Abstract
Objective
Age at onset is useful in identifying chronic back patients at an increased risk of axial SpA (axSpA). However, the majority of data on which the criterion of age at onset <45 years is based originates from Europe. Therefore it is unknown if this criterion applies in other parts of the world. We aimed to assess the age at onset of axSpA and its relationship with HLA-B27 and gender across the world.
Methods
Analyses were applied to patients from 24 countries across the world with an axSpA diagnosis and known age at onset of axial complaints. Cumulative probability plots were used to display the cumulative distribution of age at onset of axial symptoms. Linear regression models were built to assess the effect of HLA-B27 and gender on age at onset of axial symptoms.
Results
Of 2579 axSpA patients, 92% had an age at onset of axial symptoms <45 years, with only small variations across the geographical regions [Asia, n = 574 (94%); Europe and North America, n = 988 (92%); Latin America, n = 246 (89%); Middle East and North Africa, n = 771 (91%)]. Age at onset of axial symptoms was consistently lower in HLA-B27-positive patients {median 25 years [interquartile range (IQR) 19–32] vs 31 [IQR 22–39]} and male patients [median 25 years (IQR 19–33) vs 28 (IQR 21–37)], but in multivariable models an additional statistically significant effect of male gender independent of HLA-B27 was only found in Asia.
Conclusion
Around the world, the great majority of axSpA patients had an age at onset of axial disease of <45 years, with HLA-B27 and male gender associated with earlier disease onset.
Keywords: axial spondyloarthritis, age at onset, HLA-B27, gender
Rheumatology key messages.
Across the globe, 92% of axSpA patients have an age at onset <45 years.
Geographical region does not have an effect on the age at onset of axial complaints.
HLA-B27 positivity is associated with an earlier age at onset around the world.
Introduction
Axial SpA (axSpA) is a chronic, inflammatory disease predominantly affecting the sacroiliac joints and spine. HLA-B27 is the most important genetic risk factor for axSpA and has been reported to be associated with earlier onset of disease [1–4]. Data regarding the association between gender and age at onset of disease are ambiguous [5], even though there is a known difference in disease severity and disease expression between male and female patients [2, 4, 6].
AxSpA usually starts in the second or third decade of life [7, 8]. Age at onset of axSpA after 50 years appears to be uncommon [9], thus age at onset can be very useful in identifying chronic back pain patients suspected of axSpA [2], as it is an easy and accessible piece of information that can be used in the first selection of patients.
Previous research has shown that the vast majority of axSpA patients develop back pain before the age of 45 years [1, 10, 11], which formed the basis for the Assessment of Spondyloarthritis international Society (ASAS) definition of inflammatory back pain (IBP) [12] and the prominent place of age at onset in the current ASAS classification criteria for axSpA [13]. In fact, the criterion of onset before the age of 45 years is an important difference between the modified New York criteria for classification of AS [14] and the ASAS classification criteria and is even the main cause for discrepancy between the two criteria sets in classifying patients with radiographic axSpA (r-axSpA) [15]. Since the publication of the ASAS criteria for axSpA, some data have become available on the age at onset of axSpA patients in Brazil [3, 16] and China [17], but the majority of the data originate from Western Europe.
Given that both the prevalence of axSpA [18] and its main genetic risk factor of HLA-B27 [19] vary considerably throughout the world, a similar distribution in age at onset to the patients in the Feldtkeller study [1] in other parts of the world is not a given. Then again, since age at onset plays an important role in diagnosing patients with axSpA as well as in the classification of patients, the age at onset criterion should be representative of patients all around the world.
Hence the aim of this study was to assess the age at onset of axSpA as well as its relationship with HLA-B27 and gender in various regions of the world using data from the Assessment in SpondyloArthritis international Society Peripheral Involvement in Spondyloarthritis (ASAS-PerSpA) study [20].
Methods
This study was conducted using data from the ASAS-PerSpA dataset, which has been described elsewhere [20]. In brief, ASAS-PerSpA was a multicentre observational study with a cross-sectional design in which a total of 24 countries participated. Its main aim was to investigate clinical peripheral rheumatologic features in consecutively included SpA patients and evaluate the validity of existing outcome measures of peripheral rheumatologic features.
Patients
In the ASAS-PerSpA study, patients with a diagnosis of SpA (n = 4465) were included between July 2018 and February 2020, representing 24 countries in four geographical regions. The study was approved by the ethical committees in all countries (complete list available in Supplementary Data S1, available at Rheumatology online) and written informed consent was obtained from participants prior to inclusion. For this analysis, only patients with a definite diagnosis of axSpA were included, which was defined as axSpA and either r-axSpA or non-radiographic axSpA (nr-axSpA) as a disease subgroup.
Outcomes
The primary outcome of interest was the age at onset of axial symptoms across all patients with a diagnosis of axSpA and stratified by geographical region. Age at onset was ascertained from the date of first axial symptoms, as reported by the rheumatologist, and the study date. Negative values for age at onset of axial symptoms were recoded to missing values (n = 3).
Additional outcomes of interest were the association between HLA-B27 and age at onset of axial symptoms and the association between gender and age at onset of axial symptoms in the total included axSpA population and each of the geographical regions.
Analyses
Analyses were restricted to patients with a known age at onset of axial complaints. Categorical variables were reported as frequencies (proportions) and continuous variables as mean and s.d. in case of normally distributed data and as median and interquartile range (IQR) in case of non-normally distributed data.
Cumulative probability plots were used to display the cumulative distribution in age at onset of axial symptoms. Mann–Whitney U tests were used to compare the median age at onset of axial symptoms between groups stratified for HLA-B27 status or gender.
Linear regression models were built to assess the association between HLA-B27 status or gender and age at onset of axial symptoms with HLA-B27 status or gender as the independent variable and age at onset as a dependent variable. Finally, a multivariable linear regression model including both HLA-B27 status and gender as covariates was built to assess whether the association between HLA-B27 and age at onset was different for male and female patients.
Data were analysed using Stata SE version 16 (StataCorp, College Station, TX, USA). P-values <0.05 were considered statistically significant.
Results
A total of 2579 patients had a definite diagnosis of axSpA and a known age at onset of axial complaints. Patients were grouped in four previously defined geographical regions: Asia (n = 574), Europe and North America (n = 988), Latin America (n = 246) and the Middle East and North Africa (n = 771) (Supplementary Table S1, available at Rheumatology online). Overall there was only a small percentage of missing data (<5% unless indicated otherwise), with the exception of HLA-B27 status and MRI of the pelvis, where information was unavailable for a larger proportion of patients, which was especially apparent in the Middle East and North Africa population.
Across the board, 69% of included patients were male, 79% were HLA-B27 positive, the vast majority (94%) had IBP according to the ASAS definition [21], the majority (78%) had r-axSpA and the level of confidence regarding the diagnosis of axSpA was high, with very small variations between geographical regions (Table 1). Asian patients had a somewhat lower median age and shorter median symptom duration. Latin American patients more frequently had peripheral symptoms, as shown by the higher percentages of peripheral arthritis, enthesitis and dactylitis; uveitis was also more common compared with patients from the other geographical regions. Noticeably, biological DMARD use was much higher in Latin America compared with the other regions.
Table 1.
Characteristics of the axSpA patients from the ASAS-PerSpA study analysed in this study, stratified by geographical region
| Characteristics | Total (N = 2579) | Asia (n = 574) | Europe and North America (n = 988) | Latin America (n = 246) | Middle East and North Africa (n = 771) |
|---|---|---|---|---|---|
| Gender, male, % | 69 | 79 | 65 | 70 | 65 |
| Age, years, median (IQR) | 40 (31–51) | 34 (27–45) | 44 (35–53) | 42 (34–53) | 39 (32–49) |
| Symptom duration, years, median (IQR) | 11 (5–19) | 8 (4–15) | 13 (7–24) | 12 (5–20) | 10 (5–16) |
| HLA-B27 positive, % | 79** | 89* | 79** | 81** | 67*** |
| IBP (ASAS definition)a, % | 94 | 91 | 95 | 96 | 95 |
| Positive family history, % | 34 | 30 | 38 | 27 | 36 |
| Peripheral arthritis, % | 44 | 52 | 38 | 72 | 36 |
| Enthesitis, % | 45 | 53 | 37 | 71 | 42 |
| Dactylitis, % | 6 | 7 | 5 | 16 | 3 |
| Psoriasis, % | 8 | 4 | 13 | 4 | 5 |
| IBD, % | 5 | 1 | 7 | 4 | 6 |
| Acute anterior uveitis, % | 22 | 24 | 25 | 31 | 15 |
| Elevated CRP, % | 70 | 74 | 66 | 77 | 70 |
| Sacroiliitis on radiographsb, % | 78 | 85 | 73 | 75* | 79 |
| Sacroiliitis on MRIb, % | 82*** | 78*** | 77*** | 81*** | 93*** |
| SpA featuresc, mean (s.d.) | 4 (2) | 4 (1) | 4 (2) | 4 (2) | 3 (2) |
| Use of bDMARD, % | 33* | 39** | 25 | 58 | 31* |
| Use of NSAID, % | 99* | 99** | 99 | 99 | 98* |
| LOC regarding axSpA, mean (s.d.) | 8 (3) | 7 (3) | 8 (3) | 7 (4) | 9 (2) |
Four of five of the following features: onset before the age of 40 years, insidious onset, improvement with exercise, no improvement with rest, pain at night [21]. bBased on reading of local radiologists. cExcluding HLA-B27 status and sacroiliitis on imaging. *5–10% missing values **10–20% missing values ***20–40% missing values. bDMARD: biologic DMARD; LOC: level of confidence regarding the diagnosis.
Age at onset of axial symptoms
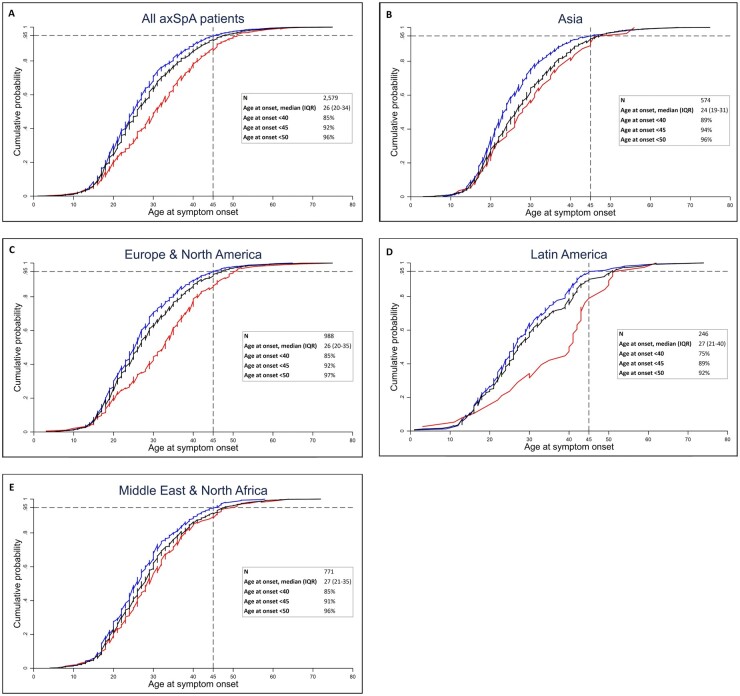
The median age at onset of axial symptoms in all included patients with axSpA was 26 years (IQR 20–34), with the lowest age at onset in Asia [24 (19–31)] followed by Europe and North America [26 (20–35)], Latin America [27 (21–40)] and Middle East and North Africa [27 (21–35)] (Fig. 1).
Fig. 1.
Cumulative distribution of the age at onset of axial symptoms, stratified by HLA-B27 status
(A) All included axSpA patients, (B) Asia, (C) Europe and North America, (D) Latin America and (E) Middle East and North Africa. The black lines represent all patients in each region, the blue lines represent HLA-B27-positive patients and the red lines represent HLA-B27-negative patients. The horizontal dashed line represents the 95% point and the vertical dashed line represents an age at onset of 45 years.
The majority (92%) of patients with axSpA had an age at onset of axial symptoms <45 years, with only a small variation across the various geographical regions (Fig. 1). This finding was even more pronounced in the HLA-B27-positive subgroup (Fig. 1 and Supplementary Table S2, available at Rheumatology online) in which 94% of patients had an age at onset of axial symptoms <45 years. Additionally, only in a very small proportion (4%) of patients did the axial complaints start after the age of 50 years.
Cumulative distribution plots showed that among all axSpA patients, 95% developed axial complaints before the age of 48 years and this was before the age of 46, 47, 51 and 48 years for the Asian, European and North American, Latin American and Middle Eastern and North African populations respectively (Fig. 1).
Patients with an onset of axial complaints at the age of ≥45 years were less often male, had a shorter median symptom duration, were less often HLA-B27 positive and had IBP less often compared with patients with an age at onset <45 years (Table 2). Elevated CRP and sacroiliitis on radiographs were also less frequent in patients with an age at onset ≥45 years.
Table 2.
Characteristics of the axSpA patients from the ASAS-PerSpA study analysed in this study, stratified by age at onset
| Characteristics | Total (N = 2579) | Age at onset <45 years (n = 2368) | Age at onset ≥45 years (n = 211) |
|---|---|---|---|
| Gender, male, % | 69 | 70 | 51 |
| Age, years, median (IQR) | 40 (31–51) | 39 (31–48) | 58 (53–64) |
| Symptom duration, years, median (IQR) | 11 (5–19) | 11 (5–20) | 6 (3–11) |
| HLA-B27 positive, % | 79** | 80** | 60*** |
| IBP ASAS definitiona, % | 94 | 95 | 87 |
| Positive family history, % | 34 | 35 | 25 |
| Peripheral arthritis, % | 44 | 43 | 50 |
| Enthesitis, % | 45 | 45 | 49 |
| Dactylitis, % | 6 | 6 | 7 |
| Psoriasis, % | 8 | 8 | 10 |
| IBD, % | 5 | 5 | 7 |
| Acute anterior uveitis, % | 22 | 23 | 17 |
| Elevated CRP, % | 70 | 71 | 61 |
| Sacroiliitis on pelvic radiographsb, % | 78 | 79 | 68 |
| Sacroiliitis on pelvic MRIb, % | 82*** | 83*** | 76*** |
| SpA featuresc, mean (s.d.) | 4 (2) | 4 (2) | 4 (2) |
| Use of bDMARD, % | 33* | 33* | 30* |
| Use of NSAID, % | 99* | 99* | 97* |
| r-axSpA, % | 79 | 80 | 73 |
| LOC regarding axSpA diagnosis, mean (s.d.) | 8 (3) | 8 (3) | 7 (3) |
Four of five of the following features: onset before the age of 40 years, insidious onset, improvement with exercise, no improvement with rest, pain at night [21]. bBased on reading of local radiologists. cExcluding HLA-B27 status and sacroiliitis on imaging. *5–10% missing values **10–20% missing values ***20–40% missing values. bDMARD: biologic DMARD; LOC: level of confidence regarding the diagnosis.
Association between HLA-B27 and age at onset
In the total included axSpA population, the median age at onset of axial symptoms of HLA-B27-positive patients was significantly lower than of HLA-B27-negative patients [25 years (IQR 19–32) vs 31 (22–39); P < 0.001]. This difference was found in each of the geographical regions: Asia 23 years (IQR 19–30) vs 28 (20–36), P = 0.009; Europe and North America 25 (19–32) vs 33 (22–40), P < 0.001; Latin America 26 (19–36) vs 40 (26–44), P < 0.001; Middle East and North Africa 25 (19–32) vs 29 (22–39), P = 0.008 (Fig. 1).
Linear regression models showed a significant effect of HLA-B27 status on the age at onset of axial symptoms in the total study population (P < 0.001) and all geographical regions (Asia, P = 0.006; Europe and North America, P < 0.001; Latin America, P < 0.001; Middle East and North Africa, P = 0.005).
Association between gender and age at onset
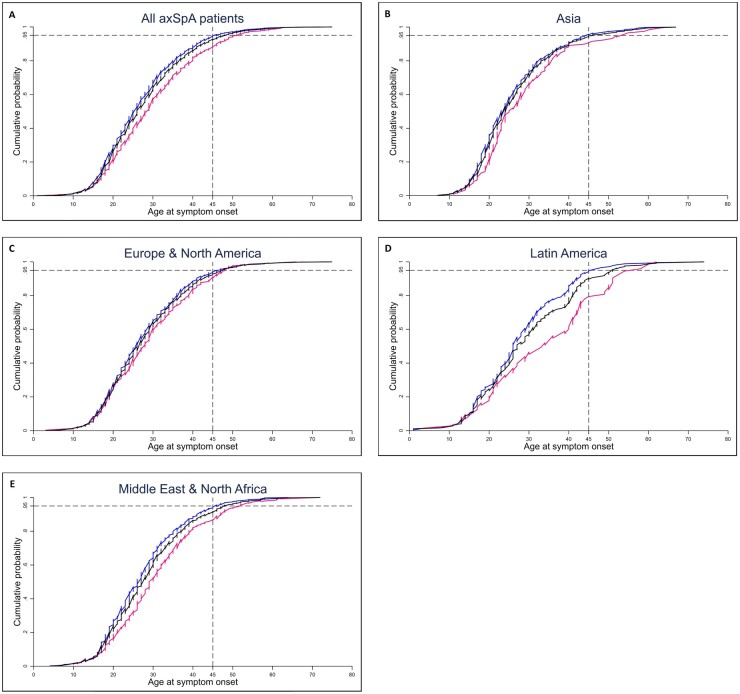
In the total included axSpA population, the median age at onset of axial symptoms of male patients [25 years (IQR 19–33)] was significantly lower than that of female patients [28 years (IQR 21–37)] (P < 0.001). This difference was seen in Asia [23 years (IQR 19–31) vs 28 (21–37), P = 0.015], Latin America [26 (19–34) vs 34 (22–43), P = 0.002] and the Middle East and North Africa [26 (20–33) vs 29 (23–37), P < 0.001], but was less pronounced in Europe and North America [26 (20–34) vs 28 (20–36), P = 0.053] (Fig. 2).
Fig. 2.
Cumulative distribution of the age at onset of axial symptoms, stratified by gender
(A) All included axSpA patients, (B) Asia, (C) Europe and North America, (D) Latin America and (E) Middle East and North Africa. The black lines represent all patients in each region, the blue lines represent male patients and the pink lines represent female patients. The horizontal dashed line represents the 95% point and the vertical dashed line represents an age at onset of 45 years.
Linear regression models showed a significant effect of gender on the age at onset of axial symptoms in the total study population (P < 0.001) and the Asian (P = 0.010), Latin American (P = 0.001) and Middle Eastern and North African (P < 0.001) populations, but just missed the significance level in the European and North American population (P = 0.054).
Multivariable model
First we tested whether there was collinearity between gender and HLA-B27 status, which was not the case, meaning gender and HLA-B27 did not have a linear relationship and could both be included in the linear regression model. In the multivariable model in the total included axSpA population, both HLA-B27 and male gender were associated with earlier disease onset. However, when stratified by region, an additional statistically significant effect of male gender independent of HLA-B27 was only found in Asia (Table 3), but a similar trend could be observed in all regions.
Table 3.
Multivariable models assessing the effect of HLA-B27 and gender on age at onset of axial symptoms
| Variable | Multivariable linear regression |
|
|---|---|---|
| β (95% CI) | P-value | |
| Total study population (N = 2063) | ||
| HLA-B27 | ||
| Negative | Ref. | |
| Positive | −4.35 (−5.45, −3.25) | <0.001 |
| Gender | ||
| Female | Ref. | |
| Male | −1.71 (−2.69, −0.74) | 0.001 |
| Asia (n = 525) | ||
| HLA-B27 | ||
| Negative | Ref. | |
| Positive | −3.68 (−6.44, −0.92) | 0.009 |
| Gender | ||
| Female | Ref. | |
| Male | −2.23 (−4.34, −0.11) | 0.039 |
| Europe and North America (n = 862) | ||
| HLA-B27 | ||
| Negative | Ref. | |
| Positive | −5.18 (−6.88, −3.48) | <0.001 |
| Gender | ||
| Female | Ref. | |
| Male | −0.92 (−2.38, 0.53) | 0.215 |
| Latin America (n = 195) | ||
| HLA-B27 | ||
| Negative | Ref. | |
| Positive | −7.30 (−11.59, −3.00) | 0.001 |
| Gender | ||
| Female | Ref. | |
| Male | −3.44 (−7.11, 0.23) | 0.066 |
| Middle East and North Africa (n = 481) | ||
| HLA-B27 | ||
| Negative | Ref. | |
| Positive | −2.44 (−4.36, −0.53) | 0.013 |
| Gender | ||
| Female | Ref. | |
| Male | −1.58 (−3.50, 0.34) | 0.106 |
Statistically significant associations are in bold.
Discussion
This study provides the first cumulative distribution of age at onset of axial symptoms in axSpA patients across the globe, showing that the vast majority of patients with axSpA have an age at onset before the age of 45 years in all parts of the world, which is consistent with the ASAS classification criteria for axSpA.
This study adds an important global perspective to what has been previously reported [1, 2, 4, 5]. Akin to what has been shown in previous studies [1, 2, 4], we found that patients with HLA-B27-negative disease had a significantly higher age at symptom onset than those with HLA-B27-positive disease and this held true in all geographical regions.
Contrary to Feldtkeller et al. [1], we showed a higher age at onset of axial symptoms in female patients compared with their male counterparts, which is in line with findings from other studies [2, 4, 5, 22, 23]. This difference may be partly explained by the fact that female patients were underrepresented in the study conducted by Feldtkeller et al. [1], possibly as a result of underdiagnosis of r-axSpA in women in the past [24]. Similar to Chung et al. [2], we found an additional effect of male gender and HLA-B27 on age at onset in multivariable analysis in the total included axSpA population, indicating a different association between HLA-B27 and age at onset for male and female patients. However, in multivariable analysis stratified by geographical region, an additional effect of male gender and HLA-B27 was only found in Asia. The current study adds important information to the work previously published, as the data presented in this study include patients with axSpA from across the globe. Also, patients had a rheumatologist-confirmed diagnosis rather than a self-reported diagnosis.
The precise pathophysiological mechanisms underlying axSpA remain unclear, but as different types of HLA-B27 are found in different parts of the world (e.g. HLA-B*27:05 in Europe and HLA-B*27:04 in Asia) and the association between HLA-B27 and axSpA varies between races [19], one might have expected to find more variation in age at onset and its association with HLA-B27 across geographical regions. Race was unavailable in the ASAS-PerSpA dataset, yet we expect the majority of the patients included in each geographical region to identify with its most prominent race, hence a clear effect of race would have been seen in the data. Additionally, many other factors are thought to have an influence on the occurrence of axSpA, such as other genetic factors and differences in the human microbiome and environmental factors, such as smoking [5, 25], which makes the relative consistency in age at onset all the more intriguing.
A potential limitation of this study is the fact that data were collected cross-sectionally based on patient records and patient-reported information, which has resulted in some missing data, especially regarding HLA-B27 status, as this was not specifically analysed for this study. Nonetheless, all geographical regions contained both patients with HLA-B27-positive and -negative disease and patients whose HLA-B27 status was unknown were not different than those with non-missing data (data not shown).
Conclusion
Irrespective of geographical region, the majority of axSpA patients had an age at onset of axial disease before the age of 45 years and being an HLA-B27 carrier and male gender were associated with earlier disease onset around the globe, yet an independent effect of male gender on top of HLA-B27 was only found in Asian patients. These results provide crucial data for diagnosis, classification and policies aimed at improving recognition of axSpA.
Supplementary Material
Acknowledgements
This study was conducted under the umbrella of the ASAS. We would like to acknowledge all the patients and investigators who participated in this research. We would like to thank the ASAS-PerSpA Steering Committee members, ClinInfo and the pharmaceutical companies supporting this initiative.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article. The ASAS-PerSpA study was conducted under the umbrella of the ASAS with unrestricted grants from AbbVie, Pfizer, Eli Lilly, Novartis, UCB, Janssen and Merck. The funders did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
For this study we used data from the ASAS-PerSpA dataset. Therefore we kindly refer any interested parties to the first author of the original ASAS-PerSpA publication.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Anne Boel, Rheumatology Department, Leiden University Medical Centre, Leiden, The Netherlands.
Clementina López-Medina, Rheumatology Department, Cochin Hospital, Assistance Publique Hôpitaux de Paris; INSERM (U1153) Clinical Epidemiology and Biostatistics, University of Paris, Paris, France; Rheumatology Department, Reina Sofia University Hospital, Maimonides Institute of Biomedical Research of Cordoba, Cordoba, Spain.
Désirée M F M van der Heijde, Rheumatology Department, Leiden University Medical Centre, Leiden, The Netherlands.
Floris Alexander van Gaalen, Rheumatology Department, Leiden University Medical Centre, Leiden, The Netherlands.
References
- 1. Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6. [DOI] [PubMed] [Google Scholar]
- 2. Chung HY, Machado P, van der Heijde D, D’Agostino M-A, Dougados M. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011;70:1930–6. [DOI] [PubMed] [Google Scholar]
- 3. Skare TL, Leite N, Bortoluzzo AB et al. Effect of age at disease onset in the clinical profile of spondyloarthritis: a study of 1424 Brazilian patients. Clin Exp Rheumatol 2012;30:351–7. [PubMed] [Google Scholar]
- 4. Rudwaleit M, Haibel H, Baraliakos X et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 5. Ciurea A, Scherer A, Weber U et al. Age at symptom onset in ankylosing spondylitis: is there a gender difference? Ann Rheum Dis 2014;73:1908–10. [DOI] [PubMed] [Google Scholar]
- 6. Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep 2018;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brophy S, Calin A. Ankylosing spondylitis: interaction between genes, joints, age at onset, and disease expression. J Rheumatol 2001;28:2283–8. [PubMed] [Google Scholar]
- 8. Braun J, Sieper J. Classification, diagnosis, and referral of patients with axial spondyloarthritis. Rheum Dis Clin North Am 2012;38:477–85. [DOI] [PubMed] [Google Scholar]
- 9. Olivieri I, Salvarani C, Cantini F, Ciancio G, Padula A. Ankylosing spondylitis and undifferentiated spondyloarthropathies: a clinical review and description of a disease subset with older age at onset. Curr Opin Rheumatol 2001;13:280–4. [DOI] [PubMed] [Google Scholar]
- 10. van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum 1984;27:241–9. [DOI] [PubMed] [Google Scholar]
- 11. Said-Nahal R, Miceli-Richard C, Berthelot JM et al. The familial form of spondylarthropathy: a clinical study of 115 multiplex families. Groupe Français d’Etude Génétique des Spondylarthropathies. Arthritis Rheum 2000;43:1356–65. [DOI] [PubMed] [Google Scholar]
- 12. Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006;54:569–78. [DOI] [PubMed] [Google Scholar]
- 13. Rudwaleit M, van der Heijde D, Landewé R et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 14. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 15. Boel A, Molto A, van der Heijde D et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. [DOI] [PubMed] [Google Scholar]
- 16. Bendahan LT, Machado NP, Mendes JG, Oliveira TL, Pinheiro MM. Performance of the classification criteria in patients with late-onset axial spondyloarthritis. Mod Rheumatol 2018;28:174–81. [DOI] [PubMed] [Google Scholar]
- 17. Chen HA, Chen CH, Liao HT et al. Clinical, functional, and radiographic differences among juvenile-onset, adult-onset, and late-onset ankylosing spondylitis. J Rheumatol 2012;39:1013–8. [DOI] [PubMed] [Google Scholar]
- 18. Dean LE, Jones GT, MacDonald AG et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–7. [DOI] [PubMed] [Google Scholar]
- 19. Khan MA. Polymorphism of HLA-B27: 105 subtypes currently known. Curr Rheumatol Rep 2013;15:362. [DOI] [PubMed] [Google Scholar]
- 20. López-Medina C, Molto A, Sieper J et al. Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: results of the worldwide, cross-sectional ASAS-PerSpA study. RMD Open 2021;7:e001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sieper J, van der Heijde D, Landewé R et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 22. Ortolan A, van Lunteren M, Ramiro S et al. Are gender-specific approaches needed in diagnosing early axial spondyloarthritis? Data from the SPondyloArthritis Caught Early cohort. Arthritis Res Ther 2018;20:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tournadre A, Pereira B, Lhoste A et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res 2013;65:1482–9. [DOI] [PubMed] [Google Scholar]
- 24. Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol 2000;12:239–47. [DOI] [PubMed] [Google Scholar]
- 25. de Koning A, Schoones JW, van der Heijde D, van Gaalen FA. Pathophysiology of axial spondyloarthritis: consensus and controversies. Eur J Clin Invest 2018;48:e12913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For this study we used data from the ASAS-PerSpA dataset. Therefore we kindly refer any interested parties to the first author of the original ASAS-PerSpA publication.