Abstract
Peptide deformylase, a bacterial enzyme, represents a novel target for antibiotic discovery. Two deformylase homologs, defA and defB, were identified in Staphylococcus aureus. The defA homolog, located upstream of the transformylase gene, was identified by genomic analysis and was cloned from chromosomal DNA by PCR. A distinct homolog, defB, was cloned from an S. aureus genomic library by complementation of the arabinose-dependent phenotype of a PBAD-def Escherichia coli strain grown under arabinose-limiting conditions. Overexpression in E. coli of defB, but not defA, correlated to increased deformylase activity and decreased susceptibility to actinonin, a deformylase-specific inhibitor. The defB gene could not be disrupted in wild-type S. aureus, suggesting that this gene, which encodes a functional deformylase, is essential. In contrast, the defA gene could be inactivated; the function of this gene is unknown. Actinonin-resistant mutants grew slowly in vitro and did not show cross-resistance to other classes of antibiotics. When compared to the parent, an actinonin-resistant strain produced an attenuated infection in a murine abscess model, indicating that this strain also has a growth disadvantage in vivo. Sequence analysis of the actinonin-resistant mutants revealed that each harbors a loss-of-function mutation in the fmt gene. Susceptibility to actinonin was restored when the wild-type fmt gene was introduced into these mutant strains. An S. aureus Δfmt strain was also resistant to actinonin, suggesting that a functional deformylase activity is not required in a strain that lacks formyltransferase activity. Accordingly, the defB gene could be disrupted in an fmt mutant.
In procaryotes and eucaryotes, protein synthesis is initiated with a methionine residue which is removed during protein maturation (13). In bacteria and mitochondria, formyltransferase, the fmt gene product, transfers a formyl group to the amino group of the methionine esterified to tRNAfMet. Consequently, nascent polypeptides have a formylated methionine at their N termini. In procaryotes the formyl moiety is removed from the growing peptide by peptide deformylase, the product of the def gene (1, 5, 13, 21). Although proteins synthesized in mitochondria are formylated, neither a def gene nor deformylase activity has been detected in these organelles (17). Searches for sequences homologous to the peptide deformylase among bacterial genomes in publicly available databases reveal the presence of shared open reading frames (ORFs) that encode homologs of Escherichia coli transformylase and deformylase proteins, indicating that the corresponding genes are widely distributed among the bacteria (9, 18, 24). It is not possible to construct null mutants of the def gene in wild-type E. coli, suggesting that the gene is essential for growth (19, 20). On the other hand, it has been shown that deletion of the transformylase-encoding gene, fmt, results in impaired growth. In this genetic background, double mutants of E. coli that lack both transformylase and deformylase can be constructed; these double mutants have the same impaired growth phenotype as the fmt single mutants (11, 19, 26).
Many successful antibiotics inhibit steps of protein synthesis; however, no antimicrobial agent that inhibits protein modification has ever been reported. The widespread occurrence, conservation, and essential nature of deformylase in bacteria, coupled with the absence of this activity in mammalian cells, make it an attractive target for antibacterial drug discovery. Very little is known about deformylase other than the E. coli deformylase. Most gram-negative organisms, including E. coli, have one chromosomal copy of the def gene; however, most gram-positive bacteria have two homologs (9). Redundancy at the genetic or biochemical level can have serious implications for the attractiveness of an enzyme as a drug target, since it provides a relatively facile means of generating resistance. This can be achieved simply through a gene dosage effect or by mutations in which one copy of the gene encodes an enzyme resistant to the antibiotic while the second copy continues to function normally. We have recently identified a potent peptide deformylase inhibitor, actinonin (8). This compound is active against gram-positive and fastidious gram-negative bacteria. The aim of this work was to investigate the suitability of bacterial deformylase as a drug target in Staphylococcus aureus. The S. aureus deformylase-encoding gene was identified and characterized. In addition, actinonin-resistant mutants were selected and the mechanism of resistance was elucidated.
MATERIALS AND METHODS
Growth conditions and strains.
The S. aureus and E. coli strains and plasmids used in this study are listed in Table 1. Bacterial cultures were incubated at 35°C unless otherwise noted. E. coli strains were grown in Luria-Bertani (LB) broth, and S. aureus strains were grown in tryptic soy broth (Difco Laboratories, Detroit, Mich.). For antibiotic selection and genetic manipulations, medium was supplemented with 100 μg of ampicillin per ml, 25 μg of kanamycin per ml, 10 μg of chloramphenicol per ml, 10 μg of tetracycline per ml, 5 μg of erythromycin per ml, or 10 μg of gentamicin per ml, as required. Actinonin, antibiotics, and other chemicals were purchased from Sigma (St. Louis, Mo.); linezolid was synthesized in-house. For growth rate determinations, cells were grown in LB broth overnight, diluted to an optical density at 600 nm of 0.04 in fresh medium, and incubated in a rotary shaker. Growth was monitored spectrophotometrically at 600 nm with a DU640 spectrophotometer (Beckman, Fullerton, Calif.).
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli JM109 | [F′ traD36 proA+B+ lacIq Δ(lacZ)M15] recA1 endA1 gyr96 hsdR17(rK− mK+) supE44 relA1 thi Δ(lac-proAB) | Promega, Madison, Wis. |
| E. coli MC1061 | F−araD139 Δ(ara leu)7697 galE15 galK16 Δ(lac)X74 rpsL hsdR2 (rK− mK+) mcrA mcrB1 | Bio-Rad, Hercules, Calif. |
| E. coli BL21(DE3)(pLysS) | F−ompT hsdSB (rB− mB−) dcm gal λ(DE3) (pLysS) | Novagen, Madison, Wis. |
| E. coli VECO2065 | MC1061 ΔPdef::PBAD; PBAD-regulated def gene | This study |
| E. coli VECO2068 | VECO2065 ΔtolC | This study |
| S. aureus ATCC 25923 | American Type Culture Collection, Manassas, Va. | |
| S. aureus 1-63 | Clinical isolate | H. F. Chambers III (12) |
| S. aureus NCTC 8325-4 | 28 | |
| S. aureus RN4220 | 14 | |
| S. aureus VSAU6011 | ATCC 25923 fmt (A108E); actinonin-resistant mutant | This study |
| S. aureus VSAU6012 | ATCC 25923 fmt (G117V); actinonin-resistant mutant | This study |
| S. aureus VSAU6013 | ATCC 25923 fmt (E157stop); actinonin-resistant mutant | This study |
| S. aureus VSAU6014 | 1-63 fmt (frameshift, codon 180); actinonin-resistant mutant | This study |
| S. aureus VSAU7108 | RN4220 ΔdefA::aac-aph (pPV158-1) | This study |
| S. aureus VSAU7136 | RN4220 Δfmt::aac-aph | This study |
| Plasmids | ||
| pAW8 | tet oriE. coli oriS. aureus; shuttle vector | 33 |
| pAW9 | tet oriE. coli; shuttle vector | 33 |
| pBAD/Myc-HisB | bla araC PBAD | Invitrogen, Carlsbad, Calif. |
| pBS-SK+ | bla lacZα | Stratagene, La Jolla, Calif. |
| pBS-SK− | bla lacZα | Stratagene, La Jolla, Calif. |
| pBS-KS+ | bla lacZα | Stratagene, La Jolla, Calif. |
| pBSII-SK+ | bla lacZα | Stratagene, La Jolla, Calif. |
| pBSL99 | kan | American Type Culture Collection, Manassas, Va. |
| pBT2 | bla cat repF(Ts); shuttle vector, temperature sensitive for replication in S. aureus | 7 |
| pET20b+ | bla PT7 | Promega, Madison, Wis. |
| pGO1 | aac-aph | 32 |
| pGO514 | aac-aph | 27 |
| pKO3 | sacB cat repA(Ts) | 16 |
| pRDC19 | erm | F. Arigoni |
| pT7Blue | bla lacZα | Novagen, Madison, Wis. |
| pUC19 | bla lacZα | New England Biolabs, Beverly, Mass. |
| pDYD11 | pBSII-SK+::kan upstreamdef | This study |
| pDYD12 | pBAD/Myc-HisB::def; PBAD-def fusion | This study |
| pDYD13 | pKO3::upstreamdef kan araC ΔPdef::PBAD-def | This study |
| pVCRΔtolC | pKO3::ΔtolC | This study |
| pPV16-1 | pBS-SK−::defB | This study |
| pPV45-1 | pET20b+::defA; PT7-defB fusion | This study |
| pPV54-2 | pT7Blue::defA | This study |
| pPV58-1 | pET20b+::defB; PT7-defA fusion | This study |
| pPV46-1 | pT7Blue::repF(Ts) | This study |
| pPV72-2 | pAW9::repF(Ts); shuttle vector, temperature sensitive for replication in S. aureus | This study |
| pPV77-1 | lacG disruption plasmid derived from pPV72-2 | This study |
| pPV92-3 | gusA disruption plasmid lacking S. aureus homology derived from pPV72-2 | This study |
| pPV120-1 | defB disruption plasmid derived from pPV72-2 | This study |
| pPV150-1 | pT7Blue::PdefA ΔdefA fmt | This study |
| pPV158-1 | pAW8::PdefA ΔdefA fmt; fmt complementation | This study |
| pPV171-1 | pBS-KS+::erm repF(Ts) shuttle vector, temperature sensitive for replication in S. aureus | This study |
| pPV172-4 | pPV46-1::erm; shuttle vector, temperature sensitive for replication in S. aureus | This study |
| pPV179-1 | pPV171-1::PdefA ΔdefA::aac-aph fmt | This study |
| pPV188-1 | pT7Blue::PdefA defA Δfmt | This study |
| pPV214-1 | pT7Blue::erm repF(Ts) PdefA defA | This study |
| Δfmt::aac-aph |
Molecular techniques, PCR, and sequence analysis.
Molecular techniques, including cloning and DNA purification from E. coli and S. aureus, were performed by standard protocols (28, 31). Oligonucleotides were synthesized at Operon Technologies (Alameda, Calif.). PCR was performed with Advantage HF Polymerase (Clontech, Palo Alto, Calif.) by using a RoboCycler instrument (Stratagene, La Jolla, Calif.). DNA sequences of cloned or PCR-amplified fragments were determined by using the dideoxy chain termination method (Sequetech, Mountain View, Calif.). Homology searches with BLAST (2) were performed at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). Preliminary sequence data were obtained from The Institute for Genomic Research website (http://www.tigr.org) and from the S. aureus Genome Sequencing Project (http://www.genome.ou.edu). Homologs of E. coli peptide deformylase retrieved from the public databases were subjected to multiple alignment and phylogenetic analyses by using the GCG software programs (Genetics Computer Group, Madison, Wis.). Plasmids were introduced into E. coli and S. aureus by electroporation with an Electroporator II apparatus (Bio-Rad, Hercules, Calif.) and by standard procedures. All constructs were confirmed by sequencing or PCR.
Construction of arabinose-dependent PBAD-def E. coli strains.
The E. coli def gene was placed under araBAD promoter control by a promoter exchange strategy. First, a kanamycin resistance-encoding gene from pBSL99 was cloned as a SacI-XbaI fragment into pBSII-SK+. Next, sequences immediately upstream of Pdef were amplified from JM109 and were cloned as a SacI-AscI fragment into this plasmid, creating pDYD11. The full-length E. coli def gene was cloned as a NcoI-BglII fragment into pBAD/Myc-HisB, yielding pDYD12. The def suicide vector was constructed by a three-way ligation of (i) the Ecl136II-NdeI fragment from pDYD11, (ii) the NdeI-BglII def-containing fragment from pDYD12, and (iii) the SmaI-BamHI-digested cloning vector, pKO3, which contains sacB and a temperature-sensitive origin of replication. The resulting clone, pDYD13, was the desired promoter replacement vector. This plasmid was used to replace, in E. coli MC1061, the endogenous def promoter with the PBAD promoter by conventional allele replacement techniques (16) with the following modifications. All plates were supplemented with kanamycin and arabinose (0.2%; wt/vol) throughout the procedure; clones were passaged twice on counterselection plates that contained sucrose (5%; wt/vol) but that lacked NaCl; and sucrose-resistant recombinants were screened for ampicillin sensitivity, chloramphenicol sensitivity, kanamycin resistance, and arabinose dependence. The resulting PBAD-def construct, E. coli VECO2065, is arabinose dependent for growth.
In order to introduce a tolC deletion mutation into VECO2065, a 2.7-kb fragment containing tolC and flanking sequences was amplified from JM109 by PCR and was subcloned into pUC19. A 700-bp internal deletion of the cloned tolC gene was created by PstI-NsiI double digestion, followed by ligation of the compatible ends. The fragment that contained the tolC deletion allele was subsequently subcloned into pKO3. The resulting plasmid, pVCRΔtolC, was used for allele replacement in E. coli VECO2065 as described above. The ΔtolC E. coli strain, VECO2068, was identified by screening of sucrose-resistant, ampicillin-sensitive clones on arabinose-supplemented MacConkey agar, which does not support the growth of tolC mutants. The presence of ΔtolC was confirmed by PCR.
Identification and cloning of S. aureus def genes.
To complete the sequence of the S. aureus defA gene, sequences upstream of the S. aureus fmt gene homolog were cloned by inverse PCR (29), taking advantage of the BsrFI restriction site within fmt. Briefly, chromosomal DNA from S. aureus NCTC 8325-4 was digested with BsrFI, religated, and subjected to PCR amplification with a pair of fmt-specific divergently oriented primers. The resulting 1.3-kb fragment was cloned and sequenced. Subsequently, a 0.7-kb fragment, extending from 260 bp upstream of the defA start codon to 20 bp downstream of its stop codon, was amplified from S. aureus RN4220, cloned into pT7Blue, and sequenced, generating pPV54-2.
An S. aureus gene that encoded a functional deformylase was cloned by complementing the arabinose-dependent phenotype of the PBAD-def E. coli strain when grown under noninducing conditions. An aliquot of a bacteriophage lambda library of S. aureus genomic DNA (Stratagene, La Jolla, Calif.) was phage amplified, and the phagemid was excised according to the manufacturer's instructions. The resulting plasmid pool was used to transform PBAD-def strain VECO2065. Recombinants carrying plasmids that complemented for a lack of endogenous deformylase activity were selected for by the ability to grow in the absence of arabinose on LB agar with ampicillin.
Overexpression of S. aureus def genes in E. coli.
The defA and defB genes were amplified from RN4220 and NCTC 8325-4 such that (i) the ATG start codon was embedded in an NdeI restriction site and (ii) the stop codon was followed by an XhoI restriction site. The primary PCR products were cloned into pT7Blue and sequenced; there were no differences in the sequences between def genes from either strain. The def genes were then subcloned, via the flanking NdeI and XhoI sites, into T7 promoter expression vector pET20b+ to create pPV58-1 and pPV45-1, which carried defA and defB, respectively. These plasmids were transformed into E. coli BL21(DE3)(pLysS). The cells were grown at 30°C in LB broth to an optical density at 600 nm of 0.5, at which point 0.2 mM isopropyl-β-d-thiogalactopyranoside was added. After 4.5 h of induction, the cells were harvested and were resuspended in 20 mM Tris (pH 8)–100 mM KCl–5 mM NiCl2. The cell suspensions were sonicated, and the deformylase enzymatic activity in the cleared lysates was determined by a formate dehydrogenase coupled assay (15). Activity was normalized to the total protein concentration (Protein Assay Kit; Bio-Rad). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis of the E. coli lysates was performed with the ReadyGel system (Bio-Rad).
Isolation of actinonin-resistant mutants.
Spontaneous actinonin-resistant mutants were isolated by plating approximately 107 CFU from an overnight culture of S. aureus ATCC 25923 or S. aureus 1-63 to each of 10 trypticase soy agar plates (TSA; Difco Laboratories) containing 100 μg of actinonin per ml. The plates were incubated overnight, and colonies that grew were passaged to TSA for further characterization.
Construction of a plasmid bearing ΔdefA fmt.
The construction of an fmt-complementing plasmid began with the amplification of ΔdefA fmt by crossover PCR (16). Specifically, the upstream and the downstream ends of the putative defA fmt operon were amplified from S. aureus NCTC 8325-4. The products of these two reactions were combined and were used as the template in a secondary PCR with flanking primers. The resulting 2.1-kb PCR product consists of PdefA ΔdefA fmt in which residues 13 to 141 of the 162-residue defA ORF have been deleted in frame; the deletion is marked by an NheI site introduced by PCR. This fragment was cloned into pT7Blue to create pPV150-1 and was subsequently subcloned into pAW8 to generate pPV158-1. Plasmid pPV158-1 carries PdefA ΔdefA fmt on a tetracycline-selectable E. coli-S. aureus shuttle vector that bears a gram-positive origin of replication that is compatible with repF(Ts)-bearing plasmids.
Construction of temperature-sensitive E. coli-S. aureus shuttle vectors.
Genetic analysis in S. aureus required the construction of E. coli-S. aureus shuttle vectors that are temperature sensitive for replication in the gram-positive host. For this purpose, 1.4-kb fragment that contained a temperature-sensitive gram-positive organism origin of replication [repF(Ts)] was amplified from pBT2 and was cloned into pT7Blue to give pPV46-1. This insert was subcloned into pAW9, yielding pPV72-2, a tetracycline-selectable shuttle vector. pPV171-1, an erythromycin-selectable shuttle vector, was constructed by subcloning the erm gene from pRDC19 and the repF(Ts) gene into pBS-KS+. A distinct erythromycin-resistant shuttle vector (pPV172-4) was constructed by subcloning the erm gene into pPV46-1. Plasmids pPV72-2, pPV171-1, and pPV172-4 are maintained as episomes when S. aureus is grown at 30°C but not at 43°C.
Gene inactivation.
To construct a strain that bears a defA deletion allele on the chromosome, a SpeI-ended aac-aph cassette (which provides resistance to gentamicin and which is from pGO514) was inserted into the compatible NheI site in pPV150-1. A fragment from this plasmid that encompasses ΔdefA::aac-aph fmt was subcloned into shuttle vector pPV171-1. The resulting plasmid, pPV179-1, was transformed at 30°C into S. aureus RN4220(pPV158-1) with selection for gentamicin and tetracycline resistance. While maintaining selection with both antibiotics, this strain was grown overnight at 43°C and was then plated at 30°C to obtain isolated colonies. A tetracycline-resistant, gentamicin-resistant, erythromycin-sensitive isolate, S. aureus VSAU7108, was selected, and the presence of ΔdefA on the chromosome was confirmed by PCR.
Construction of a strain that harbors an fmt deletion allele also used crossover PCR. A 1.4-kb fragment that encompasses PdefA defA and the start of the fmt ORF was amplified from S. aureus NCTC 8325-4; in a separate reaction, a 0.5-kb fragment that spans the downstream end of the fmt ORF was amplified from the same source. The two fragments were used as the template in a secondary PCR and were subcloned into pT7Blue, giving plasmid pPV188-1. This plasmid insert consists of PdefA defA Δfmt in which codons 4 through 164 of the 311 codon fmt ORF have been deleted. The deletion is marked by an XhoI site, into which a SalI-ended aac-aph cassette (from pGO1) was inserted. The 2.4-kb repF(Ts) erm cassette from pPV172-4 was then introduced via HindIII. The resulting plasmid, pPV214-1, is an E. coli-S. aureus shuttle vector that is temperature sensitive for replication in the gram-positive host and that carries PdefA defA Δfmt::aac-aph. S. aureus RN4220 was transformed at 30°C with pPV214-1 with selection for erythromycin resistance. The resulting transformant was passaged first at 43°C (with erythromycin) and was then passaged at 30°C (without antibiotic). Isolates that grew at 30°C were cultured on medium containing actinonin (100 μg/ml) and were rescreened for gentamicin- and actinonin-resistant, erythromycin-sensitive growth. In one such isolate, S. aureus VSAU7136, the presence of Δfmt::aac-aph on the chromosome was confirmed by PCR.
Disruption experiments with S. aureus required the construction of temperature-sensitive plasmids that bore gene fragments. An internal defB fragment (which extended from codons 17 through 139 of 183 total codons) was amplified from S. aureus NCTC 8325-4; this fragment was subcloned into pPV72-2 as a BamHI-ended fragment to create pPV120-1. In a separate experiment, the upstream half of the S. aureus lacG ORF was cloned into pAW9. The repF(Ts) gene was subcloned into this construct via HindIII, generating plasmid pPV77-1. In a distinct construction, the E. coli gusA gene was PCR amplified from E. coli JM109 and was subcloned into pPV72-2; the resulting plasmid (pPV92-3) lacks any S. aureus DNA homology.
Antibiotic susceptibility testing.
MICs were determined by the broth microdilution method with Mueller-Hinton broth (Difco Laboratories) (25). An inoculum of 0.5 × 105 to 1.0 × 105 CFU/ml was used, and the plates were incubated at 35°C for 16 to 20 h. Endpoints were determined by measuring the optical density at 600 nm with a SpectraMax 250 microtiter plate reader (Molecular Devices, Sunnyvale, Calif.). The MIC was the lowest concentration of antibiotic that yielded no visible growth. Experiments were repeated two to five times for each compound. For pPV158-1-containing strains, MICs were determined in the presence of tetracycline in order to maintain the plasmid.
Mouse thigh infection model.
A murine abscess model was used to assess the relative virulence of an actinonin-resistant mutant, VSAU6014, compared to that of its parent strain, S. aureus 1-63 (12). In this model, an abscess is created in a mouse by using a bacteria-bead suspension (10). The microcarrier beads (Cytodex 1; Sigma) were swollen in phosphate-buffered saline (PBS; 2% [wt/vol]) and were autoclaved prior to use. Log-phase cultures of each strain were grown in brain heart infusion broth (Difco Laboratories), washed, resuspended in PBS, and spectrophotometrically adjusted to the desired inoculum size. Equal volumes of the beads and the bacterial culture were mixed together, and infection was initiated by the injection of 0.1 ml of the bacteria-bead mixture into the right thigh of 25-g CD-1 female mice. After 4 days, the mice were killed and their abscessed thighs were removed, weighed, homogenized (Polytron; Brinkman, Westbury, N.Y.) and were quantitatively cultured onto TSA. The results for each group are reported as the mean ± standard deviation of the bacterial titer, expressed as the log10 number of CFU per thigh.
RESULTS
Identification of S. aureus def homologs.
The ongoing release of microbial genomic sequences has permitted the identification of peptide deformylase homologs from a range of bacteria. By using the sequence of E. coli deformylase, BLAST searches of the genomes of pathogenic and nonpathogenic bacteria were performed, and putative peptide deformylase proteins were retrieved and compared (Fig. 1). However, in the case of S. aureus, the sequence of a deformylase gene has not been published, nor is the complete genomic sequence of S. aureus yet available. To evaluate the utility of deformylase as a target for antibacterial drug discovery, the identification of an S. aureus def homolog was necessary.
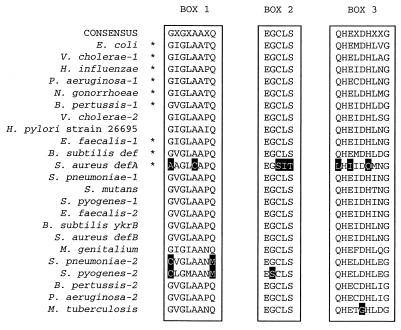
FIG. 1.
Alignment of predicted catalytic domains of deformylase. Three conserved motifs that define the catalytic domain of deformylase are shown. The positions of the motifs in the E. coli protein are as follows: box 1, G44 to Q51; box 2, E89 to S93; box 3, Q132 to G140. An asterisk next to a bacterial name indicates the presence of an fmt homolog downstream of the def homolog. Those residues that diverge from the consensus sequence are highlighted.
In other bacteria, the def gene is often located immediately upstream of fmt (Fig. 1) (18, 20). This conserved gene organization was used to identify an S. aureus def homolog, denoted defA. An fmt locus was identified by BLAST analysis of the existing S. aureus genomic sequences; the fmt ORF, located near the end of a sequence contig, was preceded by an incomplete ORF predicted to encode the C-terminal end of a deformylase-like protein. Inverse PCR was used to obtain the entire ORF. The derived fragment completed the defA ORF and overlapped other existing sequence contigs. The predicted defA gene product could be aligned with other peptide deformylases, and its sequence showed the greatest similarity to the sequences of deformylases encoded by genes with adjacent fmt loci. However, DefA lacked two of the conserved motifs, EGCLS and HEXXH (Fig. 1), diagnostic of peptide deformylases (9, 18, 24). It appears that DefA is missing consensus cysteine and histidine residues that have been shown in the E. coli enzyme to bind to a metal cofactor essential for deformylase activity (3, 4, 22–24, 30). The defA gene was therefore considered unlikely to encode a functional peptide deformylase.
The possible existence of a second S. aureus def homolog was suggested by the surprising observation that many gram-positive bacteria and some gram-negative bacteria have two def homologs (Fig. 1). To identify the S. aureus gene(s) that codes for deformylase activity, an S. aureus genomic library was screened for clones that were able to complement the arabinose-dependent phenotype of E. coli VECO2065 deprived of arabinose. This strain bears a single copy of the def gene, located on the chromosome, with expression of this gene under control of the arabinose-regulated PBAD promoter. Growth of E. coli VECO2065 is strictly arabinose-dependent in LB broth; cells do not grow in the absence of arabinose. Plasmids isolated by their ability to complement the arabinose-dependent growth phenotype were characterized by sequence, PCR, and restriction site analyses; all of these plasmids shared S. aureus sequences that included a def-homologous ORF, denoted defB. The sequence of the DefB protein conforms to the deformylase consensus sequence, including the presence of the pentapeptide motifs missing from DefA (Fig. 1). The defB gene is not adjacent to an fmt homolog, and DefB best resembles deformylases encoded by genes that also lack an adjacent fmt locus (Fig. 1).
defB encodes a functional peptide deformylase.
To assess the deformylase activity of the S. aureus def homologs, each gene was overexpressed in E. coli via a T7-regulated promoter. SDS-PAGE analysis confirmed the presence of an induced protein of the expected molecular weight in each of the lysates (data not shown). The level of deformylase activity from DefA lysates was comparable to that from the parent strain carrying the vector alone (Table 2). In contrast, overexpression of defB resulted in an approximately 1,600-fold increase in the relative amount of specific deformylase activity detected in the E. coli lysate (Table 2). Thus, in S. aureus, defB encodes a functional deformylase.
TABLE 2.
Activity of S. aureus def homolog products in E. colia
| Host E. coli strain | Arabinose in medium | Assay |
E. coli harboring:
|
||
|---|---|---|---|---|---|
| Vector alone | defA | defB | |||
| BL21(DE3) (pLysS) | NAb | Deformylase activity | 1 | 1 | 1,600 |
| VECO2068 | 0.2% | MIC (μg/ml) of actinonin | 2 | 2 | 16 |
| None | Growth | −c | − | +d | |
Enzyme activities were normalized to that of E. coli harboring vector alone.
NA, not applicable.
−, no visible growth.
+, growth.
Whole-cell assays were used to determine whether the defB homolog also conferred resistance to the deformylase-specific inhibitor actinonin. Because E. coli wild-type strains are naturally resistant to this antibiotic (8), E. coli VECO2068, which has a tolC mutation, was used. The strain was more resistant to actinonin when it harbored defB on a multicopy plasmid than when it carried either defA or vector alone (Table 2). E. coli VECO2068 additionally bears a PBAD-def fusion; the defA plasmid was unable to complement the arabinose-dependent phenotype of such a construct. In contrast, when the same strain harbored the plasmid with defB, it was able to grow even under noninducing conditions (Table 2).
Inactivation of S. aureus def homologs.
Inactivation of defA was complicated by the potential polarity of defA mutations on fmt. To avoid the effects on fmt expression, a plasmid-borne copy of S. aureus fmt was supplied in trans while defA was inactivated. Specifically, plasmid pPV158-1 carries defA fmt, from which defA codons 13 through 141, of 162 total codons, were removed. Because this deletion is in frame, the episomal copy of fmt should still be subject to the same regulation of expression seen in the intact operon. By using an S. aureus RN4220 strain carrying this fmt-complementing plasmid, the defA locus was deleted and replaced by a gene that encoded antibiotic resistance. The defA mutant showed no apparent defect in growth.
Inactivation of the defB gene was attempted by use of a shuttle vector that harbored a temperature-sensitive gram-positive organism origin of replication. As a control, a strain carrying a temperature-sensitive plasmid that included homology to the nonessential S. aureus lacG (phospho-β-galactosidase [6]) was grown at 43°C. Isolates bearing an integrated plasmid were readily recovered (Table 3). Site-specific integration at lacG was confirmed by the resulting Lac− phenotype and by PCR. This integration-disruption assay was also performed with strains carrying either of two other plasmids, one that lacked homology to S. aureus and a second that bore a fragment internal to the defB ORF. In both cases, growth was rarely observed upon a shift to the restrictive temperature (Table 3). The colonies that did appear at a low frequency at 43°C apparently harbored freely replicating plasmid or ectopically integrated plasmid, as judged by PCR. S. aureus strains disrupted in defB could not be recovered.
TABLE 3.
defB gene disruption in S. aureusa
| S. aureus strain | Disruption target | fmt genotype | Titer (log10 CFU/ml)
|
|
|---|---|---|---|---|
| 30°C | 43°C | |||
| RN4220 | lacG | fmt+ | 9.8 | 7.3 |
| RN4220 | No homology | fmt+ | 9.5 | 3.6 |
| RN4220 | defB | fmt+ | 9.6 | 3.5 |
| VSAU7136 | defB | Δfmt | 8.5 | 5.3 |
| VSAU6011 | lacG | fmt mutant | 8.7 | 4.9 |
| VSAU6011 | defB | fmt mutant | 8.8 | 4.3 |
Viable organisms recovered at permissive (30°C) or restrictive (43°C) temperatures.
Characterization of actinonin-resistant mutants.
Actinonin-resistant mutants arose in S. aureus strains at a frequency of 10−6.0 (for S. aureus ATCC 25923) to 10−6.3 (for clinical isolate 1-63). Three mutant S. aureus strains, VSAU6011, VSAU6012, and VSAU6013, were derived from S. aureus ATCC 25923; resistant S. aureus strain VSAU6014 was isolated from S. aureus 1-63. Compared with their parent strains, all of the resistant mutants were highly resistant to actinonin but remained susceptible to other classes of antibiotics, including inhibitors of protein synthesis (Table 4). These mutants were four to eight times more susceptible to kanamycin than their parent strains were. Morphologically, the mutant colonies grew to approximately half the size as their parent strains on agar plates. In accordance with this observation, the in vitro growth rates of the actinonin-resistant mutants were also substantially slower than those of the parent strains (Table 4). The in vitro growth rate of the S. aureus fmt mutants was 50% that of the wild type, similar to that found for fmt mutants in P. aeruginosa and considerably higher than that found in E. coli, for which the growth rate of fmt mutants was reported to be only 10% of that of the parent strain (11, 26). The relative virulence of S. aureus VSAU6014 was assessed in vivo by using a murine abscess model. The difference in bacterial titer between mice infected with the mutant strain (n = 4; mean = 4.11 ± 1.47 log10 CFU per thigh) and those infected with the parent strain (n = 4; mean = 8.04 ± 1.04 log10 CFU per thigh) was statistically significant (P = 0.0047; Student's unpaired t test). Thus, the ability of the mutant to establish an infection in this model was clearly attenuated. Following infection with S. aureus VSAU6014, bacteria recovered from the mice were still uniformly resistant to actinonin.
TABLE 4.
Doubling times and antibiotic susceptibilities of actinonin-resistant and -susceptible S. aureus strainsa
| S. aureus strain | T2 (min) | MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Act | Cam | Erm | Tet | Clin | Kan | Lin | Van | ||
| ATCC 25923 | 37 | 16 | 4 | 0.5 | 0.13 | 0.06 | 8 | 2 | 2 |
| VSAU6011 | 60 | >128 | 4 | 0.06 | 0.13 | 0.03 | 1 | 1 | 2 |
| VSAU6012 | 60 | >128 | 4 | 0.5 | 0.13 | 0.13 | 2 | 1 | 2 |
| VSAU6013 | ND | >128 | 4 | 0.25 | 0.13 | 0.06 | 1 | 2 | 1 |
| 1-63 | 34 | 16 | 4 | 0.5 | 0.13 | 0.06 | 2 | 2 | 0.5 |
| VSAU6014 | 62 | >128 | 4 | 0.13 | 0.06 | 0.06 | 0.5 | 1 | 0.5 |
| RN4220 | 36 | 16 | ND | ND | ND | ND | ND | ND | ND |
| VSAU7136 | 63 | >128 | ND | ND | ND | ND | ND | ND | ND |
T2, doubling time. Antibiotic susceptibilities are to actinonin (Act), chloramphenicol (Cam), erythromycin (Erm), tetracycline (Tet), clindamycin (Clin), kanamycin (Kan), linezolid (Lin), vancomycin (Van). ND, not determined.
Mechanism of resistance to actinonin.
To ascertain whether actinonin resistance resulted from an altered target, the defA and defB DNA sequences from the mutant strains were aligned with those from their parent strains. Surprisingly, the sequence comparisons revealed complete identity, even within their upstream promoter domains. In E. coli, the ability to knock out def depends on the absence of a functional fmt gene (19); thus, in the absence of formyltransferase and of formylated proteins, deformylase is no longer essential. We hypothesized that in S. aureus resistance to deformylase inhibitors could arise from mutations that altered the fmt gene. Indeed, sequence analysis indicated that all four mutants harbored alterations in the fmt gene. Two strains had single missense mutations. The other two strains were predicted to encode truncated formyltransferase proteins, one via a nonsense mutation and the other by way of a frameshift (Table 5).
TABLE 5.
Genotypic characterization of actinonin-resistant S. aureus mutantsa
| S. aureus strain | fmt genotype | MIC (μg/ml) of actinonin in the presence of:
|
|
|---|---|---|---|
| No plasmid | pPV158-1 | ||
| ATCC 25923 | Wild type | 16 | NDb |
| VSAU6011 | A108E | >128 | 4 |
| VSAU6012 | G117V | >128 | ND |
| VSAU6013 | E157Stop | >128 | ND |
| 1-63 | Wild type | 16 | 4 |
| VSAU6014 | Frameshiftc | >128 | 8 |
| RN4220 | Wild type | 16 | 2 |
| VSAU7136 | Deletion | >128 | 4 |
Values for uncomplemented strains are repeated from Table 4. For strains that harbored pPV158-1 (which supplies fmt in trans), MICs were determined in the presence of 10 μg of tetracycline per ml.
ND, not determined.
A frameshift at codon 180 results in translation termination at codon 184.
To test whether the fmt mutations are the source of the resistance phenotype, the wild-type S. aureus fmt gene was supplied in trans to the actinonin-resistant mutants. Upon transformation with plasmid pPV158-1 (which harbored fmt), the previously resistant mutants were now susceptible to actinonin (Table 5), and their in vitro growth rates were comparable to those of the wild-type parent strains (data not shown). Construction of an S. aureus fmt null mutant confirmed the mechanism of actinonin resistance. In this mutant, fmt codons 4 through 164, of 311 total codons, were deleted and replaced by a selectable marker. The resulting strain was similar in phenotype to the in vitro-selected mutants: the organism with the Δfmt construct was highly resistant to actinonin and had a decreased growth rate (Table 4); both of these phenotypes were complemented upon the introduction of the fmt-bearing plasmid (Table 5).
As noted above, def is essential in wild-type E. coli but can be mutated or knocked out in an E. coli fmt mutant strain. Inactivation of defB in the strain that harbored Δfmt confirmed that defB is nonessential in an S. aureus background that lacks fmt. Disruption of defB in VSAU6011, which has a point mutation in fmt, provided further evidence that defB is dispensable when fmt is inactivated (Table 3). In the case of both of these fmt mutants, integration at defB by the defB-disrupting plasmid was confirmed by PCR analysis.
DISCUSSION
The identification of deformylase homologs in publicly available genomes shows that peptide deformylase is widely distributed in bacteria (Fig. 1). In general, gram-negative organisms have one homolog, while gram-positive bacteria, including S. aureus, typically carry two. Our results indicate that only one of the S. aureus homologs encodes a true peptide deformylase. As in E. coli and related organisms, defA is adjacent to fmt, apparently in a dicistronic operon. However, the DefA protein sequence lacks the conserved residues of deformylase required for binding to a metal ligand that is essential for deformylase activity (Fig. 1). The significance of this structural difference was confirmed by functional assays. The defA gene could not complement the arabinose-dependent growth phenotype of E. coli PBAD-def. Similarly, defA did not provide E. coli with increased resistance to actinonin. We cannot exclude the possibility that the lack of complementation or of decreased susceptibility to actinonin was due to failure to express defA in E. coli. However, overexpression in E. coli of the defA ORF, under control of a T7 promoter, was not associated with increased deformylase activity, nor is defA essential in S. aureus, since the gene was easily inactivated even in the presence of an intact fmt gene. In contrast, the product of the second S. aureus homolog, defB, includes the conserved motifs that define the enzyme's metallo-binding site. Consistent with this sequence conservation, DefB has deformylase activity, as demonstrated by complementation by defB of the arabinose-dependent growth phenotype of an E. coli PBAD-def strain. In addition, overexpression in E. coli of the defB ORF was correlated with a large increase in deformylase activity, while an E. coli strain that overexpressed S. aureus defB displayed decreased susceptibility to actinonin. In other work that supports this observation, DefB purified from this lysate is strongly inhibited by actinonin (8). In contrast to defA, defB knockout mutants could not be constructed in wild-type S. aureus. However, strains disrupted in defB were isolated in a Δfmt background, in which no transformylase activity is expected. If formylation of proteins does not occur, then deformylation becomes a nonessential function. Since inactivation of defB, but not of defA, is dependent on the Δfmt background, then defB encodes a deformylase and defA does not. These data are consistent with the observation that fmt is epistatic to def in E. coli. That is, only when the E. coli transformylase-encoding gene is inactivated can def null alleles be constructed; the resulting double mutant has the same growth phenotype as the fmt single mutant (19). Taken together, these results indicate that the product of defB is an essential deformylase in S. aureus, while defA is a nonessential paralog of unknown function.
Actinonin-resistant mutants raised in two distinct S. aureus strains were slow growing and did not show cross-resistance to other antibiotics, suggesting a unique mechanism of resistance. Sequence analysis of the deformylase and transformylase homologs showed that these strains carry a mutation in fmt. The introduction of a plasmid with wild-type fmt restored growth, while the strain simultaneously became susceptible to the deformylase inhibitor actinonin. Phenotypically, these in vitro-isolated mutants are essentially identical to an S. aureus fmt deletion mutant. Thus, a mutation in fmt is responsible for resistance to actinonin. Presumably, a reduction in transformylase activity shrinks the pool of formylated methionyl-tRNAfMet. If unformylated methionyl-tRNAfMet is used instead for the initiation of translation, the resulting proteins will remain unformylated and the requirement for peptide deformylase will be reduced or eliminated. The implication is that a mutation in transformylase renders deformylation, and deformylase, nonessential. In fact, in S. aureus fmt mutant strains, defB was easily disrupted, in contrast to wild-type S. aureus, for which defB-disrupted strains could not be recovered.
The viability of the S. aureus actinonin-resistant mutants in vitro as well as in vivo is an important factor in predicting whether this kind of resistance will be found in clinical isolates or whether resistant strains will be selected during therapy. In S. aureus, fmt mutations arose at a high frequency in vitro, but the mutants had a substantially reduced growth rate, indicating that there is a price to pay when transformylase activity is reduced. The virulence of an fmt mutant in an abscess model, which mimics a foreign-body infection, was also assessed. In this murine model, bacterial titers for the mutant strain were significantly less than the titers obtained for the parent strain. The fmt mutation puts S. aureus at a considerable disadvantage in vivo; whether this reduction simply reflects the diminished growth rate or involves other factors is not known. The essentiality of deformylase in S. aureus and the attenuated virulence of resistant mutants suggest that deformylase, a bacterium-specific enzyme, is an attractive target for the development of novel antibacterials.
ACKNOWLEDGMENTS
We thank Gordon Archer, Fabrizio Arigoni, Reinhold Bruckner, Henry F. Chambers, and Akihito Wada for the kind gifts of strains and plasmids and Sara Lopez for technical assistance. Sequence data for bacterial genomes were obtained from The Institute for Genomic Research and the S. aureus Genome Sequencing Project.
Sequencing of the S. aureus genome has been supported by the National Institute for Allergy and Infectious Diseases and the Merck Genome Research Institute.
REFERENCES
- 1.Adams J M. On the release of the formyl group from nascent protein. J Mol Biol. 1968;33:571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker A, Schlichting I, Kabsch W, Groche D, Schultz S, Wagner A F. Iron center, substrate recognition and mechanism of peptide deformylase. Nat Struct Biol. 1998;5:1053–1058. doi: 10.1038/4162. [DOI] [PubMed] [Google Scholar]
- 4.Becker A, Schlichting I, Kabsch W, Schultz S, Wagner A F. Structure of peptide deformylase and identification of the substrate binding site. J Biol Chem. 1998;273:11413–11416. doi: 10.1074/jbc.273.19.11413. [DOI] [PubMed] [Google Scholar]
- 5.Bianchetti R, Lucchini G, Crosti P, Tortora P. Dependence of mitochondrial protein synthesis initiation on formylation of the initiator methionyl-tRNAf. J Biol Chem. 1977;252:2519–2523. [PubMed] [Google Scholar]
- 6.Breidt F J, Stewart G C. Nucleotide and deduced amino acid sequences of the Staphylococcus aureus phospho-beta-galactosidase gene. Appl Environ Microbiol. 1987;53:969–973. doi: 10.1128/aem.53.5.969-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. doi: 10.1111/j.1574-6968.1997.tb10387.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen D Z, Patel P, Hackbarth C J, Wang W, Dreyer G, Young D C, Margolis P S, Wu C, Ni Z-J, Trias J, White R J, Yuan Z. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry. 2000;39:1256–1262. doi: 10.1021/bi992245y. [DOI] [PubMed] [Google Scholar]
- 9.Dardel F, Ragusa S, Lazennec C, Blanquet S, Meinnel T. Solution structure of nickel-peptide deformylase. J Mol Biol. 1998;280:501–513. doi: 10.1006/jmbi.1998.1882. [DOI] [PubMed] [Google Scholar]
- 10.Ford C W, Hamel J C, Stapert D, Yancey R J. Establishment of an experimental model of a Staphylococcus aureus abscess in mice by use of dextran and gelatin microcarriers. J Med Microbiol. 1989;28:259–266. doi: 10.1099/00222615-28-4-259. [DOI] [PubMed] [Google Scholar]
- 11.Guillon J M, Mechulam Y, Schmitter J M, Blanquet S, Fayat G. Disruption of the gene for Met-tRNA(fMet) formyltransferase severely impairs growth of Escherichia coli. J Bacteriol. 1992;174:4294–4301. doi: 10.1128/jb.174.13.4294-4301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy S, Chambers H F. Daptomycin ( LY146032) for prevention and treatment of experimental aortic valve endocarditis in rabbits. Antimicrob Agents Chemother. 1989;33:1522–1525. doi: 10.1128/aac.33.9.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 14.Kreiswirth B N, Löfdahl S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 15.Lazennec C, Meinnel T. Formate dehydrogenase-coupled spectrophotometric assay of peptide deformylase. Anal Biochem. 1997;244:180–182. doi: 10.1006/abio.1996.9910. [DOI] [PubMed] [Google Scholar]
- 16.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahler H R, Dawidowicz K, Feldman F. Formate as a specific label for mitochondrial translational products. J Biol Chem. 1972;247:7439–7442. [PubMed] [Google Scholar]
- 18.Mazel D, Coüc E, Blanchard S, Saurin W, Marlière P. A survey of polypeptide deformylase function throughout the eubacterial lineage. J Mol Biol. 1997;266:939–949. doi: 10.1006/jmbi.1996.0835. [DOI] [PubMed] [Google Scholar]
- 19.Mazel D, Pochet S, Marliere P. Genetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 1994;13:914–923. doi: 10.1002/j.1460-2075.1994.tb06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinnel T, Blanquet S. Characterization of the Thermus thermophilus locus encoding peptide deformylase and methionyl-tRNA(fMet) formyltransferase. J Bacteriol. 1994;176:7387–7390. doi: 10.1128/jb.176.23.7387-7390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meinnel T, Blanquet S. Evidence that peptide deformylase and methionyl-tRNA (fMet) formyltransferase are encoded within the same operon in Escherichia coli. J Bacteriol. 1993;175:7737–7740. doi: 10.1128/jb.175.23.7737-7740.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinnel T, Blanquet S, Dardel F. A new subclass of the zinc metalloproteases superfamily revealed by the solution structure of peptide deformylase. J Mol Biol. 1996;262:375–386. doi: 10.1006/jmbi.1996.0521. [DOI] [PubMed] [Google Scholar]
- 23.Meinnel T, Lazennec C, Blanquet S. Mapping of the active site zinc ligands of peptide deformylase. J Mol Biol. 1995;254:175–183. doi: 10.1006/jmbi.1995.0609. [DOI] [PubMed] [Google Scholar]
- 24.Meinnel T, Lazennec C, Villoing S, Blanquet S. Structure-function relationships within the peptide deformylase family. Evidence for a conserved architecture of the active site involving three conserved motifs and a metal ion. J Mol Biol. 1997;267:749–761. doi: 10.1006/jmbi.1997.0904. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial tests for bacteria that grow aerobically. Approved standard M7-A4. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 26.Newton D T, Creuzenet C, Mangroo D. Formylation is not essential for initiation of protein synthesis in all eubacteria. J Biol Chem. 1999;274:22143–22146. doi: 10.1074/jbc.274.32.22143. [DOI] [PubMed] [Google Scholar]
- 27.Niemeyer D M, Pucci M J, Thanassi J A, Sharma V K, Archer G L. Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1996;178:5464–5471. doi: 10.1128/jb.178.18.5464-5471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 29.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopalan P T, Datta A, Pei D. Purification, characterization, and inhibition of peptide deformylase from Escherichia coli. Biochemistry. 1997;36:13910–13918. doi: 10.1021/bi971155v. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Thomas W D J, Archer G L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989;171:684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wada A, Watanabe H. Penicillin-binding protein 1 of Staphylococcus aureus is essential for growth. J Bacteriol. 1998;180:2759–2765. doi: 10.1128/jb.180.10.2759-2765.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]