Abstract
Objective
Several biological DMARDs (bDMARDs) have demonstrated anti-inflammatory effects in PsA. However, their comparative cardiovascular safety profiles remain unknown. We evaluated the risk of major adverse cardiovascular events (MACEs) in PsA patients on therapy with different classes of bDMARDs and apremilast.
Methods
This nationwide cohort study involved the administrative healthcare database of the French health insurance scheme linked to the hospital discharge database. All adults with PsA who were new users of bDMARDs/apremilast (neither in the year before the index date) during 2015–19 were included. Patients with previous cardiovascular diseases were excluded. End of follow-up was 31 December 2019. The primary endpoint was an occurrence of MACEs in a time-to-event analysis with propensity score-weighted Cox and Fine–Gray models.
Results
Between 2015 and 2019, we included 9510 bDMARD new users [mean age 48.5 (s.d. 12.7) years; 42% men], including 7289 starting a TNF inhibitor, 1058 an IL-12/23 inhibitor and 1163 an IL-17 inhibitor, with 1885 apremilast new users [mean age 54.0 (s.d. 12.5) years; 44% men]. MACEs occurred in 51 (0.4%) patients. After propensity score weighting, the risk of MACEs was significantly greater with IL-12/23 (weighted hazard ratio 2.0, 95% CI 1.3, 3.0) and IL-17 (weighted hazard ratio 1.9, 95% CI 1.2, 3.0) inhibitors than TNF inhibitors, with no significant increased risk with apremilast (weighted hazard ratio 1.3, 95% CI 0.8, 2.2). Similar results were observed with the Fine–Gray competing risks survival model.
Conclusion
Analysis of a large database revealed a small overall number of MACEs, and the risk of MACEs was greater for PsA new users of IL-12/23 and IL-17 vs TNF inhibitors.
Keywords: PsA, major adverse cardiovascular event (MACE), biologics, apremilast, National Health Data System
Rheumatology key messages.
This study provides reassuring data on cardiovascular risk in PsA patients receiving biological DMARDs/apremilast.
The risk of major adverse cardiovascular events for those with PsA was greater for new users of IL-12/23 and IL-17 inhibitors vs TNF inhibitors.
This risk did not significantly differ between apremilast new users and TNF inhibitors new users.
Introduction
PsA is a chronic inflammatory arthritis with heterogeneous manifestations that affects ∼0.01% to 0.19% of the general population and 6% to 41% of patients with psoriasis [1, 2]. PsA is associated with other diseases of the spectrum of SpA but also appears to be linked to an increased prevalence of numerous comorbidities and more specifically cardiovascular risk factors and events [3–5]. The EULAR taskforce has highlighted the need for improved screening, identification and management of cardiovascular disease (CVD) risk factors in patients with PsA [6]. Indeed, cardiovascular and cerebrovascular diseases were found to be 43% and 22% higher, respectively, than in the general population [7]. Furthermore, regardless of classical cardiovascular risk factors, the severity of psoriasis and PsA themselves seem to play an important role in increasing CVD [8–11].
Although the exact mechanism of the association is still unclear, systemic inflammation seems a central component resulting in insulin resistance, which in turn causes endothelial cell dysfunction and atherosclerosis [12, 13]. Pharmacological treatments could affect this phenomenon, and inhibition of TNF or ILs could be a potent target for atherothrombotic protection in patients with inflammatory arthritis [14].
Biological DMARDs (bDMARDs) such as TNF, IL-12/23 and IL-17 inhibitors or targeted synthetic DMARDs are recommended second-line therapies for moderate to severe PsA when standard treatments [including conventional synthetic DMARDs (csDMARDs)] fail to control disease or are not tolerated [14]. Despite an established anti-inflammatory effect, their cardiovascular safety profiles in PsA remain uncertain. Indeed, some studies report a lower risk of major adverse cardiovascular events (MACEs) [15, 16], and others show no significant risk [17–19]. Moreover, few studies have been conducted on more recently marketed bDMARDs, particularly IL-12/23 or IL-17 inhibitors, in PsA patients, and no real-world setting analysis is available [20–22]. Thus, a large study is needed to quantify the comparative cardiovascular risk associated with second-line therapies among patients with PsA outside the restricted scope of randomized controlled trials.
The aim of this study was to assess the relative comparative risk of MACEs in patients with PsA initiating bDMARDs or apremilast, after controlling for confounding factors.
Methods
Data source and study design
This French nationwide cohort study was based on health administrative data obtained from the French National Health Insurance [Système National des Données de Santé (SNDS)] covering ∼67 million individuals and linked with the national hospital discharge database (Programme de Médicalisation des Systèmes d’Information), as previously described [23, 24]. This large database has been used for several pharmacoepidemiological studies [25, 26]. Specific approval was obtained from the French data protection agency (Commission nationale de l’informatique et des libertés: SLN/CBO/AR197671) to conduct this study. No informed consent was obtained, as this was not required to use these pseudonymized data.
Study population
All adults (≥18 years old) with PsA [International Classification of Diseases, 10th Revision (ICD-10) code M07] registered in the SNDS between 2015 and 2019 were eligible for inclusion. Algorithms used to identify PsA patients were detailed previously [24]. Then, patients with at least one prescription of bDMARD or apremilast for PsA were identified. Next, we selected previously bDMARD- and apremilast-naïve patients (new users [27]), defined as those who had not filled a prescription for one of these drugs for 1 year. Finally, we excluded patients with a history of acute myocardial infarction, unstable angina, chronic ischaemic heart disease, ischaemic stoke or transient ischaemic attack identified within 5 years before the index date. The index date was the date of the first reimbursement for a bDMARD or apremilast during the study period.
Exposure definition
Biological originator and biosimilar DMARDs included etanercept, infliximab, adalimumab, certolizumab and golimumab as TNF inhibitors, ustekinumab as an IL-12/23 inhibitor (marketing authorization in October 2014), and secukinumab (June 2016) and ixekizumab (April 2018) as IL-17 inhibitors; targeted synthetic DMARDs included only apremilast (December 2015). Tofacitinib, another targeted synthetic DMARD, was only recently marketed in France (December 2018) and thus was not studied. Drugs were identified by using codes from the Anatomical Therapeutic Chemical classification.
Exposure to a molecule was defined as the time from initiation to discontinuation. We defined the discontinuation of treatment as (i) a period of >90 days without a dispensation of the same treatment after the period covered by the previous reimbursement [28], or (ii) a switch of systemic treatment (other bDMARD or apremilast). The period covered by a prescription was 30 days for all molecules except infliximab (56 days) and ustekinumab (82 days) [29]. The discontinuation date was defined as the end of the 90-day period, and the switch date was defined as the date on which another systemic treatment was first reimbursed. Only the first therapeutic sequence of bDMARD or apremilast was considered in this analysis.
Other drugs used as add-on therapies to bDMARDs or apremilast were studied: csDMARDs (MTX, LEF and SSZ), NSAIDs and prednisone. Exposure to combinations of drugs (combination of csDMARDs, NSAIDs and/or prednisone with a bDMARD or apremilast) was defined as a period of <7 days between the reimbursements of a bDMARDs or apremilast and a defined add-on therapy.
Outcome
The primary endpoint was the occurrence of a MACE, a composite outcome combining acute myocardial infarction and ischaemic stroke. Events were identified by a hospital discharge diagnosis (ICD-10 codes I21, I24 and I63, I64) with a previously validated algorithm [30, 31]. Only the first event after index date was considered in case of recurring events.
Covariates
We collected data on basic demographics, including age, sex, complementary universal health coverage and French deprivation index (geographical indicator of social disadvantage specifically adapted to health studies on the French population) [32], inflammatory diseases associated with PsA (active skin psoriasis, IBD and uveitis), cardiovascular risk biomarkers (diabetes, hypertension, dyslipidaemia, chronic obstructive pulmonary disease, dispensing of nicotine replacement therapy, varenicline or cytisine, and other hospital discharge diagnoses related to tobacco such as mental and behavioural disorders due to use of tobacco or problems related to tobacco use, low-dose antiplatelet agent reimbursement, morbid or complicated obesity defined using ICD-10 obesity-specific codes applied to in-patients or bariatric surgery procedure codes) and other comorbidities (chronic renal failure, atherosclerosis of arteries of extremities and depression). These diseases were identified by using the disease definition algorithms developed by the French National Health Insurance Fund for Employees (Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés) when available or by the presence of ICD codes and the repeated reimbursement of specific treatments (supplementary Table S1, available at Rheumatology online). We also collected the number and type of other PsA treatments (csDMARDs, NSAIDs and prednisone) and assessed care consumption (number of drugs in co-reimbursement at the index date, number of visits to the general practitioner and rheumatologist within 2 years before index date). During the follow-up, we compiled the vital status and co-reimbursement of other PsA treatments.
Statistical analyses
For descriptions of the study population, categorical data are reported as number (percentage). Quantitative data are reported as median with interquartile range (IQR) or mean (s.d.). Crude incidence rates of MACEs were calculated for the entire cohort, for each molecule and by different MACE types. These are reported per 1000 person-years (PY).
In the intention-to-treat analysis, patients were followed up to the MACE event, death from any-cause, systemic treatment switch, lost to follow-up (defined by the absence of any reimbursement for 12 consecutive months) or 31 December 2019, whichever came first.
For each systemic treatment, a cause-specific Cox proportional-hazards model was used to estimate the hazard ratio (HR) and 95% CI for the occurrence of MACEs, with the TNF inhibitor class as the reference group. The proportional-hazards assumption was tested formally by using Schoenfeld residuals and the correct specification of the model assumptions was tested using martingale residuals. To control for confounding by baseline covariates, weighted HR (HRw) values were adjusted by using inverse probability of treatment weighting. Weights were based on the propensity score, which was estimated with multinomial logistic regression including the covariates, collected at the index date, related to the primary endpoint with a P < 0.1 in the univariate analysis, but also covariates known to be risk factors for the primary endpoint and known to be associated with initiation of a therapeutic class: age, sex, diabetes, hypertension, dyslipidaemia, chronic obstructive pulmonary disease, reimbursement of low-dose antiplatelet agent, atherosclerosis of the arteries of the extremities, chronic renal failure, associated inflammatory diseases, co-prescription of csDMARDs, NSAIDs and/or prednisone, and care consumption. Covariates highly correlated with each other (correlation coefficient >0.80 or <0.80) were excluded from the multivariate analyses to ensure independence between predictors. Stabilized weights were calculated to preserve the sample size of the original data and produce an appropriate estimation of the main effect variance [33]. The balance in baseline covariates was compared with standardized differences, before and after weighting.
We performed pre-specified subgroup analyses in patients without skin psoriasis requiring topical therapies (i.e. without active skin psoriasis at the time of the study) and in patients without comorbidities related to CVD (i.e. excluding patients with diabetes, hypertension, dyslipidaemia, antiplatelet therapy, chronic renal failure or atherosclerosis of arteries of extremities).
To assess the sensitivity of the estimated HRw with respect to several possible models, we performed the following additional analyses: (i) a per-protocol analysis to try to avoid bias due to potential differential adherences to the drugs compared: follow-up was additionally censored at the time of treatment discontinuation; (ii) Fine–Gray competing risks analysis, computing inverse probability of treatment weighting subhazard ratios to account for the competing risk between all-cause out-of-hospital death and hospitalization for MACEs; and (iii) conventional multivariate Cox model computing adjusted HRs: the co-reimbursement of NSAIDs or prednisone with bDMARD or apremilast considered as time-varying variables. Finally, definitions of the study population or outcome were modified by: (iv) using a larger definition of MACE including unstable angina and transient ischaemic attack in addition to myocardial infarction and ischaemic stroke (supplementary Table S2, available at Rheumatology online); (v) modifying the new-user definition as those who had not filled a prescription for a bDMARDs or apremilast for 5 years before the index date (rather than 1 year as in the main analysis); and (vi) defining treatment discontinuation as >60 or >120 days without filling a prescription for the same treatment after the period covered by the previous prescription.
Results were considered statistically significant at P < 0.05. All analyses were performed with SAS Enterprise Guide v7.1 (SAS Institute Inc., Cary, NC, USA).
Results
Description of the cohort population
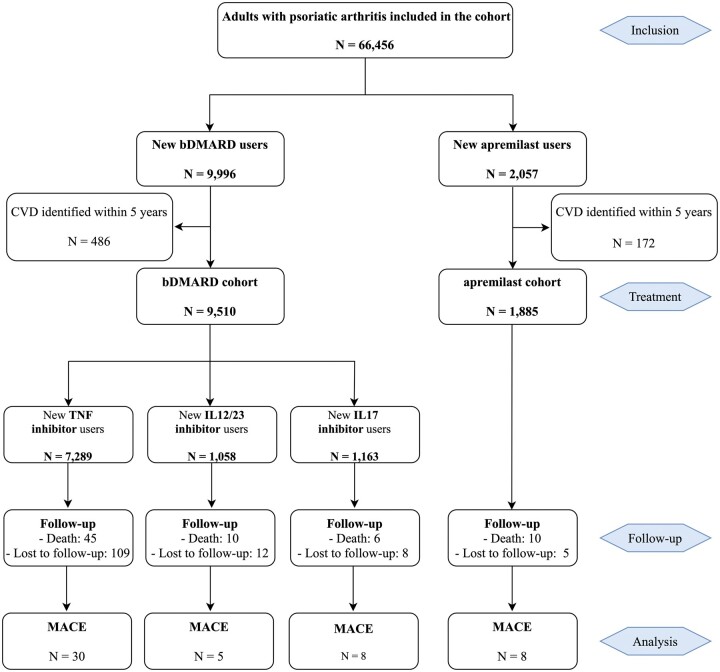
A total of 66 456 PsA patients [mean age 56.2 (s.d. 14.4) years; 46% men] were identified. After excluding those with a CVD history, analysed patients were 9510 (83%) bDMARD new users [mean age 48.5 (12.7) years; 42% men; median follow-up 12 (IQR 6–25) months], including 7289 (77%) initiating a TNF inhibitor, 1058 (11%) an IL-12/23 inhibitor and 1163 (12%) an IL-17 inhibitor (patients by molecule are in supplementary Table S3, available at Rheumatology online), and 1885 (17%) apremilast new users [mean age 54.0 (12.5) years; 44% men; median follow-up 6 (IQR 2–15) months] (Table 1). A total of 61 patients (0.6%) died during follow-up in the bDMARD cohort and 10 (0.5%) in the apremilast cohort (Fig. 1).
Table 1.
Features of the population included in the apremilast cohort, the overall cohort receiving bDMARDs and by bDMARD class
| Total bDMARDs | TNF inhibitors | IL-12/23 inhibitor | IL-17 inhibitors | Apremilast | |
|---|---|---|---|---|---|
| N = 9510 | N = 7289 (76.6%) | N = 1058 (11.1%) | N = 1163 (12.2%) | N = 1885 | |
| Follow-up, median (IQR), months | 12 (6–25) | 12 (5–26) | 14 (8–27) | 11 (5–21) | 6 (2–15) |
| Socio-demographic characteristics | |||||
| Age, mean (s.d.), years | 48.5 (12.7) | 48.2 (12.8) | 49.8 (12.8) | 49.2 (12.2) | 54.0 (12.5) |
| Men | 3959 (41.6) | 3002 (41.2) | 475 (44.9) | 482 (41.4) | 835 (44.3) |
| Complementary universal health coverage | 1192 (12.5) | 877 (12.0) | 144 (13.6) | 171 (14.7) | 197 (10.4) |
| Deprivation index, mean (s.d.) | 0.0 (0.6) | 0.0 (0.6) | 0.0 (0.6) | 0.1 (0.6) | 0.1 (0.5) |
| Associated inflammatory diseases | |||||
| Active skin psoriasis | 4497 (47.3) | 3150 (43.2) | 708 (66.9) | 639 (54.9) | 1175 (62.3) |
| IBD | 499 (5.2) | 429 (5.9) | 58 (5.5) | 12 (1.0) | 7 (0.4) |
| Uveitis | 29 (0.3) | 25 (0.3) | 1 (0.1) | 3 (0.3) | 1 (0.1) |
| Cardiovascular risk biomarkers | |||||
| Diabetes | 806 (8.5) | 572 (7.8) | 116 (11.0) | 118 (10.1) | 225 (11.9) |
| Essential hypertension | 1751 (18.4) | 1300 (17.8) | 218 (20.6) | 233 (20.0) | 464 (24.6) |
| Dyslipidaemia | 604 (6.4) | 436 (6.0) | 92 (8.7) | 76 (6.5) | 152 (8.1) |
| COPD | 802 (8.4) | 595 (8.2) | 100 (9.4) | 107 (9.2) | 202 (10.7) |
| Other hospital discharge diagnosis related to tobacco | 537 (5.6) | 373 (5.1) | 79 (7.5) | 85 (7.3) | 73 (3.9) |
| Low-dose antiplatelet agent | 471 (5.0) | 335 (4.6) | 74 (7.0) | 62 (5.3) | 143 (7.6) |
| Morbid or complicated obesity | 937 (9.9) | 648 (8.9) | 147 (13.9) | 142 (12.2) | 153 (8.4) |
| Other comorbidities | |||||
| Atherosclerosis of arteries of extremities | 74 (0.8) | 54 (0.7) | 14 (1.3) | 6 (0.5) | 24 (1.3) |
| Chronic renal failure | 60 (0.6) | 46 (0.6) | 6 (0.6) | 8 (0.7) | 23 (1.2) |
| Depression | 1589 (16.7) | 1156 (15.8) | 201 (19.0) | 232 (19.9) | 276 (14.6) |
| Other studied drugs | |||||
| csDMARDs | 3633 (38.2) | 2992 (41.0) | 305 (28.8) | 336 (28.9) | 653 (34.6) |
| NSAIDs | 1805 (19.0) | 1473 (20.2) | 144 (13.6) | 188 (16.2) | 357 (18.9) |
| Arylacetic acid derivatives | 377 (4.0) | 316 (4.3) | 21 (2.0) | 40 (3.4) | 66 (3.5) |
| Propionic acid derivatives | 1008 (10.6) | 822 (11.3) | 85 (8.0) | 101 (8.7) | 190 (10.1) |
| Coxibs | 255 (2.7) | 202 (2.8) | 21 (2.0) | 32 (2.7) | 56 (3.0) |
| Oxicam | 169 (1.8) | 143 (2.0) | 12 (1.1) | 14 (1.2) | 54 (2.9) |
| Fenamates | 8 (0.1) | 8 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nabumetone | 26 (0.3) | 17 (0.2) | 6 (0.6) | 3 (0.3) | 1 (0.1) |
| Prednisone | 921 (9.7) | 747 (10.2) | 72 (6.8) | 102 (8.8) | 160 (8.5) |
| Care consumption | |||||
| Rheumatology consultation within 2 years, mean (s.d.) | 0.8 (1.6) | 0.8 (1.5) | 0.9 (2.2) | 0.8 (1.7) | 0.8 (1.6) |
| General consultation within 2 years, mean (s.d.) | 2.4 (4.1) | 2.4 (4.1) | 2.4 (4.4) | 2.5 (3.7) | 2.5 (5.1) |
| Drugs in co-reimbursement, mean (s.d.) | 5.8 (4.3) | 5.8 (4.2) | 5.9 (4.6) | 5.8 (4.6) | 5.8 (4.1) |
Data are n (%) unless indicated. bDMARD: biological disease-modifying antirheumatic drug; IQR: interquartile range; COPD: chronic obstructive pulmonary disease; csDMARD: conventional synthetic DMARD.
Fig. 1.
Flowchart for analytic approach
Data are n. bDMARD: biological disease-modifying antirheumatic drugs; CVD: cardiovascular disease; MACE: major adverse cardiac event.
Table 1 presents characteristics for the bDMARDs and apremilast cohorts, and by class for bDMARDs. Patients initiating a bDMARD were younger and had a lower proportion of cardiovascular risk biomarkers, including lower frequency of diabetes, hypertension and dyslipidaemia, than those initiating apremilast. At the index date, among bDMARD new users, 3633 (38%), 1805 (19%) and 921 (10%) had add-on therapies with a csDMARD, NSAIDs and/or prednisone, respectively. These proportions were 653 (35%), 357 (19%) and 160 (8%) for apremilast new users.
Description of the MACEs
During follow-up, we identified 51 MACEs in the study population. The overall crude incidence rate (s.d.) was 3.4 (0.0) per 1000 PY [2.2 (0.0) per 1000 PY for acute myocardial infarction and 1.2 (0.0) per 1000 PY for cerebral infarction]: 2.8 (0.0) per 1000 PY for those initiating a TNF inhibitor, 3.1 (0.1) per 1000 PY for an IL-12/23 inhibitor, 5.9 (0.1) per 1000 PY for an IL-17 inhibitor and 5.2 (0.1) per 1000 PY for apremilast. The mean age at the time of the events ranged from 59.6 (11.4) to 66.6 (10.4) years. MACEs more frequently occurred in men than women. The median time-to-event was 12 (IQR 5–22) months in the bDMARDs cohort and 3 (IQR 2–14) months in the apremilast cohort (Table 2).
Table 2.
MACEs by therapeutic drug class
| Number of MACEs | Incidence rate per 1000 PY (95% CI) | PY | Time before MACE median (IQR), months | Age at the event, mean (s.d.), years | Sex ratio (men:women) | |
|---|---|---|---|---|---|---|
| Total bDMARDs (n = 9510) | 43 (0.5) | 3.2 (2.2, 4.1) | 13 501.6 | 12 (5–22) | 60.8 (10.3) | 29:14 |
| Acute myocardial infarction | 28 (0.3) | 2.1 (1.3, 2.8) | 14 (5–22) | 60.8 (7.7) | 19:9 | |
| Cerebral infarction | 15 (0.2) | 1.1 (0.5, 1.7) | 9 (5–22) | 59.6 (14.3) | 10:5 | |
| TNF inhibitors (n = 7289) | 30 (0.4) | 2.8 (1.8, 3.9) | 10 519.3 | 12 (5–26) | 59.6 (11.4) | 21:9 |
| Acute myocardial infarction | 19 (0.3) | 1.8 (1.0, 2.6) | 15 (4–26) | 60.8 (7.4) | 15:4 | |
| Cerebral infarction | 11 (0.1) | 1.0 (0.4, 1.7) | 9 (5–34) | 58.3 (16.3) | 6:5 | |
| IL-12/23 inhibitor (n = 1058) | 5 (0.5) | 3.1 (0.4, 5.8) | 1627.5 | 10 (5–13) | 65.0 (7.6) | 2:3 |
| Acute myocardial infarction | 5 (0.5) | 3.1 (0.4, 5.8) | 10 (5–13) | 65.0 (7.6) | 2:3 | |
| Cerebral infarction | 0 (0.0) | 0.0 (0.0, 0.0) | – | – | – | |
| IL-17 inhibitors (n = 1163) | 8 (0.7) | 5.9 (1.8, 9.9) | 1354.9 | 14 (5–18) | 61.6 (7.8) | 6:2 |
| Acute myocardial infarction | 4 (0.3) | 2.9 (0.1, 5.8) | 13 (5–17) | 60.0 (9.3) | 2:2 | |
| Cerebral infarction | 4 (0.3) | 2.9 (0.1, 5.8) | 15 (6–20) | 63.2 (7.0) | 4:0 | |
| Apremilast (n = 1885) | 8 (0.4) | 5.2 (1.6, 8.9) | 1523.9 | 3 (2–14) | 66.6 (10.4) | 7:1 |
| Acute myocardial infarction | 5 (0.3) | 3.3 (0.4, 6.1) | 6 (2–22) | 68.0 (13.4) | 5:0 | |
| Cerebral infarction | 3 (0.1) | 1.9 (0.2, 4.2) | 2 (2–3) | 64.3 (7.6) | 2:1 |
Data are n (%) unless indicated. bDMARD: biological DMARD; PY: person-years; IQR: interquartile range; MACE: major adverse cardiovascular event.
Association of treatment exposure and MACE
Before adjustment, compared with TNF inhibitors, the crude HRs associated with IL-12/23 and IL-17 inhibitors were 1.1 (95% CI 0.4, 2.9) and 2.2 (95% CI 1.0, 4.9), respectively. It was 1.9 (95% CI 0.9, 4.3) for apremilast.
After applying the stabilized propensity score, we obtained a pseudo-cohort in which the distribution of variables was similar with standardized difference <0.1 between the different treatment classes (supplementary Table S4, available at Rheumatology online).
The results of the main analysis are presented in Table 3. Risk of MACEs was significantly higher (overall P < 10−4) with the IL-12/23 inhibitor (HRw 2.0, 95% CI 1.3, 3.0) and IL-17 inhibitors (HRw 1.9, 95% CI 1.2, 3.0) but not apremilast (HRw 1.3, 95% CI 0.8, 2.2) vs TNF inhibitors. Compared with the TNF inhibitor group, the absolute differences were −0.0037, −0.0033 and −0.0010 in the IL-12/23 inhibitor, IL-17 inhibitor and apremilast groups, respectively. The cumulative incidence functions from the adjusted analysis are shown in supplementary Fig. S1, available at Rheumatology online.
Table 3.
Risk of MACEs by therapeutic class in the Cox and Fine–Gray models
| IPTW Cox |
IPTW Fine–Gray |
|||||
|---|---|---|---|---|---|---|
| HRw | 95% CI | P-value | SHRw | 95% CI | P-value | |
| Treatments (ref: TNF inhibitors) | – | – | <10−4 | – | – | <10−4 |
| IL-12/23 inhibitor | 2.0 | 1.3, 3.0 | <10−4 | 2.1 | 1.5, 2.9 | <10−3 |
| IL-17 inhibitors | 1.9 | 1.2, 3.0 | <10−3 | 2.3 | 1.5, 3.0 | <10−3 |
| Apremilast | 1.3 | 0.8, 2.2 | 0.31 | 1.4 | 0.8, 2.4 | 0.12 |
MACE: major adverse cardiovascular event; HRw: weighted hazard ratio; SHRw: weighted subhazard ratio; IPTW: inverse probability of treatment weighting.
Subgroup analyses
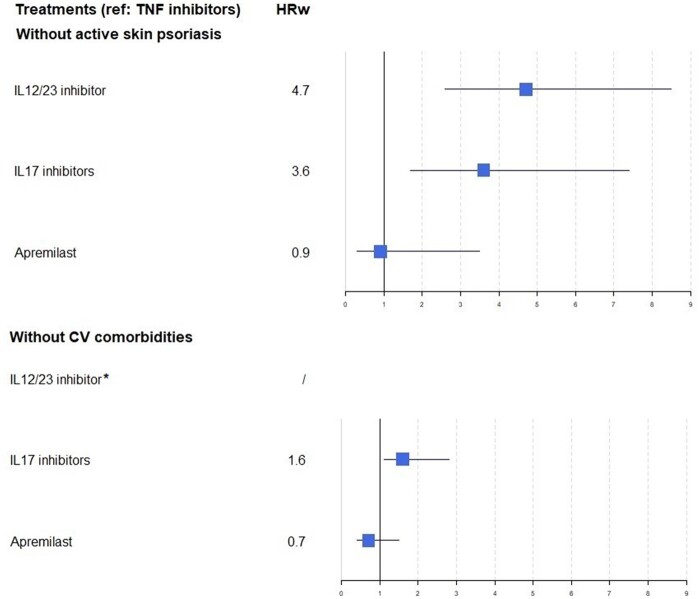
The results did not differ for patients without skin psoriasis requiring local treatment (Fig. 2). Among patients without comorbidities related to CVD (Fig. 2), the overall crude incidence was 2.0 (0.0) per 1000 PY. Risk of MACEs was significantly higher (overall P < 0.01) for IL-17 inhibitors (HRw 1.6, 95% CI 1.1, 2.8), but not apremilast (HRw 0.7, 95% CI 0.4, 1.5), than TNF inhibitors.
Fig. 2.
Forest plot of risk of major adverse cardiac events by therapeutic drug class in subgroup analyses
*Not available due to the absence of events in this class. HRw: weighted hazard ratio; CV: cardiovascular.
Sensitivity analyses
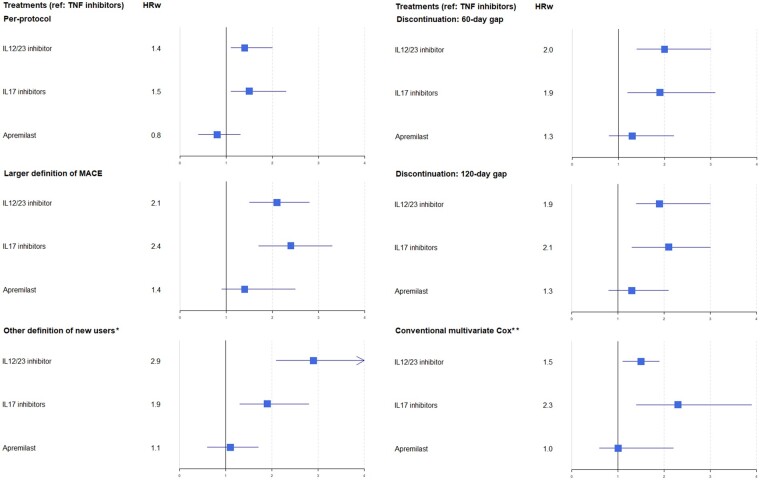
The per-protocol and additional sensitivity analyses results were consistent with those of the main analysis (Fig. 3).
Fig. 3.
Forest plot of risk of major adverse cardiac events by therapeutic drug class in sensitivity analyses
*New users defined by lack of treatment in the 5 years preceding the index date. **Adjusted on conventional synthetic DMARDs, NSAIDs, prednisone, age, sex, chronic renal failure, chronic obstructive pulmonary disease, diabetes, hypertension, dyslipidaemia, antiplatelet agent and deprivation index. HRw: weighted hazard ratio; MACE: major adverse cardiac event.
Discussion
In this nationwide PsA cohort study involving 9510 bDMARDs and 1885 apremilast new users with no history of CVD, despite an increased risk of MACE for new users of IL-12/23 and IL-17 inhibitors compared with TNF inhibitors after controlling for available confounding factors, the overall MACEs rate was low. The risk of MACEs for apremilast new users did not significantly differ from that of TNF inhibitor new users.
Our study provides reassuring information regarding the risk of MACEs in patients starting a bDMARD or apremilast for PsA. We observed an overall crude incidence of MACE (3.4 per 1000 PY) similar to that previously described in the PsA population [34–37]. Of note, we excluded patients with a history of CVD, used more recent data (considering exposure to IL-12/23 and IL-17 inhibitors and apremilast) and focused on second-line therapies, rather than all DMARDs. Previous investigations, from randomized controlled trial meta-analyses, revealed no significant change in risk of MACEs in patients with psoriasis or PsA receiving bDMARDs vs placebo [18, 38]; however, these populations were strictly selected, with younger patients and fewer comorbidities than those treated in clinical practice. In addition, bDMARDs were pooled, which could result in blurring the real but opposing effects across classes.
Our findings are important, because the comparative cardiovascular safety of each class of the molecules has not been examined in a real-world cohort. They suggest an increased risk of MACEs in new users of IL-12/23 and IL-17 inhibitors vs TNF inhibitors. The results may be due to a protective role of TNF inhibitors or an adverse effect of IL-12/23 and IL-17 inhibitors or both. In contrast to the strong evidence suggesting a beneficial effect of TNF inhibitors on cardiovascular risk in patients with RA [39, 40], such information in PsA is limited. Several studies have shown positive effects on subclinical indices of atherosclerosis (reduced progression of carotid plaques, improvement in vascular inflammation, normalization of several prothrombotic parameters) [41–43], but also on the incidence of CVD among psoriasis/PsA patients [15]. In particular, a decrease in cardiovascular risk in patients on systemic therapy, including TNF inhibitors, compared with the use of topical treatments and/or phototherapy, has been reported in well-conducted meta-analyses [16, 20, 44]. Our results may be compatible with a beneficial cardiovascular effect in PsA similar to that in RA.
The cardiovascular risk associated with exposure to IL-12/23 and IL-17 inhibitors is unclear [22, 45]. Th17 cells, inhibited by these bDMARD classes, have a key role in cardiovascular phenomena, and the balance between pro-atherogenic and atheroprotective effects seems based on the cytokine environment [46]. These cells could be essential for the stability of atherosclerotic plaques [47], and low serum level of IL-17 seems associated with increased risk of cardiovascular recurrence in patients with coronary artery disease [48]. A study had found a small but nonsignificant increase in the risk of MACE after initiation of ustekinumab vs TNF inhibitor [49]. However, in contrast to our study, patients with a diagnosis of psoriasis and PsA had been pooled and the outcome definition was less stringent (including transient ischaemic attacks and coronary revascularizations without diagnosis of myocardial infarction), resulting in a more heterogeneous population. Our results agree with a recent study showing a trigger effect on MACEs in the 6 months after ustekinumab initiation for patients at high cardiovascular risk [50]. A recent head-to-head trial evaluating secukinumab vs adalimumab, with a 52-week duration in patients with PsA [Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED)], found two MACEs in the first group (n = 426) and none in the second group (n = 427) [51].
Several studies have investigated cardiovascular-related parameters under bDMARDs. TNF inhibitors seemed to increase total cholesterol and triglycerides on one hand, but on the other hand improve insulin sensitivity [52]. IL-12/23 and IL-17 inhibitors seemed to have a neutral effect on these parameters [53–55]. However, the overall impact of these drugs is still unclear and further studies of other mechanisms are needed to try to understand this complex physiopathology.
Finally, we found that risk of MACEs in apremilast new users was not significantly increased as compared with TNF inhibitor new users, which confirms the overall good tolerance profile of this molecule. However, it should be noted that effect estimates may be imprecise and that the average conditional effect is a 30–40% increase in MACE risk. In addition, this result could partly be related to the fact that apremilast new users have milder disease, not fully captured, and therefore less inflammatory burden that could be associated with less CVD risk. Indeed, because of efficacy data and the lack of structural data, apremilast is currently reserved for patients with mild peripheral articular or enthesitic PsA, and with intolerance to or failure of DMARDs [14].
This study has limitations. First, we defined drug exposure based on healthcare reimbursement data, which are not necessarily equivalent to days of use. However, in psoriasis, adherence rates for bDMARDs are generally higher than for other treatment categories [56]. Second, although we applied a propensity score to reduce confounding bias, it may not be completely neutralized. We can only adjust on the known and actually measured characteristics of the patients and our analyses are limited by the availability of data on some individual risk factors (sedentary lifestyle, no directly available data on smoking and obesity, although proxies for severe forms were used) and familial risk factors (family history of MACE), and by the inability to account for over-the-counter NSAID use. Information on disease activity (from skin or from musculoskeletal involvement) is unavailable in our database and it cannot be completely excluded that a part of the patients with the most active psoriasis received an IL-12/23 or IL-17 inhibitor more frequently. Third, in view of the limited number of events, the dose effect could not be fully tested. However, in randomized controlled trials, no differences in adverse events were found between the different authorized doses of ustekinumab or secukinumab [38]. Finally, new users would ideally be those using a treatment for the first time (i.e. naïve patients). To assess this parameter, lifetime treatment use data would be necessary, however this framework is most often not available and in pharmacoepidemiology a washout window (period without delivery of the studied treatment) of 6–12 months is usual [57]. It must be borne in mind that some of the new user patients defined above may have received a bDMARD at an undocumented moment before the start of the study. To test the robustness of our main analysis, a sensitivity analysis was conducted by considering as new users those who had not filled a prescription for a bDMARDs or apremilast for 5 years before the index date: the results obtained were consistent. Moreover, although cumulative cardiovascular toxicity of a previously discontinued molecule could be discussed, this ‘new user’ approach, supported by the sensitivity analysis, limits the impact of this potential bias.
This study has several strengths. To our knowledge, this is the first population-based study dedicated to assessing the comparative risk of MACEs in new users of bDMARDs or apremilast in PsA. Our cohort involved a large number of patients from a national exhaustive database, with a data quality and consistency plan ensuring homogeneous data processing, and with information captured during routine medical care [23]. This framework minimizes selection bias. The definition of our PsA population was based on either ICD-10 codes for PsA applied to in-patients or on patients with fully reimbursed PsA-related care procedures. We are confident that our definition is specific: patients received bDMARDs or apremilast that are reimbursed in the context of PsA, and we have previously shown that our population had much the same characteristics as described populations of patients with moderate to severe PsA [24]. In addition, all patients treated with costly drugs, such as bDMARDs, are registered in the SNDS. The risk of misclassification is very low, especially for bDMARDs other than TNF inhibitors or apremilast, which have limited indications. Notably, the IL-12/23 inhibitor did not have marketing authorization for IBD at the time of the study in France. MACEs were detected by using comprehensive data from the national hospital discharge database, in which the outcomes were previously validated [30, 31]. Furthermore, we used a new-user design [27], and applied a propensity score method to more accurately estimate the risk of MACEs and control channelling bias [58]. Concerning this last point, it should be noted that TNF, IL-12/23 and IL-17 inhibitors are recommended second-line therapies for moderate to severe disease [14], and in France, each physician is free to choose either of the biologics labelled for PsA. Due to the greater use experience, the majority of patients initiate a TNF inhibitor [59]. However, except in minority cases where an extra-articular manifestation (very active psoriasis, IBD, severe or repeated acute anterior uveitis) guides the choice of prescriber, no factor (and in particular neither the activity nor severity of the rheumatism) is today likely to influence this prescription at population level since no study has demonstrated a better benefit/risk ratio of one therapeutic class over another. In this way, we can note that in our study, the different groups were comparable on a large number of criteria (Table 1). Overall, we feel that a potential channelling bias could not have a significant influence in our results. Finally, several sensitivity analyses were performed and supported the integrity of our results.
Conclusion
Given the relatively small number of events, our study provides reassuring data regarding the risk of MACEs in patients with PsA initiating a bDMARD or apremilast. Our results suggest an increased risk of MACEs in new users of IL-12/23 and IL-17 inhibitors vs TNF inhibitors in PsA. If these results are confirmed by further studies using other data sources, they could encourage physicians to adapt the therapeutic journey of PsA patients by preferentially prescribing TNF inhibitors as the first second-line therapy, especially in patients at high cardiovascular risk, because they appear to have a better cardiovascular effect than other available IL inhibitors.
Supplementary Material
Acknowledgements
L.P.V. received a Master 2 grant from the French Society of Rheumatology (Bourse Master 2ème Année 2019).
Funding: There are no funders to report for this submission.
Disclosure statement: L.P.V., P.L.C., L.P., M.P. and E.S. have no conflict of interest to declare.
P.C. has received consulting fees from Abbvie, Pfizer, Roche-Chugai, Bristol-Myers Squibb, MSD, UCB, Novartis, Janssen, Lilly and Celgene (<$10 000 each), and has been an investigator for Roche Chugai, Sanofi Aventis, Celgene, Pfizer, MSD, Novartis and BMS.
Data availability statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Laura Pina Vegas, EpiDermE, Université Paris Est Créteil; Service de Rhumatologie, AP-HP.
Philippe Le Corvoisier, Inserm, Centre d’investigation clinique 1430, Hôpital Henri Mondor; Inserm, U955-IMRB, Équipe 03, UPEC, Ecole Nationale Vétérinaire d’Alfort, Créteil.
Laetitia Penso, EpiDermE, Université Paris Est Créteil; EPI-PHARE Scientific Interest Group in Epidemiology of Health Products from the French National Agency for the Safety of Medicines and Health Products and the French National Health Insurance, Saint Denis.
Muriel Paul, EpiDermE, Université Paris Est Créteil; Service de Pharmacie.
Emilie Sbidian, EpiDermE, Université Paris Est Créteil; Inserm, Centre d’investigation clinique 1430, Hôpital Henri Mondor; Service de Dermatologie, AP-HP, Hôpital Henri Mondor, Créteil, France.
Pascal Claudepierre, EpiDermE, Université Paris Est Créteil; Service de Rhumatologie, AP-HP.
References
- 1. Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:28–34. [DOI] [PubMed] [Google Scholar]
- 2. Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinnathurai P, Buchbinder R, Hill C, Lassere M, March L. Comorbidity in psoriatic arthritis and rheumatoid arthritis. Intern Med J 2018;48:1360–8. [DOI] [PubMed] [Google Scholar]
- 4. Jafri K, Bartels CM, Shin D, Gelfand JM, Ogdie A. Incidence and management of cardiovascular risk factors in psoriatic arthritis and rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) 2017;69:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulati AM, Semb AG, Rollefstad S et al. On the HUNT for cardiovascular risk factors and disease in patients with psoriatic arthritis: population-based data from the Nord-Trøndelag Health Study. Ann Rheum Dis 2016;75:819–24. [DOI] [PubMed] [Google Scholar]
- 6. Agca R, Heslinga SC, Rollefstad S et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 7. Polachek A, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2017;69:67–74. [DOI] [PubMed] [Google Scholar]
- 8. Lai YC, Yew YW. Psoriasis as an independent risk factor for cardiovascular disease: an epidemiologic analysis using a national database. J Cutan Med Surg 2016;20:327–33. [DOI] [PubMed] [Google Scholar]
- 9. Szentpetery A, Healy GM, Brady D et al. Higher coronary plaque burden in psoriatic arthritis is independent of metabolic syndrome and associated with underlying disease severity. Arthritis Rheumatol 2018;70:396–407. [DOI] [PubMed] [Google Scholar]
- 10. Ahlehoff O, Gislason GH, Charlot M et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011;270:147–57. [DOI] [PubMed] [Google Scholar]
- 11. Gelfand JM, Neimann AL, Shin DB et al. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735–41. [DOI] [PubMed] [Google Scholar]
- 12. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 2011;20:303–7. [DOI] [PubMed] [Google Scholar]
- 13. Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74. [DOI] [PubMed] [Google Scholar]
- 14. Gossec L, Baraliakos X, Kerschbaumer A et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahlehoff O, Skov L, Gislason G et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med 2013;273: 197–204. [DOI] [PubMed] [Google Scholar]
- 16. Yang ZS, Lin NN, Li L, Li Y. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol 2016;51:240–7. [DOI] [PubMed] [Google Scholar]
- 17. Kavanaugh A, Gladman DD, Edwards CJ et al. Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1–3 pooled analysis. Arthritis Res Ther 2019;21:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Champs B, Degboé Y, Barnetche T et al. Short-term risk of major adverse cardiovascular events or congestive heart failure in patients with psoriatic arthritis or psoriasis initiating a biological therapy: a meta-analysis of randomised controlled trials. RMD Open 2019;5:e000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu JJ, Poon KYT. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol 2014;13:932–4. [PubMed] [Google Scholar]
- 20. Roubille C, Richer V, Starnino T et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liew JW, Ramiro S, Gensler LS. Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol 2018;32:369–89. [DOI] [PubMed] [Google Scholar]
- 22. Dommasch ED, Troxel AB, Gelfand JM. Major cardiovascular events associated with anti-IL 12/23 agents: a tale of two meta-analyses. J Am Acad Dermatol 2013;68:863–5. [DOI] [PubMed] [Google Scholar]
- 23. Tuppin P, Rudant J, Constantinou P et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique 2017;65(Suppl 4):S149–67. [DOI] [PubMed] [Google Scholar]
- 24. Pina Vegas L, Sbidian E, Penso L, Claudepierre P. Epidemiologic study of patients with psoriatic arthritis in a real-world analysis: a cohort study of the French health insurance database. Rheumatology (Oxford) 2021;60:1243–51. [DOI] [PubMed] [Google Scholar]
- 25. Meyer A, Rudant J, Drouin J et al. Effectiveness and safety of reference infliximab and biosimilar in Crohn disease: a French equivalence study. Ann Intern Med 2019;170:99–107. [DOI] [PubMed] [Google Scholar]
- 26. Neumann A, Weill A, Ricordeau P et al. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia 2012;55:1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–20. [DOI] [PubMed] [Google Scholar]
- 28. Warren RB, Smith CH, Yiu ZZN et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015;135:2632–40. [DOI] [PubMed] [Google Scholar]
- 29. Sbidian E, Billionnet C, Weill A, Maura G, Mezzarobba M. Persistence of apremilast in moderate-to-severe psoriasis: a real-world analysis of 14 147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br J Dermatol 2020;182:690–7. [DOI] [PubMed] [Google Scholar]
- 30. Giroud M, Hommel M, Benzenine E et al. ; FRESCO Study. Positive predictive value of French hospitalization discharge codes for stroke and transient ischemic attack. Eur Neurol 2015;74:92–9. [DOI] [PubMed] [Google Scholar]
- 31. Bezin J, Girodet PO, Rambelomanana S et al. Choice of ICD-10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol 2015;29:586–91. [DOI] [PubMed] [Google Scholar]
- 32. Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu S, Ross C, Raebel MA et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010;13:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lauper K, Courvoisier DS, Chevallier P, Finckh A, Gabay C. Incidence and prevalence of major adverse cardiovascular events in rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis. Arthritis Care Res (Hoboken) 2018;70:1756–63. [DOI] [PubMed] [Google Scholar]
- 35. Bengtsson K, Forsblad-d’Elia H, Lie E et al. Are ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis associated with an increased risk of cardiovascular events? A prospective nationwide population-based cohort study. Arthritis Res Ther 2017;19:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogdie A, Yu Y, Haynes K et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2015;74:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li L, Hagberg KW, Peng M et al. Rates of cardiovascular disease and major adverse cardiovascular events in patients with psoriatic arthritis compared to patients without psoriatic arthritis. J Clin Rheumatol 2015;21:405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta‐analysis of randomized controlled trials. Br J Dermatol 2017;176:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karpouzas GA, Ormseth SR, Hernandez E, Budoff MJ. Biologics may prevent cardiovascular events in rheumatoid arthritis by inhibiting coronary plaque formation and stabilizing high-risk lesions. Arthritis Rheumatol 2020;72:1467–75. [DOI] [PubMed] [Google Scholar]
- 40. Ozen G, Pedro S, Michaud K. The risk of cardiovascular events associated with disease-modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol 2021;48:648–55. [DOI] [PubMed] [Google Scholar]
- 41. Eder L, Joshi AA, Dey AK et al. Association of tumor necrosis factor inhibitor treatment with reduced indices of subclinical atherosclerosis in patients with psoriatic disease. Arthritis Rheumatol 2018;70:408–16. [DOI] [PubMed] [Google Scholar]
- 42. Di Minno MND, Iervolino S, Peluso R et al. Carotid intima-media thickness in psoriatic arthritis: differences between tumor necrosis factor-α blockers and traditional disease-modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol 2011;31:705–12. [DOI] [PubMed] [Google Scholar]
- 43. Beinsberger J, Heemskerk JWM, Cosemans JMEM. Chronic arthritis and cardiovascular disease: altered blood parameters give rise to a prothrombotic propensity. Semin Arthritis Rheum 2014;44:345–52. [DOI] [PubMed] [Google Scholar]
- 44. Ahlehoff O, Skov L, Gislason G et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol 2015;29:1128–34. [DOI] [PubMed] [Google Scholar]
- 45. von Stebut E, Boehncke WH, Ghoreschi K et al. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol 2019;10:3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erbel C, Dengler TJ, Wangler S et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res Cardiol 2011;106:125–34. [DOI] [PubMed] [Google Scholar]
- 47. Taleb S, Romain M, Ramkhelawon B et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med 2009;206:2067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simon T, Taleb S, Danchin N et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J 2013;34:570–7. [DOI] [PubMed] [Google Scholar]
- 49. Lee MP, Desai RJ, Jin Y et al. Association of ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol 2019;155:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poizeau F, Nowak E, Kerbrat S et al. Association between early severe cardiovascular events and the initiation of treatment with the anti-interleukin 12/23p40 antibody ustekinumab. JAMA Dermatol 2020;156:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McInnes IB, Behrens F, Mease PJ et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020;395:1496–505. [DOI] [PubMed] [Google Scholar]
- 52. Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-alpha blockade on metabolic syndrome components in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther 2009;22:61–73. [DOI] [PubMed] [Google Scholar]
- 53. Wang HN, Huang YH. Changes in metabolic parameters in psoriatic patients treated with secukinumab. Ther Adv Chronic Dis 2020;11:2040622320944777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Egeberg A, Wu JJ, Korman N et al. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J Am Acad Dermatol 2018;79:104–9.e8. [DOI] [PubMed] [Google Scholar]
- 55. Marovt M, Marko PB, Pirnat M, Ekart R. Effect of biologics targeting interleukin-23/-17 axis on subclinical atherosclerosis: results of a pilot study. Clin Exp Dermatol 2020;45:560–4. [DOI] [PubMed] [Google Scholar]
- 56. Aleshaki JS, Cardwell LA, Muse ME, Feldman SR. Adherence and resource use among psoriasis patients treated with biologics. Expert Rev Pharmacoecon Outcomes Res 2018;18:609–17. [DOI] [PubMed] [Google Scholar]
- 57. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lobo FS, Wagner S, Gross CR, Schommer JC. Addressing the issue of channeling bias in observational studies with propensity scores analysis. Res Social Adm Pharm 2006;2:143–51. [DOI] [PubMed] [Google Scholar]
- 59. Wendling D, Lukas C, Prati C et al. 2018 update of French Society for Rheumatology (SFR) recommendations about the everyday management of patients with spondyloarthritis. Joint Bone Spine 2018;85:275–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request.