Abstract
Objectives
Psoriasis impacts health-related quality of life (HRQoL) in PsA patients. However, this is not adequately measured with a general HRQoL questionnaire. The aim of this study was to quantify the degree of psoriasis evolution in PsA patients over the first year of follow-up and to evaluate whether the impact of psoriasis on HRQoL can be adequately measured with a dermatology-specific HRQoL questionnaire.
Methods
Data were used from PsA patients in the Dutch south west Early Psoriatic Arthritis cohort. Psoriasis severity was measured with the Psoriasis Area and Severity Index (PASI). Dermatology-specific HRQoL was assessed with the Skindex-17 questionnaire. We used a Sankey diagram to illustrate the evolution of psoriasis severity during the first year of follow-up. To assess the association between psoriasis severity and the symptoms and psychosocial subscale of the Skindex-17, a linear regression analysis with hierarchical variable selection and zero-inflated negative binominal regression analysis were performed, respectively.
Results
We included 644 patients; 109 (17%) patients had no psoriasis (PASI = 0), 456 (71%) had mild psoriasis (PASI < 7), 56 (9%) had moderate psoriasis (PASI 7–12) and 23 (4%) had severe psoriasis (PASI > 12). Psoriasis severity did not fluctuate much during the first year. PASI was significantly associated with both subscales of the Skindex-17 at baseline and 12 months.
Conclusion
Psoriasis severity in PsA patients is mostly mild but impacts HRQoL when measured using a dermatology-specific HRQoL questionnaire. For optimal management of PsA patients, we recommend rheumatologists acquire information on skin burden by using a dermatology-specific HRQoL questionnaire.
Keywords: PsA, health-related quality of life, psoriasis
Rheumatology key messages.
Psoriasis severity in psoriatic arthritis patients is mostly mild with minor fluctuations during follow-up.
Skin burden in psoriatic arthritis patients is adequately measured with a dermatology-specific health-related quality of life questionnaire.
Introduction
PsA is an auto-inflammatory disorder affecting multiple parts of the body. Key symptoms include arthritis, enthesitis, dactylitis, spondylitis and psoriasis [1]. In up to 30% of patients with psoriasis in secondary care, PsA will eventually develop [2].
Both psoriasis and PsA are known to impact health-related quality of life (HRQoL) [3, 4]. A recent study by Strober et al. in a real-world cohort of psoriasis patients reported an association between severity of psoriasis and HRQoL, mainly on the domains of fatigue, itch and pain. Work productivity impairment was associated with increased disease activity [3]. When assessing HRQoL in PsA patients, more disease manifestations should be taken into account, compared with psoriasis patients. PsA patients report high levels of pain and functional disability, as well as a high prevalence of anxiety and depression symptoms, ranging from 7.9% to 36.6% [5]. Patients with PsA have a poorer HRQoL compared with patients with only psoriasis, given the added burden of additional disease manifestations [6].
Previous research by our group showed that general HRQoL, measured with the Short Form-36 (SF-36), was not negatively affected by psoriasis severity, but mainly influenced by inflammatory pain stemming from tender joints, entheses and the back [7]. With PsA being a multifaceted disease, it is plausible that psoriasis severity influences general HRQoL to a lesser degree than pain.
However, the effect of psoriasis on HRQoL within PsA should not be neglected. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) advises rheumatologists to involve a dermatologist in the management of PsA patients with severe psoriasis [8]. Because mild psoriasis in PsA patients may respond well to the applied DMARD treatment, assessment of psoriasis may not always be a priority for rheumatologists in daily practice. To date, the extent of psoriasis involvement and its impact on HRQoL within PsA patients is not completely understood.
Therefore, in order to assess the burden of psoriasis in early PsA patients and whether more attention from rheumatologists is warranted, the aim of this study is (i) to quantify the degree of psoriasis evolution in patients with early psoriatic arthritis during the first year of follow-up and (ii) to evaluate whether the impact of psoriasis on HRQoL in PsA can be adequately measured with a dermatology-specific HRQoL questionnaire.
Methods
Patients and setting
Data from the first year of follow-up were used from patients included in the Dutch south-west Early Psoriatic ARthritis (DEPAR) cohort between July 2013 and March 2020. The DEPAR cohort consists of PsA patients who were newly diagnosed by their treating rheumatologist. All patients received usual care. The study design of the DEPAR study was described earlier [9]. This study was approved by the local medical research ethics committee of the University Medical Center Rotterdam (MEC-2012–549). Written informed consent was obtained for all study participants according to the Declaration of Helsinki.
Data collection
Clinical data, including PASI score, were collected by trained research nurses at baseline, 3, 6, 9 and 12 months. Data on demographics, medical history, comorbidities, family history and medication was also collected by trained research nurses during each visit.
Health-related quality of life
Patients completed multiple questionnaires within one week before or after their research-nurse visits. Psoriasis severity was assessed using the Psoriasis Area and Severity Index (PASI) and categorized as: no psoriasis (PASI = 0), mild psoriasis (PASI < 7), moderate psoriasis (PASI 7–12) and severe psoriasis (PASI > 12) [10]. General HRQoL was assessed with the Short-Form 36 (SF-36), consisting of a physical component scale and mental component scale. A higher score indicates a better HRQoL [11].
Dermatology-specific health-related quality of life
Dermatology-specific HRQoL was measured with the Skindex-17 questionnaire. This questionnaire consists of 17 questions that patients answer on a 5-point Likert scale. A symptoms subscale and a psychosocial subscale are then generated. The symptoms subscale ranges from 1 to 10 and has to be interpreted as follows: 0–4 few symptoms; 5–10 a lot of symptoms. The psychosocial subscale ranges from 0 to 24 and has to be interpreted as follows: 0–4 little impairment; 5–9 moderate impairment; 10–24 high impairment [12]. We opted for the Skindex-17 questionnaire above the Dermatology Life Quality Index due to the two separate scores it computes on symptoms and psychosocial skin burden.
Patient-reported outcomes (PROs)
The included patient-reported outcomes (PROs) were fatigue, anxiety, depression and pain. Fatigue was measured with the Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ) [13]. Anxiety and depression were measured with the Hospital Anxiety and Depression Scale (HADS) questionnaire [14] and pain with a Visual Analogue Scale (VAS) score [15].
Statistical analysis
Simple descriptive statistics were used for demographics and to quantify the degree of psoriasis involvement. Evolution of psoriasis severity was measured at baseline, 3, 6, 9 and 12 months and illustrated using a Sankey diagram.
To evaluate the association between psoriasis severity and the symptoms subscale of the Skindex-17 questionnaire, a linear regression model with hierarchical variable selection of complete cases was performed at baseline and 12 months. Three blocks of variables were added consecutively, namely demographic characteristics, clinical characteristics and the aforementioned relevant PROs.
To assess the relationship between psoriasis severity and the psychosocial subscale of the Skindex-17 questionnaire, a zero-inflated negative binominal regression analysis of complete cases was performed at baseline and 12 months. We opted for a zero-inflated model due to overdispersion of zero-values, which violated the assumptions of a linear regression model. In this model, the three aforementioned blocks of variables were added in three consecutive stages. All statistical analyses were performed in STATA 15.1 (StataCorp, College Station, TX, USA).
Results
Patients
In total, 644 patients were included. Mean (s.d.) age was 49.7 (13.6) years and 49% (n = 316) were male (Table 1). A total of 109 (17%) patients had no measurable psoriasis, 456 (71%) had mild psoriasis, 56 (9%) had moderate psoriasis and 23 (3%) patients had severe psoriasis.
Table 1.
Baseline characteristics patient sample (n = 644)
| Psoriasis severityi |
||||||
|---|---|---|---|---|---|---|
| Total | None | Mild | Moderate | Severe | ||
| Demographic characteristics | ||||||
| Number of patients, n (%) | 644 (100) | 109 (17) | 456 (71) | 56 (9) | 23 (4) | |
| Age, mean (s.d.) | 49.7 (13.6) | 50.5 (13.5) | 50.3 (13.5) | 44.8 (13.1) | 46.2 (15.4) | |
| Male, n (%) | 316 (49) | 43 (39) | 225 (49) | 31 (55) | 17 (74) | |
| Psoriasis complaints preceding PsA diagnosis, years, median (IQR)b | 10.0 (3.0–20.7) | 10.6 (2.4–23.4) | 10.0 (2.9–20.3) | 9.8 (4.9–23.2) | 14.6 (6.2–21.1) | |
| Duration of joint complaints, months, median (IQR) | 11.0 (3.6–33.8) | 12.0 (4.0–36.0) | 11.0 (3.5–34.2)a | 11.5 (2.8–32.2) | 9.4 (3.3–34.5) | |
| Clinical characteristics | ||||||
| PASI, median (IQR) | 2.0 (0.4–4.4) | 0 (0–0) | 2.1 (0.8–3.6) | 8.4 (7.7–9.6) | 14 (13.1–18.2) | |
| Swollen joint count (66), median (IQR)c | 2 (0–4) | 1 (0–4) | 2 (1–4)c | 1 (0–4) | 5 (2–6)c | |
| Tender joint count (68), median (IQR) | 3 (1–7) | 2 (1–6) | 3 (1–7)c | 4 (1–8) | 5 (1–11)c | |
| DAPSA score, median (IQR)d | 15.9 (10.4–23.1) | 15.2 (11.1–21) | 15.3 (10–23.4) | 19.3 (12.6–23.2) | 21.3 (15–26.6) | |
| Health Related Quality of Life | ||||||
| General | ||||||
| SF-36e | Physical component scale, mean (s.d.) | 39.2 (8.6) | 39.2 (8.3) | 39.2 (8.8) | 39.4 (8.5) | 39.3 (8.1) |
| Mental component scale, median (IQR) | 49.6 (40.6–55.9) | 49.6 (39.9–55.0) | 49.8 (41.1–56.0) | 46.2 (40.6–55.1) | 47.3 (39.8–57.4) | |
| Dermatology specific | ||||||
| VAS psoriasis, median (IQR)f | 22 (6–46) | 5 (1–19) | 24 (8–46) | 47 (21–64) | 49 (27–76) | |
| Skindex-17 | Symptoms, median (IQR)e | 4 (2–6) | 2 (0–3) | 4 (2–6) | 6 (4–8) | 7 (5–9) |
| Psychosocial, median (IQR)g | 2 (0–8) | 0 (0–1) | 2 (0–8) | 7 (3–13) | 8 (5–16) | |
| Disease impact | ||||||
| Anxiety and depression | HADSh | |||||
| Anxiety score, median (IQR) | 4 (2–7) | 4 (3–6) | 4 (2–7) | 5 (3–8) | 6 (3–8) | |
| Anxiety score >8, n (%) | 99 (19) | 10 (11) | 75 (19) | 10 (24) | 4 (22) | |
| Depression score, median (IQR) | 3 (1–6) | 3 (1–6) | 3 (1–6) | 4 (2–7) | 3.5 (2–10) | |
| Depression score >8, n (%) | 85 (16) | 10 (11) | 64 (17) | 6 (14) | 5 (28) | |
4 missing.
62 missing.
1 missing.
171 missing.
94 missing.
95 missing.
96 missing.
110 missing.
No psoriasis (PASI 0), mild psoriasis (PASI < 7), moderate psoriasis (PASI 7–12) and severe psoriasis (PASI > 12).
DAPSA: Disease Activity in Psoriatic Arthritis; HADS: Hospital Anxiety and Depression Scale; IQR: interquartile range; PASI: Psoriasis Area and Severity Index; SF-36: Short Form 36; VAS: Visual Analogue Scale.
Psoriasis complaints preceded PsA diagnosis with a median of 10.0 years (IQR 3.0–20.7). Median (IQR) PASI score was 2 (0.4–4.4) in the entire group. Median (IQR) duration of joint complaints prior to PsA diagnosis was 11.0 (3.6–33.8) months. Patients had a median (IQR) swollen joint count (66) of 2 (0–4) and tender joint count (68) of 3 (1–7).
Patients had a mean (s.d.) score of 39.2 (8.6) on the physical component scale of the SF-36. Median (IQR) score on the mental component scale was 49.6 (40.6–55.9). On the symptoms and psychosocial subscale of the Skindex-17, patients had a median (IQR) score of 4 (2–6) and 2 (0–8), respectively. Of the patients who filled in the Skindex-17 questionnaire, 21% (n = 116) reported never having had itch in the previous week. The majority, however, reported itch: 13% (n = 73) rarely; 32% (n = 175) sometimes; 27% (n = 146) often; and 7% (n = 39) always. SF-36 scores and Skindex-17 scores across psoriasis severity categories are shown in Fig. 1. Median (IQR) HADS anxiety score was 4 (2–7) and median (IQR) depression score was 3 (1–6). At baseline, respectively, 19% and 16% of patients scored ≥8 regarding HADS anxiety and depression. These patients are more suggestive of the presence of an anxiety disorder or depression.
Fig. 1.
Short Form-36 scores and Skindex-17 scores across psoriasis severity categories
(A, B) Mean SF36 Physical component scale score and median Mental component scale score per psoriasis severity category at baseline. (C, D) Median Skindex-17 psychosocial score and median symptoms score per psoriasis severity category at baseline. No psoriasis (PASI = 0), mild psoriasis (PASI < 7), moderate psoriasis (PASI 7–12) and severe psoriasis (PASI > 12).
Psoriasis evolution
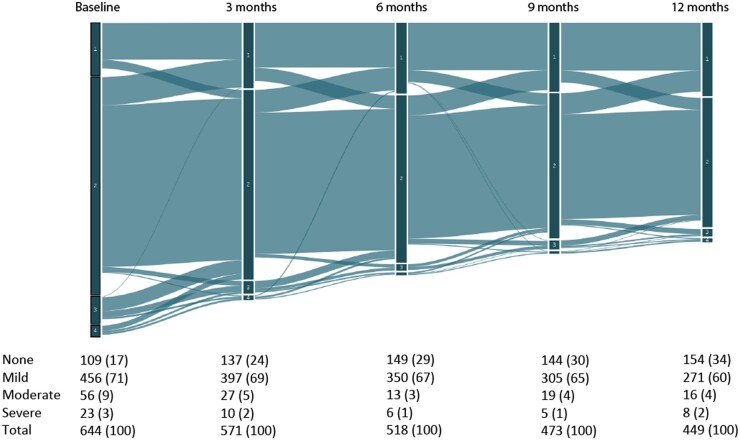
The changes in psoriasis severity over the course of the first year are shown in the Sankey diagram (Fig. 2). The group of patients with no psoriasis increased from 109 patients (17%) at baseline to 154 patients (34%) at 12 months. The percentage of patients with severe psoriasis remained stable with 3% (n = 23) at baseline and 2% (n = 8) at 12 months. The majority of patients remained in the same category of psoriasis severity throughout the year with the majority of patients fluctuating between having no psoriasis and having mild psoriasis. The group of patients with moderate and severe psoriasis was the smallest and also decreased in size over the course of the first year.
Fig. 2.
Sankey diagram of changes in psoriasis severity during the first year of follow-up
Divided into PASI severity category 1: no psoriasis (PASI = 0); 2: mild psoriasis (PASI < 7); 3: moderate psoriasis (PASI 7–12); 4: severe psoriasis (PASI > 12).
Psoriasis and skindex-17
Symptoms subscale
Complete cases were available for 465 (72%) patients at baseline and 339 (53%) patients at 12 months. Correlations between the variables and regression statistics are reported in Table 2. At baseline, age (ß −0.02, 95% CI −0.04, −0.00), sex (ß 0.89, 95% CI 0.43, 1.34) and BMI (ß 0.09, 95% CI 0.04, 0.13) were significantly associated with the Skindex-17 symptoms subscale when only demographics were added to the model. Age lost its significance when clinical characteristics and PROs were added, but sex and BMI did not. Psoriasis severity remained significant throughout the entire model, irrespective of the number of blocks added. Besides psoriasis severity (PASI), sex and BMI, also swollen joint count (ß −0.08, 95% CI −0.12, −0.03), tender joint count (ß 0.06, 95% CI 0.01, 0.10) and anxiety (ß 0.13, 95% CI 0.05, 0.20) were associated with the symptoms subscale of the Skindex-17. The variation of the model increased from 7% with only demographics added to 38% when all three blocks of variables were added.
Table 2.
Linear regression model with hierarchical variable selection at baseline (n = 465) and 12 months (n = 339) of the symptoms subscale of the Skindex-17
| Baseline |
12 months |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step | 1 |
2 |
3a |
1 |
2 |
3a |
||||||
| Independent variables | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI | ß | 95% CI |
| Demographic variables | ||||||||||||
| Age (years) | −0.02 | −0.04 to−0.00 | −0.01 | −0.03, 0.00 | −0.01 | −0.02, 0.01 | −0.01 | −0.03,0.01 | 0.00 | −0.02, 0.02 | 0.01 | −0.01, 0.03 |
| Sex | 0.89 | 0.43, 1.34 | 0.94 | 0.52, 1.36 | 0.71 | 0.30, 1.13 | 0.31 | −0.24, 0.83 | 0.31 | −0.17, 0.80 | 0.10 | −0.39, 0.59 |
| BMI | 0.09 | 0.04, 0.13 | 0.06 | 0.02, 0.10 | 0.05 | 0.01, 0.09 | −0.01 | −0.03, 0.02 | −0.02 | −0.04, 0.01 | −0.02 | −0.04, 0.01 |
| Clinical characteristics | ||||||||||||
| Swollen joint count (66) | −0.08 | −0.13 to −0.03 | −0.08 | −0.12 to −0.03 | −0.10 | −0.23, 0.02 | −0.08 | −0.20, 0.03 | ||||
| Tender joint count (68) | 0.08 | 0.04, 0.12 | 0.06 | 0.01, 0.10 | 0.11 | 0.05, 0.16 | 0.05 | −0.01, 0.11 | ||||
| Enthesitis (LEI/MASES) | 0.13 | 0.05, 0.21 | 0.07 | −0.00, 0.15 | 0.13 | 0.02, 0.24 | 0.07 | −0.03, 0.18 | ||||
| Dactylitis (LDI) | 0.28 | −0.38, 0.93 | 0.45 | −0.18, 1.08 | −0.24 | −1.81, 1.33 | −0.44 | −1.96, 1.08 | ||||
| Psoriasis (PASI) | 0.34 | 0.28, 0.40 | 0.32 | 0.26, 0.38 | 0.39 | 0.31, 0.47 | 0.37 | 0.29, 0.45 | ||||
| Patient reported outcomes | ||||||||||||
| Fatigue (BRAF MDQ) | −0.01 | −0.05, 0.03 | 0.02 | −0.01, 0.06 | ||||||||
| Anxiety (HADS) | 0.13 | 0.05, 0.20 | 0.04 | −0.05, 0.12 | ||||||||
| Depression (HADS) | −0.06 | −0.14, 0.03 | −0.00 | −0.11, 0.11 | ||||||||
| Pain (VAS) | −0.01 | −0.03, 0.00 | 0.00 | −0.02, 0.02 | ||||||||
| BRAF*Pain(VAS) | 0.00 | 0.00, 0.00 | 0.00 | −0.00, 0.00 | ||||||||
| R2 | 0.07 | 0.30 | 0.38 | 0.01 | 0.26 | 0.33 | ||||||
Final model; the reference category for sex is male; for dactylitis is none. Significant coefficients in bold.
BRAF MDQ: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire; HADS: Hospital Anxiety and Depression Scale; LDI: Leeds Dactylitis Index; LEI/MASES: Leeds Enthesitis Index/Maastricht Ankylosing Spondylitis Enthesitis Score; PASI: Psoriasis Area and Severity Index; VAS: Visual Analogue Scale.
At 12 months, demographic characteristics was not associated with the symptoms subscale. Tender joint count (ß 0.11, 95% CI 0.05, 0.16) and enthesitis (ß 0.13, 95% CI 0.02, 0.24) had a significant association when only demographic and clinical characteristics were added to the model, but lost their association when PROs were added. Psoriasis severity (PASI) was associated with the symptoms subscale of the Skindex-17 and kept its statistical significance irrespective of how many other covariates were added. The variation of the model increased from 1% to 33% when all three blocks of variables were added.
Psychosocial subscale
Complete cases were available for 465 (72%) patients at baseline and 339 (53%) patients at 12 months. Results are shown in Table 3. At baseline, 37% of the patients had a zero-value on the Skindex17 psychosocial. The odds of having a zero value decreased by 0.52 (OR; 95% CI: 0.41, 0.66) per PASI point increase when only adding the demographic variable block. The strength of the PASI association was similar when adding the other two variable blocks. For those patients that had a Skindex17-psychosocial value higher than zero, no demographic covariates were associated with higher levels of the Skindex17-psychosocial score. Of the clinical characteristics, tender joint count [incidence rate ratio (IRR) 1.04, 95% CI 1.01, 1.06] and PASI (IRR 1.07, 95% CI 1.03, 1.10) were associated with higher values. When adding the PROs, higher levels of anxiety (IRR 1.07, 95% CI 1.04, 1.11) were associated with higher Skindex17-psychosocial scores. With all covariates in the model, the strength of the associations slightly changed but all kept their statistical significance, and dactylitis score became significantly associated. We did not observe associations with age, sex, BMI, self-reported pain and fatigue.
Table 3.
Zero-inflated negative binominal regression analysis at baseline (n = 465) and 12 months (n = 339) of the psychosocial subscale of the Skindex-17
| Baseline |
12 months |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | IRR | (95% CI) | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | IRR | 95% CI |
| Demographic variables | ||||||||||||
| Age (years) | 0.99 | 0.99, 1.00 | 0.99 | 0.99, 1.00 | 1.00 | 0.99, 1.00 | 0.99 | 0.98, 1.01 | 1.00 | 0.98, 1.01 | 1.00 | 0.99, 1.02 |
| Sex | 1.12 | 0.90, 1.40 | 1.13 | 0.91, 1.42 | 0.99 | 0.81, 1.20 | 0.99 | 0.70, 1.42 | 0.97 | 0.68, 1.37 | 0.97 | 0.72, 1.32 |
| BMI | 1.01 | 0.99, 1.03 | 1.01 | 0.99, 1.03 | 1.01 | 0.99, 1.02 | 1.00 | 0.96, 1.03 | 1.00 | 0.96, 1.03 | 1.00 | 0.97, 1.04 |
| Clinical characteristics | ||||||||||||
| Swollen joint count (66) | 0.98 | 0.96, 1.01 | 0.98 | 0.96, 1.00 | 0.97 | 0.88, 1.07 | 0.99 | 0.92, 1.07 | ||||
| Tender joint count (68) | 1.04 | 1.01, 1.06 | 1.02 | 1.00, 1.04 | 1.05 | 1.01, 1.09 | 1.01 | 0.97, 1.04 | ||||
| Enthesitis (LEI/MASES) | 1.03 | 0.99, 1.07 | 0.99 | 0.96, 1.03 | 1.03 | 0.96, 1.11 | 1.02 | 0.96, 1.09 | ||||
| Dactylitis | 1.15 | 0.82, 1.62 | 1.39 | 1.05, 1.84 | 0.14 | 0.03, 0.78 | 0.23 | 0.04, 1.21 | ||||
| Psoriasis (PASI) | 1.07 | 1.03, 1.10 | 1.05 | 1.02, 1.08 | 1.11 | 1.04, 1.18 | 1.10 | 1.05, 1.15 | ||||
| Patient reported outcomes | ||||||||||||
| Fatigue (BRAF MDQ) | 1.01 | 1.00, 1.02 | 1.01 | 1.00, 1.03 | ||||||||
| Anxiety (HADS) | 1.07 | 1.04, 1.11 | 1.07 | 1.02, 1.12 | ||||||||
| Depression (HADS) | 1.03 | 0.99, 1.07 | 1.04 | 0.98, 1.10 | ||||||||
| Pain (VAS) | 1.00 | 1.00, 1.01 | 1.00 | 0.99, 1.01 | ||||||||
| Zero-inflated: PASIb | 0.52 | 0.41, 0.66 | 0.52 | 0.39, 0.68 | 0.54 | 0.42, 0.68 | 0.36 | 0.19, 0.70 | 0.36 | 0.15, 0.86 | 0.43 | 0.24, 0.78 |
Final model; the reference category for sex is male; for dactylitis is none. Significant values in bold.
Reported as odds ratio.
BRAF MDQ: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire; HADS: Hospital Anxiety and Depression Scale; LDI: Leeds Dactylitis Index; LEI/MASES: Leeds Enthesitis Index/Maastricht Ankylosing Spondylitis Enthesitis Score; PASI: Psoriasis Area and Severity Index; VAS: Visual Analogue Scale.
At 12 months, 50% of the patients scored 0 on the Skindex17-psychosocial. The odds of having a zero value decreased by 0.36 (OR; 95% CI: 0.19, 0.70) per PASI point with only demographics entered. For those that had scores above 0, we observed similar results when evaluating the demographic variables and clinical variables. However, when adding all the variables to the model only PASI (IRR 1.10, 95% CI 1.05, 1.15) and anxiety (IRR 1.07, 95% CI 1.02, 1.12) were significantly associated.
Discussion
In order to assess whether psoriasis deserves more attention from rheumatologists in daily clinical practice, we firstly quantified the degree of psoriasis involvement in a cohort of patients with early psoriatic arthritis. Secondly, we evaluated whether the impact of psoriasis on health-related quality is adequately measured with the dermatology-specific Skindex-17 questionnaire, because a general HRQoL such as the SF-36 questionnaire does not measure this impact.
Regarding the evolution of psoriasis during the first year of follow-up, the majority of PsA patients had mild psoriasis (PASI < 7) and remained in this category. The group of patients with no psoriasis (PASI = 0) grew from 109 patients (17%) at baseline to 154 patients (34%) at 12 months and the group of severe psoriasis remained stable. This shows that overall, psoriasis severity improved over the first year of follow-up, although the majority still had mild psoriasis.
When measured with the dermatology-specific Skindex-17 questionnaire, in our sample of PsA patients, impaired HRQoL was seen with increasing psoriasis severity. The group of patients with no psoriasis reported having ‘few symptoms’ on the symptoms subscale and ‘little impairment’ on the psychosocial subscale, while the group with severe psoriasis reported ‘a lot of symptoms’ and ‘moderate impairment’, respectively.
PASI score was significantly associated with both subscales of the Skindex-17 at baseline and 12 months. At baseline, other variables such as tender joint count and HADS anxiety score showed an association with the Skindex-17. At 12 months, only PASI score was associated with the Skindex-17 symptoms subscale, meaning this questionnaire indeed measured the impact of psoriasis severity on dermatology-specific HRQoL. This time-related difference may be due to the fact that at baseline patients recently received their PsA diagnosis and are not yet fully able to cope with their condition. Besides PASI, HADS anxiety score was also associated with the psychosocial subscale at 12 months. It is known that patients with psoriasis report a higher degree of anxiety than healthy controls and that anxiety disorders impair quality of life [16–18]. It is worth mentioning that the HADS questionnaire is a screening tool. Nonetheless, it is interesting that impact of anxiety is also detected in a dermatology-specific HRQoL questionnaire [14].
Seeing that PASI was the only variable that correlated well with the Skindex-17 symptoms subscale and psychosocial subscale at both baseline and 12 months, and that there was no clear relation with any musculoskeletal PsA features, it indicates that the Skindex-17 is a good tool for evaluating skin burden in PsA patients. The SF-36 does not seem to be influenced by psoriasis severity, indicating that rheumatologists should use a dermatology-specific HRQoL questionnaire or at least specifically ask patients about their skin burden. We found it interesting to see that even severe psoriasis was not reflected in the SF-36 questionnaire by means of a lower score. Therefore, general HRQoL questions will probably reflect different disease features, such as joint inflammation and pain and will not capture the impact of skin burden.
To our knowledge, the burden of psoriasis in early psoriatic arthritis patients has not been reported to this extent before. In a recent observational real-world PsA cohort study from Athanassiou et al., the burden of psoriasis was evaluated in 222 patients, using the Dermatology Life Quality Index questionnaire. When corrected for arthritis activity with the DAS28-CRP, an increase in mean Dermatology Life Quality Index score was observed, leading the researchers to conclude that increasing psoriasis severity negatively impacts dermatology-specific HRQoL [19]. These observations are in accordance with our study.
The effect of psoriasis on HRQoL in psoriasis patients has been studied more elaborately. A systematic review by de Korte et al. reported mean (s.d.) summary scores of the SF-36 physical and mental component scale of 41.2 (14.2)–55.5 (14.4) and 45.2 (12.1)–50.9 (9.3), respectively, with a score of 50 being the average HRQoL of the general population [20]. We observed similar SF-36 scores in our sample.
In another study among psoriasis patients, researchers looked at the association between the eight subscales of the SF-36 and components of the PASI score in assessing HRQoL. In this study, no association was seen between the compound scores of the SF-36 and PASI scores. They did find a significant correlation between desquamation on the upper limbs and mental health and bodily pain (r =−0.23 and r =−0.28, respectively) and between desquamation on the scalp and mental health (r =−0.29) [21]. This study among psoriasis patients highlighted that the burden of psoriasis is not fully captured when only compound scores of disease severity are used. In our study, however, we did see that compound scores of psoriasis severity and the Skindex-17 subscales were positively correlated.
In our study, a relatively small number of patients with severe psoriasis (PASI > 12) was included. This could be considered a limitation. However, as this is a real-world cohort, our results are applicable to rheumatology practices in the Netherlands. Strengths of this study include the large number of patients with a follow-up of one year. To our knowledge, this is also the first study to assess psoriasis to this extent in a cohort of PsA patients. Further research could focus on assessing what questions from dermatology-specific questionnaires are most relevant for rheumatologists to ask in daily clinical practice.
In conclusion, psoriasis severity in PsA patients is mostly mild, but impacts HRQoL when measured using a dermatology-specific HRQoL questionnaire. For optimal management of PsA patients, we therefore recommend rheumatologists to additionally acquire information on the degree of psoriatic involvement and not merely ask patients about their general wellbeing. In our opinion, this information is valuable for the adequate assessment of HRQoL.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Fazira R Kasiem, Department of Rheumatology, Erasmus Medical Center.
Marc R Kok, Department of Rheumatology and clinical immunology, Maasstad Hospital, Rotterdam.
Jolanda J Luime, Department of Rheumatology, Erasmus Medical Center.
Ilja Tchetverikov, Department of Rheumatology, Albert Schweitzer Hospital, Dordrecht.
Kim Wervers, Department of Rheumatology, Erasmus Medical Center.
Lindy-Anne Korswagen, Department of Rheumatology, Franciscus Gasthuis & Vlietland, Rotterdam.
Nastasja H A M Denissen, Department of Rheumatology, Amphia Hospital, North Brabant.
Yvonne P M Goekoop-Ruiterman, Department of Rheumatology, Haga Hospital, Zuid-holland.
Maikel van Oosterhout, Department of Rheumatology, Groene Hart Ziekenhuis, Gouda.
Faouzia Fodili, Department of Rheumatology, Reumazorg Zuid West Nederland, Roosendaal, Netherlands.
Johanna M W Hazes, Department of Rheumatology, Erasmus Medical Center.
Marijn Vis, Department of Rheumatology, Erasmus Medical Center.
Data availability statement
The data can be shared on reasonable request to the corresponding author (f.kasiem@erasmusmc.nl).
References
- 1. Mease PJ, Karki C, Palmer JB et al. Clinical and patient-reported outcomes in patients with Psoriatic Arthritis (PsA) by body surface area affected by psoriasis: results from the Corrona PsA/Spondyloarthritis registry. J Rheumatol 2017;44:1151–8. [DOI] [PubMed] [Google Scholar]
- 2. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 3. Strober B, Greenberg JD, Karki C et al. Impact of psoriasis severity on patient-reported clinical symptoms, health-related quality of life and work productivity among US patients: real-world data from the Corrona Psoriasis Registry. BMJ Open 2019;9:e027535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolli SS, Amin SD, Pona A, Cline A, Feldman SR. Psychosocial impact of psoriasis: a review for dermatology residents. Cutis 2018;102:21–5. [PubMed] [Google Scholar]
- 5. Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018;14:405–17. [DOI] [PubMed] [Google Scholar]
- 6. Rosen CF, Mussani F, Chandran V et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology 2012;51:571–6. [DOI] [PubMed] [Google Scholar]
- 7. Wervers K, Luime JJ, Tchetverikov I et al. Influence of disease manifestations on health-related quality of life in early psoriatic arthritis. J Rheumatol 2018;45:1526–31. [DOI] [PubMed] [Google Scholar]
- 8. Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology 2020;59:i37–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kasiem FR, Luime JJ, Vis M et al. Lessons learned from clinical phenotypes in early psoriatic arthritis: the real-world Dutch south west Early Psoriatic ARthritis study. Scand J Rheumatol 2021;50:124–31. [DOI] [PubMed] [Google Scholar]
- 10. Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 11. Leung YY, Ho KW, Zhu TY et al. Testing scaling assumptions, reliability and validity of medical outcomes study short-form 36 health survey in psoriatic arthritis. Rheumatology 2010;49:1495–501. [DOI] [PubMed] [Google Scholar]
- 12. Nijsten TE, Sampogna F, Chren MM, Abeni DD. Testing and reducing skindex-29 using Rasch analysis: skindex-17. J Invest Dermatol 2006;126:1244–50. [DOI] [PubMed] [Google Scholar]
- 13. Hewlett S, Dures E, Almeida C. Measures of fatigue: bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res 2011;63(Suppl 11):S263–86. [DOI] [PubMed] [Google Scholar]
- 14. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011;63(Suppl 11):S240–52. [DOI] [PubMed] [Google Scholar]
- 16. Golpour M, Hosseini SH, Khademloo M et al. Depression and anxiety disorders among patients with psoriasis: a hospital-based case-control study. Dermatol Res Pract 2012;2012:381905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleming P, Bai JW, Pratt M et al. The prevalence of anxiety in patients with psoriasis: a systematic review of observational studies and clinical trials. J Eur Acad Dermatol Venereol 2017;31:798–807. [DOI] [PubMed] [Google Scholar]
- 18. Beard C, Weisberg RB, Keller MB. Health-related Quality of Life across the anxiety disorders: findings from a sample of primary care patients. J Anxiety Disord 2010;24:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Athanassiou P, Theodoridou A, Koukli EM et al. Psoriasis impact on patient-reported outcomes in psoriatic arthritis in a real-world setting: results from the APOPSIS Study. Arthritis Rheumatol 2019;71(Suppl 10):abstract 2509. [Google Scholar]
- 20. de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc 2004;9:140–7. [DOI] [PubMed] [Google Scholar]
- 21. Heydendael VM, de Borgie CA, Spuls PI et al. The burden of psoriasis is not determined by disease severity only. J Investig Dermatol Symp Proc 2004;9:131–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be shared on reasonable request to the corresponding author (f.kasiem@erasmusmc.nl).