
| HR2 | 40,148,898 | 37,688,849 | 0.02 | 98.26 | 94.86 | 62.46 |
| HR3 | 43,391,501 | 40,465,965 | 0.02 | 98.35 | 95.09 | 62.28 |
| Total | 243,625,045 | 227,005,816 | 62.72 |
Fig. 1.
Characteristics of circRNAs in the sheep in the ovine liver tissue with High-RFI and Low-RFI. A The proportion of introns, exons and intergenic of circRNAs. B The number of circRNAs within different spliced-length ranges. C Chromosomal distribution of circRNAs. D The Venn diagram of two comparisons and the number of annotated circRNAs
Differentially expressed analysis of circRNAs
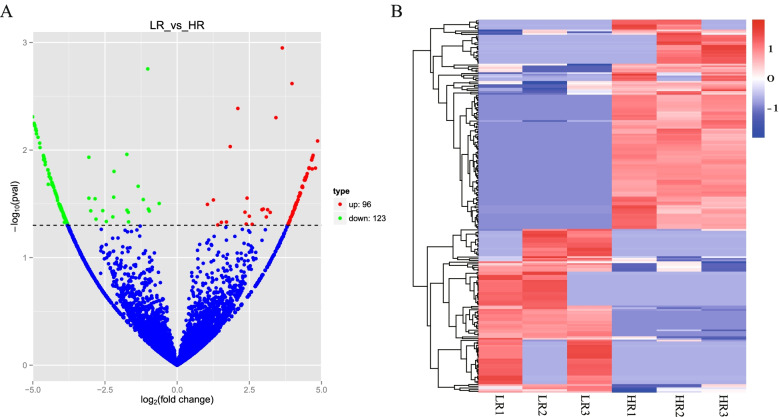
From the six liver samples, we obtained 15,026 and 11,648 circRNAs from the Low-RFI and High-RFI groups, respectively. Of all circRNAs, 5945 circRNAs were co-expressed in the liver of both the Low-RFI and High-RFI lambs (Fig. 1D), 219 circRNAs were significantly differentially expressed (|log2Fold Change|≥ 1 and p-value < 0.05) when comparing Low-RFI and High-RFI groups. Compared to High-RFI group, the expression levels of 96 circRNAs were up-regulated in Low-RFI group, while 123 circRNAs were down-regulated (Fig. 2A). The hierarchical clustering heatmap analysis suggested that the expression patterns of the differentially expressed circRNAs were clearly differentiated and aggregated between Low-RFI and High-RFI groups (Fig. 2B).
Fig. 2.
Differentially expressed gene analysis in the ovine liver tissue with High-RFI and Low-RFI. A Volcano plot of circRNAs differential expression results. B Clustering heat-map of the DE circRNAs
Validation of differentially expressed circRNAs
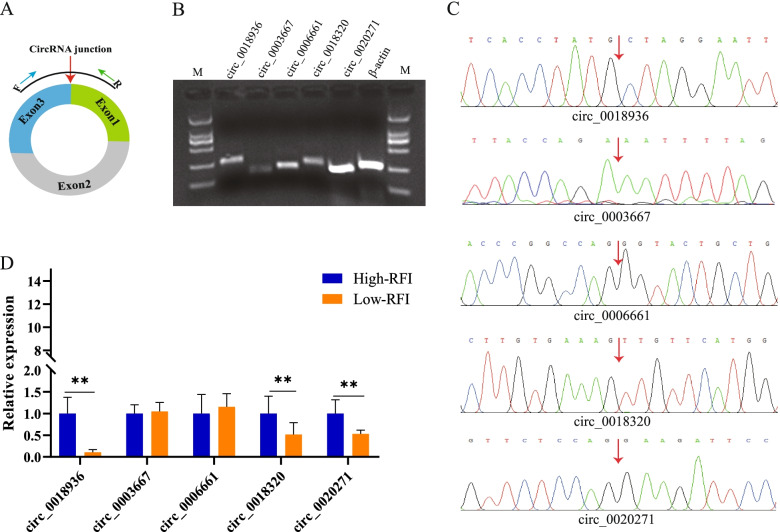
To validate the presence of circRNAs circular structures (Fig. 3A), we randomly selected five DE circRNAs to perform RT-PCR amplification and DNA sequencing. The 1.5% agarose gel electrophoresis results showed all five circRNAs can be checked and have a single band of the expected size (Fig. 3B). Simultaneously, the RT-PCR amplification product was sequenced using Sanger sequencing, the sequencing results was consistent with the sequences from RNA-Seq, which confirmed the presence of head-to-tail back-splicing of these circRNAs (Fig. 3C). Finally, the five circRNAs were subjected to quantitative RT-PCR (real-time quantitative PCR) analysis using the same primer as RT-PCR amplification, the results from the RT-qPCR for five circRNAs was consistent with the trends obtained from the RNA-seq analysis (Fig. 3D). These data demonstrated that the RNA-seq result and expression levels of candidate circRNAs are reliable in the present study.
Fig. 3.
Verification of circRNAs data from transcriptome sequencing. A Divergent primers used to amplify the circular junctions. B Visualization of RT-PCR amplification of circRNAs on a 1.5% agarose electrophoresis gel. C Head-to-tail junctions were confirmed by Sanger sequencing. D The expression levels of DE circRNAs in liver tissues of groups with different residual feed intakes. Values are means ± SE. * P < 0.05, ** P < 0.01
Enrichment analysis of for source genes of differentially expressed circRNAs
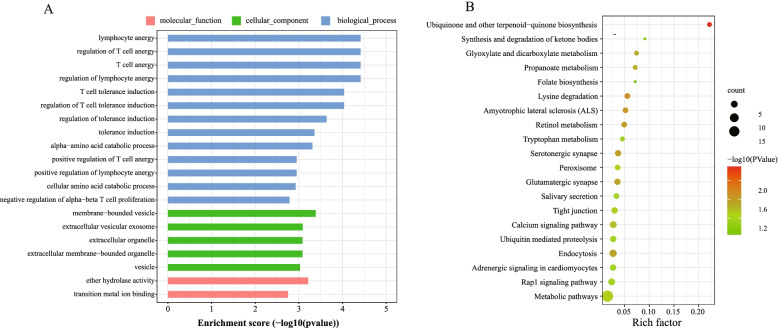
To further investigate biological functional of host genes of the differentially expressed circRNAs in the Low-RFI group and the High-RFI group, we performed GO and KEGG pathways enrichment analysis for 219 DE circRNAs hosting genes. The GO analyses showed a total of 233 terms, including 133 about biological process (BP), 25 about cellular component (CC), and 75 GO about molecular function (MF) were significantly enriched (P < 0.05), Fig. 4A shows the top 20 most enriched categories that were related to immunity response, such as the lymphocyte anergy, regulation of T cell anergy as well as T cell energy and so on. KEGG pathway assessment indicated that the host genes for DE circRNAs were significantly enriched in ubiquinone and other terpenoid-quinone biosynthesis, lysine degradation, retinol metabolism, glyoxylate and dicarboxylate metabolism, propanoate metabolism and other signaling pathways (Fig. 4B), and some derived genes were engaged in the metabolic pathway, but not significantly, which suggest that some circRNAs are involved in the process of energy metabolic, and then play a regulatory role.
Fig. 4.
Function enrichment analysis for the source genes of circRNAs. A Gene ontology (GO) distribution of source genes. B Kyoto Encyclopedia of Genes and Genomes (KEGG) classification of source genes
Analysis of circRNA-miRNA-mRNA networks
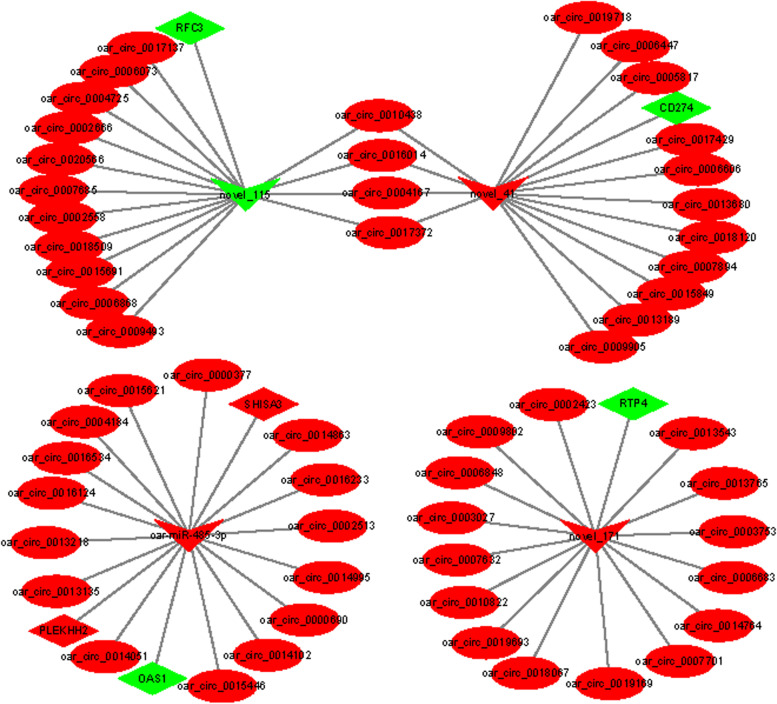
The biological roles of DE circRNA could be obtained by exploring the function of target miRNAs and mRNAs. We constructed ceRNA regulatory networks of circRNA-miRNA-mRNA by the integration of our previous mRNA data and the unpublished miRNA data. A total of 590 interactions between 147 circRNAs and 4 DE miRNAs were identified (Supplementary Table S1). Notably, some circRNAs (circ_0010438, circ_0016014, circ_0017372 and circ_0004167) contained at least two potential binding sites for four differentially expressed miRNAs (Fig. 5), and the four miRNAs have six encoding protein genes (SHISA3, PLEKHH2, RTP4, CD274, OAS1, and RFC3), these genes play a role in metabolism process and immunity function. thereby suggesting that these circRNAs may be involved in metabolic and immunity process of liver by sponging multiple miRNAs.
Fig. 5.
The interaction network of circRNA–miRNA–mRNA. Diamond represent mRNA, ellipse represent circRNAs, V represent miRNA, the green and red indicate down-regulated and up-regulated, respectively
SNP identification and association analysis
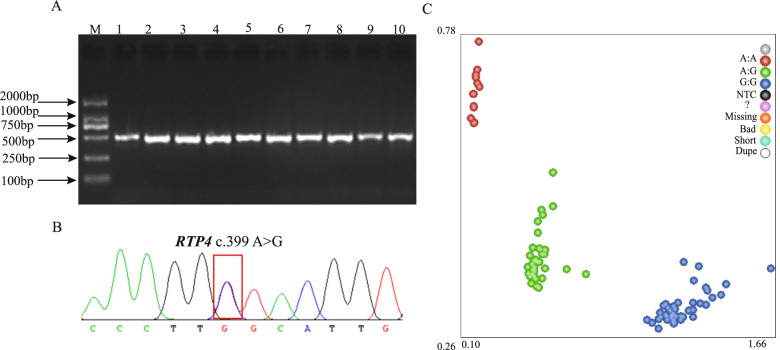
To further verify the effect of target genes of our above network results on the feed efficiency in the enlarged experimental population. The sequences of 486 bp PCR fragments were amplified from the mixed DNA from 20 Hu sheep as templates by using PCR primer pairs in Table 4 (Fig. 6A), the PCR products were sequenced by Tsingke (Xi’an, China) to scan for SNPs (single nucleotide polymorphisms) in the target genes. The sequencing results showed that a novel SNP (c.399 A > G) was identified in ovine the RTP4 gene (Fig. 6B), and subsequently genotyped with KAspar assays in the enlarged experimental population (n = 1220), three genotypes were obtained: the red, green and blue dots representing AA, AG and GG genotypes, respectively (Fig. 6C). A total of 1194 individuals were genotyped successfully and used for association analysis, the result of the association analysis found that the polymorphism RTP4 c.399 A > G was not associated with FCR (P > 0.05), whereas the polymorphism RTP4 c.399 A > G was significantly associated with RFI (P < 0.05) (Table 2), and in this locus, sheep with the GG genotypes were significantly greater than AA genotype carriers on RFI. However, the difference between AG individuals and AA and GG individuals was not significant. This result showed that allele G contributes to higher phenotype values compared with that in the allele A.
Table 4.
The primers used for SNP identification and KASPar detection
| primer's purpose | primer name | Primer sequence (5'–3') |
|---|---|---|
| Polymorphism | RTP4-F | CCCAGGCAGCTTGACTTCCA |
| RTP4-R | GGTCAACACAGGACGCACAA | |
| KAspar | RTP4_AlleleX | GAAGGTGACCAAGTTCATGCTCTGACCTTGATGTGGCCCTTG |
| RTP4_AlleleY | GAAGGTCGGAGTCAACGGATTTCTGACCTTGATGTGGCCCTTA | |
| RTP4_Common | CCACAGCCCACCAGAGGGAC |
Fig. 6.
Single nucleotide polymorphism identification of the RTP4 Genes. A PCR amplification of mixed DNA of RTP4 gene. B Sequencing peak images for RTP4 gene. C Genotyping of RTP4 c.399 A > G SNPs using Kaspar technology
Table 2.
Association results between genotypes at c.399 A > G polymorphism of the ovine RTP4 and feed efficiency traits
| Gene/loci | Genotype | No | RFI | FCR |
|---|---|---|---|---|
| RTP4 c.399 G > A | AA | 135 | -0.00962 ± 0.090b | 5.935 ± 0.679 |
| AG | 513 | -0.00083 ± 0.089ab | 5.919 ± 0.644 | |
| GG | 546 | 0.00983 ± 0.087a | 5.988 ± 0.866 |
Note. Letters with different genotypes in same trait (a, b) means significantly at P < 0.05
Discussion
As is widely known, the RFI is a vital and accurate index that is increasingly recognized as an alternate evaluate of feed efficiency of livestock [16, 17]. Considering the importance of RFI to evaluate feed efficiency on the economics of sheep production, and the estimated heritability of RFI in sheep populations is moderate, it is difficult to change this feature through traditional selection. Therefore, it is of great significance to elucidate the genetic mechanisms underlying RFI at the molecular level to improve feed efficiency.
With breakthroughs in high-throughput sequencing techniques, RNA-seq technology has been used successfully for identifying and characterizing circular RNAs [18]. In recent years, though many unique circRNAs have been identified in a variety of species based on the reference genome has been assembled, and annotated. However, there are no studies concering the role of circRNAs in the process of RFI in sheep. Herein, we identified and described the expression patterns of circRNAs in extreme RFI sheep liver tissue using RNA-seq, as well as bioinformatics analysis. A total of 219 DE circRNAs was found in the Low-RFI and High-RFI groups. The total of number of circRNAs here was less than other tissues of sheep, for example, Li et al. identified that 5086 circRNAs were differentially expressed in longissimus dorsi muscle of sheep at different development periods [19]. This result is consistent with previous studies in pigs [20] and humans [21] that have found circRNAs have specific expression patterns in differential tissues. These circRNAs in the present study were mainly distributed over 1 to 3 chromosomes, which was associated with the length of these chromosomes, and the distribution of circRNAs was consistent with what was described by Li et al. [22]. Furthermore, the RT-PCR, DNA sequencing as well as RT-qPCR were performed to confirm the particular circular structure and sequencing data of these circRNAs. We results demonstrated the presence of circRNAs circular structure and the reliability of sequencing data.
Previous studies showed that circRNAs may affect the biological functions by regulating the expression level of their host gene [23]. Functional enrichment analysis was performed for the host genes of differentially expressed circRNAs. The GO annoation results indicated the main functions for the source genes of circRNAs were involved in immune response. Existing literature showed that activating the immune system requires considerable energy [24], and the transfer of nutrients from growth to immune-related processes may increase maintenance requirements in animals during the immune response [25]. And many studies showed the immune response may be important factors contributing to the difference in feed efficiency [26, 27]. KEGG enrichment indicated that the parental genes of circRNAs were mainly related to metabolism-related pathways, which may be related to the metabolic ability of the liver, because the liver is the largest metabolic organ, and plays an important role in protein, carbohydrate, amino acid as well as fat metabolism [28, 29]. This is consistent with previous studies that have found that energy is the limiting factor that affects the feed efficiency of animals.
Finally, to further understand how circRNAs is to regulate feed efficiency. We constructed circRNA-miRNA-mRNA interaction network based on competing endogenous RNAs (ceRNAs) to reveal the main functions for these DE circRNAs. Some literatures demonstrated that another major function of circRNAs is to act as miRNA sponges to indirectly regulate expression of downstream target gene for miRNAs [30]. In the interaction network, 219 DE circRNAs targeting 4 DE miRNAs were found, interestingly, we found that some circRNAs contain two target sites of miRNAs (novel_41, novel_115, novel_171 and oar-miR-485-3p), consistent with the previous findings that some circRNAs have multiple binding sites for miRNAs [31]. Simultaneously, 6 targeted key genes (SHISA3, PLEKHH2, RTP4, CD274, OAS1, and RFC3) were identified from 4 miRNAs, and these genes were mainly related to metabolism process and immunity function. Energy metabolism and immune response are important process that affect the feed efficiency [2]. In the present study, two target genes (SHISA3 and PLEKHH2) have been identified as related to metabolism process. SHISA3 (shisa family member 3) gene encodes a single-transmembrane protein which is one of nine members of a family of transmembrane adaptors. Li et al., reported SHISA3 gene is associated with intramuscular fat percentage of beef, suggesting the function of SHISA3 might be related with adipose deposition [32]. PLEKHH2 (Pleckstrin homology, MyTH4 and FERM domain containing H2) is a protein coding gene, and play a role in linking podocyte foot processes to the glomerular basement membrane. However, few studies have reported the function of PLEKHH2. RTP4 (Receptor transporter protein 4) as an innate antiviral effector [33], it was related to the flavivirus replication, binds viral RNA, and inhibits viral genome amplification [34], and plays a role in diverse viral infections [35]. In addition, Li et al. reported that RTP4 was associated with immune cell infiltration [36]. This suggests that RTP4 might be involved in immunity response process, while previous study indicated that activation of the immune response results in low feed efficiency due to increased maintenance requirements [26, 37]. In addition, nutrients shifted away from growth toward the immune-related processes may reduce feed efficiency in animals during the immune response [25]. In the present study, we found that a nonsynonymous SNP c.399 A > G mutation in the RTP4 gene was significantly associated to RFI. Therefore, we speculated circRNAs, as a miRNA sponge, by regulating the expression of downstream target gene, thus affect immune response and metabolic functions of the liver. Nonetheless, the specific mechanisms need to be studied in future via experiments.
Conclusion
In the present study, we identified and examined the expression patterns, genomic characteristics, and length distribution of circRNAs in sheep liver. Meanwhile, Function enrichment indicated that the source genes of circRNAs were mainly related to immunity response and metabolism-related pathways. Interestingly, the polymorphism (c.399 A > G) in the target gene RTP4 were significantly associated with RFI, which lay the foundation for the study of the regulation feed efficiency by circRNAs.
Methods
Data collection and samples obtained
In the present study, the animal performance and liver tissues of circular RNA sequencing were the same as those investigated in our previous study [38]. Briefly, 137 healthy male Hu lambs were raised according to the procedures described by Zhang et al. (2019), and the feed intake (FI), initial body weight (IBW) and final body weight (FBW) of all lambs were determined in the experimental period. Simultaneously, the average daily feed intake (ADFI), average daily gain (ADG), mid-test metabolic body weight (MBW), feed conversion ratio (FCR) and residual feed intake (RFI) were calculated, the formulae used was: ADFI = FI/N, ADG = (IBW—FBW)/N, MBW = [0.5*(IBW + FBW)]0.75, FCR = ADFI/ADG, and the RFI was calculated using a linear regression model according to the data on ADG, MBW and ADFI, the basic model used was: Yi = β0 + β1MBWi + β2ADGi + ei, in the formulae and model above, N is the experimental period (days), Yi is the ADFI of the ith sheep; β0 is the regression intercept; β1 is the regression coefficient for MBW; β2 is the regression coefficient for ADG and ei is the random error associated with the ith animal. At the end of the experimental period, all lambs were slaughtered and the liver tissue were immediately frozen in liquid N2, and stored at − 80℃ until RNA isolation. For RNA-sequencing, two extreme groups were selected with both High-RFI (0.20 ± 0.02, n = 3) and Low-RFI (-0.25 ± 0.05, n = 3) sheep according to the RFI value from these individuals. The phenotype data of the other batches were collected after RNA sequencing to expand the validation experimental population of association analysis. Briefly, a total of 1220 male Hu sheep were randomly selected from Lanzhou Tianxin Sheep Industry Co. Ltd., Gansu Sanyangjinyuan Husbandry Co. Ltd., Shandong Runlin Sheep Industry Co. Ltd., Gansu Zhongsheng Huamei Sheep Industry Development Co. Ltd., Gansu Zhongtian Sheep Industry Co. Ltd., and Wuwei Pukang Sheep Industry Co. Ltd. To collect phenotypic data, all lambs were transferred to Minqin Defu Agriculture Co. Ltd. after weaning at 56 days old, and raised and housed indoors in individual pens (0.8 × 1 m) until 6 months old age. Throughout the experiment, all lambs have the same feeding model and management conditions according to our previously studies. At the end of the experimental period, venous blood samples were collected for extracting DNA.
Library construction and circRNAs sequencing analysis
Total RNA from each liver tissue was isolated TRIzol (Invitrogen, Waltham, MA, USA) based on the manufacturer's procedure. The total RNA purity and quality was detected using the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and RNA Nano 6000 Assay Kit, respectively. For circRNA sequencing, firstly, we take approximately 5 µg high quality RNA per sample as input material and use the 15 U Rnase R (Epicentre, USA) for each RNA sample to digest linear RNA. Secondly, ribosomal RNA was removed by Epicentre Ribozero™ rRNA Removal Kit (Epicentre, USA). The remaining RNAs were used to construct a cDNA library of circRNAs, and the average fragment size for final cDNA library was 250–300 bp. Finally, the cDNA library of circRNAs were 150 bp paired-end sequenced using an Illumina HiSeq 2500 platform (Illumina San Diego, CA, USA) by Beijing Novogene Science and Technology Co., Ltd. (Beijing, China). The adaptor sequences and low quality reads with quality scores < Q20 were removed, the remaining reads were mapped to the Ovis aries reference genome (Oar_v4.0) using TOPHAT (v2.0.9) (tophat -o./ file1.fastq,file2.fastq Bowtie2Index/genome). Subsequently, the Find_circ software (python find_circ.py -G chomosomes.fa -p prefix -s find_circ.sites.log > find_circ.sites.bed 2 > find_circ.sites.reads) was used to identify circRNA following the steps. The 20 bp anchor sequences from both ends for each unmapped read aligns again with the Ovis aries reference sequence. The 5' end of the anchor sequence is aligned to the reference sequence, meanwhile, the 3' end of the anchor sequence is aligned to the upstream of this site, and there are splicing sites (GT-AG) between the breakpoints of the reference sequence, which this read is considered as candidate circRNA. Finally, the characterization of circRNAs was statistically analyzed, the expression level of each identified circRNAs were calculated using TPM method [39], the DESeq R package (https://github.com/nyudanin/RNASeq_DESeq2/blob/master/2017-11-03_DESeq2.R) was performed to identify DE circRNAs between Low-RFI and High-RFI liver tissue. The significant DE circRNAs were selected using the parameters of a |log2Fold Change|≥ 1 and p-value < 0.05.
Validation of circRNAs presence using RT-PCR amplification and Sanger sequencing
To validate the presence of circRNAs circular structures. Total RNAs were extracted from liver tissues of sheep using TRIzol reagent (Invitrogen), one microliter of total RNA was used as templates to synthesize complementary DNA (cDNA) with a EvoM-MLV RT kit with gDNA Clean for qPCR (Accurate Biotechnology Co., Lth, Hunan, China). Five divergent primers used for circRNAs PCR were designed using Oligo 7.0 software, and were synthesized by Tsingke (Xi’an, China) and listed in Table 3. The cDNA products were amplified using circRNA PCR primers, the amplified products were checked by agarose gel (1.5%) electrophoresis and then sequenced using Sanger sequencing. The sequences data of amplified products were aligned to the RNA-seq data and the sheep reference genome with DNAMAN (v 9.0) software to determine the authenticity of the location of the junction sites in the circular RNAs.
Table 3.
RT-PCR primers and annealing temperatures for DE circRNAs
| CircRNAs | Forward 5' → 3' | Reverse 5' → 3' | product length (bp) | Tm/°C |
|---|---|---|---|---|
| circ_0018936 | AGTCCCTGAGTTACGCCTT | TGGCCTTACCGATGATGACA | 253 | 55 |
| circ_0003667 | TCCTTCTCTGGGGTTCGAAA | TTGCATAAAACTCCTCCCGCTA | 136 | 57 |
| circ_0006661 | ACAGGAGCCGATATTTAGAGACC | TGCTGCCATAGCGACTTGCC | 173 | 58 |
| circ_0020271 | TAATCCGCTGAAGATCCCT | CTCATCACACTGTACCCAT | 205 | 54 |
| circ_0018320 | CTCTAGAAGCATTGGAGCAC | GCAGATGAAGAAAATACGCTCA | 222 | 55 |
| β-actin | TCCGTGACATCAAGGAGAAGC | CCGTGTTGGCGTAGAGGT | 267 | 52–65 |
Validation of the RNA-seq datas using RT-qPCR analysis
To verify the authenticity of RNA-seq datas. According to the manufacturer's protocols, five DE circRNAs that were the same as those analyzed using RT-PCR were subjected to real-time quantitative PCR (RT-qPCR) using 2 × the TransStart® Tip Green qPCR SuperMix (TransGen Biotech, Beijing, China) on an applied Roche LightCycler 480 (Roche Applied Science) platform. The reaction system and conditions of RT-qPCR were described by our previous article [38], the RNA samples and primers used for the RT-qPCR were the same as those used for the RT-PCR. The gene ACTB (encodes beta-actin) was used as housekeeping gene for standardization and the relative expression data were calculated with the 2−∆∆Ct method [40].
Functions enrichment analysis and interaction network construction
Gene ontology (GO) functions and Kyoto encyclopedia of genes and genomes (KEGG) enrichment pathways were analyzed to explore the important function of the host genes of DE circRNAs using GOseq R package and KOBAS-i software, respectively. P-value was < 0.05 was used as a threshold for the significantly enriched. For miRNA sequencing, total RNA was isolated from the above same liver tissue, six small RNA libraries were generated using NEBNext® Multiplex small RNA library Prep Set for Illumina® (NEB, USA). After quality control, libraries were sequenced on an Illumina Hiseq 2500 platform and 50 bp single-end reads were obtained. Clean data were obtained by removing reads containing adapters and low-quality. Then, the clean data were used to perform all the downstream analyses. In the present study, the differentially expressed miRNAs for (P ≤ 0.05 and |Log2 FC|≥ 1) were identified using the DEGseq R package. The target microRNAs of circRNAs were identified by miRanda (v.3.3a), the differentially expressed genes, circRNAs and their target microRNAs were used to build an interaction network based on our previous mRNA data (NCBI SRA accessions for mRNA sequencing data: SRR9291141, SRR9301148, SRR9301110, SRR9302163, SRR9302192, and SRR9302375) [38] and the unpublished miRNA data from the same liver samples. The circRNA-miRNA-gene network was visualized by using Cytoscape (v3.6.1) software.
Identification and genotyping of target gene SNPs
DNA isolation for 1120 individuals were extracted from was performed using an EasyPure Blood Genomic DNA Kit (TransGen Biotech) according to the manufacturer's instructions. The PCR primers of the target genes designed in Table 4 using the Oligo7.0 software, and the specific PCR fragments were amplified using 20 mixed samples selected randomly from the above Hu sheep DNA samples, subsequently, the PCR products were sequenced to identify potential SNPs loci in the target genes. Finally, the identification of polymorphic loci in the target genes was carried out using KASPar assays according to a previous study [41]. The appropriate primers for the genotyping are listed in Table 4.
Data analyses
The results of the experimental data were statistically analyzed by using SPSS 21.0 for Windows Software. The differences of expression level were analyzed using a t-test for two groups. The association between genotypes and trait were analyzed by a linear model with the fixed effects: Yijk = μ + Gi + Fj + Bk + εijk, where Yijk is the phenotypic observation value, μ is the overall population mean, Gi is the effect of genotype, Fj is the effect of the jth farm (j = 1, 2…6), Bk is the batch effect (k = 1, 2…5), ε is the random error. The data were indicated as means ± SD (standard deviation). A P-value < 0.05 was known as the statistically significant criterion. The Prism software (v5.0) was used to draw the bar graphs.
Supplementary Information
Acknowledgements
We would like to thank the staff at our laboratory for their ongoing assistance.
Abbreviations
- CircRNA
Circular RNA
- CeRNAs
Competing endogenous RNAs
- FI
Feed intake
- IBW
Initial body weight
- FBW
Final body weight
- ADFI
Average daily feed intake
- ADG
Average daily gain
- MBW
Mid-test metabolic body weight
- FCR
Feed conversion ratio
- RFI
Residual feed intake
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- SNP
Single nucleotide polymorphism
Authors’ contributions
D. Y. Zhang and W. M Wang designed the work. D. Y Zhang, X. X Zhang, F. D Li, X. L Li, Y. Zhao, Y. K Zhang and L. M Zhao, J. H Wang, D. Xu collected the sample and performance data, D. Y Zhang, L. M Zhao, J. H Wang, D. Xu, X. B Yang and P. P Cui contributed to DNA and RNA extraction. D. Y Zhang, X. X Zhang and W. M Wang perform the experiments and analyzed the data. D. Y Zhang wrote the manuscript. X. X Zhang and W. M Wang revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31760651), the West Light Foundation of the Chinese Academy of Sciences (CN), and the National for joint research on improved breeds of livestock and poultry (19200140).
Availability of data and materials
The sequencing reads of each sample in the present study have been deposited at GenBank with the Bioproject accession number PRJNA738639.
Declarations
Ethics approval and consent to participate
The whole study has been approved by the Institutional Animal Care and Use Committee of Gansu Agriculture University (Lanzhou, China; permit number: Approval number: NO.2012–2-159). All experimental procedures and sample collection methods were performed in accordance with approved guidelines to ensure animal welfare. In this study, written informed consent was obtained from Gansu Agricultural University and Minqin Defu Agriculture Co. Ltd. to use these animals. Meanwhile, the study is in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no have any conflict of interest for this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang X, Wang W, Mo F, La Y, Li C, Li F. Association of residual feed intake with growth and slaughtering performance, blood metabolism, and body composition in growing lambs. Sci Rep. 2017;7(1):12681. doi: 10.1038/s41598-017-13042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantalapiedra-Hijar G, Abo-Ismail M, Carstens GE, Guan LL, Hegarty R, Kenny DA, McGee M, Plastow G, Relling A, Ortigues-Marty I. Review: biological determinants of between-animal variation in feed efficiency of growing beef cattle. Animal. 2018;12(s2):s321–s335. doi: 10.1017/S1751731118001489. [DOI] [PubMed] [Google Scholar]
- 3.Koch RM, Swiger LA, Doyle C, Gregory KE. efficiency of feed use in beef cattle. J Anim Sci. 1963;2:486–494. doi: 10.1109/TAU.1963.1161733. [DOI] [Google Scholar]
- 4.Chen Y, Gondro C, Quinn K, Herd RM, Parnell PF, Vanselow B. Global gene expression profiling reveals genes expressed differentially in cattle with high and low residual feed intake. Anim Genet. 2011;42(5):475–490. doi: 10.1111/j.1365-2052.2011.02182.x. [DOI] [PubMed] [Google Scholar]
- 5.Gunsett FC. Linear index selection to improve traits defined as ratios. J Anim Sci. 1984;59(5):1185–1185. doi: 10.2527/jas1984.5951185x. [DOI] [Google Scholar]
- 6.Tortereau F, Marie-Etancelin C, Weisbecker JL, Marcon D, Bouvier F, Moreno-Romieux C, Franois D. Genetic parameters for feed efficiency in Romane rams and responses to single-generation selection. Animal. 2020;14(4):681–687. doi: 10.1017/S1751731119002544. [DOI] [PubMed] [Google Scholar]
- 7.Herd RM, Bishop SC. Genetic variation in residual feed intake and its association with other production traits in British Hereford cattle. Livest Prod Sci. 2000;63(2):111–119. doi: 10.1016/S0301-6226(99)00122-0. [DOI] [Google Scholar]
- 8.Santos A, Giráldez F, Trevisi E, Lucini L, Frutos J, Andrés S. Liver transcriptomic and plasma metabolomic profiles of fattening lambs are modified by feed restriction during the suckling period. J Anim Sci. 2018 doi: 10.1093/jas/sky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tizioto PC, Coutinho LL, Decker JE, Schnabel RD, Rosa KO, Oliveira PS, Souza MM, Mourão GB, Tullio RR, Chaves AS. Global liver gene expression differences in Nelore steers with divergent residual feed intake phenotypes. BMC Genomics. 2015;16(1):242. doi: 10.1186/s12864-015-1464-x.s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanger HL, Klotz G, Riesner D, Gross HJ. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci. 1976;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Y, Tang J, Di R, Wang X, Chu M. Differential expression of circular RNAs in polytocous and monotocous uterus during the reproductive cycle of sheep. Animals. 2019;9(10):797. doi: 10.3390/ani9100797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Li C, Wei J, Ni W, Xu Y, Yao R, Zhang M, Li H, Liu L, Dang H. Comprehensive expression profiling analysis of pituitary indicates that circRNA participates in the regulation of sheep estrus. Genes. 2019;10(2). 10.3390/genes10020090. [DOI] [PMC free article] [PubMed]
- 13.Yang, Yang, Cun-Yuan, Hazi, Wureli, Sheng-Wei, Zhi-Jin, Jun-Chang, Wang, Da-Wei. Expression profiles of circular RNAs in sheep skeletal muscle. Asian-Australas J Anim Sci 2018. 10.5713/ajas.17.0563. [DOI] [PMC free article] [PubMed]
- 14.Wang J, Zhou H, Hickford JGH, Hao Z, Gong H, Hu J, Liu X, Li S, Shen J, Ke N, et al. Identification and characterization of circular RNAs in mammary gland tissue from sheep at peak lactation and during the nonlactating period. J Dairy Sci. 2021;104(2):2396–2409. doi: 10.3168/jds.2020-18911. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R, Liu N, Han F, Li H, e JH. Identification and characterization of circRNAs in the skin during wool follicle development in Aohan fine wool sheep. 2020, 21(1):187. 10.1186/s12864-020-6599-8. [DOI] [PMC free article] [PubMed]
- 16.Jie L, Liu R, Jie W, Zhang Y, Jie W. exploring genomic variants related to residual feed intake in local and commercial chickens by whole genomic resequencing. Genes. 2018;9(2):57. doi: 10.3390/genes9020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manuel M, Cavani L, Millen D, Andrighetto C, Fonseca RD. Estimation of genetic parameters for weight traits and Kleiber Index in a Brahman cattle population. Sciagric. 2019 doi: 10.1590/1678-992x-2018-0062. [DOI] [Google Scholar]
- 18.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Li X, Yang Y, Ma Q, Hu S. Genome-wide analysis of circular RNAs in prenatal and postnatal pituitary glands of sheep. Rep. 2017;8(57):97165–97177. doi: 10.1038/s41598-017-16344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang M, Shen Y, Mao H, Chen L, Xu N. Circular RNA expression profiles in the porcine liver of two distinct phenotype pig breeds. Asian Australas J Anim Sci. 2018;31(6):812–819. doi: 10.5713/ajas.17.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rybak-Wolf A, Stottmeister C, Glaar P, Jens M, Pino N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed – science direct. Mol Cell. 2015;58(5):870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Luo R, Wang X, Wang H, Ma Y. Unraveling stage-dependent expression patterns of circular RNAs and their related ceRNA modulation in ovine postnatal testis development. Front Cell Dev Biol. 2021;9:627439. doi: 10.3389/fcell.2021.627439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Vigors S, O'Doherty JV, Ryan M, Sweeney T. Analysis of the basal colonic innate immune response of pigs divergent in feed efficiency and following an ex vivo lipopolysaccharide challenge. Physiol Genomics. 2019;51(9):443–448. doi: 10.1152/physiolgenomics.00013.2019. [DOI] [PubMed] [Google Scholar]
- 25.Horodyska J, Hamill RM, Reyer H, Trakooljul N, Lawlor PG, McCormack UM, Wimmers K. RNA-Seq of liver from pigs divergent in feed efficiency highlights shifts in macronutrient metabolism, hepatic growth and immune response. Front Genet. 2019;10:117. doi: 10.3389/fgene.2019.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patience JF, Rossoni-Serão MC, Gutiérrez NA. A review of feed efficiency in swine: biology and application. J Anim Sci Biotechnol. 2015;6(1):33. doi: 10.1186/s40104-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, He T, Xiong F, Chen X, Fan X, Jin S, Geng Z. Identification of key genes and pathways associated with feed efficiency of native chickens based on transcriptome data via bioinformatics analysis. BMC Genomics. 2020;21(1):292. doi: 10.1186/s12864-020-6713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138(2):397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 29.Andus T, Holstege A. Cytokines and the liver in health and disease. Effects on liver metabolism and fibrogenesis. Acta Gastroenterol Belg. 1994;57(3–4):236–244. [PubMed] [Google Scholar]
- 30.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 31.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Wang M, Li Q, Gao Y, Li Q, Li J, Cao Y. Transcriptome profiling of longissimus lumborum in Holstein bulls and steers with different beef qualities. PLoS One. 2020;15(6):e0235218. doi: 10.1186/s41065-021-00183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen JR, Lazear HM. Antiviral Effector RTP4 Bats against Flaviviruses. Immunity. 2020;53(6):1133–1135. doi: 10.1016/j.immuni.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boys IN, Xu E, Mar KB, De La Cruz-Rivera PC, Eitson JL, Moon B, Schoggins JW. RTP4 is a potent IFN-inducible anti-flavivirus effector engaged in a host-virus arms race in bats and other mammals. Cell Host Microbe 2020, 28(5). 10.1016/j.chom.2020.09.014. [DOI] [PMC free article] [PubMed]
- 35.Zarei Ghobadi M, Mozhgani S-H, Farzanehpour M, Behzadian F. Identifying novel biomarkers of the pediatric influenza infection by weighted co-expression network analysis. Virol J. 2019;16(1):124. doi: 10.1186/s12985-019-1231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Qi J, Yang J. RTP4 is a novel prognosis-related hub gene in cutaneous melanoma. Hereditas. 2021;158(1):22. doi: 10.1186/s41065-021-00183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins MG, Kenny DA, Fitzsimons C, Blackshields G, Coyle S, McKenna C, McGee M, Morris DW, Waters SM. The effect of breed and diet type on the global transcriptome of hepatic tissue in beef cattle divergent for feed efficiency. BMC Genomics. 2019;20(1):525. doi: 10.1186/s12864-019-5906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deyin Z, Xiaoxue F, Chong Y, Futao G, Yukun X. transcriptome analysis identifies candidate genes and pathways associated with feed efficiency in Hu Sheep. Front Genet. 2019;10:1183–1183. doi: 10.3389/fgene.2019.01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L, Chen J, Li Z, Li X, Hu X, Yi H, Zhao X, Liang C, Wang Y, Sun L. Integrated profiling of microRNAs and mRNAs: MicroRNAs located on Xq273 associate with clear cell renal cell carcinoma. PloS One. 2010;5(12):e15224. doi: 10.1371/journal.pone.0015224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjl A, Tds B. Analysis of relative gene expression data using real-time quantitative PCR and the 2 ΔΔ C T Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, La Y, Zhou X, Zhang X, Li F, Liu B. The genetic polymorphisms of TGFβ superfamily genes are associated with litter size in a Chinese indigenous sheep breed (Hu sheep) Anim Reprod Sci. 2018;189:19–29. doi: 10.1016/j.anireprosci.2017.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing reads of each sample in the present study have been deposited at GenBank with the Bioproject accession number PRJNA738639.