Abstract
The penetration of two antibiotics, ampicillin and ciprofloxacin, through biofilms developed in an in vitro model system was investigated. The susceptibilities of biofilms and corresponding freely suspended bacteria to killing by the antibiotics were also measured. Biofilms of Klebsiella pneumoniae were developed on microporous membranes resting on agar nutrient medium. The susceptibilities of planktonic cultures and biofilms to 10 times the MIC were determined. Antibiotic penetration through biofilms was measured by assaying the concentration of antibiotic that diffused through the biofilm to an overlying filter disk. Parallel experiments were performed with a mutant K. pneumoniae strain in which β-lactamase activity was eliminated. For wild-type K. pneumoniae grown in suspension culture, ampicillin and ciprofloxacin MICs were 500 and 0.18 μg/ml, respectively. The log reductions in the number of CFU of planktonic wild-type bacteria after 4 h of treatment at 10 times the MIC were 4.43 ± 0.33 and 4.14 ± 0.33 for ampicillin and ciprofloxacin, respectively. Biofilms of the same strain were much less susceptible, yielding log reductions in the number of CFU of −0.06 ± 0.06 and 1.02 ± 0.04 for ampicillin and ciprofloxacin, respectively, for the same treatment. The number of CFU in the biofilms after 24 h of antibiotic exposure was not statistically different from the number after 4 h of treatment. Ampicillin did not penetrate wild-type K. pneumoniae biofilms, whereas ciprofloxacin and a nonreactive tracer (chloride ion) penetrated the biofilms quickly. The concentration of ciprofloxacin reached the MIC throughout the biofilm within 20 min. Ampicillin penetrated biofilms formed by a β-lactamase-deficient mutant. However, the biofilms formed by this mutant were resistant to ampicillin treatment, exhibiting a 0.18 ± 0.07 log reduction in the number of CFU after 4 h of exposure and a 1.64 ± 0.33 log reduction in the number of CFU after 24 h of exposure. Poor penetration contributed to wild-type biofilm resistance to ampicillin but not to ciprofloxacin. The increased resistance of the wild-type strain to ciprofloxacin and the mutant strain to ampicillin and ciprofloxacin could not be accounted for by antibiotic inactivation or slow diffusion since these antibiotics fully penetrated the biofilms. These results suggest that some other resistance mechanism is involved for both agents.
Bacterial biofilms are frequently observed on the surfaces of tissues (12, 30, 31) and biomaterials (5, 10, 39, 42, 47) at the site of persistent infections (8). Medical implants are particularly susceptible to biofilm formation because immune responses are markedly reduced in the proximity of foreign bodies (7, 42). In fact, biofilm formation is a major cause of implant failure and often limits the lifetimes of many indwelling medical devices (24). Once in the biofilm, extracellular polymeric substances shield bacteria from opsonization and phagocytosis (8, 23). In addition, in vitro experiments have demonstrated that the bacteria in biofilms are considerably less susceptible to antibiotics than their planktonic counterparts (3, 10, 14, 19, 28, 41). Treatment of an infection after the biofilm is established is frequently futile with current remedies (8). Often, the only solution is mechanical removal of the biofilm or implant, which is costly and traumatic to the patient (8).
Two principal hypotheses have been formulated to explain the reduced susceptibility of biofilms to antibiotics. The first hypothesis, which could be termed penetration limitation, suggests that only the surface layers of a biofilm are exposed to a lethal dose of the antibiotic due to a reaction-diffusion barrier that limits transport of the antibiotic into the biofilm (17, 19, 25, 26, 28, 34, 35, 38). This mechanism has been demonstrated for reactive oxygen species such as hypochlorite (11, 44) and hydrogen peroxide (26). Synthesis of an antibiotic-degrading enzyme, such as a β-lactamase enzyme (17, 28, 35), or sorption of an antibiotic to biofilm components (25, 35) could also give rise to such a situation. The transport properties of numerous antibiotics, including ciprofloxacin (33, 40, 41), levofloxacin (33), ofloxacin (33, 44, 46), sparfloxacin (33), gentamicin (25, 33, 45), imipenem (33), piperacillin (19, 33), and vancomycin (10, 14), through biofilms have been examined. Some investigators report retarded penetration of antibiotics (19, 25, 33), while others indicate rapid penetration (10, 14, 25, 33, 40, 41). The second hypothesis for reduced biofilm susceptibility, which could be termed physiological limitation, proposes that some microorganisms within the biofilm exist in a more recalcitrant phenotypic state (6, 9, 34). The roles of various physiological factors, such as growth rate (13, 15, 16), biofilm age (2), and starvation (22, 27), have been examined. Gradients in the physiological status of cells within biofilms have been demonstrated recently (20, 21, 37, 43).
The objective of the work presented here was to evaluate the significance of penetration limitation as a mechanism of biofilm resistance to two antibiotics, ampicillin and ciprofloxacin. The antibiotic susceptibilities of membrane-supported Klebsiella pneumoniae biofilms were compared to the susceptibilities of corresponding planktonic cultures. Permeation of active antibiotic through membrane-supported biofilms was tracked by a novel bioassay technique. The role of transport limitation was assessed for the two antibiotics by comparing the susceptibility and permeability data.
MATERIALS AND METHODS
Bacterial strains, media, and antimicrobials.
Pure cultures of K. pneumoniae Kp1 and Kp102M were used in the experiments. Kp1, an environmental isolate, was obtained from the culture collection at the Center for Biofilm Engineering. Kp102M is a β-lactamase-deficient mutant isolated by nitrosoguanidine mutagenesis of Kp1, followed by screening for ampicillin-sensitive mutants. Escherichia coli ATCC 25922 (provided by Barbara Hudson, Montana State University) served as the antibiotic-sensitive microorganism in antibiotic bioassays.
The culture medium for K. pneumoniae contained 6.25 g of anhydrous dextrose, 0.360 g of NH4Cl, 0.100 g of MgSO4 · 7H2O, 5.68 g of Na2HPO4 (anhydrous), 5.44 g of KH2PO4, 250 μl of trace elements (defined below), and 15.0 g of Bacto Agar (Difco Laboratories, Detroit, Mich.) per liter of deionized water. Glucose was dissolved in deionized water and was filter sterilized into the autoclaved medium. The trace element solution contained 1.28 g of nitrilotriacetic acid, 0.00896 g of molybdic acid, 0.9088 g of ZnSO4 · 7H2O, 0.07296 g of MnSO4 · H2O, 0.01792 g of CuSO4 · 5H2O, 0.00896 g of Na2B4O7 · 10H2O, 0.0145 g of Co(NO3)2 · 6H2O, and 1.018 g of FeSO4 · 7H2O per liter of deionized water. The culture broth was identical to the culture medium but lacked the Bacto Agar. The chloride-free medium was analogous to the culture medium but contained 0.444 g of NH4SO4 per liter in place of the ammonium chloride. Phosphate-buffered water (PBW) was prepared as described elsewhere (1) and was used for culture dilutions. E. coli was grown in Luria-Bertani (LB) broth (Difco Laboratories). Ampicillin sodium salt was purchased from Sigma Chemical Company (St. Louis, Mo.). The Bayer Corporation (Leverkusen, Germany) donated ciprofloxacin hydrochloride powder. Powdered antibiotics were dissolved in sterile nanopure water, and the antibiotic solutions were added to molten culture medium (∼50°C) to create antibiotic-amended agar for biofilm experiments or were added to culture broth for planktonic experiments.
Biofilm preparation.
Overnight planktonic cultures of K. pneumoniae were diluted to an optical density (600 nm, 1-cm path length) of 0.200 in PBW. One 5-μl drop (for susceptibility experiments) or five 10-μl drops (for permeability and antibiotic degradation studies) of diluted planktonic culture were used to inoculate individual sterile, black, polycarbonate membrane filters (diameter, 25 mm; pore size, 0.2 μm; Poretics Corp., Livermore, Calif.) resting on agar culture medium. The membranes were sterilized by UV exposure (15 min per side) prior to inoculation. The plates were inverted and incubated at 35°C for 48 h, with the membrane-supported biofilms transferred to fresh culture medium every 8 to 10 h.
Planktonic susceptibility.
Overnight planktonic cultures of K. pneumoniae were diluted to an optical density (600 nm) of 0.200 with PBW. One milliliter of diluted culture was used to inoculate 100 ml of culture broth for a final population of ca. 106 CFU per ml. After removing the time-zero sample, 0.500 g of ampicillin or 180 μg of ciprofloxacin, dissolved in sterile nanopure water, was added to the subculture to attain antibiotic concentrations of 5,000 and 1.8 μg/ml, respectively. The culture was placed on a 35°C orbital shaker and was sampled every 30 min for 4 h. The sampling procedure was as follows. A 1.5-ml sample of the culture was pipetted into a 2.0-ml conical microcentrifuge tube (Fisher Scientific, San Francisco, Calif.). The bacteria were pelleted at 10,000 rpm for 7.5 min at room temperature with a Micro14 microcentrifuge (Fisher Scientific). The supernatant was removed with a pipette. The bacteria were washed with 1.5 ml of PBW and were repelleted as described above, and the supernatant was removed and discarded. The bacteria were suspended in 1.5 ml of PBW and were then serially diluted in PBW. Viable bacteria were enumerated as described below.
The MICs of ampicillin and ciprofloxacin for Kp1 and Kp102M were estimated as follows. Aliquots of culture broth containing 5 × 105 CFU/ml were treated with 50 to 700 μg of ampicillin per ml or 0.02 to 0.35 μg of ciprofloxacin per ml and were incubated for 20 h at 35°C. The optical density was measured at 600 nm with a Spectronic Genesys spectrophotometer (Spectronic Instruments, Inc., Rochester, N.Y.), and viable bacteria were quantified as described below. The MIC was taken as the minimum antibiotic concentration at which no increase in the number of viable bacteria after treatment was observed.
Biofilm susceptibility.
The biofilms were transferred to antibiotic-containing agar, and the agar plates were incubated at 35°C. The biofilms were sampled every 30 min for 4 h and after 24 h. When sampled, each membrane-supported biofilm was placed in 9.0 ml of PBW, and the mixture was vortexed at high speed for 2.0 min with a Maxi Mix II Vortex mixer (Barnstead/Thermolyne, Dubuque, Iowa) and then serially diluted in PBW. The viable bacteria were enumerated as described below.
Viable bacteria enumeration and comparison.
Serially diluted samples were plated onto R2A agar (Difco Laboratories) by the drop plating method (18, 32), and the plates were incubated at 35°C for 18 to 20 h. The change in the number of CFU was normalized for each experiment by converting the measurements of the number of viable bacteria to the log reduction of the number of CFU. The log reduction of the number of CFU, or simply log reduction, at a particular sampling time was defined as the negative log10 of the quotient of the number of CFU at that time and the number of CFU prior to treatment. A positive log reduction represents a decrease in the number of CFU. The log reduction values at each time point for identical experiments were averaged, and the standard error of the mean was calculated. The mean log reductions for the various experiments were compared by a two-tailed, two-sample t test with the assumption of unequal variances.
Antibiotic penetration.
Black polycarbonate membrane filters (diameter, 13 mm; pore size, 0.2 μm; Poretics Corp.) were placed on top of 48-h-old K. pneumoniae biofilms. A concentration disk (catalog no. 1599-33-6; Difco Laboratories) was moistened with 24 μl of culture broth prior to placement on top of the 13-mm-diameter membranes. Wetting of the disk helped prevent capillary action of the antibiotic medium through the biofilm. The biofilm sandwiched between membranes and the moistened disk was transferred to antibiotic-containing agar culture medium (Fig. 1). The disk was removed after specified exposure times, sealed in parafilm (American National Can, Chicago, Ill.), and stored at 4°C. After collection of all samples, the disks were placed on Mueller-Hinton (Difco Laboratories) plates spread with 100 μl of planktonic E. coli. The E. coli strain was grown in LB broth at 35°C, and the growth was diluted with LB broth to an optical density of 0.050 prior to plating. The plates were incubated at 35°C for 18 to 20 h. The zone of inhibited growth was measured and was used to determine the concentration of active antibiotic in the disk. The effective bulk agar antibiotic concentration was estimated by averaging the equilibrated concentration observed in disks placed on the two-membrane system, i.e., the unit without the bacterial biofilm. The concentration that penetrated the biofilms was divided by the bulk agar concentration to provide a normalized penetration curve.
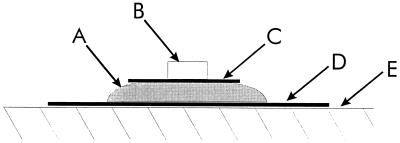
FIG. 1.
Schematic depiction of the experimental system used to track the penetration of antibiotics through the biofilms. The biofilm (A) was developed on a 25-mm-diameter microporous polycarbonate membrane (D) resting on agar culture medium. A 13-mm-diameter microporous polycarbonate membrane (C) was placed on top of the biofilm. A moistened concentration disk (B) was placed on top of the 13-mm-diameter membrane. The entire unit, components A through D, was transferred to antibiotic-containing agar (E) with sterile forceps.
Chloride penetration.
Black polycarbonate membrane filters (diameter, 13 mm; pore size, 0.2 μm; Poretics Corp.) were placed on top of 48-h-old K. pneumoniae biofilms grown on chloride-free culture medium. Concentration disks were moistened with 24 μl of chloride-free culture broth and were placed on top of the 13-mm-diameter membranes. The entire units were transferred to chloride-containing agar culture medium. The disks were removed after specified exposure times and were placed in 1.0 ml of sterile nanopure water. The resulting chloride concentration in the water was measured with a DX-500 ion chromatograph (Dionex, Sunnyvale, Calif.). The bulk agar chloride concentration was estimated by averaging the equilibrated concentration observed in the disks placed on the two-membrane system exposed to chloride-containing agar. The concentration that penetrated the biofilms was divided by the bulk agar concentration to provide a normalized penetration curve.
Antibiotic degradation.
Antibiotic degradation by the bacteria within the biofilms was examined qualitatively. Forty-eight-hour-old Kp1 and Kp102M biofilms were placed on culture medium plates containing 500 μg of ampicillin per ml or 0.18 μg of ciprofloxacin per ml, and the plates were incubated at 35°C. After 24 h, the biofilms were removed and the plates were spread with 100 μl of a planktonic Kp1 culture (∼108 CFU/ml). The spread plates were incubated at 35°C for 48 h and were then analyzed for zones of growth.
The rates of antibiotic degradation for Kp1 and Kp102M were quantified in planktonic cultures. Antibiotics were added to 100 ml of turbid overnight planktonic cultures (∼109 CFU/ml) for a final antibiotic concentration of 1,000 μg of ampicillin per ml or 1.8 μg of ciprofloxacin per ml. A 1.5-ml sample from each culture was taken every 30 min for 4 h and was placed in a 2.0-ml microcentrifuge tube. The bacteria were pelleted at 7,000 × g for 7.5 min at room temperature. A concentration disk was inoculated with 24 μl of the supernatant. The antibiotic concentration was estimated by the zone-of-inhibition bioassay described above. The natural log of the antibiotic concentration was plotted with respect to time. An apparent first-order rate coefficient of antibiotic degradation was estimated from least-squares linear regression of the data. Analysis of variance was used to determine whether the estimated reaction rates were statistically different from zero.
RESULTS
Antibiotic susceptibility.
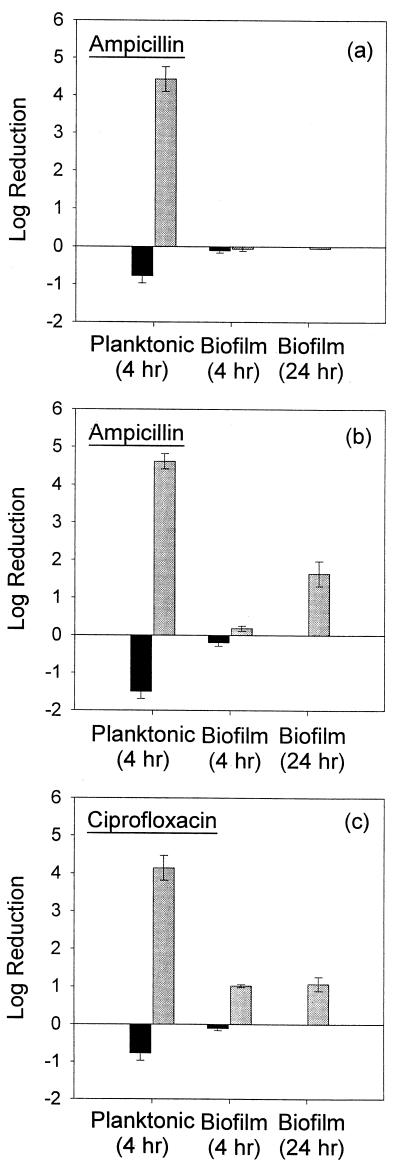
The observed MICs of ampicillin for planktonic Kp1 and Kp102M were 500 and 2 μg/ml, respectively. The MIC of ciprofloxacin was 0.18 μg/ml for both strains. Planktonic cultures and biofilms of both the wild-type and mutant strains were challenged with 10 times the MIC of each antibiotic for the wild-type strain: 5,000 μg/ml for ampicillin and 1.8 μg/ml for ciprofloxacin. Planktonic bacteria were rapidly killed by these treatments. The log reductions in the number of CFU were 4.43 ± 0.33 and 4.14 ± 0.33 after 4 h of ampicillin and ciprofloxacin treatment, respectively, for planktonic wild-type bacteria (Fig. 2a and c). Planktonic cultures of the β-lactamase-deficient mutant experienced a 4.62 ± 0.20 log reduction after exposure to 5,000 μg of ampicillin per ml for 4 h (Fig. 2b). However, the number of CFU in biofilms challenged with ampicillin remained virtually unchanged after 4 h of treatment. Kp1 (Fig. 2a) and Kp102M (Fig. 2b) biofilms exhibited −0.06 ± 0.06 and 0.18 ± 0.07 log reductions, respectively. Both log reductions were statistically different from those for the analogous planktonic treatments (P = 0.001 and P = 0.030, respectively). The log reduction observed for Kp1 biofilms was not statistically different from that for the untreated control (P = 0.63). Challenge of Kp1 biofilms with ciprofloxacin for 4 h resulted in a 1.02 ± 0.04 log reduction in the number of CFU, a result statistically different (P = 0.011) from that for the treated planktonic culture (Fig. 2c). The response of Kp102M biofilms treated with ciprofloxacin for 4 h was not statistically different from that of the wild type (P = 0.37) (data not shown).
FIG. 2.
Susceptibilities of planktonic cultures and biofilms to antibiotics as the observed log reduction in the number of CFU after exposure to 5,000 μg of ampicillin per ml (a and b) or 1.8 μg of ciprofloxacin per ml (c). (a and c) Susceptibility of Kp1 to ampicillin and ciprofloxacin, respectively. (b) Ampicillin treatment of Kp102M. █, untreated controls; , antibiotic-challenged cultures. Error bars reflect ±1 standard error of the mean.
Biofilms of the mutant strain retained their relative resistance to ampicillin compared to that of a planktonic culture after 24 h of treatment. Kp1 biofilms were unaffected by a 24-h ampicillin treatment (log reduction, −0.05; Fig. 2a). Kp102M biofilms exhibited a 1.64 ± 0.33 log reduction in the number of CFU after 24 h (Fig. 2b), which indicated that they were statistically significantly less susceptible than planktonic cultures treated for only 4 h (P = 0.016). A log reduction of 1.07 ± 0.18 was observed for Kp1 biofilms challenged with ciprofloxacin for 24 h (Fig. 2c). This reduction was statistically different from that for 4-h planktonic treatment (P = 0.004). Kp102M biofilms treated with ciprofloxacin for 24 h responded the same as the wild-type bacteria (data not shown).
Antibiotic penetration.
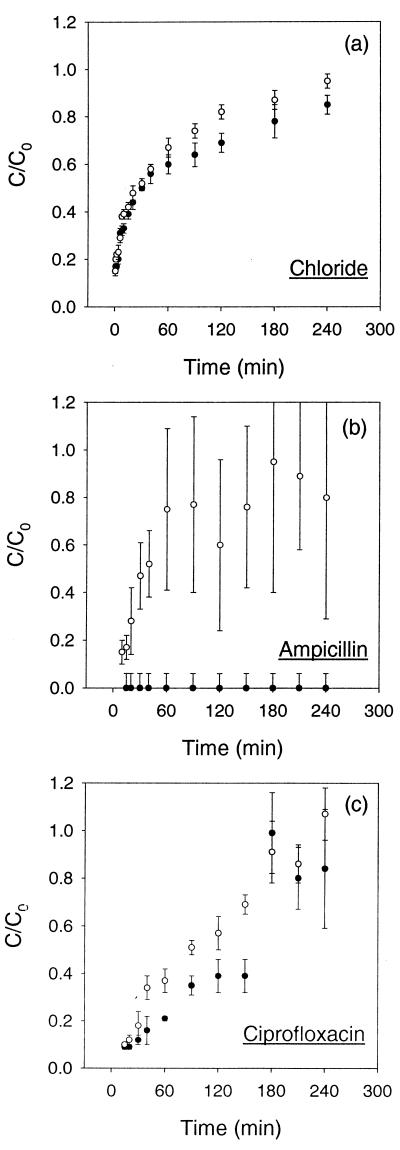
Chloride acted as a nonreactive tracer, allowing visualization and qualitative comparison of solute transport properties within Kp1 and Kp102M biofilms. Chloride quickly penetrated biofilms of either strain with a penetration profile characteristic of one described by Fick's second law (Fig. 3a). In less than 30 min, the chloride concentration at the distal edges of biofilms of either strain was at least 50% of the bulk concentration of 6.7 mM. The transport dynamics of chloride were nearly identical for Kp1 and Kp102M biofilms.
FIG. 3.
Solute transport through the biofilms as the measured fraction of bulk chloride (a), ampicillin (b), and ciprofloxacin (c) that penetrated Kp1 (●) and Kp102M (○) biofilms. Error bars reflect ±1 standard error of the mean. The relatively large error bars in panel b were due to the poor sensitivity of the bioassay above an ampicillin concentration of 1,000 μg/ml. C, concentration on distal edge of biofilm; C0, applied concentration.
The rate of penetration of ampicillin through Kp1 and Kp102M biofilms differed significantly (Fig. 3b). The concentration of ampicillin in the concentration disks was below the detection limit during the 4-h experiment for Kp1 biofilms. On the other hand, ampicillin rapidly penetrated the β-lactamase-deficient Kp102M biofilms. The ampicillin concentration exceeded 500 μg/ml, or 250 times the MIC for the mutant, at the distal edge of the biofilm in less than 10 min. Within 40 min, the ampicillin concentration was approximately 50% of the bulk concentration, i.e., over 1,000 times the MIC for the mutant. The sensitivity of the bioassay used in this study was poor at ampicillin concentrations above 1,000 μg/ml, resulting in the relatively large error bars.
Ciprofloxacin readily penetrated the biofilms of either strain (Fig. 3c). In less than 20 min, the ciprofloxacin concentration at the distal edge of the biofilm exceeded the MIC (1.8 μg/ml). The ciprofloxacin concentration was roughly 50% of the bulk concentration within 120 min. Ciprofloxacin permeation rates were similar for both strains.
Antibiotic degradation.
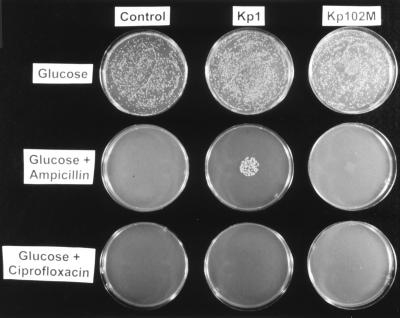
Degradation of the antibiotics by Kp1 and Kp102M biofilms was investigated qualitatively (Fig. 4). The control plates, not exposed to biofilms, demonstrated that antibiotic instability was not sufficient to significantly decrease the antibiotic concentration in the plates and permit growth. The zone of growth directly under the biofilm's former location on an ampicillin plate exposed to a Kp1 biofilm suggested that the wild-type bacteria were capable of neutralizing enough ampicillin to enable growth of a susceptible strain. The lack of such a zone on the ampicillin plate exposed to a Kp102M biofilm suggested that the mutant strain had little capacity to degrade ampicillin. The experiment also indicated that neither strain was capable of appreciably degrading ciprofloxacin.
FIG. 4.
Antibiotic depletion in agar plates. The plates in the left column (Control) were not exposed to biofilms. The plates in the middle and right columns were exposed to wild-type and mutant biofilms, respectively, placed in the center of each plate for 24 h. The plates in the top row were agar culture medium plates without antibiotics. The plates in the middle and bottom rows contained 500 μg of ampicillin per ml and 0.18 μg of ciprofloxacin per ml, respectively. All plates were spread with a lawn of Kp1 bacteria. A spot of growth on the ampicillin-amended plate exposed to a Kp1 biofilm (center plate) indicated local neutralization of the ampicillin.
The rate of antibiotic degradation measured in planktonic cultures was consistent with the observations presented above. The first-order degradation rate of ampicillin by planktonic cultures with 109 CFU/ml was approximately 0.73 h−1, a rate statistically different (P = 0.007) from zero (Fig. 5a). The rate of ampicillin degradation by Kp102M cultures was not statistically different (P = 0.655) from zero (Fig. 5b). Neither Kp1 (P = 0.992) nor Kp102M (P = 0.364) could degrade ciprofloxacin at a rate statistically different from zero (data not shown).
FIG. 5.
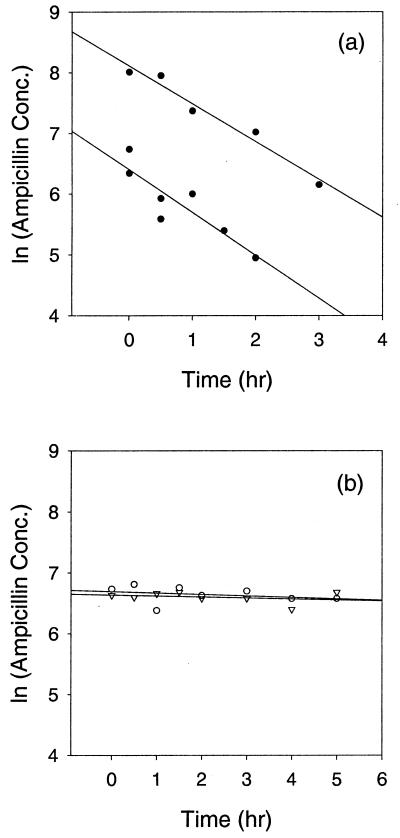
Ampicillin degradation rate in planktonic cultures. Kp1 (●) cultures that contained ∼109 CFU/ml degraded ampicillin at a rate statistically different from zero (a). The top series in panel a illustrates the degradation of ampicillin with an initial concentration of 5,000 μg/ml. Least-squares linear regression estimated a degradation rate of 0.63 h−1 with a correlation coefficient (R2) of 0.967. The bottom series in panel a depicts degradation of ampicillin with an initial concentration of 1,000 μg/ml. Linear least-squares regression predicted a degradation rate of 0.83 h−1 with a correlation coefficient (R2) of 0.922. The two rates were averaged to provide an estimated ampicillin degradation rate of 0.73 h−1 for Kp1. The ampicillin degradation rates for Kp102M cultures (○) and the sterile control (▿) were not statistically different from zero (b).
Mutant characteristics.
Despite the nonspecific procedure used to generate the β-lactamase-deficient mutant, Kp102M appeared to behave much like Kp1 with the exception of ampicillin-degrading activity. The MIC of ciprofloxacin was nearly identical for both strains. Kp102M responded to ciprofloxacin treatment in a manner statistically indistinguishable from that for Kp1 when in planktonic or biofilm state. The two strains also had very similar properties for chloride and ciprofloxacin penetration. In addition, the maximum growth rates of the two organisms were within 20% of each other, with the mutant having a slightly faster growth rate (data not shown). Furthermore, the structures and densities of Kp1 and Kp102M biofilms were virtually indistinguishable via microscopy (data not shown).
DISCUSSION
The biofilms formed by wild-type K. pneumoniae resisted killing by ampicillin and ciprofloxacin. This simple model system, while surely an imperfect representation of a real biofilm, does capture the characteristic antibiotic resistance of biofilm bacteria. Membrane-supported biofilms are attractive as in vitro systems for investigation of biofilm resistance because many biofilm samples can be cultured in parallel with relative ease. A particular advantage of this system is that it permits physical access to both sides of the biofilm, a feature that allowed us to directly measure the penetration of solutes through the model biofilms. Although not intended to simulate a particular disease, these colony biofilms might be considered primitive models of some infections.
While ciprofloxacin and chloride ion readily penetrated the biofilm, ampicillin was unable to penetrate a wild-type K. pneumoniae biofilm in the 4-h treatment period (Fig. 3). This result showed that the biofilm matrix did not pose an inherent barrier to solute mobility, an interpretation that is consistent with experimentally measured effective diffusion coefficients in biofilms (36). We hypothesized that the failure of ampicillin to penetrate biofilms was instead due to its deactivation in the surface layers of the biofilm faster than it could diffuse in. This reaction-diffusion mechanism of penetration limitation has previously been mathematically modeled and has been experimentally demonstrated for reactive oxidants such as hypochlorous acid (11, 35, 38, 44) and hydrogen peroxide (26, 35, 38).
To further test the role of reactive neutralization in limiting biofilm penetration of ampicillin, we isolated a β-lactamase-deficient mutant of our wild-type K. pneumoniae strain. The wild-type strain degraded ampicillin in a batch experiment (Fig. 5a) at a statistically significant rate (P = 0.007). In the same batch test with the mutant strain, there was no statistically discernible degradation of ampicillin (P = 0.655) (Fig. 5b). Furthermore, in a qualitative visualization of the potential of biofilms to deplete the ampicillin in agar medium, the wild-type K. pneumoniae strain locally neutralized ampicillin, whereas the mutant strain did not (Fig. 4). The wild-type and β-lactamase-deficient mutant strains exhibited similar specific growth rates, ciprofloxacin susceptibilities, and microscopic biofilm structures, and the chloride and ciprofloxacin penetrations were similar for both strains. These measurements and observations suggest that the mutant strain was deficient in β-lactamase activity or synthesis but was not otherwise altered in any way that would significantly affect our experiments.
Ampicillin penetrated biofilms of the β-lactamase-deficient mutant strain (Fig. 3b). Its penetration through biofilms of the mutant strain was similar to the penetration of chloride ion through biofilms of both the mutant and wild-type strains. The restoration of ampicillin penetration by deletion of β-lactamase activity supported the contention that penetration breakdown in the wild-type results from a reaction-diffusion interaction. This mechanism was further supported by the Thiele modulus estimate of 6.7 for the ampicillin-challenged wild-type biofilm. This value was calculated by using equation 20 of Stewart (35), with values of 0.727/h for the first-order reaction rate constant, 3.86 × 10−10 m2/s for the effective diffusion coefficient of ampicillin, and 0.465 mm for the biofilm thickness. The Thiele modulus is a dimensionless parameter that compares the relative rates of reaction and diffusion (4, 38). When the Thiele modulus exceeds 1, the diffusion rate is slower than the reaction rate and the system can be considered transport limited. A Thiele modulus of 6.7 is sufficient to account for the observed magnitude of reduced biofilm susceptibility according to mathematical analysis of the reaction-diffusion-disinfection problem (38).
In contrast to the situation with ampicillin, transport limitation probably played a very minor role in protecting the biofilms from ciprofloxacin. The concentration of ciprofloxacin reached the MIC throughout the biofilm in less than 20 min and reached 50% of the bulk agar concentration within 2 h (Fig. 3c). These measurements were consistent with literature reports of relatively facile penetration of fluoroquinolone agents into biofilms (25, 33, 40, 41). We attribute the ability of ciprofloxacin to penetrate a biofilm to its low reactivity. Enzymes that chemically deactivate fluoroquinolones, analogous to the β-lactamases that cleave penicillins, have not been described. Fluoroquinolones can be exported from the cell by efflux pumps (29), but this would not be expected to change the ability of the antibiotic to penetrate the biofilm.
In summary, antibiotics can fail to penetrate microbial biofilms. The ability of an antibiotic to penetrate a biofilm depends on the rate at which it is deactivated in the biofilm. Agents that experience reactive neutralization in the biofilm are prone to penetration failure. On the other hand, agents that are not deactivated in the biofilm will penetrate a biofilm in a matter of minutes or hours. Mathematical models of the underlying reaction-diffusion interaction exist (28, 34, 35, 38) and could be used as quantitative frameworks to test this mechanism of antimicrobial resistance of biofilms with other systems.
The data reported here provide evidence for a mechanism of biofilm resistance other than incomplete antimicrobial penetration. Ciprofloxacin penetrated biofilms within a few hours, but bacteria were not killed even after 24 h of treatment (log reduction, 1.07 ± 0.18). A biofilm formed by a β-lactamase-deficient mutant was fully penetrated by ampicillin but, likewise, was not effectively killed. A planktonic β-lactamase-deficient mutant K. pneumoniae strain exposed to ampicillin experienced a 4.62 ± 0.20 log reduction in the number of CFU within 4 h of treatment. Biofilms, on the other hand, were reduced by only 1.64 ± 0.33 log CFU after 24 h of antibiotic exposure, even though the ampicillin concentration was over 1,000 times the MIC for the mutant for at least 23 of the 24 h. Localized regions of slow growth or starvation within the biofilm interior may be at the root of this resistance. Slowly growing or nongrowing bacteria are known to be less susceptible to many antibiotics (13, 15, 16, 27). Spatial variation in the physiological status of bacteria within biofilms has recently been demonstrated by fluorescence staining approaches (20, 21, 37, 43). Membrane-supported biofilms of K. pneumoniae similar to those used in this study also exhibit pronounced physiological heterogeneity over distances as short as 10 μm (20, 43). Ongoing studies in our laboratory are using the membrane-supported biofilm model system to investigate the role of nutrient-limited physiology in mediating K. pneumoniae biofilm resistance to ampicillin and ciprofloxacin.
ACKNOWLEDGMENTS
This work was supported through cooperative agreement EEC- 8907039 between the National Science Foundation and Montana State University and by the industrial partners of the Center for Biofilm Engineering.
We thank Matthew Jackson for helping with the isolation of a β-lactamase-deficient K. pneumoniae mutant.
REFERENCES
- 1.American Public Health Association, American Water Works Association, and Water and Environment Federation. Standard methods for the examination of water and wastewater. Washington, D.C.: American Public Health Association; 1995. [Google Scholar]
- 2.Anwar H, Strap J L, Costerton J W. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob Agents Chemother. 1992;36:1347–1351. doi: 10.1128/aac.36.7.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar H, Strap J L, Costerton J W. Kinetic interaction of biofilm cells of Staphylococcus aureuswith cephalexin and tobramycin in a chemostat system. Antimicrob Agents Chemother. 1992;36:890–893. doi: 10.1128/aac.36.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey J E, Ollis D F. Biochemical engineering fundamentals. New York, N.Y: McGraw-Hill Book Co.; 1986. [Google Scholar]
- 5.Blaser J, Vergeres P, Widmer A F, Zimmerli W. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob Agents Chemother. 1995;39:1134–1139. doi: 10.1128/aac.39.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M R W, Gilbert P. Sensitivity of biofilms to antimicrobial agents. J Appl Bacteriol. 1993;74(Suppl.):87S–97S. doi: 10.1111/j.1365-2672.1993.tb04345.x. [DOI] [PubMed] [Google Scholar]
- 7.Christensen G D, Baddour L M, Hasty D L, Lowrance G H, Simpson W A. Microbial and foreign body factors in the pathogenesis of medical device infections. In: Bison A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C.: American Society for Microbiology; 1989. pp. 27–59. [Google Scholar]
- 8.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 9.Costerton J W, Cheng K-J, Geesey G G, Ladd T J, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche R O, Dhir A, Miller A J, Landon G C, Raad I I, Musher D M. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis. 1994;170:720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 11.DeBeer D, Srinivasan R, Stewart P S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol. 1994;60:4339–4344. doi: 10.1128/aem.60.12.4339-4344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic V, Schurr M J, Boucher J C, Martin D W. Conversion of Pseudomonas aeruginosato mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J Bacteriol. 1994;176:2773–2780. doi: 10.1128/jb.176.10.2773-2780.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duguid I G, Evans E, Brown M R W, Gilbert P. Growth-rate-independent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis; evidence for a cell-cycle dependency. J Antimicrob Chemother. 1992;30:791–802. doi: 10.1093/jac/30.6.791. [DOI] [PubMed] [Google Scholar]
- 14.Dunne W M, Jr, Mason E O, Kaplan S L. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidisbiofilm. Antimicrob Agents Chemother. 1993;37:2522–2526. doi: 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans D J, Allison D G, Brown M R W, Gilbert P. Susceptibility of Pseudomonas aeruginosa and Escherichia colibiofilms towards ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother. 1991;27:177–184. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert P, Collier P J, Brown M R. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990;34:1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giwercman B, Jensen E T, Hoiby N, Kharazmi A, Costerton J W. Induction of β-lactamase production in Pseudomonas aeruginosabiofilm. Antimicrob Agents Chemother. 1991;35:1008–1010. doi: 10.1128/aac.35.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoben H J, Somasegaran P. Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobiumspp. in inoculants made from presterilized peat. Appl Environ Microbiol. 1948;44:1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyle B D, Alcantara J, Costerton J W. Pseudomonas aeruginosabiofilm as a diffusion barrier to piperacillin. Antimicrob Agents Chemother. 1992;36:2054–2056. doi: 10.1128/aac.36.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C-T, Xu K D, McFeters G A, Stewart P S. Spatial patterns of alkaline phosphatase expression within bacterial colonies and biofilms in response to phosphate starvation. Appl Environ Microbiol. 1998;64:1526–1531. doi: 10.1128/aem.64.4.1526-1531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C-T, Yu F P, McFeters G A, Stewart P S. Nonuniform spatial patterns of respiratory activity with biofilms during disinfection. Appl Environ Microbiol. 1995;61:2252–2256. doi: 10.1128/aem.61.6.2252-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins D E, Schultz J E, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen E T, Kharazmi A, Lam K, Costerton J W, Hoiby N. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosagrown in biofilms. Infect Immun. 1990;58:2383–2385. doi: 10.1128/iai.58.7.2383-2385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khardori N, Yassien M. Biofilms in device-related infections. J Ind Microbiol. 1995;15:141–147. doi: 10.1007/BF01569817. [DOI] [PubMed] [Google Scholar]
- 25.Kumon H, Tomochika K, Matunaga T, Ogawa M, Ohmori H. A sandwich cup method for the penetration assay of antimicrobial agents through Pseudomonas exopolysaccharides. Microbiol Immunol. 1994;38:615–619. doi: 10.1111/j.1348-0421.1994.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Roe F, Jesaitis A, Lewandowski Z. Resistance of biofilms to the catalase inhibitor 3-amino-1,2,4-triazole. Biotechnol Bioeng. 1998;59:156–162. [PubMed] [Google Scholar]
- 27.McLeod G I, Spector M P. Starvation- and stationary-phase-induced resistance to the antimicrobial peptide polymyxin B in Salmonella typhimurium is RpoS (ςS) independent and occurs through both phoP-dependent and -independent pathways. J Bacteriol. 1996;178:3683–3688. doi: 10.1128/jb.178.13.3683-3688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols W W, Evans M J, Slack M P, Walmsley H L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989;135:1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- 29.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 30.Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837–1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 31.Rayner M G, Zhang Y, Gorry M C, Chen Y, Post J C, Ehrlich G D. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279:296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 32.Reed R W, Reed G B. “Drop plate” method of counting viable bacteria. Can J Res. 1948;26:317–326. [Google Scholar]
- 33.Shigeta M, Tanaka G, Komatsuzawa H, Sugai M, Suginaka H, Usui T. Permeation of antimicrobial agents through Pseudomonas aeruginosabiofilms: a simple method. Chemotherapy (Basel) 1997;43:340–345. doi: 10.1159/000239587. [DOI] [PubMed] [Google Scholar]
- 34.Stewart P S. Biofilm accumulation model that predicts antibiotic resistance of Pseudomonas aeruginosabiofilms. Antimicrob Agents Chemother. 1994;38:1052–1058. doi: 10.1128/aac.38.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart P S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 1996;40:2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart P S. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng. 1998;59:261–271. doi: 10.1002/(sici)1097-0290(19980805)59:3<261::aid-bit1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Stewart P S, Griebe T, Srinivasan R, Chen C-I, Yu F P, DeBeer D, McFeters G A. Comparison of respiratory activity and culturability during monochloramine disinfection of binary population biofilms. Appl Environ Microbiol. 1994;60:1690–1692. doi: 10.1128/aem.60.5.1690-1692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart P S, Raquepas J B. Implications of reaction-diffusion theory for the disinfection of microbial biofilms by reactive antimicrobial agents. Chem Eng Sci. 1995;50:3099–3104. [Google Scholar]
- 39.Stickler D J, Morris N S, McLean R J C, Fuqua C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl Environ Microbiol. 1998;64:3486–3490. doi: 10.1128/aem.64.9.3486-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suci P A, Mittelman M W, Yu F P, Geesey G G. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosabiofilms. Antimicrob Agents Chemother. 1994;38:2125–2133. doi: 10.1128/aac.38.9.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrany J D, Stewart P S, Suci P A. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by Pseudomonas aeruginosabiofilms displaying rapid-transport characteristics. Antimicrob Agents Chemother. 1997;41:1352–1358. doi: 10.1128/aac.41.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward K H, Olson M E, Lam K, Costerton J W. Mechanism of persistent infection associated with peritoneal implants. J Med Microbiol. 1992;36:406–413. doi: 10.1099/00222615-36-6-406. [DOI] [PubMed] [Google Scholar]
- 43.Wentland E J, Stewart P S, Huang C-T, McFeters G A. Spatial variations in growth rate within Klebsiella pneumoniaecolonies and biofilms. Biotechnol Prog. 1996;12:316–321. doi: 10.1021/bp9600243. [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Stewart P S, Chen X. Transport limitation of chlorine disinfection of Pseudomonas aeruginosaentrapped in alginate beads. Biotechnol Bioeng. 1996;49:93–100. doi: 10.1002/(SICI)1097-0290(19960105)49:1<93::AID-BIT12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda H, Ajiki Y, Koga T, Kawada H, Yokota T. Interaction between biofilms formed by Pseudomonas aeruginosaand clarithromycin. Antimicrob Agents Chemother. 1993;37:1749–1755. doi: 10.1128/aac.37.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda H, Ajiki Y, Koga T, Yokota T. Interaction between clarithromycin and biofilms formed by Staphylococcus epidermidis. Antimicrob Agents Chemother. 1994;38:138–141. doi: 10.1128/aac.38.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Younger J J, Christensen G D, Bartley D L, Simmons J C H, Barrett F F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987;156:548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]