Abstract
Background and aims
Problematic smartphone use (PSU) is growing rapidly among teens. It has similar presentations as other behavioral addictions in terms of excessive use, impulse control problems, and negative consequences. However, the underlying neurobiological mechanisms remain undiscovered. We hypothesized that structural changes in the striatum might serve as an important link between alteration in glutamate signaling and development of PSU.
Methods
Among 88 participants, twenty (F:M, 12:8; age 16.2 ± 1.1) reported high scores in the smartphone addiction proneness scale (SAPS) with a cut-off score of 42; the other 68 (F:M, 19:49; age 15.3 ± 1.7) comprised the control group. Sociodemographic data and depression, anxiety, and impulsivity traits were measured. Striatal volumes (caudate, putamen, and nucleus accumbens) were estimated from T1 imaging data. Serum glutamate levels were estimated from peripheral blood samples. Group comparisons of each data were performed after controlling for age and gender. Mediation analyses were conducted to test the indirect effects of glutamate level alteration on PSU through striatal volumetric alteration.
Results
The PSU group showed a decrease in both caudate volumes than the control group. Left caudate volume was positively correlated with serum glutamate level, and negatively with impulsivity traits and SAPS scores. The mediation model revealed a significant indirect effect of serum glutamate on SAS scores through the reduced left caudate volume.
Discussion and conclusions
This study suggests that altered glutamatergic neurotransmission may be associated with PSU among teens, possibly through reduced left caudate volume. Current findings might support neural mechanisms of smartphone addiction.
Keywords: problematic smartphone use, caudate nucleus, glutamate, adolescents, structural MRI
Introduction
A survey on smartphone overdependence in South Korea showed that the overall high-risk population of smartphone overdependence has increased every year since 2012, and reached 2.9% in 2019 (Korea Internet & Security Agency, 2019). Among all age groups, overdependence rates were highest among adolescents aged 10–19 years, among whom 3.9% were classified as high-risk for problematic smartphone use (PSU) and 26.4% were deemed as a potential risk group.
PSU is characterized by self-control failure (Jiang & Zhao, 2016; H. J. Kim, Min, Min, Lee, & Yoo, 2018), increased salience to mobile devices (Csibi, Griffiths, Demetrovics, & Szabo, 2019), and continuation of smartphone use despite the occurrence of negative consequences (Y. H. Lin et al., 2014), which are similar presentations as exhibited by other behavioral addictions. Recent literature has focused on identifying the clinical characteristics of people with PSU, and have demonstrated the role of depression, social anxiety, and stress in PSU (Y. J. Kim, Jang, Lee, Lee, & Kim, 2018; Rho et al., 2019). However, PSU is still not widely accepted as a psychiatric disorder, and has different definitions and nomenclature (e.g., addiction, overuse, overdependence) by various research groups (Haug et al., 2015; Jeong & Oh, 2020; H. K. Lee et al., 2017).
Still, little is known about the neurobiological mechanisms of PSU. Considering its rapid growth and negative consequences in youth, PSU needs serious attention as a novel condition warranting further clinical research, as with how internet gaming disorder (IGD) is receiving attention (S.-G. Kim et al., 2019).
Few neuroimaging studies have been conducted to explore the underlying neurobiological mechanisms of PSU. People with PSU have been associated with having reduced gray matter volume in their frontal and anterior cingulate cortex (ACC) (Horvath et al., 2020), smaller subgenual ACC (Montag et al., 2018), and right lateral orbitofrontal cortex (D. Lee, Namkoong, Lee, Lee, & Jung, 2019). These findings may support the theoretical models of PSU in which PSU is characterized by impaired executive function, attention, and inhibitory control (Chen, Liang, Mai, Zhong, & Qu, 2016; Chun et al., 2018; Hadar et al., 2017). The striatal region, where the nucleus accumbens is found, is well known to play a crucial role in addictive disorder as a core of the reward circuitry that delivers dopaminergic transmission (Chun et al., 2018; Di Chiara et al., 2004). Recent neuroimaging studies of IGD revealed the implication of striatum on behavioral addiction including increased volume of caudate (Cai et al., 2016; C. H. Park et al., 2017; J. W. Seok & J. H. Sohn, 2018; Wee et al., 2014) and nucleus accumbens (Cai et al., 2016; C. H. Park et al., 2017; Wee et al., 2014), and aberrant fronto-striatal connectivity (Wang et al., 2019; Wee et al., 2014) in adult participants. In an adolescent study, delayed developmental trajectories of the fronto-limbic region have been found (Takeuchi et al., 2016). However, morphological alteration of caudate or putamen among adolescents with PSU are yet to be investigated.
Glutamate plays a pivotal role in modulating frontostriatal circuits with dopamine. Altered glutamate neurotransmissions have been associated with addictive behaviors, such as pathological gambling and IGD (S.-H. Paik, M. R. Choi, S. M. Kwak, S. H. Bang, & D.-J. Kim, 2018; Pettorruso et al., 2014). A release of glutamate can induce augmented responsiveness of the nucleus accumbens, which results in sensitization to the stimulus (Tzschentke & Schmidt, 2003). In addition, compulsive, repetitive behaviors in addiction associated with corticostriatal circuits are modulated by glutamate inputs received by the caudate from the sensory motor cortex, and dopamine inputs from the substantia nigra (Kalivas, 2007). Considering that the active pruning of excitatory synapses during adolescence results in significant morphologic changes in the brain (Selemon, 2013), structural changes in the striatum might serve as an important link between alteration in glutamate signaling and PSU in the adolescent population.
In this study, we hypothesized that there would be significant indirect effects of altered glutamatergic transmission on adolescents with PSU via volumetric alteration in striatal regions. Altered glutamatergic neurotransmission, resulting from differential susceptibility to early life stress, could induce neuronal loss in striatum, and presented as PSU eventually. Still, there is no clear boundary between smartphone use as a pleasurable activity and an addiction. We postulated that neurochemical-neurobiological-behavioral changes might exist on a continuum. To test this hypothesis, we particularly measured volumes in striatal regions (i.e., the caudate, putamen, and nucleus accumbens) and serum glutamate concentrations from teenagers with PSU and typically developing adolescents.
Methods
Participants
Adolescents aged 12 to 18 were recruited to answer an online survey on smartphone use. The survey was distributed via a link that was posted on social media websites by the polling agency “Embrain.” The survey was administered for three and a half months from May to mid-August 2017, and 1,631 adolescents participated in it. In the course of the survey, 801 adolescents responded, and 131 adolescents and their parents left their contact information to participate further in magnetic resonance imaging (MRI) acquisition and blood sampling. Subsequently, we asked them to visit our research facility. Participants were divided into two groups (i.e., adolescents with problematic smartphones use, PSU, and typically developing children, TDC) according to an assessment by a psychiatrist based on the Korean Smartphone Addiction Proneness Scale (SAPS) (D. Kim, Lee, Lee, Nam, & Chung, 2014) for Youth. We delegated the PSU group of participants who reported a SAPS score of 42 and higher into one group and the remaining were the TDC.
Measures
MRI acquisition
High-resolution T1 images were obtained using the 3-Tesla MAGNETOM Verio system (Siemens, Erlangen, Germany). The acquisition of the T1-weighted images was performed with three-dimensional (3D) magnetization-prepared rapid gradient-echo (MPRAGE) imaging using the following parameters: TR = 2,300 ms, TE = 2.22 ms, 176 slices, slice thickness = 1 mm, flip angle = 9°, voxel size = 1 × 1 × 1 mm, image matrix = 256 × 256, and FOV = 256 mm2.
Each participant's three-dimensional structural images were preprocessed with the volume-based stream built in the FreeSurfer imaging suite processing pipeline. Preprocessing includes the following steps: Talairach atlas registration, bias field correction, skull stripping, intensity normalization, linear and non-linear volumetric registration, volumetric labeling and statistics (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999). Both right and left striatal volumes (caudate nucleus, putamen, nucleus accumbens) were estimated and used in the statistical analysis.
Blood collection and immunoassay
Blood samples collected from all participants were centrifuged at 1,000×g for 20 minutes. The upper phase that contained the serum was transferred into a fresh tube and stored at -80 °C until use. Glutamate concentrations were analyzed using a human glutamate ELISA kit (ARG80453; Arigo Biolaboratories, Hsinchu, Taiwan). Serum was preprocessed by extraction and derivatization before glutamate assay according to the manufacturer's instructions. One hundred μL of standards or the preprocessed serum was applied to a glutamate-coated microtiter plate and 50 μL of glutamate antiserum was added. The plate was incubated overnight at 4 °C. After washing the plate, 100 μL of enzyme-conjugated antibody was added and the plate was incubated for 30 minutes at room temperature. One hundred μL of TMB reagent was added and the plate was incubated for 30 minutes in the dark. After adding 100 μL of stop solution, the plate was read at 450 nm to measure neurotransmitter concentrations.
Procedure
The participants were asked to visit the psychiatry outpatient clinic at Seoul St. Mary's Hospital, and were screened for comorbid diagnoses with the Korean Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL); the validity and reliability of the original and Korean versions of the K-SADS-PL have been established (Kaufman et al., 1997; Y. S. Kim et al., 2004). The exclusion criteria for participation were: (1) an IQ < 70, (2) currently suffering from any major medical disorders (e.g., diabetes mellitus), (3) neurological disorders (e.g., seizure disorders, head injury), or (4) psychiatric disorders (e.g., major depressive disorder, anxiety disorders, and Attention-Deficit/Hyperactivity Disorder (ADHD)). Based on the screening interviews, one participant was identified as having major depressive disorder and was excluded from the study. IQ was measured using the Korean Wechsler Intelligence Scale for Children-fourth edition (K-WISC-IV) by a board-certified clinical psychologist.
Sociodemographic data including age, gender, and the educational background of both parents were collected from each participant and their parents. Participants were also asked to fill-up the Dickman Functional and Dysfunctional Impulsivity Inventory (DFII), to measure their own functional and dysfunctional impulsivity. The DFII is a self-report measure containing a total of 23 items, 11 assess functional impulsivity and 12 assess dysfunctional impulsivity (Dickman, 1990). To screen for the presence of depression and anxiety, participants were also asked to respond to the Patient Health Questionnaire-9 (PHQ-9) (S.-J. Park, Choi, Choi, Kim, & Hong, 2010) and the Generalized Anxiety Disorder-7 (GAD-7) (Ahn, Kim, & Choi, 2019), respectively.
Statistical analysis
Among volunteers, MRI acquisition was completed from 109 subjects, and blood samples were successfully analyzed in 104 subjects. We matched both neuroimaging and serum data from a total of 93 participants. Two participants were excluded because of depressive disorder. Additionally, data from three participants were excluded because of severe head motion during acquisition. Finally, 88 subjects were included in the statistical analysis.
Sociodemographic characteristics were analyzed using student's t-test and χ2 test according to the characteristics of each variable. Comparisons of each striatal volume and serum glutamate level were conducted using analysis of covariance (ANCOVA) with age, gender, and intracranial volume (ICV) as covariates.
In addition, we assessed the correlation between striatal volume data and clinical measures of participants. However, we noticed that there was a significant correlation between serum glutamate level and ICV. Controlling of ICV effect in the linear regression model could also deteriorate a potential association of the serum glutamate on the left caudate. Thus, we decided to exclude ICV from covariates in the correlation and mediation analyses to investigate the glutamate-associated susceptibility of each striatal region. We adopted the Benjamini-Hochberg's approach (BH) to control the false discovery rate when performing multiple comparisons.
Finally, we speculated the indirect effect through volume reduction of the caudate nucleus on the path between serum glutamate level and PSU. Although there was no significant direct effect of serum glutamate level on PSU, we postulated that serum glutamate concentration was affected from earlier neurobiological changes. Altered glutamatergic neurotransmission could be mediated by distal process, such as morphological changes in susceptible regions in striatum. Then, these neurobiological changes finally presented as addictive behaviors (Yager, Garcia, Wunsch, & Ferguson, 2015).
To test this hypothesis, we performed mediation analyses (adjusted for age and gender) using the SPSS PROCESS macro (Hayes, 2012) with model 4 (single mediator model). Using bootstrapping, 95% bias-corrected bootstrap confidence intervals [CI] for the indirect effects based on 10,000 bootstrap resamples were calculated. Considering the symptom continuum from a pleasurable activity to an addictive smartphone use, all participants were included in the mediation analysis.
Considering disparity of clinical characteristics between PSU and TDC groups, we further added PHQ-9, GAD-7, and DFII scores as covariates in the sensitivity analysis. Additionally, we have attempted to identify whether mediation effect remained significant in an age and gender matched subgroup by propensity scoring.
Statistical analyses were performed using IBM SPSS Statistics software (version 18 for Windows; IBM Co., Armonk, NY, USA). P values of less than 0.05 were regarded as statistically significant.
Ethics
The study protocol employed was performed in accordance with the ethical standards delineated in the 1964 Declaration of Helsinki and approved by the institutional review board of Seoul St. Mary's Hospital. We relayed to participants and their parents the study's purpose and the details of the study procedure. Each participant provided written informed consent prior to the experiment.
Results
Demographic and clinical characteristics
Demographic and clinical variables are presented in Table 1. Significant differences were found in age (t86 = −2.92, P = 0.005) and gender (χ2 = 6.96, P = 0.008) between the PSU and TDC groups. The PSU group showed high SAPS scores (t86 = −17.50, P < 0.001), impulsivity (DFII, t86 = −3.27, P = 0.002), depressive mood (PHQ-9, t83 = −3.86, P = 0.001) and anxiety (GAD-7, t83 = −2.87, P = 0.009) compared with the TDC group. Partial correlation analysis revealed that SAPS scores were positively correlated with DFII scores (r = 0.30, P = 0.006, BH adjusted P = 0.015), GAD-7 (r = 0.46, P < 0.001, BH adjusted P = 0.01) and PHQ-9 (r = 0.48, P < 0.001, BH adjusted P = 0.005).
Table 1.
Demographic and Clinical characteristics of the Participants
| Variables | PSU (n = 20) | TDC (n = 68) | t or χ2 |
| Age, y | 16.20 ± 1.11 | 15.26 ± 1.68 | −2.92** |
| Gender (Male: Female) | 8:12 | 49:19 | 6.96** |
| Full scale IQ | 100.35 ± 16.14 | 103.09 ± 13.74 | 0.75 |
| SAPS | 45.70 ± 3.23 | 27.43 ± 6.22 | −17.50*** |
| DFII | 12.00 ± 4.41 | 8.69 ± 3.84 | −3.27** |
| PHQ-9 | 7.20 ± 4.81 | 2.82 ± 2.94 | −3.86** |
| GAD-7 | 5.60 ± 5.16 | 2.14 ± 2.68 | −2.87** |
*P < 0.05, **P < 0.01, ***P < 0.001.
PSU, Problematic Smartphone Use; TDC, Typically Developing Children; SAPS, Korean Smartphone Addiction Proneness Scale; DFII, Dickman Functional and Dysfunctional Impulsivity Inventory; PHQ-9, Patient Health Questionnaire-9; GAD-7, Generalized Anxiety Disorder-7.
Group difference in striatal volume and serum glutamate level
ANCOVA analyses adjusting age, gender and ICV revealed that the volume of the left (F = 6.18, P = 0.015) and right (F = 4.43, P = 0.038) caudate nucleus were smaller in the PSU group than those in the TDC group (Table 2). No significant differences in volume of the putamen, nucleus accumbens and were found between the two groups.
Table 2.
Total Intracranial volume, Striatal volume and serum glutamate level of the Participants
| Variables | PSU (n = 20) | TDC (n = 68) | F |
| Total Intracranial Volume, cm3 | 1515.41 ± 148.14 | 1575.64 ± 150.39 | 0.15 |
| Left Caudate, mm3 | 3508.67 ± 370.39 | 3853.36 ± 449.23 | 6.18* |
| Left Putamen, mm3 | 5152.70 ± 647.95 | 5535.87 ± 623.92 | 0.72 |
| Left Nucleus Accumbens, mm3 | 535.39 ± 67.31 | 579.11 ± 99.12 | 0.92 |
| Right Caudate, mm3 | 3570.91 ± 473.12 | 3910.64 ± 473.12 | 4.43* |
| Right Putamen, mm3 | 5338.99 ± 600.72 | 5696.36 ± 617.25 | 0.64 |
| Right Nucleus Accumbens, mm3 | 564.04 ± 82.12 | 611.28 ± 81.85 | 2.51 |
| Serum Glutamate, mg/dl | 27.23 ± 4.35 | 26.95 ± 6.22 | 0.69 |
*P < 0.05, **P < 0.01, ***P < 0.001.
PSU, Problematic Smartphone Use; TDC, Typically Developing Children.
The group differences for volume of both caudates remained significant when controlling age and gender only (left caudate, F = 5.61, P = 0.020; right caudate, F = 4.23, P = 0.043).
A comparison of serum glutamate levels did not find any meaningful difference between the PSU and TDC groups.
Correlation analyses among striatal volume, psychological measures, and glutamate level
A correlation analysis revealed a strong correlation between serum glutamate level and ICV (r = 0.36, P = 0.001). When controlling age, gender, and ICV, there was no significant association between the serum glutamate and each caudate volume (left caudate, r = 0.09, P = 0.401; right caudate, r = 0.09, P = 0.410).
Further correlation analyses adjusted effects from age and gender only. Serum glutamate level was significantly correlated with volume of the left caudate (r = 0.23, P = 0.039, BH adjusted P = 0.025) and right caudate (r = 0.22, P = 0.048, BH adjusted P = 0.03).
SAPS scores were significantly correlated with left caudate volume (r = −0.24, P = 0.033, BH adjusted P = 0.02), but not with right caudate volume (r = 0.21, P = 0.061, BH adjusted P = 0.04) or serum glutamate level (r = 0.05, P = 0.684, BH adjusted P = 0.05). DFII scores showed marginal correlations with the left caudate (r = −0.21, P = 0.050, BH adjusted P = 0.035), but not with right caudate volume (r = −0.19, P = 0.080, BH adjusted P = 0.045).
However, none of the correlation results for caudate volume remained significant after correcting for multiple comparisons by BH test.
Mediation analysis
The mediation model revealed a significant indirect effect of serum glutamate on PSU through the left caudate volume (effect size: −0.09, 95% Bootstrap CI [-0.21, -0.00]), but no direct effect from serum glutamate level on PSU was found (Fig. 1).
Fig. 1.
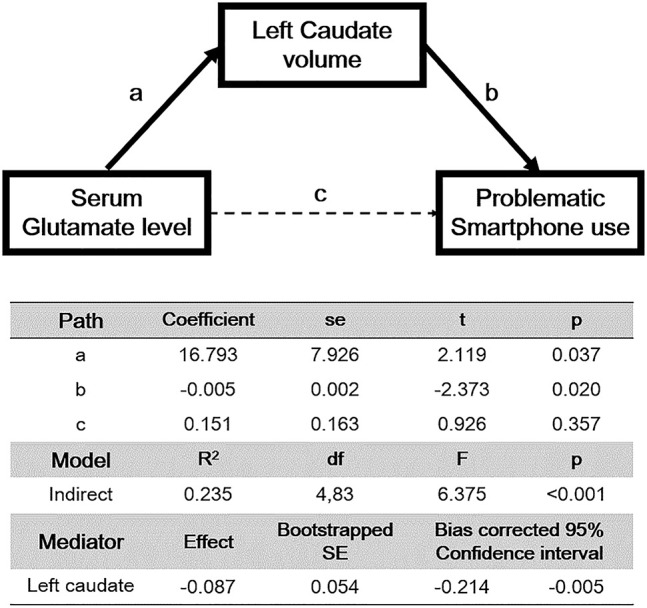
Standardized regression coefficients for the relationship between serum glutamate level and severity of problematic smartphone use as mediated by left caudate volume
However, a model using right caudate volume as a mediator could not demonstrate significant direct and indirect effects (effect size, −0.08; 95% Bootstrap CI [−0.20, 0.00]) from serum glutamate on PSU.
Sensitivity analysis
Volume differences of the left and right caudate remained significant after adding the DFII, PHQ-9, and GAD-7 scores as covariates (left caudate: F = 6.41, P = 0.013; right caudate: F = 5.10, P = 0.027).
Mediation effects were observed between serum glutamate levels and the PSU through the left caudate volume, which remained significant when corrected for age, gender, impulsivity, and depressive symptoms (effect size, −0.10; 95% Bootstrap CI [−0.23, −0.01]). Still, there was no significant indirect effect of serum glutamate level on PSU that was mediated by the right caudate volume (effect size, −0.08; 95% Bootstrap CI [−0.21, 0.00]).
In a subgroup analysis, significant volume difference of left caudate was found (F = 6.41, P = 0.013). Indirect effect of left caudate volume remained significant (effect size: −0.58, 95% Bootstrap CI [−1.19, −0.09]) in a mediation model using age and gender as covariates. However, its significance was lost when adding DFII, PHQ-9, and GAD-7 scores in a covariate matrix. More details of subgroup results are described in the supplementary materials.
Discussion
To date, while growing attention has been paid to PSU, its pathophysiology is still veiled. To our knowledge, this study is the first to investigate the clinical characteristics, neuroimaging, and biochemistry data and their association with the pathophysiology of PSU among adolescents. The PSU group showed high impulsivity, depressive mood, and anxiety compared with the TDC group. Reduced volumes of both caudate nuclei were found in the PSU group, but the serum glutamate level was not significantly different from that of the TDC group. Notably, there was a significant indirect effect of serum glutamate on PSU severity, which was mediated by left caudate volume loss among adolescents. These findings remained significant after controlling for the effects of demographic and clinical variables.
Glutamate signaling has been implicated as important in drug addiction including alcohol (Dodd, Beckmann, Davidson, & Wilce, 2000), nicotine (Reid, Fox, Ho, & Berger, 2000), and opiates (Aghajanian, Kogan, & Moghaddam, 1994). In addition, altered glutamatergic transmission have been suggested as a potential pathophysiological mechanism of behavioral addiction such as pathological gambling (Potenza, 2013), and IGD (S. H. Paik, M. R. Choi, S. M. Kwak, S. H. Bang, & D. J. Kim, 2018) as well as its treatment (Grant, Odlaug, & Schreiber, 2014). However, we could not find significant differences in serum glutamate level between PSU and TDC groups. One possible explanation is large individual heterogeneity in pre-existing glutamate concentration. Individual levels of excitatory and inhibitory neurotransmitter could be affected by age-related development, interaction with environmental stimulus, circadian rhythm, and hormonal changes (Krause & Cohen Kadosh, 2014). Considering neurobiological basis of genes-brain-behavior on addiction (Tzschentke & Schmidt, 2003), changes in glutamate level might be multifactorial, and distal to the development of PSU.
Nevertheless, links between glutamatergic dysfunction and addictive behavior have yet to be well understood. Previous literature demonstrated that glutamatergic signals from the medial prefrontal cortex to the caudate critically affects inhibitory modulation of reward-seeking behavior (Jentsch & Taylor, 1999). Thus, frontostriatal dysfunction might have a critical role in terms of impaired self-control in PSU. To the best of our knowledge, this is the first study that demonstrated that the effect of serum glutamate level on smartphone use severity was completely mediated by the left caudate among adolescents.
The striatum has been implicated in the pathophysiology of addictive disorders (Di Chiara et al., 2004; Yager et al., 2015) as a part of the reward circuit. Previous literature highlighted the role of nucleus accumbens in addiction in that it relays dopaminergic transmission from the ventral tegmental area to the frontal cortex (Di Chiara et al., 2004; Yager et al., 2015). However, the implication of the caudate or putamen in addictive disorders has been less studied in human studies. Some animal studies implicate the caudate and putamen in the compulsive aspects of addiction, such as drug-seeking behavior (Everitt & Robbins, 2005; Everitt & Wolf, 2002; Hearing, Schwendt, & McGinty, 2011). These findings are parallel with those of a study with obsessive-compulsive disorder patients, who were associated with alteration of the structure, connectivity, and activity of the caudate nucleus (Fan et al., 2012; Guehl et al., 2008; Sakai et al., 2011). Studies on individuals with IGD, who show similar characteristics with individuals with PSU, demonstrated an association of reduced caudate volume with disease severity (J.-W. Seok & J.-H. Sohn, 2018). Taking evidence into account, the role of the caudate is to mediate the compulsive nature of behavioral addiction, which is also a frequently presented characteristic of people with PSU (Y. Lin et al., 2017).
Gray matter reductions in the caudate, inferior frontal gyrus, and insula have been identified among cocaine-dependent people (Moreno-López et al., 2012). Among the regions of the inhibitory control network, the caudate is one of the core regions that control impulsive choice (Dalley, Everitt, & Robbins, 2011). Another study suggested that the smaller left relative to the right caudate volume was related with higher impulsiveness among people with attention deficit hyperactivity disorder (Dang et al., 2016). These studies support a correlation between high impulsivity and the left caudate nucleus in the PSU group. In addition, a smaller caudate and aberrant connectivity in the frontostriatal network have been reported in several studies with depressed adolescents (Forbes et al., 2009; Gabbay et al., 2013; Matsuo et al., 2008). These studies suggested that altered caudate volume might be affected by high impulsivity and depressive symptoms in individuals with PSU compared with TDC. However, the volume difference and mediation effect of the left caudate remained significant in the sensitivity analysis after controlling for the effects of the DFII and PHQ-9 scores. Accordingly, indirect mediation effects from the left caudate in the current study may be specific to the pathophysiology of PSU.
Normalization of regional volumes by ICV is a common approach when analyzing brain volume, but the results might vary by confounding variables (Hyatt et al., 2020). In our ANCOVA model, statistical significance was changed a little by adding or excluding ICV into a covariate matrix. However, a strong correlation between serum glutamate level and ICV was present and substantial effects of glutamate-associated regional susceptibility were regressed out after covarying for ICV. As our primary aim was to investigate glutamate effect on striatal volume, ICV was excluded from covariate matrix in our mediation analysis. Further evidences would be needed to estimate the potential effect of the ICV on glutamate-associated volume alteration.
The current study has several limitations. First, there were significant differences in demographic variables such as age and gender. These disparities in demographic characteristics could have been due to selection bias in the recruitment of volunteers. Although we analyzed all data after controlling for age and gender effects, further studies may be required to minimize the effects of demographic variables. Second, the sample size was relatively small, particularly, the PSU group. Third, correlations between left caudate – SAPS score, and left caudate – serum glutamate level became less significant after multiple comparison correction. To validate the current findings, a sample with more adolescents with PSU would be needed. Fourth, there was no convincing evidence that the serum glutamate level was correlated with that circulating in the brain. We recommend the estimation of glutamate from cerebrospinal fluids or magnetic resonance spectroscopy for future studies. Fifth, ADHD symptoms were not measured in our study. They could partially account for the striatal volume differences, given the strong evidence indicating cortico-striato-thalamo-cortical alterations in ADHD. Finally, our findings resulted from structural MRI only, and lacked evidences from a task-based fMRI experiment. Therefore, findings from the present study are not yet conclusive and need more supportive evidences.
Conclusions
This study revealed that left caudate volume is a significant mediator that links aberrant serum glutamate levels with PSU. This finding provides a neurobiological model of PSU and may support the compulsive nature of smartphone use in youth. Further neuroimaging studies would be necessary to investigate structural and functional dysfunctions within PSU and to develop methods for advanced treatment.
Funding sources
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014M3C7A1062893). This study was supported by Research Fund of Seoul St.Mary's Hospital, The Catholic University of Korea. This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Authors' contribution
D-J.K. and J-W.C. contributed to the conception and design of study. J-Y.K. and J.C. contributed to the acquisition of imaging data. M.R.C. contributed to estimate serum glutamate levels. H.C. undertook the clinical assessments. J.H.Y., J-W.C. and M.R.C. performed analysis of imaging and biochemical data and wrote the manuscript including the figures and tables. D-J.K. assisted with the explanation of data and contributed to the final draft of the manuscript. All authors contributed to the manuscript and have approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary material
The online version of this article offers supplementary material https://doi.org/10.1556/2006.2021.00024.
References
- Aghajanian, G. K., Kogan, J., & Moghaddam, B. (1994). Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: An in vivo microdialysis study. Brain Research, 636(1), 126-130. [DOI] [PubMed] [Google Scholar]
- Ahn, J.-K., Kim, Y., & Choi, K.-H. (2019). The Psychometric properties and clinical utility of the korean version of GAD-7 and GAD-2. Frontiers in Psychiatry, 10, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, C., Yuan, K., Yin, J., Feng, D., Bi, Y., Li, Y., et al. (2016). Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging and Behavior, 10(1), 12–20. 10.1007/s11682-015-9358-8. [DOI] [PubMed] [Google Scholar]
- Chen, J., Liang, Y., Mai, C., Zhong, X., & Qu, C. (2016). General deficit in inhibitory control of excessive smartphone users: Evidence from an event-related potential study. Frontiers in Psychology, 7, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, J. W., Choi, J., Cho, H., Choi, M. R., Ahn, K. J., Choi, J. S., et al. (2018). Role of frontostriatal connectivity in adolescents with excessive smartphone use. Front Psychiatry, 9, 437. 10.3389/fpsyt.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibi, S., Griffiths, M. D., Demetrovics, Z., & Szabo, A. (2019). Analysis of problematic smartphone use across different age groups within the ‘components model of addiction’. International Journal of Mental Health and Addiction, 1–16. [Google Scholar]
- Dale, A. M., Fischl, B., & Sereno, M. I.(1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dalley, J. W., Everitt, B. J., & Robbins, T. W. (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron, 69(4), 680-694. [DOI] [PubMed] [Google Scholar]
- Dang, L. C., Samanez-Larkin, G. R., Young, J. S., Cowan, R. L., Kessler, R. M., & Zald, D. H. (2016). Caudate asymmetry is related to attentional impulsivity and an objective measure of ADHD-like attentional problems in healthy adults. Brain Structure & Function, 221(1), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara, G., Bassareo, V., Fenu, S., De Luca, M. A., Spina, L., Cadoni, C., et al. (2004). Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology, 47, 227–241. [DOI] [PubMed] [Google Scholar]
- Dickman, S. J. (1990). Functional and dysfunctional impulsivity: Personality and cognitive correlates. Journal of Personality and Social Psychology, 58(1), 95–102. 10.1037//0022-3514.58.1.95. [DOI] [PubMed] [Google Scholar]
- Dodd, P. R., Beckmann, A. M., Davidson, M. S., & Wilce, P. A. (2000). Glutamate-mediated transmission, alcohol, and alcoholism. Neurochemistry International, 37(5–6), 509–533. [DOI] [PubMed] [Google Scholar]
- Everitt, B. J., & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–1489. [DOI] [PubMed] [Google Scholar]
- Everitt, B. J., & Wolf, M. E. (2002). Psychomotor stimulant addiction: A neural systems perspective. Journal of Neuroscience, 22(9), 3312-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Q., Yan, X., Wang, J., Chen, Y., Wang, X., Li, C., et al. (2012). Abnormalities of white matter microstructure in unmedicated obsessive-compulsive disorder and changes after medication. PLoS One, 7(4), e35889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B., & Dale, A. M.(2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B., Sereno, M. I., & Dale, A. M.(1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl, B., Sereno, M. I., Tootell, R. B., & Dale, A. M.(1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 2722–2844. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E. E., Hariri, A. R., Martin, S. L., Silk, J. S., Moyles, D. L., Fisher, P. M., et al. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166(1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay, V., Ely, B. A., Li, Q., Bangaru, S. D., Panzer, A. M., Alonso, C. M., et al. (2013). Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 628–641. e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J. E., Odlaug, B. L., & Schreiber, L. R. (2014). Pharmacological treatments in pathological gambling. British Journal of Clinical Pharmacology, 77(2), 375–381. 10.1111/j.1365-2125.2012.04457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl, D., Benazzouz, A., Aouizerate, B., Cuny, E., Rotgé, J.-Y., Rougier, A., et al. (2008). Neuronal correlates of obsessions in the caudate nucleus. Biological Psychiatry, 63(6), 557–562. [DOI] [PubMed] [Google Scholar]
- Hadar, A., Hadas, I., Lazarovits, A., Alyagon, U., Eliraz, D., & Zangen, A. (2017). Answering the missed call: Initial exploration of cognitive and electrophysiological changes associated with smartphone use and abuse. PLoS One, 12(7), e0180094. 10.1371/journal.pone.0180094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug, S., Castro, R. P., Kwon, M., Filler, A., Kowatsch, T., & Schaub, M. P. (2015). Smartphone use and smartphone addiction among young people in Switzerland. Journal of Behavioral Addictions, 4(4), 299–307. 10.1556/2006.4.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2012). Process: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. University of Kansas, KS. [Google Scholar]
- Hearing, M. C., Schwendt, M., & McGinty, J. F. (2011). Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. International Journal of Neuropsychopharmacology, 14(6), 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, J., Mundinger, C., Schmitgen, M. M., Wolf, N. D., Sambataro, F., Hirjak, D., et al. (2020). Structural and functional correlates of smartphone addiction. Addictive Behaviors, 105, 106334. 10.1016/j.addbeh.2020.106334. [DOI] [PubMed] [Google Scholar]
- Hyatt, C. S., Owens, M. M., Crowe, M. L., Carter, N. T., Lynam, D. R., & Miller, J. D. (2020). The quandary of covarying: A brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. NeuroImage, 205, 116225. 10.1016/j.neuroimage.2019.116225. [DOI] [PubMed] [Google Scholar]
- Jentsch, J. D., & Taylor, J. R. (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology, 146(4), 373–390. 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jeong, Y. W., & Oh, J. (2020). Pattern of smartphone usage and psychosocial factors affecting smartphone overdependence in middle-aged women. Journal of Addictions Nursing, 31(1), 39–46. 10.1097/JAN.0000000000000323. [DOI] [PubMed] [Google Scholar]
- Jiang, Z., & Zhao, X. (2016). Self-control and problematic mobile phone use in Chinese college students: The mediating role of mobile phone use patterns. BMC Psychiatry, 16(1), 416. 10.1186/s12888-016-1131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas, P. W. (2007). Cocaine and amphetamine-like psychostimulants: Neurocircuitry and glutamate neuroplasticity. Dialogues in Clinical Neuroscience , 9(4), 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, J., Birmaher, B., Brent, D., Rao, U., Flynn, C., Moreci, P., et al. (1997). Schedule for affective disorders and Schizophrenia for school-age children-present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim, Y. S., Cheon, K. A., Kim, B. N., Chang, S. A., Yoo, H. J., Kim, J. W., et al. (2004). The reliability and validity of kiddie-schedule for affective disorders and schizophrenia-present and Lifetime version- Korean version (K-SADS-PL-K). Yonsei Medical Journal, 45(1), 81–89. 10.3349/ymj.2004.45.1.81. [DOI] [PubMed] [Google Scholar]
- Kim, Y. J., Jang, H. M., Lee, Y., Lee, D., & Kim, D. J. (2018). Effects of internet and smartphone addictions on depression and anxiety based on propensity score matching analysis. International Journal of Environmental Research and Public Health. International Journal of Environmental Research and Public Health, 15(5). 10.3390/ijerph15050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D., Lee, Y., Lee, J., Nam, J. K., & Chung, Y. (2014). Development of Korean Smartphone addiction proneness scale for youth. PLoS One, 9(5), e97920. 10.1371/journal.pone.0097920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J., Min, J. Y., Min, K. B., Lee, T. J., & Yoo, S. (2018). Relationship among family environment, self-control, friendship quality, and adolescents' smartphone addiction in South Korea: Findings from nationwide data. PLoS One, 13(2), e0190896. 10.1371/journal.pone.0190896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.-G., Park, J., Kim, H.-T., Pan, Z., Lee, Y., & McIntyre, R. S. (2019). The relationship between smartphone addiction and symptoms of depression, anxiety, and attention-deficit/hyperactivity in South Korean adolescents. Annals of General Psychiatry, 18(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, B., & Cohen Kadosh, R. J. F. i. s. n. (2014). Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. K., Kim, J. H., Fava, M., Mischoulon, D., Park, J. H., Shim, E. J., et al. (2017). Development and validation study of the smartphone overuse screening Questionnaire. Psychiatry Research, 257, 352–357. 10.1016/j.psychres.2017.07.074. [DOI] [PubMed] [Google Scholar]
- Lee, D., Namkoong, K., Lee, J., Lee, B. O., & Jung, Y.-C. (2019). Lateral orbitofrontal gray matter abnormalities in subjects with problematic smartphone use. Journal of Behavioral Addictions, 8(3), 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. H., Chang, L. R., Lee, Y. H., Tseng, H. W., Kuo, T. B., & Chen, S. H. (2014). Development and validation of the smartphone addiction inventory (SPAI). PLoS One, 9(6), e98312. 10.1371/journal.pone.0098312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., Lin, Y., Lin, S.-H., Lee, Y., Lin, P., Chiang, C., et al. (2017). To use or not to use? Compulsive behavior and its role in smartphone addiction. Translational Psychiatry, 7(2), e1030–e1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo, K., Rosenberg, D. R., Easter, P. C., MacMaster, F. P., Chen, H.-H., Nicoletti, M., et al. (2008). Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. Journal of Child and Adolescent Psychopharmacology, 18(2), 121–131. [DOI] [PubMed] [Google Scholar]
- Montag, C., Zhao, Z., Sindermann, C., Xu, L., Fu, M., Li, J., et al. (2018). Internet communication disorder and the structure of the human brain: Initial insights on WeChat addiction. Scientific Reports, 8(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López, L., Catena, A., Fernández-Serrano, M. J., Delgado-Rico, E., Stamatakis, E. A., Pérez-García, M., et al. (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence, 125(3), 208–214. [DOI] [PubMed] [Google Scholar]
- Paik, S.-H., Choi, M. R., Kwak, S. M., Bang, S. H., & Kim, D.-J. (2018). Decreased serum glutamate levels in male adults with internet gaming disorder: A pilot study. Clinical Psychopharmacology and Neuroscience, 16(3), 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik, S. H., Choi, M. R., Kwak, S. M., Bang, S. H., & Kim, D. J. (2018). Decreased serum glutamate levels in male adults with internet gaming disorder: A pilot study. Clin Psychopharmacol Neurosci, 16(3), 276–281. 10.9758/cpn.2018.16.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.-J., Choi, H.-R., Choi, J.-H., Kim, K.-W., & Hong, J.-P. (2010). Reliability and validity of the Korean version of the patient Health questionnaire-9 (PHQ-9). Anxiety and Mood, 6(2), 119–124. [Google Scholar]
- Park, C. H., Chun, J. W., Cho, H., Jung, Y. C., Choi, J., & Kim, D. J. (2017). Is the Internet gaming-addicted brain close to be in a pathological state? Addiction Biology, 22(1), 196–205. 10.1111/adb.12282. [DOI] [PubMed] [Google Scholar]
- Pettorruso, M., De Risio, L., Martinotti, G., Di Nicola, M., Ruggeri, F., Conte, G., et al. (2014). Targeting the Glutamatergic System to Treat Pathological Gambling: Current Evidence and Future Perspectives. BioMed Research International, 2014, 109786. 10.1155/2014/109786 [DOI] [PMC free article] [PubMed]
- Potenza, M. N. (2013). Neurobiology of gambling behaviors. Current Opinion in Neurobiology, 23(4), 660–667. 10.1016/j.conb.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, M. S., Fox, L., Ho, L. B., & Berger, S. P. (2000). Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: Neuropharmacological characterization. Synapse, 35(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Rho, M. J., Park, J., Na, E., Jeong, J.-E., Kim, J. K., Kim, D.-J., et al. (2019). Types of problematic smartphone use based on psychiatric symptoms. Psychiatry Research, 275, 46–52. [DOI] [PubMed] [Google Scholar]
- Sakai, Y., Narumoto, J., Nishida, S., Nakamae, T., Yamada, K., Nishimura, T., et al. (2011). Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. European Psychiatry, 26(7), 463–469. [DOI] [PubMed] [Google Scholar]
- Korea Internet & Security Agency, K. C. C. (2019). Survey on smartphone overdependence. Retrieved from Seoul. [Google Scholar]
- Selemon, L. D. (2013). A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry Electronic Resource, 3, e238. 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok, J.-W., & Sohn, J.-H. (2018). Altered prefrontal and inferior parietal activity during a stroop task in individuals with problematic hypersexual behavior. Frontiers in Psychiatry, 9, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok, J. W., & Sohn, J. H. (2018). Altered gray matter volume and resting-state connectivity in individuals with internet gaming disorder: A voxel-based morphometry and resting-state functional magnetic resonance imaging study. Front Psychiatry, 9, 77. 10.3389/fpsyt.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H., Taki, Y., Hashizume, H., Asano, K., Asano, M., Sassa, Y., et al. (2016). Impact of videogame play on the brain's microstructural properties: Cross-sectional and longitudinal analyses. Molecular Psychiatry, 21(12), 1781–1789. 10.1038/mp.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke, T. M., & Schmidt, W. J. (2003). Glutamatergic mechanisms in addiction. Molecular Psychiatry, 8(4), 373–382. 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Liu, X., Hu, Y., Zheng, H., Du, X., & Dong, G. (2019). Altered brain functional networks in internet gaming disorder: Independent component and graph theoretical analysis under a probability discounting task. CNS Spectrums, 24(5), 544–556. 10.1017/S1092852918001505. [DOI] [PubMed] [Google Scholar]
- Wee, C. Y., Zhao, Z., Yap, P. T., Wu, G., Shi, F., Price, T., et al. (2014). Disrupted brain functional network in internet addiction disorder: A resting-state functional magnetic resonance imaging study. PLoS One, 9(9), e107306. 10.1371/journal.pone.0107306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager, L. M., Garcia, A. F., Wunsch, A. M., & Ferguson, S. M. (2015). The ins and outs of the striatum: Role in drug addiction. Neuroscience, 301, 529–541. 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


