Abstract
Background
Hypertension is more prevalent and clinically severe among African–Americans than whites. Several health behaviors influence blood pressure (BP) control, but effective, accessible, culturally sensitive interventions that target multiple behaviors are lacking.
Purpose
We evaluated a culturally adapted, automated telephone system to help hypertensive, urban African–American adults improve their adherence to their antihypertensive medication regimen and to evidence-based guidelines for dietary behavior and physical activity.
Methods
We randomized 337 hypertensive primary care patients to an 8-month automated, multi-behavior intervention or to an education-only control. Medication adherence, diet, physical activity, and BP were assessed at baseline and every 4 months for 1 year. Data were analyzed using longitudinal modeling.
Results
The intervention was associated with improvements in a measure of overall diet quality (+3.5 points, p<0.03) and in energy expenditure (+80 kcal/day, p<0.03). A decrease in systolic BP between groups was not statistically significant (−2.3 mmHg, p=0.25).
Conclusions
Given their convenience, scalability, and ability to deliver tailored messages, automated telecommunications systems can promote self-management of diet and energy balance in urban African–Americans.
Keywords: Behavioral intervention, Cultural adaptation, Hypertension
Introduction
In the USA, the prevalence of hypertension is significantly higher among African–Americans compared to Whites. Although the majority of US Blacks who have hypertension receive medical care and antihypertensive medication, only 58% of them achieve adequate blood pressure control [1].
Multiple well-designed studies have shown that lifestyle interventions delivered in-person can improve adherence to antihypertensive medication regimens, to regular physical activity, to a healthful diet, and to weight control recommendations—all of which can reduce elevated blood pressure [2–4]. Nonetheless, these programs are not suitable for use on a public health scale for reasons including their high cost of personnel, facilities, and materials and the burden on participants to attend multiple counseling sessions in the face of other life priorities. Minority groups of low socioeconomic status (SES) may encounter additional barriers including logistical obstacles (e.g., unreliable transportation), cost considerations, and cultural incongruence of intervention content or delivery [5, 6].
The current public health challenge is to design and implement health programs that can be delivered easily to the millions of patients with hypertension in a practical, low-cost manner. Tailoring these programs to the needs, barriers, and ethno-cultural characteristics of specific populations can help maximize their impact [7].
Studies from our group and others have shown that computer-based, telephone intervention systems can be efficacious in changing patients’ health-related behaviors, including medication adherence for hypertensive patients [8], physical activity [9, 10], and dietary behaviors [11]. However, most of these have targeted single rather than multiple behaviors, and few have been culturally adapted. The purpose of this study was to evaluate an automated, culturally adapted, multi-behavior intervention that can be used on a public health scale to promote hypertension self-management in urban African–Americans of low socioeconomic status. The primary study hypothesis was that the intervention group would experience greater improvements in these behaviors than the control group. Our secondary hypothesis was that mean blood pressure (BP) would decrease more in the intervention than control group.
Methods
We conducted a two-armed randomized controlled trial (intervention vs. usual care control) of a novel automated telephone counseling system designed to promote three health-related behaviors that affect blood pressure control: (a) adhering to the antihypertensive medication regimen, (b) following the dietary approaches to stop hypertension (DASH) diet [2], and (c) engaging in regular physical activity [12].
Participants were drawn from the adult primary care practices of a large, urban safety-net hospital and from four affiliated community health centers. Inclusion criteria were as follows: (a) African–American by self-report; (b) a diagnosis of hypertension on the active problem list of the patient’s medical chart; (c) a current prescription for ≥1 antihypertensive medications; (d) ≥1 primary care office visits in the previous 2 months; (e) two elevated clinic BP readings in the previous 6 months (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg among non-diabetic patients, and ≥130/80 among diabetic patients); and (f) age ≥35 years. Patients were ineligible if they were pregnant, or had a medical condition that precluded moderate exercise or for which the DASH diet was contraindicated. Patients were also excluded if they scored 7 out of 7 on a modified version of the 8-item Morisky Medication Adherence Scale, indicating high baseline medication adherence [13], had a terminal illness, psychiatric or cognitive disorder, or no access to a telephone.
Potentially eligible patients were identified by searching the electronic medical records of the participating clinical sites for patients who met the inclusion criteria (a–f) above. Primary care providers were then asked to review lists of their potentially eligible patients and remove those with any exclusion. The remaining patients were sent a letter on behalf of their primary care provider that described the study and were asked to return a postage-paid postcard if they did not want to be contacted by study staff. Those who did not opt-out were contacted by phone, the study was explained to them, and they were screened for eligibility. All participants provided informed consent. Based on power analyses and projected attrition, we sought to randomize 360 patients expecting 300 to complete the 8-month study assessment thus providing sufficient power to analyze the three primary behavioral outcomes.
Intervention: Telephone-Linked Care for Hypertension in African–Americans
The intervention was a totally automated, computer-based, interactive telephone counseling system called Telephone-Linked-Care, designed to monitor, educate, and counsel African–American adults with hypertension and to provide summary data regularly to the patient’s primary care provider [8–10]. The intervention incorporated principles of social cognitive theory [14], the transtheoretical model of behavioral change [15], and motivational interviewing [16], and was tailored to the user’s values. Content was also adapted to cultural characteristics of culturally African–American adults (i.e., not Caribbean–American, Black–Hispanic, etc.). This group-level cultural adaptation was guided by a conceptual framework targeting “surface” and “deep” structural domains, and by ethnic mapping [17] in focus groups of potential users. Surface structure adaptation involves matching intervention materials and messages to observable, “superficial” characteristics of a target population including familiar or preferred people, places, language, music, food, locations, and clothing. In this study, surface adaptation included using pre-recorded speech from African–American voice professionals to deliver the automated telephone counseling messages. Deep structure can be viewed as the underlying rationale for surface structure preferences. It denotes the cultural values, social, historical, and psychological forces that influence how a given health behavior is viewed by the target population. For example, inviting an African–American adult to join a neighborhood walking group may have greater deep structure appeal than suggesting he/she walk alone due to the cultural priority of community well-being observed in African–American subgroups [18].
In ethnic mapping, we presented focus group members with foods, activities, and health recommendations and asked to rate them on a continuum from “Mostly a black thing” (e.g., dancing) to “Mostly a white thing” (e.g., skiing, yoga) and we included material in automated telephone scripts, accordingly. These scripts were then pre-tested by potential users of the system and their feedback was incorporated. New script elements emerged from focus group material related to topics such as dance as an effective method to combine fun and healthy exercise, and using low-cost community resources, home-based exercise, or mall-walking to achieve a safe place to exercise. Another new element addressed concerns over “sweating out” one’s hairdo as a barrier to exercise:
“Some hairstyles can get messed when you sweat or swim in a pool. It’s nice to look good, but it’s also important to be healthy. Consider a low-maintenance style that will look good on you and hold up well with an active lifestyle, like a short natural cut or braids. Ask your hairstylist’s opinion the next time you visit. And remember—regular exercise can help you look good too.”
The automated telephone intervention delivered one call per week for 32 weeks. The first three calls introduced the three targeted behaviors and their role in BP control, and described the system. Subsequent calls were arranged as modules on medication adherence, physical activity, and diet, and were delivered in the order chosen by the participant. Each call consisted of (a) an introduction, (b) a section for reporting health information collected on study-issued home measurement devices (pedometers, sphygmomanometers, weight scales), and (c) theory-based interactive education and counseling on the targeted behavior.
The physical activity module consisted of 12 calls to increase levels of moderate-or-greater intensity physical activity and was adapted from our previously evaluated automated telephone systems to promote physical activity [9, 10]. The diet module consisted of nine calls—one overview call and two calls for each of four topics: fruits and vegetables, fiber, sodium, and fat. The content of these calls was designed to promote the DASH diet [2]. The medication adherence module consisted of eight calls and was adapted from a successful prototype from our group [8]. Study staff monitored participant use of the system and contacted those who did not call to assist or re-engage them with the system. In addition, participants and their primary care providers received printouts of the participant’s progress in each health behavior. These were sent at the beginning and end of each of the three behavioral modules and were designed to reinforce the intervention.
Randomization Groups
Patients who screened eligible by telephone attended an in-home study visit for the purpose of health education, baseline assessments, and randomization. At this visit, participants received a 75-page resource manual that described hypertension, listed dietary recommendations, heart healthy food recipes, and local resources for exercise, and provided information to support antihypertensive medication adherence. They received a 20-min education session based on the content of this manual, and were given a pedometer and a digital weight scale (Healthometer, model # HDR900KD01). Participants completed baseline study assessments and were then randomized to the automated, telephone-linked behavioral intervention plus standard primary medical care, or to the control condition of standard primary medical care alone. Randomization was accomplished using a random number generator to assign subjects to one of the two groups. Neither participants nor research assistants knew the group assignment until after baseline assessments were complete. After group assignment was revealed, intervention group members received a digital home BP monitor (Omron, model # HEM711AC), were trained to use it, and were trained to use the automated telephone system.
Assessments
Assessments were completed by trained research assistants at the participant’s home except for a handful done in the study office for logistical reasons. The three primary study outcomes were change in (a) diet quality, (b) leisure time physical activity of moderate-or-greater intensity, and (c) adherence to the antihypertensive medication regimen from baseline to the end of the 8-month intervention period. The secondary outcome was change in BP. All outcomes were assessed at baseline (randomization), 4, 8 (end-of-intervention), and 12 months. Other assessments included weight, height, psychosocial characteristics, and users’ perceptions of the automated telephone system (see Table 1 for a list of demographic, medical, and social characteristics assessed, and for information about how they were measured and categorized).
Table 1.
Characteristics of the sample at baseline by randomization group
| Variable | Group | |
|---|---|---|
|
|
||
| Automated telephone intervention (n =169) | Control (n = 168) | |
|
| ||
| Gender (% female) | 65.7 | 75.0 |
| Age, years, mean (SD) | 56.3 (10.6) | 56.8 (11.4) |
| Education, years, mean (SD) | 12.0 (2.5) | 12.4 (2.8) |
| Body mass index, kg/m2, mean (SD) | 34.4 (8.6) | 34.3 (8.4) |
| Married or living together (%) | 35.7 | 34.7 |
| Employed full- or part-time (%) | 38.9 | 40.4 |
| Median household income | $10—$20 K/year | $10-$20 K/year |
| Income perception (%) | ||
| Comfortable with enough for “extras” | 19.2 | 20.3 |
| Enough for bills but not “extras” | 19.2 | 20.3 |
| Cut back in order to pay bills | 23.7 | 24.2 |
| Not enough to pay all bills | 37.8 | 35.3 |
| Insurance (%) | ||
| Medicare/medicaid | 53.8 | 49.4 |
| Free carea | 19.5 | 25.3 |
| Otherb | 24.9 | 24.7 |
| Self-pay | 1.8 | 0.6 |
| Medications, total numberc, mean (SD) | 5.1 (3.0) | 5.0 (3.3) |
| Medications, number for blood pressure, mean (SD) | 1.9 (0.8) | 1.9 (0.8) |
| Diabetes (%) | 40.7 | 35.8 |
| Stroke (%) | 11.2* | 4.2* |
| Co-morbidity indexd, mean (SD) | 5.8 (3.8) | 6.2 (4.7) |
| Health literacy scoree, mean (SD) | 51.7 (20.5)g | 54.3 (18.4)g |
| Primary outcomes | ||
| Diet index score, mean (SD) | 53.9 (17.6) | 55.8 (17.0) |
| Moderate-or-greater intensity leisure time physical activity, min/week, mean (SD) | 162.4 (169.0)* | 126.3 (144.3)* |
| >150 min per week (%) | 38.5* | 26.2* |
| Energy expenditure, kcals/day | 3234.7 (860.7) | 3188.5 (820.3) |
| Medication Adherence Scoref, mean (SD) | 4.93 (1.6) | 4.77 (1.4) |
| Secondary outcomes | ||
| Systolic blood pressure, mean (SD) | 130.6 (19.8) | 131.8 (18.6) |
| Diastolic blood pressure, mean (SD) | 80.9 (12.5) | 80.3 (11.8) |
p<0.05
Health Safety Net (Free Care) funding program for underinsured Massachusetts state residents
Employee-sponsored, self-insured, and other products
Total number of self-reported prescription medications
Oliver Co-morbidity Index [33]
REALM questionnaire [34]
From the 7-item version of the 8-item Morisky Medication Adherence Scale [13]
Scores correspond to a 7th–8th grade reading level
Physical activity outcomes were assessed with the interviewer-administered 7-day physical activity recall [19]. The main physical activity outcomes were (a) minutes per week of moderate-or-greater intensity physical activity, (b) total energy expenditure per day (kcal/day) [20], and (c) whether the participant met the joint Centers of Disease Control and Prevention—American College of Sports Medicine recommendation of at least 150 min of moderate-or-greater physical activity per week [12]. In order to validate self-reported physical activity, 48 participants were randomly selected to wear an accelerometer (CSA Actigraph, model # W7164LS) for 3 days before the baseline and 8-month assessments.
Diet outcomes were assessed with the picture-sort Food Frequency Questionnaire [21]. Five diet outcomes were derived and were analyzed as continuous variables and as categorical variables denoting whether or not the participant reported consuming recommended levels. The outcomes and their categorical cut-offs are as follows [22]: (a) percent of total calories derived from fat (≤30%); (b) percent of total calories derived from saturated fat (≤7%); (c) grams of fiber (≥25 g/day); (d) servings of fruit and vegetables (F&V ≥5 servings); and (e) milligrams of sodium (NA ≤2,400 mg/day).
A composite diet quality score was constructed that combined the five diet outcomes using an approach similar to that used in previous research [11]. Each diet outcome variable was converted into a score from 0 to 100, with higher scores indicating healthier dietary intake. Participants received a score of 100 if they met or exceeded the dietary recommendations described above for total fat, saturated fat, and fiber. However, for fruit and vegetables and sodium intake, a score of 100 points was based on stricter DASH diet recommendations [2]: ≥10 fruit and vegetable servings/day and ≤1,500 mg NA/day. As there are no published criteria to anchor the unhealthy end of the five component dietary outcome scales listed above, we assigned a score of zero to values ≥95th percentile for fat and sodium intake in baseline data, and ≤5th percentile for fiber and F&V. These values were 50.5% of calories from total fat, 16.9% of calories from saturated fat, 9.2 g/day of fiber, 1.5 servings/day of F&V, and 7,663 mg NA/day. This approach captured most of the variation in the sample and reduced the effect of outliers. Next, scores between 0 and 100 were set using a linear transformation (e.g., a value halfway between the criteria for 0 and 100would receive a score of 50). The overall Diet Quality Score was set as the mean of these five component scores and, therefore, also had a possible range of 0–100.
Medication adherence was assessed using a 7-item version of the 8-item Morisky Medication Adherence Scale [13]. Scores could range from 0 to 7, with higher scores indicating better adherence.
BP was measured with the Welch Allyn Spot Vital Signs Monitor (Model # 4020–00) by trained research assistants during each home visit using a research protocol similar to other studies [23]. These BP monitors were calibrated at the beginning of the study and annually thereafter. Participants were instructed to sit quietly for 5 min before each reading. At the first home visit a reading was taken in each arm, and the arm with the highest blood pressure was chosen for all subsequent readings. At each visit, three readings were taken in the designated arm and the mean of the last two was used for analysis. Systolic (SBP) and diastolic blood pressure (DBP) were analyzed as continuous outcome variables in separate statistical models. Each was also analyzed as the dichotomous variable (“high” or “controlled”) according to national guidelines [24]. Specifically, among non-diabetic participants SBP values ≥140 mmHg and DBP values ≥90 mmHg were considered “high”. The corresponding BP cut-off for diabetic participants was 130/80 mmHg. We examined another dichotomous BP outcome that considered BP “controlled” when both the SBP and DBP values were below the recommended cut-off, and “high” when one or both of these values exceeded its cut-off.
Data Analysis
The distributions of the primary outcome variables were examined and adjustments were made to normalize the data when appropriate. For diet outcomes, we eliminated as unreliable all Food Frequency Questionnaire results where the total caloric intake appeared unrealistically low (<800 cal/day) or high (>6,000 cal/day). Consequently, 15 (1.3%) were eliminated for low values and 30 (2.6%) for high values. Results from the remaining sample across the five dietary behaviors were normally distributed except for a few high outliers. These remaining outliers were capped at three standard deviations from the mean, which affected only 32 (0.6%) of the 5,705 total observations.
The distribution of minutes of moderate-or-greater physical activity had a large positive skew; therefore, log-transformed values were used in analyses. Non-transformed means are presented for ease of interpretation. At baseline, the distribution of total energy expenditure (kcal/day) and medication adherence scores were approximately normal.
Preliminary analyses found a higher rate of drop out in the intervention group than among controls, and our primary outcome analyses used an intent-to-treat approach to minimize this potential bias [25]. Missing data were imputed using the last value carried forward. For cases where data were available at time points before and after the missing value, the mean of these two values was used. Results of analyses that considered only participants who were assessed at the 8-month, end-of-intervention time point (i.e., the available-cases analyses) were similar to results from the intent-to-treat analyses.
The two study groups were compared on demographic and outcome variables at baseline using t tests for continuous characteristics and chi-square tests for categorical characteristics. Regression models for longitudinal data were used to analyze the effect of randomization group on the change in primary outcomes from baseline to each of the three follow-up assessment time points (i.e., 4, 8, and 12 months). As we hypothesized that intervention effects would be greatest at the end of the intervention, our primary focus was on changes in outcome through the 8-month assessment. Variables on which the groups differed significantly at baseline (e.g., stroke history) or that were likely to independently affect the outcome were included as covariates in each analysis. These included gender, age, perceived financial well-being, education, diabetic status, and stroke history for all analyses (see Table 1). Other covariates were included for particular outcomes as appropriate (e.g., season for physical activity and diet outcomes). Random effects linear regression models were used for continuous outcome measures. Generalized estimating equations logistic regression models were used for categorical outcomes. SAS version 9 was used for all analysis. Dose–response analyses were conducted for participants randomized to the treatment group by calculating the Pearson correlation between the number of completed automated telephone calls and outcomes at 8 months. Analyses were repeated separately for the subset of patients with type 2 diabetes.
Results
Study Participants
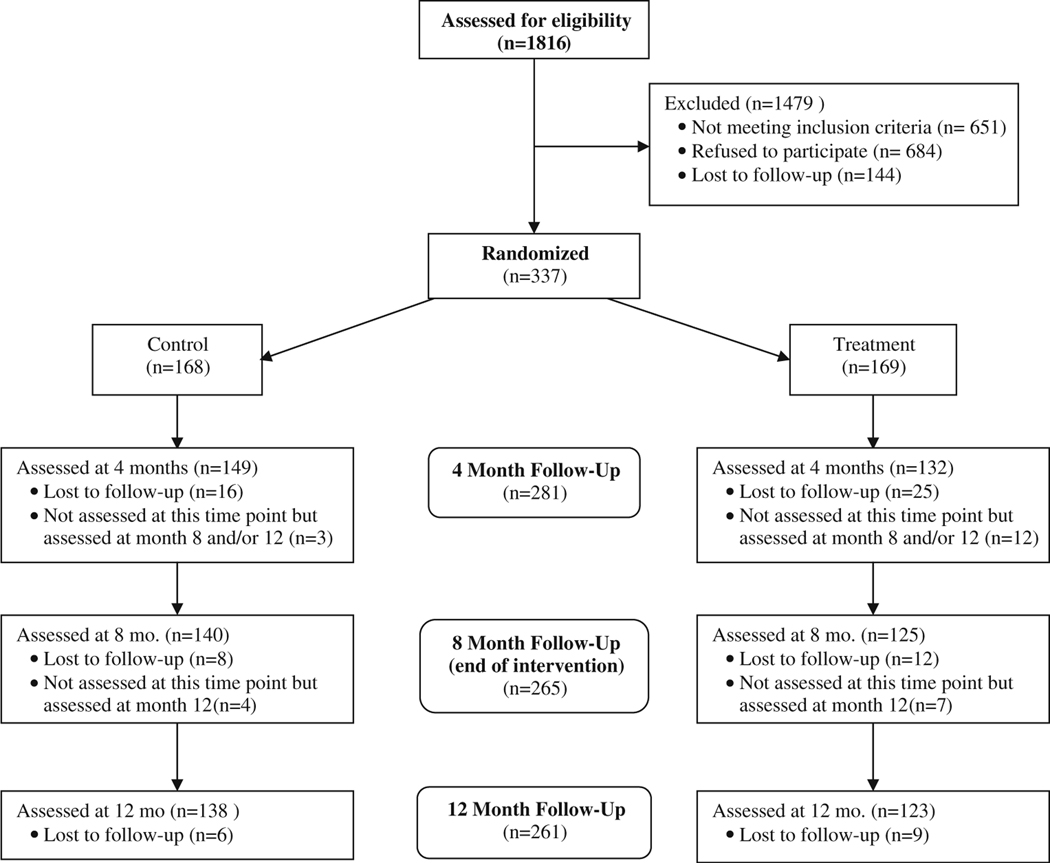
Participant recruitment and follow-up occurred from October 2003 to July 2007. Figure 1 displays the flow of potentially eligible participants through the study. Of the 1,816 individuals who were contacted by phone and invited to participate in the study, 684 (35.8%) refused to participate, 651 (37.7%) did not meet eligibility criteria, and 144 (7.9%) were lost to follow-up before the baseline assessment. Of those not meeting eligibility requirements, 161 (24.7%) were excluded because of a perfect medication adherence score on the 7-item Morisky Medication Adherence Scale at the eligibility screening telephone call. The remaining 337 (18.6%) were confirmed to be eligible and were randomized. Table 1 displays sample characteristics at baseline. Of those randomized, 70% were female, 35% had a live-in partner and 37% reported a diagnosis of type 2 diabetes. Of the 99% who reported health insurance, 79% had Medicare/Medicaid or state-subsidized coverage. The sample’s SES status was low: 84% had an annual income <$30,000, 27% worked full time, and the average duration of education was 12.2 years. Mean medication adherence was low in both groups, but almost 20% of participants had an adherence score of six or greater at baseline indicating higher adherence [13]. Intervention group participants reported more moderate-or-greater physical activity per week than controls (162.4 min. vs. 126.3 min., p=0.04), and a greater percentage of the intervention group met national moderate-or-greater physical activity recommendations (38.5% vs. 26.2%, p<0.02). In addition, more intervention group participants than controls reported a history of stroke (11.2% vs. 4.2%, p<0.02).
Fig. 1.
Consort diagram
Participant Attrition
By the 8-month assessment, 61 (18.1%) of the 337 randomized participants had become lost to follow-up resulting in a sample size of 276. Sixty-seven percent (n=41) of these had dropped out by the 4-month visit. The dropout rate was higher in the intervention group than in the control group (21.9% vs. 14.3%, p =0.07), and all of these differential dropouts occurred by the 4-month assessment. We compared dropouts to completers on the characteristics displayed in Table 1 and found that participants who dropped out by the 8-month assessment were more likely to be male (44.3% vs. 26.4% of completers, p=0.006). Those who met recommendations for physical activity at baseline dropped out at the same rate across groups, but those who did not meet the recommendation dropped out at twice the rate from the intervention group as from the control group (25.0% vs. 12.1%, p=0.01). Lastly, we examined if those who dropped out from the intervention group were different from control group dropouts on other factors. The only significant difference was the control group dropouts were more likely to be diabetic than intervention group dropouts (50% vs. 22.2%, p=0.03).
Use of the Automated Telephone System
Overall use of the automated telephone system was modest: 26 (15.4%) did not call the system at all, and 8 (4.5%) connected with it but did not complete a call. The mean number of completed automated telephone calls was 9.0 of the 32 planned calls, and for those who completed at least one call the mean number of completed calls was 11.4. Eight percent of the intervention group completed at least 80% of the 32 planned calls. Participants with diabetes completed 24% more calls than non-diabetic participants (10.8 vs. 8.2, p =0.08). Use of the three modules was nearly identical. Among intervention group members, 75 (44.4%) completed at least one diet module call. For medication adherence and physical activity the numbers were 75 (44.4%) and 77 (45.6%), respectively. Participants completed a mean of 5.03 of 9 diet calls (56%), 5.85 of 12 physical activity calls (49%), and 5.05 of 8 medication adherence calls (63%). Their choice of which module to do first was equally distributed across modules.
We examined whether any of the following 11 patient characteristics predicted patterns of system use: age, gender, income, perceived financial well-being, education, health literacy, number of medications, presence of diabetes or stroke, total number of comorbidities, employment status, presence of a live-in partner, and number of household occupants. We found that none of these variables significantly predicted whether or not an individual used the system. Among intervention group participants who completed at least one automated telephone call, age (r=0.22, p=0.009) and greater perceived financial well-being (r=0.17, p=0.05) correlated positively with the number of calls made. In a multiple linear regression model containing all 11 patient characteristics, only perceived financial well-being (p=0.02) was an independent predictor of number of completed automated telephone calls.
Multivariable Analysis of Outcome Measures
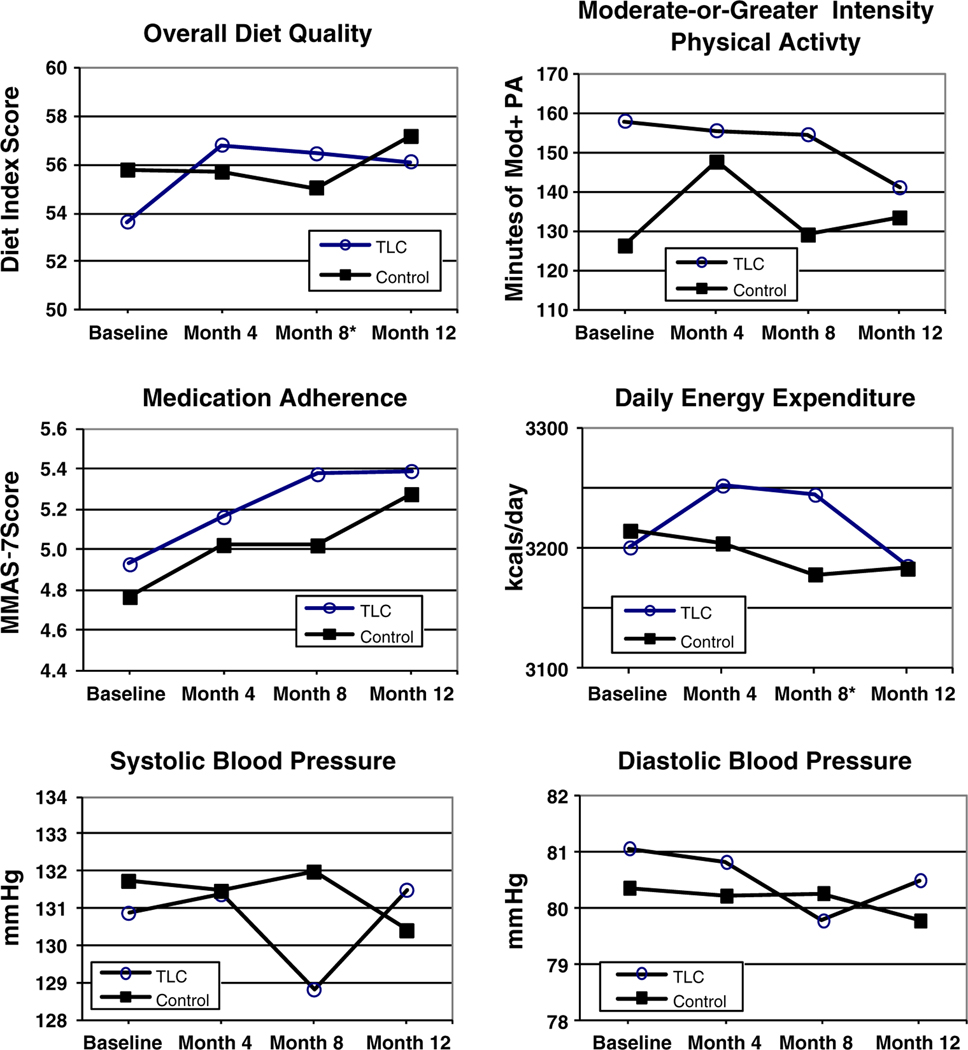
Table 2 presents the change in adjusted means and proportions from baseline to the 8-month (end of intervention) assessment for the primary and secondary outcome variables by study group. No significant intervention effects were observed at the 4- and 12-month assessments. Adjusted group means for continuous outcome variables across all four assessment time points are graphically presented in Fig. 2.
Table 2.
Change in the primary and secondary outcomes from baseline to the end of the intervention period (month 8) within and between groups
| Variables | Within-group change scores from baseline to the end of intervention | Difference in change scores between groups | |
|---|---|---|---|
|
|
|||
| Automated telephone intervention (n =169) | Control (n=168) | ||
|
| |||
| Primary outcomesd | |||
| Overall diet quality score | 2.8 | −0.74 | 3.54* |
| MOD+PAa (min/week) | −3.44 | 2.77 | −6.21 |
| >150 min/week MOD+PA (%) | −2 | 5 | 7 |
| Total energy expenditure (kcal/day) | 43.8 | −36.2 | 80.0* |
| Medication Adherence Scoreb | 0.45 | 0.26 | 0.19 |
| Secondary outcomesd | |||
| Systolic blood pressure (mmHg) | −2.06 | 0.25 | −2.31 |
| Achieved controlc (%) | 0.7 | 1.9 | −1.2 |
| Diastolic blood pressure (mmHg) | −1.28 | −0.1 | −1.18 |
| Achieved controlc (%) | 6.4 | 5.1 | 1.3 |
| Achieved control of both systolic and diastolic blood pressure (%) | 5.8 | 5.0 | 0.8 |
p<0.05
Mod+PA—moderate-or-greater intensity leisure time physical activity
From the 7-item version of the 8-item Morisky Medication Adherence Scale [13]
Systolic blood pressure control defined as <140 mmHg (<130 mmHg if diabetic). Diastolic blood pressure control defined as <90 mmHg (<80 mmHg if diabetic)
Means and proportions are adjusted for covariates used in the specific regression model (see text)
Fig. 2.
Primary and secondary outcomes across all time points. Asterisks difference between groups in change in outcome from baseline to 8 months significant at p <0.05. TLC telephone-linked-care, the name of the automated telephone intervention
Dietary Outcomes
At the 8-month assessment, there was a significant improvement of 3.5 points (or 0.25 SD, p<0.03) in the overall diet quality score in the intervention group vs. controls. Among the five diet component subgroups, fiber intake improved significantly in the intervention group vs. controls (relative improvement of 2.29 g/day, p<0.02). Favorable changes were also observed for other diet components, but none of these reached statistical significance.
Physical Activity
Actigraph readings from the subsample at baseline and at 8 months correlated with self-reported leisure time moderate-or-greater physical activity (r=0.33, p<0.05), thus providing objective validation of the 7-Day Physical Activity Recall questionnaire data. There was no significant difference between groups in leisure time moderate-or-greater physical activity or in the percentage meeting the recommended 150 min/week, but there was a significant increase in energy expenditure of 80 kcal/day in the intervention group vs. controls at the 8-month assessment (p=0.02). This finding was primarily due to a relative increase in work-related activity in the intervention group.
Medication Adherence
Although the treatment group’s adjusted 7-item Morisky Medication Adherence Scale scores improved by 0.19 points relative to controls, this change was not statistically significant (p=0.25) in the intent-to-treat analysis. When this analysis was done on available cases, the relative change was larger, at 0.35 points (p=0.08).
Blood Pressure
When analyzed as continuous variables, the largest decrease in both SBP and DBP in the intervention group vs. the control group occurred at the 8-month assessment (−2.3 mmHg, and − 1.2 mmHg, respectively); however, neither difference was statistically significant. Similarly, there was no statistical difference between groups in the proportion who achieved control of SBP, DBP, or both.
Diabetics
Though there were no pre-specified hypotheses for diabetic participants, we examined intervention effects in this subgroup both because the automated telephone intervention contained material tailored specifically for diabetics with hypertension, and because BP control is especially important in this population. In the intervention group, participants who reported type 2 diabetes used the automated telephone system more and had a lower attrition rate than those without diabetes. Among all diabetics, the intervention was associated with greater absolute improvements in most primary and secondary outcomes relative to controls than for non-diabetics, but the differences were not statistically significant given the small subgroup size (n=127). For example, in the full sample there was no appreciable effect on minutes of moderate-or-greater physical activity, but among diabetics there was an increase of 37 min/week in the intervention group vs. controls at 8 months (p<0.18). Similarly, among diabetics mean SBP decreased by 6.21 mmHg and DBP 1.51 mmHg in the intervention group vs. controls (p=0.1 and p=0.43, respectively).
Dose Response
There was a dose–response relationship between the number of completed diet calls and improvement in the overall diet quality score (r=0.31, p=0.008). There were no significant dose–response relationships for the other outcomes.
Acceptability of the Automated Telephone System
At the 8-month assessment, participants who completed at least one intervention call were surveyed on their experience with the automated system [26]. Responses to key summary questions were on a scale from 1 to 5 with 5 being the most favorable rating. The mean score for the question “How satisfied were you with the automated telephone system?” was 3.7. For “How helpful did you find the system?” it was 4.1, and for “How helpful did you find the information the system provided?” it was 4.2. The most frequent reasons reported for missing or skipping automated calls were participant related such as not being home when the system called (77%), being too busy (56%), or having major life disruptions (36%). By contrast, system-related reasons for missed calls were reported less often. Examples include “The system wasn’t right for me” (16%), and participants having problems accessing the system (14%). Several baseline participant characteristics correlated with users’ opinions of the automated telephone system. Those with lower educational attainment rated it as more enjoyable, helpful, useful, friendly, personal, and informative (r=0.19–0.29, p=0.002–0.02). After controlling for educational attainment, the system was rated as more flexible by users with lower vs. higher perceived financial well-being (r=0.19, p=0.04).
Discussion
The intervention was associated with statistically significant improvements in the primary outcomes of overall diet quality and energy expenditure, and with modest improvements in BP that were not statistically significant (Fig. 2). For all outcomes, positive effects did not persist 4 months after the intervention had ended. There was a dose–response effect on diet quality in the intervention group. Diabetic subjects in the intervention group had a significantly lower dropout rate than non-diabetics. They also tended towards greater use of the intervention and greater improvements in most outcomes than non-diabetics though these differences were not statistically significant.
In prior studies from our group, single automated telephone interventions targeting physical activity, diet, or medication adherence alone were each found effective [8, 9, 11]. Although the current intervention combined content from these successful single-behavior prototypes, the observed effects were smaller overall. This difference may relate to differences in the study sample (e.g., proactive recruitment in this study versus volunteer-based in our prior studies) and study protocols (greater participant burden and attrition due to the need to assess multiple behaviors vs. a single behavior). It may also reflect fundamental differences in adherence caused by the effort and complexity of making more than one lifestyle change [27]. Nevertheless, a recent meta-analysis found that computer-tailored interventions targeting up to three health behaviors can be as effective as those targeting only one [28].
With respect to multi-behavior interventions, a further consideration is the relative impact of delivering each module sequentially vs. simultaneously. The scant literature on this issue is mixed. In one study comparing simultaneous and sequential interventions among 289 blacks with hypertension, there was evidence that the simultaneous approaches were superior for sodium reduction and smoking cessation [29]. In contrast, findings from another trial among 315 female smokers supported a sequential over a simultaneous approach to multiple behavior changes including diet, exercise, and smoking cessation [30]. In our study we delivered each module sequentially, but required all participants to check and report their home blood pressure readings throughout follow-up. A subsample also chose to measure and report their weight regularly. Such longitudinal self-monitoring constituted a “simultaneous” intervention that spanned the three behavioral modules and may have helped participants form connections between the content of each module.
In this study, maximal intervention effects were seen at 8 months for several outcomes despite the fact that each behavioral module lasted only 2–3 months. This suggests that subsequent modules may have reinforced positive changes initiated during earlier modules, even though each module targeted a different behavior. This phenomenon is akin to the “co-variation” of effects described in other multi-behavior interventions where improvements in a targeted health behavior are associated with improvements in other targeted or even non-targeted behaviors [31]. Indeed, the first three automated calls were designed not only to introduce the three behavioral modules, but also to emphasize the complementary role of each behavior in blood pressure control. Making these explicit connections between the three behavioral modules early may have enabled mutual reinforcement of behavioral messages later on. More research is needed to elucidate how messages targeting one behavior can be used to reinforce messages targeting other behaviors in multi-behavior change interventions whether modules are delivered sequentially or simultaneously.
What is clear from the literature and from our results is that positive behavioral changes decay toward baseline levels once the intervention is withdrawn [32]. A more intensive intervention or booster sessions following an intervention can slow this decay [32]. An advantage of automated counseling systems such as the one we report here is that they can be easily modified to deliver a more intensive intervention (i.e., to increase content and contact frequency), and to help maintain positive behavior change by providing booster contacts after the primary intervention ends. In retrospect, providing intermittent reinforcement of positive behavior changes throughout the intervention may have maintained or increased earlier improvements and been more effective.
The low overall rates of system use in this study likely limited intervention effects. Factors such as lower income and educational attainment and the presence of social–environmental obstacles have been shown to predict lower subject participation in research studies [5, 6]. There was little socio-demographic variation in our study sample and no baseline characteristic predicted system use vs. non-use, but participants who reported greater perceived financial hardship used the system less often than those who felt more financially secure. This is despite the fact that those with greater perceived financial hardship actually rated the system as more flexible than subjects who felt more financially secure. This suggests that those who experience greater financial stress and its attendant life disruptions may particularly appreciate the flexibility and accessibility of computerized health promotion systems such as ours. Even though their life circumstances may make it difficult for them to fully utilize these systems, it is conceivable that their engagement in more rigid, traditional, face-to-face programs would be even lower. Similarly, we found that participants with lower educational attainment rated the system more favorably than more educated users in the areas of information content, applicability, and in their overall experience. Efforts were made to develop an intervention that was acceptable to a wide range of literacy levels. The system’s ability to identify and address the user’s knowledge gaps and to allow users to repeat conversations within modules may have enhanced its appeal among participants with less formal education. Further qualitative work is needed to identify barriers to engagement in automated behavior change programs.
Several additional issues may have reduced observed intervention effects. First, baseline values for several outcomes were unexpectedly high, which made it more difficult to achieve substantial improvements in the intervention group on these outcomes. Despite selection criteria designed to create a sample with uncontrolled BP and low adherence to self-care recommendations, mean baseline BP was only 131/81; 36% and 37% met recommendations for fruit and vegetable intake, respectively; and 32% met recommended guidelines for physical activity. In fact, 47% more participants in the intervention group met physical activity guidelines than in the control group (38.5% vs. 26.3%). This chance occurrence meant that a greater proportion of intervention group participants were advised to maintain rather than increase their levels of physical activity, and that downward regression to the mean for physical activity was more likely in this group. Additionally, since participants with low baseline physical activity were twice as likely to drop out of the intervention group than were control participants, the potential impact of upward regression to the mean was decreased in intent to treat analysis, also reducing physical-activity-related intervention effects.
The limited impact of the intervention on our three behavioral outcomes made it especially difficult to see an intervention effect on BP—a secondary outcome of this study—especially in the context of limited power. In our sample, there was less than 80% power to detect a difference in SBP of 7 mmHg between the experimental groups. The relationship between behavior change and resulting physiologic change is complex and depends on both the magnitude and duration of the observed behavioral changes. Further, BP reductions achieved by multi-behavior interventions are typically smaller than the sum of the reductions observed for their components when deployed alone. For example, the Trials of Hypertension Prevention Phase II study [27] showed an intervention combining weight loss and sodium restriction was associated with a smaller reduction in diastolic BP vs. controls at 6 months (−2.8 mmHg, p<0.001) than the sum of the two component interventions when deployed singly (−2.7 mmHg for weight loss alone, p<0.001, −1.6 for sodium alone, p<0.001). In the PREMIER trial [4], adding the original DASH diet to an “established” regimen consisting of weight loss, limited sodium and alcohol intake, and increased physical activity did not significantly reduce systolic BP further (−0.6 mmHg vs. “established”, p=0.43).
Conclusion
This study is the first to evaluate a culturally adapted, computer-based telephone counseling system for African–Americans that targets three health-related behaviors for the self-management of hypertension. It was associated with modest improvements in several study outcomes, but effects were hampered by high baseline attainment of goal levels for several principal study outcomes and by low system use. Nevertheless, computerized technologies like this one provide an opportunity to deliver behavior change programs on a public health scale at relatively low cost, and to tailor health messages to both ethnic/racial group and individual-level characteristics. Although our automated system was developed using input from African–Americans in Boston, it was designed to address obstacles that are likely to pose barriers to a broader segment of urban African–American of low socioeconomic status as well. As such, our results can be generalized to other urban settings in the USA. In addition, the content of the intervention can be further culturally adapted to reflect the cultural themes of specific African–American communities. More research is needed to better understand what combination of system features and user characteristics may lead to improved hypertension self-management among urban African–Americans of low SES.
Acknowledgments
This study was funded by a grant from the National Heart, Lung, and Blood Institute (R01 HL69395). We would like to thank Kenneth Resnicow, PhD for his assistance in conceptualizing and designing this study; Lisa Marks, Adena Cohen-Barak, and Julia Gefter for their dedication to seeing this project through; and Amy Bachand, PhD for her assistance with the accelerometer data analysis.
Study registered at ClinicalTrials.gov with ID: NCT00207194
Footnotes
Conflict of interest Dr. Friedman has stock ownership and a consulting agreement with InfoMedics, the company that owns commercial rights to the TLC technology used in the computerized intervention. He is also a member of its Board of Directors. None of the other authors has any potential conflicts of interest to disclose.
Contributor Information
Jeffrey P. Migneault, Medical Information Systems Unit, General Internal Medicine, Boston University School of Medicine, Boston, MA, USA
Julien J. Dedier, Medical Information Systems Unit, General Internal Medicine, Boston University School of Medicine, Boston, MA, USA
Julie A. Wright, Department of Exercise and Health Science, University of Massachusetts, Boston, MA, USA
Timothy Heeren, Medical Information Systems Unit, General Internal Medicine, Boston University School of Medicine, Boston, MA, USA
Marci Kramish Campbell, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA
Donald E. Morisky, Department of Community Health Sciences, UCLA School of Public Health, Los Angeles, CA, USA
Peter Rudd, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA
Robert H. Friedman, Medical Information Systems Unit, General Internal Medicine, Boston University School of Medicine, Boston, MA, USA
References
- 1.Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control—Continued disparities in adults: United States, 2005–2006. NCHS Data Brief No. 3. Hyattsville, MD: National Center for Health Statistics; 2008. [PubMed] [Google Scholar]
- 2.Appel L, Moore T, Obarzanek E, Vollmer W, Svetkey L, Sacks F, et al. A clinical trial of the effects of dietary patterns on blood pressure. New England Journal of Medicine 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Archives of Internal Medicine. 1997;157:657–67. [PubMed] [Google Scholar]
- 4.Svetkey L, Harsha D, Vollmer W, Stevens V, Obarzanek E, Elmer P et al. Premier: A clinical trial of comprehensive lifestyle, Ann Epidemiol 2003;13:462–71. [DOI] [PubMed] [Google Scholar]
- 5.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist 2003;43:18–26. [DOI] [PubMed] [Google Scholar]
- 6.French S, Jeffery R, Story M, Neumark-Sztainer D. Perceived barriers to and incentives for participation in a weight-loss program among low-income women in WIC. J Am Diet Assoc. Jan 1998;98:79–81. [DOI] [PubMed] [Google Scholar]
- 7.Noar S, Benac C, Harris M. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol. Bull 2007;133:673–93. [DOI] [PubMed] [Google Scholar]
- 8.Friedman R, Kazis L, Jette A, Smith M, Stollerman J, Torgerson J, Carey K. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens. 1996;9:285–92. [DOI] [PubMed] [Google Scholar]
- 9.Pinto B, Friedman R, Marcus B, Kelley H, Tennstedt S, Gillman M. Effects of a computer-based, telephone-counseling system on physical activity. American Journal of Preventive Medicine 2002;23:113–20. [DOI] [PubMed] [Google Scholar]
- 10.King A, Friedman R, Marcus B, Castro C, Napolitano M, Ahn D, Baker L. Ongoing physical activity advice by humans versus computers: The community health advice by telephone (CHAT) trial. Health Psychology. 2007; 26:718–727. [DOI] [PubMed] [Google Scholar]
- 11.Delichatsios H, Friedman R, Glanz K, Tennstedt S, Smigelski C, Pinto B, et al. Randomized trial of a “talking computer” to improve adults’ eating habits. Am J Health Promot 2001;15:215–24. [DOI] [PubMed] [Google Scholar]
- 12.Pate R, Pratt M, Blair S, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273:402–7. [DOI] [PubMed] [Google Scholar]
- 13.Morisky D, Alfonso A, Krousel-Wood M, Ward H. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice Hall, 1977 [Google Scholar]
- 15.Prochaska J, DiClemente C. Stage and processes of self-change in smoking: Towards an integrative model of change J Consult Clin Psych 1983;51:390–5. [DOI] [PubMed] [Google Scholar]
- 16.Miller W, Rollnick S, editors. Motivational interviewing: Preparing people for change. New York: The Guilford Press; 2002. p. 284–98. [Google Scholar]
- 17.Resnicow K, Baranowski T, Ahluwalia J, Braithwaite R. Cultural sensitivity in public health: Defined and demystified. Ethnicity and Disease 1999;9:10–21. [PubMed] [Google Scholar]
- 18.Nobles W. African philosophy: Foundations for black psychology. In: Jones R, editor. Black Psychology, 2nd Ed. New York: Harper & Row; 1980. p. 23–36. [Google Scholar]
- 19.Blair SN, Haskell W, Ho P, Paffenbarger R Jr., Vranizan K, Farquhar J, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804. [DOI] [PubMed] [Google Scholar]
- 20.Sarkin J, Campbell L, Gross L, et al. Project GRAD seven-day physical activity recall interviewer’s manual. Med Sci Sports Exerc 1997;29:S91–S102. [Google Scholar]
- 21.Yaroch A, Resnicow K, Davis M, Davis A, Smith M, Khan L. Development of a modified picture-sort food frequency questionnaire administered to low-income, overweight, African–American adolescent girls. J Am Diet Assoc 2000;100:1050–6. [DOI] [PubMed] [Google Scholar]
- 22.Krauss R, Eckel R, Howard B, et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102;2284–99 [DOI] [PubMed] [Google Scholar]
- 23.The Trials of Hypertension Prevention Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. JAMA. 1992;267:1213–20. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 Report. JAMA 2003;289:2560–71. [DOI] [PubMed] [Google Scholar]
- 25.Lachin JM. Statistical considerations in the intent-to-treat principle. Controlled Clinical Trials 2000; 21:167–189. [DOI] [PubMed] [Google Scholar]
- 26.Stevens A, Migneault J, Fava J, Friedman R, Farzanfar R. Health technology questionnaire: Psychometric properties of a health behavior change measure. Annals of Behavioral Medicine. Annual meeting supplement, 2006; 31:S126. [Google Scholar]
- 27.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high normal blood pressure: The trials of hypertension prevention, phase II. Arch Intern Med. 1997;157:657–67. [PubMed] [Google Scholar]
- 28.Krebs P, Prochaska J, Rossi J. A meta-analysis of computer-tailored interventions for health behavior change. Preventive Medicine 51;2010:214–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyman D, Pavlik V, Taylor W, Goodrick G, Moye L. Simultaneous vs. sequential counseling for multiple behavior change. Arch Intern Med. 2007;167:1152–8. [DOI] [PubMed] [Google Scholar]
- 30.Spring B, Pagoto S, Pingitore R, Doran N, Schneider K, Hedeker D. Randomized controlled trial for behavioral smoking and weight control treatment: Effect of concurrent versus sequential intervention. J Consult Clin Psychol. 2004;72:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prochaska J. Multiple health behavior research represents the future of preventive medicine. Preventive Medicine. 2008;46:281–5 [DOI] [PubMed] [Google Scholar]
- 32.Artinian N, Fletcher G, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:406–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangha O, Stucki G, Liang M, Fossel A, Katz J. The self-administered co-morbidity questionnaire: A new method to assess co-morbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–63. [DOI] [PubMed] [Google Scholar]
- 34.Davis T, Long S, Jackson R, et al. Rapid estimate of adult literacy in medicine: A shortened screening instrument. Fam Med 1993;25:391–5. [PubMed] [Google Scholar]