Abstract
Objective
To compare the safety and efficacy of switching from reference adalimumab to adalimumab biosimilar CT-P17 with continuing reference adalimumab/CT-P17 in active RA.
Methods
This double-blind, phase III study randomized (1:1) subjects with active RA to receive 40 mg (100 mg/ml) CT-P17 or European Union-sourced reference adalimumab subcutaneously every 2 weeks (Q2W) until week (W) 24 [treatment period (TP) 1]. Thereafter, subjects receiving reference adalimumab were randomized (1:1) to continue reference adalimumab or switch to CT-P17 from W26 (both Q2W until W48; TP2). Subjects receiving CT-P17 in TP1 continued CT-P17. W0–W24 results were previously reported; we present W26–W52 findings. End points were efficacy (including joint damage progression), pharmacokinetics, safety and immunogenicity.
Results
Of 607 subjects who initiated TP2 treatment, 303 continued CT-P17, 153 continued reference adalimumab and 151 switched to CT-P17. Efficacy improvements up to W24 were maintained during TP2; efficacy was comparable among groups. At W52, 20% improvement in ACR response rates were 80.5% (continued CT-P17), 77.8% (continued reference adalimumab) and 82.2% (switched to CT-P17). Joint damage progression was minimal. Mean trough serum adalimumab concentrations were similar among groups. CT-P17 and reference adalimumab safety profiles were numerically similar and switching did not affect immunogenicity. At W52, 28.4% (continued CT-P17), 27.0% (continued reference adalimumab) and 28.3% (switched to CT-P17) of subjects were anti-drug antibody-positive.
Conclusion
Efficacy, pharmacokinetics, safety and immunogenicity of CT-P17 and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to CT-P17.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT03789292.
Keywords: adalimumab, biosimilar, CT-P17, efficacy, immunogenicity, rheumatoid arthritis, safety, switching, tumour necrosis factor inhibitors
Rheumatology key messages.
CT-P17 and reference adalimumab had comparable efficacy, safety and immunogenicity during 1 year of treatment.
Switching from reference adalimumab to CT-P17 had no adverse impact on efficacy, safety or immunogenicity.
Sustained comparability of CT-P17 and reference adalimumab reinforces the equivalent efficacy demonstrated at week 24.
Introduction
CT-P17 is a citrate-free biosimilar of the high-concentration (100 mg/ml) formulation of the TNF inhibitor adalimumab that has received regulatory approval from the European Commission [1]. To date, two randomized phase I studies have evaluated the pharmacokinetics (PK) and safety of CT-P17 in healthy adults. These studies compared CT-P17 with reference adalimumab sourced from the European Union (EU) and USA [2], and evaluated CT-P17 administration by autoinjector or prefilled syringe [3].
Equivalent efficacy of CT-P17 and EU-sourced reference adalimumab was demonstrated in this randomized, phase III study in subjects with active RA [4]. The proportion of subjects achieving a 20% improvement by ACR criteria (ACR20) response at week (W) 24, which was the primary end point, was achieved by 82.7% of subjects in both CT-P17 and reference adalimumab groups. Both the 95% and 90% confidence intervals for the estimate of treatment difference (−5.94–5.94 and −4.98–4.98, respectively) were contained entirely within the corresponding predefined equivalence margins {−15–15 [European Medicines Agency (EMA) assumption] and −12–15 [US Food and Drug Administration (FDA) assumption]}. Comparability of responses to CT-P17 and reference adalimumab was also demonstrated up to W24 for each of the secondary end points of efficacy, PK, usability and overall safety.
Here, efficacy, PK, safety and immunogenicity findings until the end of study (EOS) at W52 are reported for subjects who continued treatment with CT-P17 or reference adalimumab or who switched from reference adalimumab to CT-P17 at W26.
Methods
Study design and procedures
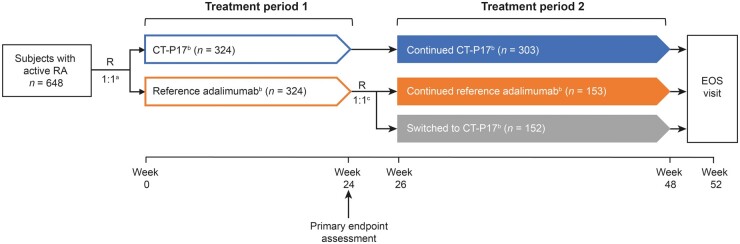
This was a randomized, double-blind, active-controlled, multicentre, phase III study (NCT03789292). The study centres and study design to the end of treatment period 1 (W24) are reported in the primary manuscript [4]; Fig. 1 depicts the full study design. Briefly, before dosing at W0, subjects were randomized (1:1) to receive 40 mg (100 mg/ml) of CT-P17 (Celltrion, Inc., Incheon, Republic of Korea) or EU-sourced reference adalimumab (Humira®, AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany) every 2 weeks (Q2W) until W24. Prior to dosing at W26, subjects in the reference adalimumab group were randomized (1:1) to continue reference adalimumab (continued reference adalimumab group) or to switch to CT-P17 (switched to CT-P17 group). Subjects receiving CT-P17 during treatment period 1 underwent the second randomization process to maintain study blinding and continued to receive CT-P17 (continued CT-P17 group). Randomization was performed using an interactive web response system (IWRS) and was stratified by disease activity per Simplified Disease Activity Index (SDAI) at W24, in terms of remission (SDAI ≤3.3) vs non-remission (SDAI >3.3). Similar to the first randomization [4], IWRS randomization at W26, linking sequential subject randomization numbers to treatment codes, was generated by the biostatistics team using Rave Randomization and Trial Supply Management (Medidata Solutions, New York, NY, USA). Randomization was by permuted block with a block size of four. From W26–W48 (treatment period 2), study drugs were administered Q2W. Throughout, study drugs were administered with concomitant methotrexate and folic acid [4]. Subjects attended an EOS visit at W52. As previously reported [4], subjects received CT-P17 or reference adalimumab by subcutaneous injection by prefilled syringe, which could be self-administered at home [4].
Fig. 1.
Study design
aRandomization prior to week 0 study drug administration; details of randomization methods including stratification factors have been published previously (Kay et al., Arthritis Res Ther 2021;23:51). bSubjects received 40 mg (100 mg/ml) CT-P17 or reference adalimumab, as shown, every 2 weeks with concomitant methotrexate and folic acid (Kay et al., Arthritis Res Ther 2021;23:51). cRandomization prior to week 26 study drug administration was stratified by disease activity per SDAI at week 24, in terms of remission (SDAI ≤3.3) versus non-remission (SDAI >3.3). EOS: end-of-study; R: randomization; SDAI: Simplified Disease Activity Index.
Due to the coronavirus disease 2019 (COVID-19) pandemic, some study procedures (affecting W48 and EOS visits only) were amended per FDA and EMA guidance [5, 6], to prioritize subject safety and data validity. Changes were approved by the independent data safety monitoring board. The study drug could be delivered to subjects at home by courier and/or relatives, rather than collected from the study centre, due to restrictions on visits. Based on known stability information, study drugs were stable for this alternative distribution procedure. Chest X-ray, interferon-γ release assay (IGRA) and clinical laboratory parameters could be analysed locally rather than centrally, if required. Major safety assessments [IGRA; chest, hand and foot X-rays; 12-lead electrocardiogram; serum pregnancy and hepatitis B virus tests (if required)] could be performed at the last treatment visit (W48) instead of the EOS visit. If this occurred, the EOS visit could be replaced by a telephone call that included safety follow-up. Alternatively, the on-site EOS visit window could be extended by a maximum of 2 weeks before the planned EOS visit date or, if a subject could not attend the on-site EOS visit due to travel restrictions, the EOS visit was rescheduled to the earliest time point after the site was released from quarantine.
As reported [4], the study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All national, state and local laws or regulations were followed. Before study initiation, the study protocol was reviewed and approved by the independent ethics committee/institutional review board at each site. All subjects provided written informed consent.
Subjects
Full eligibility criteria are described in the primary publication [4]. Briefly, subjects were adults (aged 18–75 years) with active RA who were receiving a stable methotrexate dose by a consistent administration route. Active RA was defined by the presence of ≥6 swollen joints, ≥6 tender joints and elevation of the ESR to >28 mm/h or serum CRP to >1.0 mg/dl at screening. Prior biologic DMARD or targeted synthetic DMARD treatments for RA were not permitted; prior TNF inhibitor treatment was not permitted for any purpose. Exclusion criteria also included history of or current serious infection or tuberculosis, or a known malignancy within 5 years before study drug administration (except completely excised and cured squamous cell carcinoma of the uterine cervix in situ, cutaneous basal cell carcinoma or cutaneous squamous cell carcinoma).
Study end points
The primary end point was reported previously [4]. Efficacy end points evaluated during treatment period 2 included the proportions of subjects achieving clinical response according to 20%, 50% or 70% improvement by ACR criteria from baseline (ACR20, ACR50 and ACR70, respectively), hybrid ACR response, Disease Activity Score in 28 joints (DAS28)-CRP response, EULAR response, SDAI and Clinical Disease Activity Index (CDAI) response rates, and 36-item Short Form Health Survey (SF-36) physical and mental component scores. Joint damage progression was evaluated using the modified total Sharp score. DAS28-CRP, SDAI, CDAI and Boolean remission rates were analysed post hoc. Trough serum concentrations (Ctrough) were measured every 4 weeks during treatment period 2 between W26 and W46. Safety, immunogenicity and injection-site pain were also assessed.
Study assessments
Full details of study assessments are shown in Supplementary Table S1, available at Rheumatology online. As in treatment period 1, efficacy assessments were performed before study drug administration by blinded independent joint count assessors at each study centre; blood samples for PK assessments were obtained immediately before study drug administration [4]. Safety assessments performed throughout treatment period 2 included treatment-emergent adverse events (TEAEs), treatment-emergent adverse events of special interest (TEAESIs), immunogenicity, clinical monitoring for tuberculosis, and review of prior and concomitant medications, as described for treatment period 1 [4]. Anti-drug antibodies (ADAs) were detected using a validated electrochemiluminescent bridging assay with acid dissociation. Binding specificity and ADA titre were measured in ADA-positive samples. A validated electrochemiluminescent assay with affinity capture elution was used to analyse ADA-positive samples for neutralising antibodies (NAbs). Injection-site pain was assessed using a 100-mm visual analogue scale (VAS) at all study visits other than at W28 and W40. Subjects who stopped taking study drug continued to attend study centre visits up to W52 for safety and efficacy assessments.
Statistical analyses
Sample size calculations and statistical analysis for the primary end point have been described [4]. Analysis populations for treatment period 2 are described in the Supplementary Methods, available at Rheumatology online. Post hoc analyses were conducted to compare selected parameters between treatment groups (continued CT-P17 with continued reference adalimumab, and continued reference adalimumab with switched to CT-P17), using P-values generated by the Wald test (for proportional values) or t test (for mean values), for overall treatment groups or subgroups of ADA-positive and ADA-negative subjects. All statistical analyses were performed using SAS software, Version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Subject disposition
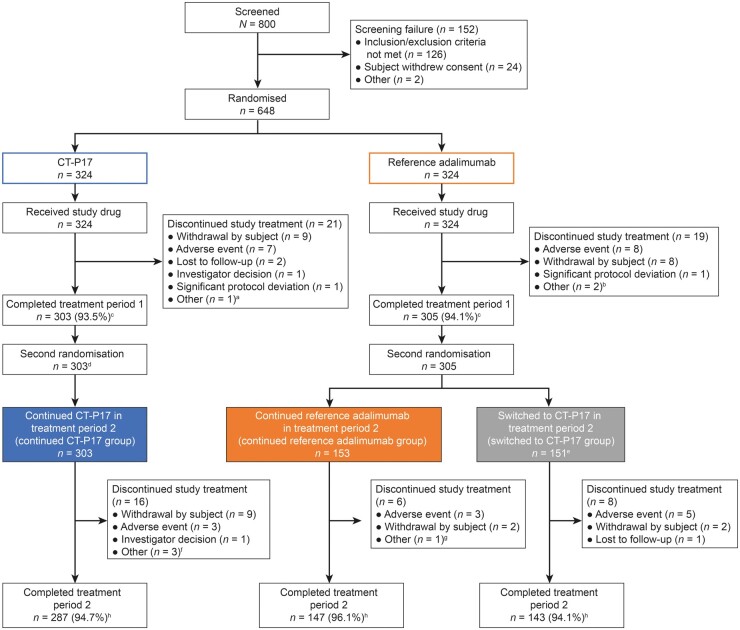
As previously reported [4], subjects were recruited between 5 December 2018 and 25 April 2019. The last study centre visit for the last subject was on 24 April 2020. Fig. 2 presents the disposition of subjects. In the treatment period, 2607 subjects initiated study treatment (continued CT-P17: 303; continued reference adalimumab: 153; switched to CT-P17: 151). Consistent with findings for treatment period 1 [4], the most frequent reason for study drug discontinuation overall was withdrawal from the study [13 (2.1%) subjects], followed by adverse event [12 (2.0%) subjects]. Similar proportions of subjects in each group completed the study [continued CT-P17: 287 (94.7%); continued reference adalimumab: 147 (96.1%); switched to CT-P17: 143 (94.1%)].
Fig. 2.
Subject disposition (intention-to-treat population)
Figure adapted from Kay et al. (Arthritis Res Ther 2021;23:51) as permitted under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/). aSubject discontinued CT-P17 treatment because of significant dose delay due to adverse event. bTwo subjects discontinued reference adalimumab treatment because of subject decision due to adverse event. cDenominator is the number of subjects assigned to the treatment group in the first randomization. dAll subjects receiving CT-P17 during treatment period 1 continued to receive CT-P17 during treatment period 2 following a mock randomization process to maintain the study blind. eOne subject randomized to switch to CT-P17 did not initiate study drug in treatment period 2 due to adverse event. fOne subject discontinued CT-P17 treatment because of significant dose delay due to adverse event and two subjects discontinued as they were unable to visit the study site due to the COVID-19 pandemic. gOne subject discontinued reference adalimumab as they were unable to visit the study site due to the COVID-19 pandemic. hDenominator is the number of subjects assigned to the treatment group in the second randomization. COVID-19: coronavirus disease 2019.
During the study, 79 (26.1%) subjects in the continued CT-P17 group, 44 (28.8%) in the continued reference adalimumab group and 37 (24.3%) in the switched to CT-P17 group had protocol deviations related to COVID-19 (Supplementary Table S2, available at Rheumatology online). However, no subject was excluded from an analysis population because of a COVID-19-related protocol deviation and the study was completed within 7 weeks of the COVID-19 pandemic being declared by the World Health Organization. No subjects were positive for severe acute respiratory syndrome coronavirus 2 as far as is known.
Baseline demographics and disease characteristics were balanced among treatment groups following both the first [4] and second randomization (Table 1). There were no significant differences (P > 0.05) between treatment groups in any baseline characteristic.
Table 1.
Demographics and baseline disease characteristics (intention-to-treat population—treatment period 2 subset, unless otherwise specified)
| Characteristica | Continued CT-P17 (n = 303) | Continued reference adalimumab (n = 153) | Switched to CT-P17 (n = 152) |
|---|---|---|---|
| Age, years, median (range) | 53.0 (18–75) | 53.0 (19–75) | 53.0 (20–73) |
| Sex, n (%) | |||
| Male | 71 (23.4) | 31 (20.3) | 28 (18.4) |
| Female | 232 (76.6) | 122 (79.7) | 124 (81.6) |
| Race, n (%) | |||
| White | 280 (92.4) | 138 (90.2) | 141 (92.8) |
| Mestizo | 22 (7.3) | 15 (9.8) | 11 (7.2) |
| Native Peruvian | 1 (0.3) | 0 | 0 |
| RA disease duration, years | 6.70 (6.81) | 6.61 (6.92) | 6.37 (6.52) |
| SDAI | 39.8 (11.7) | 39.6 (10.4) | 39.9 (11.7) |
| CDAI | 38.8 (11.2) | 38.3 (10.1) | 38.9 (11.3) |
| DAS28-CRP | 5.530 (0.8833) | 5.545 (0.8012) | 5.547 (0.8992) |
| Tender joint count | 20.3 (10.4) | 19.6 (9.7) | 20.1 (10.3) |
| Swollen joint count | 14.0 (6.4) | 14.2 (6.6) | 13.9 (6.4) |
| Subject’s assessment of painb | 69.6 (19.0) | 68.6 (16.9) | 71.7 (15.9) |
| Subject’s global assessment of disease activityb | 69.7 (17.9) | 68.7 (15.9) | 70.8 (17.0) |
| Physician’s global assessment of disease activityb | 67.3 (15.0) | 67.2 (15.4) | 67.1 (15.8) |
| HAQ estimate of physical ability | 1.41 (0.58) | 1.44 (0.56) | 1.54 (0.56) |
| CRP, mg/dlc | 0.997 (1.63) | 1.26 (2.24) | 0.960 (1.54) |
| ESR, mm/hc | 42.3 (16.22) | 42.2 (16.54) | 43.2 (17.36) |
| Total Sharp score | 25.7 (37.8) | 24.9 (38.9) | 27.0 (48.6) |
Data are mean (s.d.) unless stated otherwise.
Assessed by 100-mm visual analogue scale.
Evaluated in the pharmacodynamic population—treatment period 2 subset. CDAI: Clinical Disease Activity Index; DAS28-CRP: Disease Activity Index in 28 joints–CRP; SDAI: Simplified Disease Activity Index.
Clinical efficacy
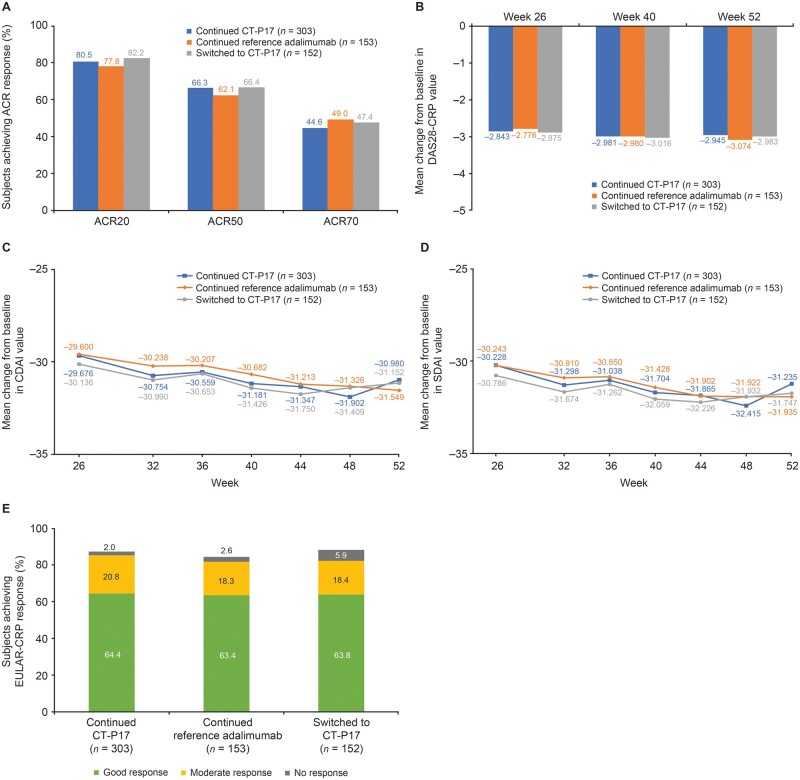
During treatment period 2, comparable efficacy was maintained until W52. ACR20, ACR50 and ACR70 response rates were similar among groups at W52 (Fig. 3A). Mean changes from baseline in DAS28-CRP, CDAI and SDAI values were maintained, with slight further decreases during treatment period 2; results at W52 were similar between treatment groups (Table 2; Fig. 3B–D). Mean hybrid ACR scores increased slightly during treatment period 2; scores at W52 were similar between treatment groups (Table 2). At W52, EULAR (CRP) response rates were comparable among treatment groups (Fig. 3E). CDAI, SDAI, DAS28-CRP and Boolean remission rates at W52 were comparable between treatment groups (Table 2).
Fig. 3.
Clinical efficacy during treatment period 2 (intention-to-treat population—treatment period 2 subset)
(A) ACR response rates at week 52; (B) Mean change from baseline in DAS28-CRP value during treatment period 2; (C) Mean change from baseline in CDAI value during treatment period 2; (D) Mean change from baseline in SDAI value during treatment period 2; (E) EULAR-CRP response rates at week 52. ACR20/50/70: 20%/50%/70% improvement according to ACR criteria; CDAI: Clinical Disease Activity Index; DAS28-CRP: Disease Activity Score in 28 joints–CRP; SDAI: Simplified Disease Activity Index.
Table 2.
Efficacy results at week 52 (intention-to-treat population—treatment period 2 subset)
| Parametera | Continued CT-P17 (n = 303) | Continued reference adalimumab (n = 153) | Switched to CT-P17 (n = 152) |
P-value |
|
|---|---|---|---|---|---|
| Continued CT-P17 vs continued reference adalimumab | Continued reference adalimumab vs switched to CT-P17 | ||||
| Change from baseline in CDAI value | −30.980 (11.5170) | −31.549 (11.4058) | −31.152 (13.5302) | 0.6462 | 0.7982 |
| CDAI remission rate, n (%) | 94 (31.0) | 52 (34.0) | 48 (31.6) | 0.5249 | 0.6541 |
| Change from baseline in SDAI value | −31.235 (11.6048) | −31.935 (11.6042) | −31.747 (13.8054) | 0.5771 | 0.9057 |
| SDAI remission rate, n (%) | 89 (29.4) | 56 (36.6) | 51 (33.6) | 0.1234 | 0.5767 |
| Change from baseline in DAS28-CRP | −2.945 (1.1273) | −3.074 (1.1926) | −2.983 (1.2529) | 0.2967 | 0.5504 |
| DAS28-CRP remission rate, n (%) | 152 (50.2) | 80 (52.3) | 70 (46.1) | 0.6684 | 0.2752 |
| Boolean remission rate, n (%) | 76 (25.1) | 47 (30.7) | 43 (28.3) | 0.2088 | 0.6417 |
| Hybrid ACR score | 64.5 (22.3) | 67.0 (22.4) | 65.5 (26.0) | 0.3051 | 0.6052 |
| Change from baseline in total Sharp scoreb | 0.24 (1.99) | 0.34 (2.51) | 0.04 (0.963) | 0.6946 | 0.2074 |
Data are mean (s.d.) unless stated otherwise.
Hand and foot X-ray results were included in the week 52 result even if the subject’s end-of-study visit was performed earlier than planned (week 52) due to the COVID-19 pandemic. CDAI: Clinical Disease Activity Index; DAS28-CRP: Disease Activity Score in 28 joints–CRP; SDAI: Simplified Disease Activity Index.
Mean SF-36 physical and mental component scores at W52 were similar among treatment groups. Mean (s.d.) changes from baseline in physical component scores were 9.635 (7.5102), 10.703 (8.5358) and 9.745 (8.8442) in the continued CT-P17, continued reference adalimumab and switched to CT-P17 groups, respectively. Corresponding mean (s.d.) changes from baseline in mental component scores at W52 were 5.909 (9.8506), 7.732 (10.4129) and 7.195 (10.5759).
Joint damage progression
The mean increase from baseline in total Sharp radiographic joint damage scores was similar between treatment groups (Table 2). Progression of joint damage was negligible and not clinically significant.
Pharmacokinetics
Mean adalimumab Ctrough values were similar for all treatment groups at each time point evaluated during treatment period 2 (Supplementary Table S3, available at Rheumatology online).
Safety
During treatment period 2, similar proportions of subjects in each treatment group experienced TEAEs, TEAEs considered by the investigator to be study drug related, or TEAEs leading to study drug discontinuation (Table 3). Most TEAEs were grade 1–2 in intensity; similar proportions of subjects in each treatment group experienced ≥1 grade 3 or grade 4 TEAEs. The most frequently reported TEAEs were neutropenia in the continued CT-P17 and switched to CT-P17 groups [continued CT-P17: 15 (5.0%) subjects; continued reference adalimumab: six (3.9%); switched to CT-P17: eight (5.3%)] (Table 3). Upper respiratory tract infection was the most frequently reported TEAE in the continued reference adalimumab group [11 (7.2%) subjects vs 10 (3.3%) in the continued CT-P17 group and six (3.9%) in the switched to CT-P17 group] (Table 3). While safety profiles were generally comparable at the System Organ Class (SOC) level, there were small numerical differences in the incidence of TEAEs in some SOCs (Supplementary Table S4, available at Rheumatology online). TEAEs of infections and infestations were reported by a greater proportion of subjects in the continued reference adalimumab group [41 (27.0%) subjects] compared with the continued CT-P17 [54 (17.8%)] and switched to CT-P17 [28 (18.4%)] groups. In contrast, the incidence of TEAEs of blood and lymphatic system disorders was slightly lower in the reference adalimumab group [eight (5.3%) subjects] than in the continued CT-P17 [22 (7.3%)] and switched to CT-P17 [12 (7.9%)] groups. There was a higher incidence of TEAEs in the investigations SOC in the switched to CT-P17 group [17 (11.2%) subjects] than in the continued CT-P17 [20 (6.6%)] and continued reference adalimumab [four (2.6%)] groups.
Table 3.
TEAEs during treatment period 2 (safety population—treatment period 2 subset)
| TEAEa | Continued CT-P17 (n = 303) | Continued reference adalimumab (n = 152) | Switched to CT-P17 (n = 152) |
|---|---|---|---|
| Subjects with ≥1 TEAE | 121 (39.9)b | 69 (45.4) | 73 (48.0) |
| Study drug-related | 48 (15.8) | 27 (17.8) | 36 (23.7) |
| TEAEs reported in ≥3% of subjects in any treatment group | |||
| Neutropenia | 15 (5.0) | 6 (3.9) | 8 (5.3) |
| Upper respiratory tract infection | 10 (3.3) | 11 (7.2) | 6 (3.9) |
| Urinary tract infection | 9 (3.0) | 5 (3.3) | 3 (2.0) |
| Alanine aminotransferase increased | 8 (2.6) | 1 (0.7) | 7 (4.6) |
| Leukopenia | 8 (2.6) | 0 | 7 (4.6) |
| Nasopharyngitis | 6 (2.0) | 5 (3.3) | 3 (2.0) |
| Diarrhoea | 2 (0.7) | 3 (2.0) | 5 (3.3) |
| Subjects with ≥1 TESAE | 6 (2.0) | 3 (2.0) | 5 (3.3) |
| Subjects with ≥1 TEAE leading to study drug discontinuation | 3 (1.0) | 2 (1.3) | 5 (3.3) |
| Subjects with ≥1 TEAE classified as hypersensitivity/allergic reactions | 2 (0.7) | 1 (0.7) | 0 |
| Subjects with ≥1 TEAE classified as ISR | 1 (0.3) | 4 (2.6) | 1 (0.7) |
| Subjects with ≥1 TEAE classified as infection | 54 (17.8) | 41 (27.0) | 28 (18.4) |
| Subjects with ≥1 TEAE classified as malignancy | 0 | 1 (0.7) | 0 |
| Total number of TEAEs leading to death | 0 | 0 | 0 |
Data are n (%) unless stated otherwise.
Includes a grade 1 TEAE of lung disorder for which causality was assessed as unknown by the investigator since the event occurred after the end-of-study visit and diagnosis was not completed at the time of the last report. ISR: injection-site reaction; TEAE: treatment-emergent adverse event; TESAE: treatment-emergent serious adverse event.
Treatment-emergent serious adverse events (TESAEs; Table 3) were reported by similar proportions of subjects in each treatment group during treatment period 2. Comparable proportions of subjects in each treatment group experienced TEAEs classified as hypersensitivity/allergic reactions or injection-site reactions (ISRs) during treatment period 2 (Table 3). One (0.7%) subject in the continued reference adalimumab group experienced a TEAE classified as malignancy (grade 2 basal cell carcinoma), which was considered by the investigator to be unrelated to study drug. No active tuberculosis was reported during treatment period 2, but one (0.7%) subject in the continued reference adalimumab group had a new positive IGRA conversion at W52. Latent tuberculosis (positive IGRA result with negative examination of chest X-ray) was reported in this subject, who subsequently began tuberculosis prophylaxis.
During the overall study period (from baseline to W52), proportions of subjects experiencing TEAEs, TESAEs, TEAEs leading to study drug discontinuation or TEAESIs were comparable among the continued CT-P17, continued reference adalimumab and switched to CT-P17 groups (Supplementary Table S5, available at Rheumatology online). Most TEAEs were grade 1–2 in intensity. No deaths or pregnancies were reported up to W52.
Mean (s.d.) VAS scores for injection-site pain were comparable among groups at W26 [continued CT-P17: 4.60 (9.168); continued reference adalimumab: 3.55 (5.627); switched to CT-P17: 3.47 (7.615)]. Corresponding scores at W48 were also comparable among groups: 4.19 (7.313), 3.64 (7.184) and 3.44 (6.727).
Immunogenicity and ADA subgroup analysis
Similar proportions of subjects in each treatment group were positive for ADAs and NAbs at W52, comparable to those prior to dosing at W26 (Supplementary Table S6, available at Rheumatology online). At W52, 28.4% (continued CT-P17), 27.0% (continued reference adalimumab) and 28.3% (switched to CT-P17) of subjects were ADA positive. During treatment period 2, the proportions of subjects who newly developed ADAs were 9.9%, 6.1% and 11.1% in the continued CT-P17, continued reference adalimumab and switched to CT-P17 groups, respectively. Corresponding proportions of subjects who developed NAbs during treatment period 2 were 9.1%, 3.6% and 6.8%.
Among ADA-positive and ADA-negative subgroups, there were no significant differences (P > 0.05) between treatment groups in terms of efficacy (ACR20 or change from baseline in DAS28-CRP at W52), PK (Ctrough at W46) or safety parameters (TEAEs classified as hypersensitivity/allergic reactions or ISRs up to W52) (Supplementary Table S7, available at Rheumatology online). ADA positivity was associated with substantially lower Ctrough at W46, while there were no clear differences in efficacy and safety parameters between ADA-positive and ADA-negative subgroups.
Discussion
In this study, efficacy, PK, safety and immunogenicity results between W26 and W52 were similar for subjects with RA who received continued treatment with CT-P17 or reference adalimumab, or who switched from reference adalimumab to CT-P17 at W26. This indicates sustained comparability between CT-P17 and reference adalimumab over 52 weeks of treatment and no adverse effect of a single switch from reference adalimumab to CT-P17. These results reinforce the finding of equivalent efficacy and comparable safety and immunogenicity identified between CT-P17 and reference adalimumab between W0 and W24 (treatment period 1) [4].
Efficacy observed in treatment period 1 [4] was sustained from W26–W52 (comprising treatment period 2 and the EOS visit) across efficacy end points. ACR20/50/70 response rates were maintained through W52, with little change in all groups. EULAR-CRP good response rates and CDAI, SDAI, DAS28-CRP and Boolean remission rates were also all maintained or increased slightly from W24–W52.
Mean Ctrough values were generally maintained at the concentrations observed at the end of treatment period 1, with the difference between CT-P17 and reference adalimumab groups decreasing during treatment period 2 [4]. Mean Ctrough levels remained relatively stable following the switch from reference adalimumab to CT-P17, and concentrations were within the therapeutic range for subjects with RA (5–8 μg/ml [7]) throughout treatment period 2.
The overall safety profile of CT-P17 was similar to that of reference adalimumab, including following a single switch from reference adalimumab. Safety findings were consistent with the known safety profile of reference adalimumab [8] and no new or unexpected safety findings arose during the study. During treatment period 2, a slightly greater proportion of subjects in the switched to CT-P17 group experienced increased alanine aminotransferase (ALT) concentrations [seven (4.6%) subjects] or leukopenia [seven (4.6%)] than in the other treatment groups. However, this was considered not to be a clinically significant finding for CT-P17, for several reasons. All subjects experiencing these TEAEs during treatment period 2 had at least one predisposing factor or had relevant TEAEs/abnormal laboratory results during treatment period 1, other than for one subject who reported grade 1 neutropenia and leukopenia. Predisposing factors included anaemia or steroid treatment (for leukopenia) and use of hepatoxic drugs (for ALT increases). All subjects were receiving concomitant methotrexate, which is known to be associated with haematological changes and hepatotoxicity [9, 10]. Furthermore, the slightly higher proportion of subjects with increased ALT in the switched to CT-P17 group was not correlated with an increased incidence of TEAEs in the SOC of hepatobiliary disorders (0.7% in each group during treatment period 2). Despite a higher incidence of leukopenia in the switched to CT-P17 group, the proportion of subjects with TEAEs in the SOC of infections and infestations was higher in the continued reference adalimumab group than in the switched to CT-P17 group. While the incidence of infections and infestations was highest in the continued reference adalimumab group during treatment period 2, TEAEs in this SOC were mostly grade 1–2 infections; there were no clinically meaningful differences among groups in terms of grade ≥3 infections.
Immunogenicity findings were comparable among treatment groups, with no increase in the proportion of subjects with ADAs or NAbs following the switch from reference adalimumab to CT-P17. Within the ADA-positive and ADA-negative subgroups, there were no statistically significant differences among continued CT-P17, continued reference adalimumab and switched to CT-P17 groups in terms of efficacy (ACR20 or change from baseline in DAS28-CRP at W52), PK (Ctrough at W46) or safety parameters (TEAEs classified as hypersensitivity/allergic reactions or ISRs up to W52). The clear relationship between ADA positivity and PK results, and the lack of association between ADA results and efficacy and safety parameters, is consistent with findings up to W24 [4]. The efficacy [11], PK [12] and safety [13] findings are also consistent with reports for other adalimumab biosimilars up to 24 or 52 weeks.
The comparability of efficacy, safety and immunogenicity among continued treatment with reference adalimumab or CT-P17 and switching to CT-P17 is consistent with findings of previous RA studies that compared licensed adalimumab biosimilars with reference adalimumab [12–16]. As described for the primary end point of ACR20 response rate at W24 [4], ACR20 response rates at W52 for subjects who continued treatment with reference adalimumab or CT-P17, or switched to CT-P17, were comparable to rates reported between 48 and 54 weeks in other studies that evaluated reference adalimumab or adalimumab biosimilars (which ranged from ∼60–90%) [12–16]. The comparability of PK profiles identified in our study is also consistent with that observed for licensed adalimumab biosimilars and reference adalimumab evaluated in subjects with RA [14, 17].
This study evaluated a single switch from reference adalimumab to CT-P17, providing data relevant to the use of CT-P17 in clinical practice. One study limitation is that the number of subjects in the continued reference adalimumab and switched to CT-P17 groups was smaller than that in the continued CT-P17 group, since subjects receiving reference adalimumab during treatment period 1 were re-randomized to continue reference adalimumab or to switch to CT-P17, whereas subjects receiving CT-P17 continued CT-P17 throughout. Another limitation is that statistical comparisons between groups were conducted post hoc for treatment period 2; however, this study was not powered to assess equivalence between groups for the secondary end points. The COVID-19 pandemic emerged during the latter half of the study period, which required adjustments to some procedures and necessitated remote conduct of W48 and EOS visits for some subjects. Safety follow-up was completed as much as possible, and the safety of study subjects was not affected. Thus, the impact of COVID-19 on study validity was limited. In addition, there were no TEAEs of COVID-19 infection reported during the study. As discussed previously, the generalizability of our findings may be limited by the predominance of subjects from Eastern European countries, especially Poland; however, this should be viewed in the context of the objectives of a biosimilar study and the global scope of the CT-P17 clinical development programme [4].
In conclusion, after 52 weeks of treatment, CT-P17 was comparable to reference adalimumab in terms of efficacy, PK, safety and immunogenicity. Efficacy, safety and immunogenicity following the switch from reference adalimumab to CT-P17 were comparable to findings with continued treatment with reference adalimumab or CT-P17. Thus, this study affirms the biosimilarity of CT-P17 to reference adalimumab and provides clinical evidence for switching from reference adalimumab to CT-P17.
Supplementary Material
Acknowledgements
Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, collating author comments, copyediting, fact checking and referencing, was provided by Beatrice Tyrrell, DPhil, at Aspire Scientific Limited (Bollington, UK). Funding for medical writing support for this article was provided by Celltrion, Inc. (Incheon, Republic of Korea). D.E.F., J.K. and E.K. contributed to study design and data analysis or interpretation. J.J., R.W., P.W., A.D., M.K., S.J., A.Z., J.T., K.B-M., M.K-W. and P.A.K. contributed to data collection. S.J.L., S.H.K., Y.J.B., G.E.Y. and J.K.Y. contributed to study design, data collection and data analysis or interpretation. All authors reviewed and critically revised the manuscript, approved the final draft and are accountable for accuracy and integrity of the manuscript. Patient consent for publication was not required. An abstract reporting selected data up to W52 has been published in the abstract book for the European League Against Rheumatism (EULAR) 2021 Congress (Ann Rheum Dis 2021;80(Suppl 1):1123).
Funding: This work was supported by Celltrion, Inc. (Incheon, Republic of Korea).
Disclosure statement: D.E.F. has received grant/research support from Corbus Pharmaceuticals, CSL Behring, Galapagos NV, Gilead Sciences, Inc., GlaxoSmithKline, Kadmon Corporation, Pfizer and Talaris Therapeutics, Inc., and consultancies from AbbVie Inc., Amgen Inc., Boehringer Ingelheim, Corbus Pharmaceuticals, CSL Behring, Galapagos NV, Gilead Sciences, Inc., Novartis, Pfizer, Roche/Genentech and Talaris Therapeutics, Inc. R.W. has received honoraria for lectures from Eli Lilly and Company, Merck Sharp & Dohme and Novartis. P.W. has received consultancies and honoraria from Boehringer Ingelheim GmbH, Eli Lilly and Company, Gilead Sciences, Inc., Novartis International AG, Pfizer Inc., Sandoz Inc. and Sun Pharmaceutical Industries Ltd. S.J.L., S.H.K., Y.J.B., G.E.Y. and J.K.Y. are employed by Celltrion, Inc. J.K. has received research support paid to the University of Massachusetts Medical School from Novartis Pharmaceuticals Corp. and Pfizer Inc., and has received consultancies and honoraria from AbbVie Inc., Boehringer Ingelheim GmbH, Celltrion Healthcare Co. Ltd, Merck & Co., Inc., Pfizer Inc., Samsung Bioepis Co., Ltd, Sandoz Inc., and UCB Inc. E.K. has received research funding from Amgen Inc., Merck, Pfizer and PuraPharm Corporation Ltd, has consulting agreements or advisory board membership with AbbVie Inc., Amgen Inc., Bristol Myers Squibb, Celltrion, Eli Lilly and Company, F. Hoffman-La Roche Inc., Gilead Sciences, Inc., Janssen Inc., Merck, Myriad Autoimmune, Pfizer Inc., Sandoz Inc., Sanofi Genzyme and Samsung Bioepis Co., Ltd, and has speaker honoraria agreements with Amgen Inc., AbbVie Inc., Celltrion, F. Hoffman-La Roche Inc., Gilead Sciences, Inc., Janssen Inc., Merck, Novartis, Pfizer and Sanofi Genzyme. J.J., A.D., M.K., S.J., A.Z., J.T., K.B-M., M.K-W. and P.A.K. have nothing to disclose.
Data availability statement
Available data and methodological information for this study are included in this article and accompanying supplementary materials.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Daniel E Furst, Division of Rheumatology, Department of Medicine, University of California, Los Angeles, Los Angeles, CA; Division of Rheumatology, Department of Medicine, University of Washington, Seattle, WA, USA; Department of Medicine, University of Florence, Florence, Italy.
Janusz Jaworski, Department of Rheumatology, Reumatika-Centrum Reumatologii, Warsaw.
Rafal Wojciechowski, Department of Rheumatology and Connective Tissue Diseases, University Hospital No 2, Bydgoszcz.
Piotr Wiland, Department of Rheumatology and Internal Diseases, Medical University, Wrocław.
Anna Dudek, Department of Rheumatology, Centrum Medyczne AMED, Warsaw.
Marek Krogulec, Rheumatology Clinic, NZOZ Lecznica MAK-MED, Nadarzyn.
Slawomir Jeka, Department of Rheumatology and Connective Tissue Diseases, Nasz Lekarz Przychodnie Medyczne, Toruń.
Agnieszka Zielinska, Medycyna Kliniczna.
Jakub Trefler, Reuma Centrum, Warsaw.
Katarzyna Bartnicka-Maslowska, Department of Rheumatology, Centrum Medyczne Amed, Łódź.
Magdalena Krajewska-Wlodarczyk, Department of Internal Medicine, University of Warmia and Mazury, Olsztyn.
Piotr A Klimiuk, Department of Rheumatology and Internal Diseases, Medical University of Bialystok and Gabinet Internistyczno-Reumatologiczny Piotr Adrian Klimiuk, Białystok, Poland.
Sang Joon Lee, Clinical Development Division, Celltrion, Inc., Incheon, Republic of Korea.
Sung Hyun Kim, Clinical Development Division, Celltrion, Inc., Incheon, Republic of Korea.
Yun Ju Bae, Clinical Development Division, Celltrion, Inc., Incheon, Republic of Korea.
Go Eun Yang, Clinical Development Division, Celltrion, Inc., Incheon, Republic of Korea.
Jae Kyoung Yoo, Clinical Development Division, Celltrion, Inc., Incheon, Republic of Korea.
Jonathan Kay, Division of Rheumatology, Department of Medicine, University of Massachusetts Medical School and UMass Memorial Medical Center; Division of Epidemiology, Department of Population and Quantitative Health Sciences, University of Massachusetts Medical School, Worcester, MA, USA.
Edward Keystone, Department of Rheumatology, University of Toronto, Toronto, ON, Canada.
References
- 1.European Medicines Agency. Yuflyma summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/yuflyma-epar-product-information_en.pdf (15 April 2021, date last accessed).
- 2. Yu K-S, Jang I-J, Lim H-S et al. Pharmacokinetic equivalence of CT-P17 to high-concentration (100 mg/mL) reference adalimumab: a randomized phase I study in healthy subjects. Clin Transl Sci 2021; doi:10.1111/cts.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davidson A, Brimhall D, Kay J et al. Randomised, phase I pharmacokinetic study of adalimumab biosimilar CT-P17 (40 mg/0.4 mL) by autoinjector and prefilled syringe in healthy subjects. Br J Clin Pharmacol 2021; doi: 10.1111/bcp.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kay J, Jaworski J, Wojciechowski R et al. Efficacy and safety of biosimilar CT-P17 versus reference adalimumab in subjects with rheumatoid arthritis: 24-week results from a randomized study. Arthritis Res Ther 2021;23:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Conduct of clinical trials of medical products during COVID-19 public health emergency: Guidance for industry, investigators, and institutional review boards. 2020. https://www.fda.gov/media/136238/download (15 April 2021, date last accessed).
- 6.European Medicines Agency. Guidance on the management of clinical trials during the COVID-19 (coronavirus) pandemic. Version 4. 2021. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf (15 April 2021, date last accessed).
- 7. Pouw MF, Krieckaert CL, Nurmohamed MT et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 2015;74:513–8. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. Humira summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf (15 April 2021, date last accessed).
- 9.European Medicines Agency. Nordimet summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/nordimet-epar-product-information_en.pdf (15 April 2021, date last accessed).
- 10.European Medicines Agency. Jylamvo summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/jylamvo-epar-product-information_en.pdf (15 April 2021, date last accessed).
- 11. Cohen S, Genovese MC, Choy E et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis 2017;76:1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen SB, Alonso-Ruiz A, Klimiuk PA et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis 2018;77:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinblatt ME, Baranauskaite A, Dokoupilova E et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol 2018;70:832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Genovese MC, Glover J, Greenwald M et al. FKB327, an adalimumab biosimilar, versus the reference product: results of a randomized, Phase III, double-blind study, and its open-label extension. Arthritis Res Ther 2019;21:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiland P, Jeka S, Dokoupilová E et al. Switching to biosimilar SDZ-ADL in patients with moderate-to-severe active rheumatoid arthritis: 48-week efficacy, safety and immunogenicity results from the phase III, randomized, double-blind ADMYRA study. BioDrugs 2020;34: 809–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen S, Pablos JL, Pavelka K et al. An open-label extension study to demonstrate long-term safety and efficacy of ABP 501 in patients with rheumatoid arthritis. Arthritis Res Ther 2019;21:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang J, Eudy-Byrne RJ, Mondick J et al. Population pharmacokinetics of adalimumab biosimilar adalimumab-adbm and reference product in healthy subjects and patients with rheumatoid arthritis to assess pharmacokinetic similarity. Br J Clin Pharmacol 2020;86:2274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available data and methodological information for this study are included in this article and accompanying supplementary materials.