ABSTRACT
Animals, including humans, detect odours and use this information to behave efficiently in the environment. Frequently, odours consist of complex mixtures of odorants rather than single odorants, and mixtures are often perceived as configural wholes, i.e. as odour objects (e.g. food, partners). The biological rules governing this ‘configural perception’ (as opposed to the elemental perception of mixtures through their components) remain weakly understood. Here, we first review examples of configural mixture processing in diverse species involving species-specific biological signals. Then, we present the original hypothesis that at least certain mixtures can be processed configurally across species. Indeed, experiments conducted in human adults, newborn rabbits and, more recently, in rodents and honeybees show that these species process some mixtures in a remarkably similar fashion. Strikingly, a mixture AB (A, ethyl isobutyrate; B, ethyl maltol) induces configural processing in humans, who perceive a mixture odour quality (pineapple) distinct from the component qualities (A, strawberry; B, caramel). The same mixture is weakly configurally processed in rabbit neonates, which perceive a particular odour for the mixture in addition to the component odours. Mice and honeybees also perceive the AB mixture configurally, as they respond differently to the mixture compared with its components. Based on these results and others, including neurophysiological approaches, we propose that certain mixtures are convergently perceived across various species of vertebrates/invertebrates, possibly as a result of a similar anatomical organization of their olfactory systems and the common necessity to simplify the environment's chemical complexity in order to display adaptive behaviours.
KEY WORDS: Odour-guided behaviour, Odour object, Elemental perception, Comparative olfaction, Vertebrates, Invertebrates
Summary: Based on recent, convergent data from multiple animal species, including humans, we argue that some odour mixtures are spontaneously processed configurally, i.e. as odour objects, whereas others are not.
Introduction
In complex sensory environments, the extraction of information is a prerequisite to survival. For adult animals, odours (see Glossary) are critically involved in behaviour but are rarely experienced as single odorants (see Glossary). Animals must rapidly extract pertinent information from the mass of environmental molecules and assign meaning to certain mixtures before responding. To cope with this complexity, the olfactory system either breaks down a complex stimulus into its elements – elemental odour perception (see Glossary) – or combines the elements into new, synthetic information – configural odour perception (see Glossary). The elemental strategy involves responding to certain (or all of) the odorants within a mixture, i.e. to key odorants (e.g. Laloi et al., 2000; Reinhard et al., 2010) or certain pheromones (e.g. Renou et al., 2015; Wyatt, 2015). By contrast, the configural strategy results in the attribution of additional or unique information (weak or robust configural odour perception, respectively; see Glossary) to a whole mixture, which carries a value distinct from that of its component values (Kay et al., 2005; Lei and Vickers, 2008). This contributes to the elaboration of stable, background-detached representations of complex signals as meaningful objects (Stevenson and Wilson, 2007). Configural processing in olfaction may thus allow complex patterns of stimuli to be grouped into perceptual units and facilitates the representation of complex sensory ‘objects’ (e.g. partners, prey). These processes are probably vital for organisms to maintain constant perception of certain information despite changes over time (e.g. circadian, seasonal changes) and to allow individuals to focus on the most salient stimuli. These considerations about the adaptive advantages of each perception mode are largely theoretical, and key questions remain unanswered about the biological mechanisms that support elemental/configural perception, e.g. under what circumstances and through which mechanisms do complex odour stimuli constitute for the receiver a sum of elements or blend into a unique percept, and for what benefit?
Here, we begin by discussing configural processing of odour mixtures that seem to be species specific and then consider non-species-specific configural processing. Further, we present some models of configural odour perception and possible neural mechanisms underlying this perception. We conclude with perspectives on the convergent configural perception of odour mixtures between species, and future prospects for the field.
Glossary.
Configural odour perception
Perception of an odour mixture wherein the mixture evokes a specific odour, distinct from the odours of its individual components.
Electro-olfactograms
Negative electrical potential recorded at the surface of the olfactory epithelium. It represents primarily/exclusively the summated generator potential in the olfactory receptor neurons.
Elemental odour perception
Perception of an odour mixture wherein the perceptual quality of the mixture matches one or the other of the components, and/or allows identification of the components.
Hyper-addition
A case of odour mixture interaction observed when the magnitude of the response (sensory or electrophysiological) for a mixture is higher than the sum of responses to its components.
Hypo-addition
A case of odour mixture interaction observed when the magnitude of the response (sensory or electrophysiological) for a mixture is lower than the sum of responses to its components.
Odorant
A volatile chemical compound or mixture of chemical compounds that induces an olfactory percept.
Odour
The perceptual quality emergent from odorants. Most natural odours are composed of multiple different chemical compounds, many or all of which may have unique perceptual qualities if experienced alone.
Partial addition
A case of odour mixture interaction observed when the magnitude of the response (sensory or electrophysiological) for a mixture is higher than the response to the stronger component, but lower than the sum of responses to the components.
Robust configural odour perception
Perception of a mixture through the unique quality of the mixture itself, to the detriment of the qualities of the mixture components.
Weak configural odour perception
Perception of a mixture through the quality of the mixture itself in addition to the qualities of one or several of the mixture components.
Configural odour perception in the animal kingdom
Many species of invertebrates and vertebrates show evidence of configural perception (see Table 1 for some examples). Configural processing allows behavioural responses to specific stimuli in a species-specific manner, i.e. an organism from a given species can be ‘tuned’ to respond (e.g. lay eggs, mate, feed) to a specific combination of molecules presented in a specific ratio. Thus, a mixture processed configurally by one species to evoke a particular behaviour may be processed elementally by other species and trigger no or a different behaviour.
Table 1.
Examples of configural odour processing in invertebrates and vertebrates
Several factors may alter the perception of odour mixtures, although their respective roles remain to be clearly established. First, the chemical nature of the mixed odorants plays a role: with the same number of components, some mixtures are perceived configurally and others elementally, sometimes as a result of particular odorants being more salient than others (e.g. Thomas-Danguin et al., 2014). This is the case for some food aromas (Laska and Hudson, 1993). A similar effect has been found in rats, where removing an individual odorant can affect the odour quality of the whole mixture (Chapuis and Wilson, 2011). Second, the ratio of components can affect mixture perception. For example, rats discriminate binary mixtures according to the molar ratios of their components (Kay et al., 2003), which ensures mixture recognition at higher/lower concentrations (Uchida and Mainen, 2008). The ratio of odorants in binary mixtures is also the driving factor for configural perception in insects (Clifford and Riffell, 2013), catfish (Valentincic et al., 2000) and humans (Olsson, 1998). Third, the perception of odour mixtures is affected by the number of components: humans rarely identify more than 4 odorants in a mixture (e.g. Livermore and Laing, 1998) and are thought to perceive an ‘olfactory white’ in artificial 30-component mixtures that span the physicochemical odorant space (Weiss et al., 2012). Adult rats show difficulties identifying components within mixtures including more than 3–4 components, whereas adult honeybees and newborn rabbits display higher elemental abilities (e.g. Laloi et al., 2000; Sinding et al., 2013).
The mechanisms underlying the neural processing of elemental versus configural perception remain to be clarified. Neuroanatomical similarities that exist between the olfactory systems of vertebrates and invertebrates (Hildebrand and Shepherd, 1997; Eisthen, 2002) suggest, however, that commonalities in the processing of odour mixtures may exist (Box 1).
Box 1. Organisation of the olfactory system in vertebrates and in invertebrates and possible neural structures involved in configural processing.
In the figure, numbers indicate structures/neurons where mixture-specific properties can emerge (see ‘Potential neural mechanisms underlying configural processing’ for details). During olfaction, odorants are sampled by olfactory receptors (ORs) located in the cilia of olfactory sensory neurons (OSNs) in the olfactory epithelium of vertebrates or cuticular sensilla of insects (ORs from mammals and insects belong to different receptor families). In the brain, OSNs enter the olfactory bulb (OB; in mammals) or antennal lobe (AL; in insects) and connect with second-order neurons [the mitral cells (mammals) or projection neurons (insects)] (Buonviso and Chaput, 1990; Vosshall et al., 2000). OSNs expressing the same OR converge onto the same glomerulus, giving rise to odorant-specific maps in the OB/AL (Joerges et al., 1997; Johnson and Leon, 2007; see also Friedrich and Korsching, 1997, for results in fishes). Regarding superior brain areas, mammalian mitral cells project mainly to the anterior olfactory nucleus, anterior and posterior piriform cortex (aPC, pPC), lateral entorhinal cortex and amygdala (Mori and Sakano, 2011). In insects, higher-order centres comprise the mushroom bodies (MBs) and the lateral horn (Mobbs, 1982; Jefferis et al., 2007). The MBs are composed of numerous intrinsic neurons, the Kenyon cells, which are each highly selective to the activation of a different combination of projection neurons (Perez-Orive et al., 2002). Thus, the architecture of the olfactory system presents similarities in vertebrates and invertebrates from the periphery to higher-order levels. Hipp, hippocampus; Tha, thalamus; Amyg, amygdala; Hypo, hypothalamus; NLOT, nucleus of the lateral olfactory tract; OT, olfactory tubercle; AON, anterior olfactory nucleus; TT, taenia tecta; KC, Kenyon cells; PN, projection neuron; LH, lateral horn; Lo, lobula; Me, medulla.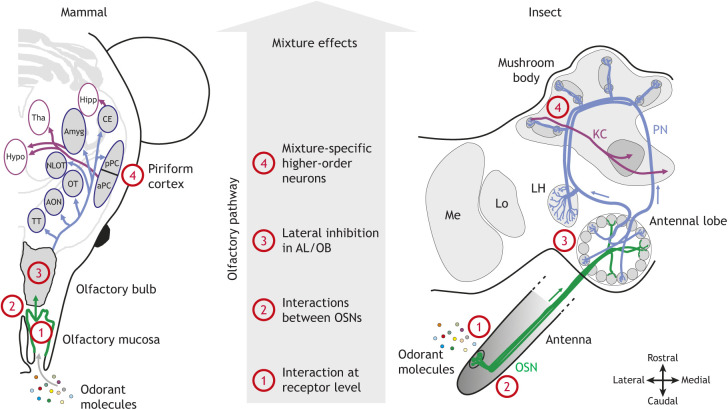
In summary, specific mixtures are perceived as the sum of odour elements, whereas others are perceived as configural odour objects in terrestrial/aquatic vertebrates and invertebrates, which present remarkable similarities in their olfactory architecture. Could there be common rules for complex odour processing across animals? Below, we consider work that has recently addressed this question by studying configural perception of the same mixtures in five different species.
Non-species-specific configural processing
Cross-species analyses of defined perceptual functions can provide unique insights to the basic processes underlying critical behaviours (Jourjine and Hoekstra, 2021). We have used behavioural assays that differ between species in order to test the robustness of the suspected inter-species conservation of configural perception. Here, we summarize the main findings obtained so far and their generalization.
A model binary mixture, the AB mixture
We explored configural processing primarily using the binary AB odour mixture, which has been designed to evoke configural perception in human adults, i.e. the perception of a pineapple odour completely different from the odours of the components A (ethyl isobutyrate, strawberry odour) and B (ethyl maltol, caramel odour). The perception of AB was compared between humans, rabbits, rodents and bees, despite the lack of known biological significance in the non-human species. The goal was to evaluate whether AB could, by its physicochemical properties, generate configural perception not only in humans but also in the other species.
Experiments in humans
As in every species, humans are exposed to odour cues that support the categorization of objects and detection of noxious sources or environments, and contribute to driving behaviours such as food choices (Prescott, 2015) and inter-personal communication (de Groot et al., 2017). Because most of these odours rely on the perception of complex mixtures of odorants, we have been developing a series of experiments to gain insight into the processing of odour mixtures.
As configural processing should confer on a mixture an odour quality that is not (or is less) present in the components, the sensory paradigm used was based on a typicality rating task with the pineapple odour as the target for the AB mixture (Le Berre et al., 2008a, 2010; Barkat et al., 2012). Participants had to rate typicality of distinct stimuli, delivered by a static olfactometric method, according to the following question: ‘is this odour a good or a poor example of the odour of pineapple?’.
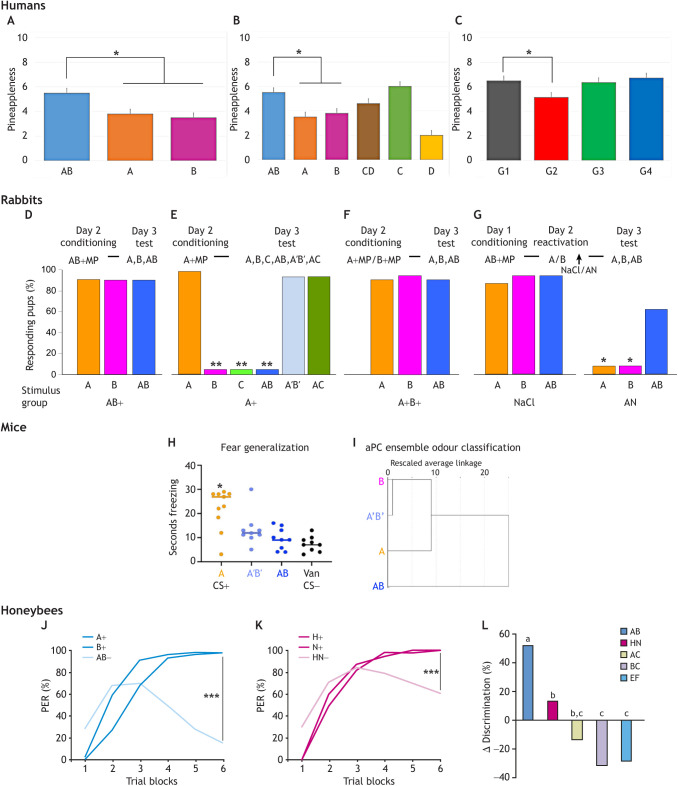
The AB mixture, at the specific 30/70 ratio of A/B, evoked an odour quality more typical of pineapple than of its components (Fig. 1A) (Le Berre et al., 2008a). Not all mixtures of fruity and caramel odours produced the configural perception of pineapple. Indeed, in a binary mixture of ethyl caproate (fruity green-banana odour) and furaneol (caramel odour), the pineapple odour typicality of ethyl caproate itself was actually the highest (Fig. 1B) (Barkat et al., 2012). Moreover, a very small variation of component proportions impaired the mixture configuration and induced a decrease in pineapple typicality (Le Berre et al., 2008a).
Fig. 1.
Demonstration of configural perception of the AB mixture in four different species. In humans: (A) mean pineappleness of the AB mixture compared with its components; (B) replication including the CD mixture (ethyl caproate+furaneol); (C) mean pineappleness of AB in participants pre-exposed to AB (group G1), A and B separately (G2), a control mixture (G3), or the components of the control mixture (G4). In rabbits: (D,E,F) proportions of rabbit pups responding orally to odorants A, B or C and to the AB, A′B′ or AC mixtures 24 h after the learning of AB (D), A (E) or A then B (F); (G) responses 48 h after the learning of AB then re-exposure to A and B followed by NaCl or anisomycin (AN) injection. MP, mammary pheromone. In mice: (H) freezing of mice exposed to the conditioned stimulus (CS)+ odorant A, the elemental mixture A′B′, the configural mixture AB, and the CS– odorant vanillin (Van); (I) hierarchical cluster analysis of mouse single-unit ensembles recorded in the anterior piriform cortex (aPC) after exposure to A, B, the AB mixture or the A′B′ mixture (similar results were observed in rats, not shown). In honeybees: (J,K) proboscis extension responses (PER) in a protocol in which bees received rewarded presentations of mixture components (A/B, or hexanol/nonanol H/N) but non-rewarded presentations of the mixture (AB or HN); (L) comparison of differentiation scores (sum of responses to the rewarded elements minus those to the mixture over the whole procedure) for the AB and HN mixtures, and the control AC, BC and EF mixtures. *P<0.05, **P<0.01, ***P<0.001.
These results underline the specificity of configural odour processing as a function of the stimulus chemical features. Humans' perceptual experience/expertise and attentional processes can also modulate configural processing (Le Berre et al., 2008b; Barkat et al., 2012; Sinding et al., 2015). For instance, pre-exposure to the single components A and B further altered configural processing of the AB mixture, a result not found after pre-exposure to control components or mixtures (Fig. 1C) (Sinding et al., 2015).
Experiments in rabbits
Olfaction allows rabbit neonates to suck during the once per day nursing (Zarrow et al., 1965; Hudson and Distel, 1982). The mammary pheromone (MP; 2-methylbut-2-enal) emitted by adult females triggers neonatal orocephalic movements used for nipple location/seizing (e.g. Coureaud, 2001; Coureaud et al., 2010; Schaal et al., 2003). The MP is perceived among 150 volatiles that compose the rabbit milk, which highlights the ability of pups to perceive at least some mixtures elementally. Moreover, as the MP promotes the learning of new odours through single-trial associative conditioning (Coureaud et al., 2006), we used it to induce learning by the pups of odorants A and B, and of mixtures of A/B, before later testing their orocephalic responsiveness.
The AB mixture appeared to be perceived configurally in pups at the same 30/70 ratio as in humans: after conditioning to AB, the pups responded to both AB and its components (Fig. 1D), whereas after conditioning to A they responded to A (not to B or C; where C is guaïacol), A′B′ (68/32 A/B ratio) and AC, but not to AB (Fig. 1E) (Coureaud et al., 2008, 2009, 2011; Sinding et al., 2011). The pups therefore perceive AB in the weak configural way (perception of 3 cues: the AB odour in addition to the A and B odours) and A′B′ and AC in the elemental way (perception of 2 cues only: the element odours). After conditioning to A, the pups did not respond to AB because it included 2 unfamiliar cues (out of 3) compared with A′B′ and AC (1 out of 2); conversely, when conditioned to AB, the pups learned the 3 cues and could later respond to the mixture and its elements. After learning of A then B, the pups responded to AB (Fig. 1F) because they knew 2 of the 3 mixture cues (Coureaud et al., 2008).
The weak configural perception of AB has been confirmed (1) behaviourally: after conditioning to AB, pups displayed a longer memory of A and B compared with the AB configuration (Coureaud et al., 2014b); (2) pharmacologically: after conditioning to AB, reactivation of the memory of A and B, then injection of anisomycin (a blocker of reconsolidation), pups became amnesic for A and B but still responded to AB (Fig. 1G); with A′B′, the same experiment ended with no response to A, B or A′B′. This demonstrated the independent perception and memory of the AB configuration compared with the A/B components, although A′B′ was perceived and retained as its components (Coureaud et al., 2014a).
Recent results highlight that the perception of AB changes from weak to robust configural between postnatal days 2 and 9, whereas that of A′B′ becomes weak configural at postnatal day 24, i.e. close to weaning (Coureaud et al., 2020).
Experiments in mice and rats
Rodents have been used extensively for understanding odour perception and memory. They have excellent odour discrimination ability (Laska and Shepherd, 2007), though rodent odour discrimination is shaped by both past experience (Chapuis and Wilson, 2011; Chen et al., 2015; Rabin, 1988) and the nature of the odour discrimination assay (Cleland et al., 2002). Discrimination of odour mixtures from each other and from their components by rodents is influenced by the identity and relative proportions of the molecules involved in the mixture (Kay et al., 2003).
Evidence for configural processing of the AB mixture (30/70 A/B ratio) has been found in mice using a standard odour-specific fear conditioning task with odour A as the CS+ (i.e. predicts shock). Animals froze significantly more in response to A than to the configural odour AB, with some generalization to the elemental odour mixture A′B′ (68/32 A/B ratio) (Wilson et al., 2020) (Fig. 1H). Similarly, single-unit neural ensembles in the anterior piriform cortex (aPC) – a region critically involved in odour perception and configural processing (Gottfried, 2010; Wilson and Sullivan, 2011) – processed AB as distinct from both A′B′ and the components A and B as assessed with hierarchical cluster analysis in both rats and mice (Wilson et al., 2020) (Fig. 1I). Interestingly, this pattern was not observed in posterior piriform cortical ensembles, consistent with the known distinct roles of the anterior and posterior piriform in odour processing (Kadohisa and Wilson, 2006; Howard et al., 2009).
Similar results were found with odour habituation/cross-habituation assays (Coureaud and Wilson, 2019), a procedure widely used as a metric of odour discrimination (e.g. in humans: Rabin, 1988, Goyert et al., 2007; in rodents: Fletcher and Wilson, 2001; Cleland et al., 2002). The rate of habituation can also be used to extract information about the stimulus. For example, in both humans (Caron and Caron, 1968, 1969; Cohen et al., 1975; Hunter et al., 1982) and animal models (Brennan et al., 1984), the rate of habituation is shaped by stimulus complexity: perceptually more complex stimuli induce slower habituation. We hypothesize that an odour perceived configurally should induce more rapid habituation than an odour perceived elementally. In fact, in mice, the rate of habituation to the elemental A′B′ mixture was significantly slower compared with that to the configural AB mixture (Coureaud and Wilson, 2019). Thus, the more rapid habituation to AB is consistent with the hypothesis of a configural perception of AB (i.e. simple stimulus) and elemental perception of A′B′ (i.e. complex stimulus). These results also suggest that associative training or familiarity are not required for the expression of this perceptual discrimination between these two mixtures.
Experiments in honeybees
Configural processing of olfactory mixtures in honeybees has been demonstrated using Pavlovian conditioning of the proboscis extension response (Bitterman et al., 1983; Menzel, 1999; Giurfa and Sandoz, 2012), in which bees learn to associate odours (conditioned stimulus, CS) with a sucrose reward (unconditioned stimulus, US). Negative patterning, a special type of differential conditioning task, involves discriminating between a binary mixture and each of its components. In this procedure, two odorants are rewarded when presented alone (X+, Y+) but not when presented in a mixture (XY–; Deisig et al., 2001). Negative patterning is a difficult task for bees and only a small proportion manage to differentiate the stimuli at the end of training (Deisig et al., 2001, 2002, 2003; Komischke et al., 2005; Devaud et al., 2015). Indeed, each element (X or Y) is presented as often with the sucrose reward as without, so configural processing of the mixture is necessary to solve this task (Deisig et al., 2001; Devaud et al., 2015). Recently, bees' negative patterning performances were compared between AB (91/9 – the ratio was adapted so that bees detected the two components equally well) and a list of control mixtures (Wycke et al., 2020).
The control mixtures included two mixtures known to be elementally perceived in newborn rabbits: AC (67/33 ethyl isobutyrate and guaïacol) and BC (17/83 ethyl maltol and guaïacol) (Coureaud et al., 2009); and two mixtures often used in bees (Guerrieri et al., 2005; Schubert et al., 2015): HN (50/50 1-hexanol and 1-nonanol) and EF (50/50 2-octanone and octanal). The conditioning procedure included 6 blocks of 4 trials. In each block, bees received a presentation of each single odorant alone with a reward and two unrewarded presentations of the mixture (1A+, 1B +, 2AB−). In the case of the AB mixture (Fig. 1J), bees quickly differentiated between the components and the mixture. At the end of training, responses to AB were much lower than responses to A and B. These performances were compared with those obtained with the four other mixtures. The second-best performances were observed with the HN mixture (Fig. 1K). At the end of training, the bees responded more to the elements than to the mixture, but differentiation was less marked than for the AB mixture. Likewise, for the 3 other mixtures, differentiation at the end of training was generally low or non-existent. The amount of differentiation observed between the elements and the mixture throughout the training [responses to the components (CS+) minus responses to the mixture (CS−)], was higher for AB than for all other tested mixtures (Fig. 1L). The same was observed when comparing differentiation at the end of training. Thus, in honeybees too, the mixture of A and B has remarkable qualities which suggest configural processing of this mixture.
Generalization of the results
In humans and rabbits, results very similar to those observed with the AB mixture have been obtained with a senary mixture. This mixture, robust-configurally perceived in humans (called RC because it smells like red cordial) is perceived in the weak configural mode in rabbit pups at the same ratio of odorants as in humans (Sinding et al., 2013; Romagny et al., 2014, 2018; Coureaud et al., 2020). Whereas the 6 odorants appear to contribute equivalently to the configural perception of RC in rabbits (Romagny et al., 2014), in humans, 2 odorants mainly contribute to it (Romagny et al., 2018). The fact that a limited number of key odorants may promote configural perception has also been shown in psychophysical human food studies (e.g. Rochelle et al., 2018).
Theoretical models of configural odour processing
Two main models have been proposed to explain configural odour perception (Harris, 2006). The unique-cue theory proposes that an XY mixture activates the representations of the X and Y elements but also of a ‘U’ (unique cue) percept, specific to the mixture (Rescorla et al., 1985). During learning, X, Y and U separately receive associative strength, allowing the animal to differentiate between mixtures and elements. Another theory (Pearce, 1994) states that the mixture gives rise to a single configural unit ‘XY’, and does not evoke elemental units. During learning, animals may respond to the X/Y elements through a generalization rule.
Our experiments generally support the unique-cue theory, which predicts that during conditioning to the AB mixture, three main units A, B and U are engaged. In rabbits, after conditioning to AB, pups distinctively retain AB compared with A and B, and A and B can be forgotten but not AB (Coureaud et al., 2014a,b). In humans, the configural perception of AB is lower after learning of its odorants, suggesting that the subjects’ attention is then mainly focused on the elements' odours (Le Berre et al., 2008b). Behavioural work in honeybees also supports the unique-cue account (Deisig et al., 2003; Lachnit et al., 2004) and imaging experiments show that before any training, the neural representation of a mixture XY is not the pure sum of the representations of X and Y (Deisig et al., 2006; Chen et al., 2015). This difference may be used by the brain as a unique cue. As associative learning modifies odorant representations in the bee brain (Faber et al., 1999; Sandoz et al., 2003; Fernandez et al., 2009; Chen et al., 2015; Locatelli et al., 2016), the particular treatment of AB observed in our negative patterning experiment may result from learning-induced plasticity. A similar explanation could support the mouse fear conditioning results and piriform mixture coding.
According to Pearce's theory, mixtures are represented as single configural units, which can support rapid acquisition of mixture-specific behaviours (Pearce, 1994). Some of our results fit into this framework. Thus, humans spontaneously perceive a pineapple odour in the AB mixture, distinct from the strawberry/caramel odours of A/B (such distinction does not appear with other binary mixtures, or senary mixtures in Sinding et al., 2013). In rabbits, after single conditioning to A, pups do not respond to AB, suggesting spontaneous perception in the mixture of a cue different from the sum of its elements. Similarly, the habituation rate shows different processing of the AB and A′B′ mixtures in mice, with the simpler configural mixture inducing more rapid habituation than the elemental one.
Actually, we propose that odour mixture processing could lie in between the two theoretical frameworks. Thus, because of the stimulus properties (e.g. structural features of odorants targeting specific olfactory receptors, concentration of odorants shaping receptor response) and/or coding processes, some mixtures would be perceived elementally and others configurally. Then, under the effect of experience and odour learning/attentional processes, the initial perception may be tuned to either the elements or the configuration. In this respect, it has been shown in rats with mixtures other than AB/A′B′ that elemental perception can be conditioned such that sniffing can modulate which odours in a mixture are perceived, i.e. that the physical properties of both the odorants and the mucosa contribute to the ease with which odorants can be detected in a mixture (Rojas-Líbano and Kay, 2012). Ultimately, odour mixture processing may allow organisms to decrease environmental complexity by building experience-dependent perceptual associations (e.g. Wilson and Stevenson, 2003; Deisig et al., 2003; Gerber et al., 2011; Coureaud et al., 2014a).
Potential neural mechanisms underlying configural processing
The neural substrates underlying configural perception remain to be clearly determined, though some clues exist at different levels within the olfactory system.
At the periphery, each olfactory receptor (OR) and olfactory sensory neuron (OSN) responds to a variety of odorants so that molecular identity is encoded by the combination of activated ORs/OSNs (e.g. Malnic et al., 1999; Duchamp-Viret et al., 2000). The overlapping responses and proximity of OSNs favour interactions during mixture processing. Electrophysiological comparisons between OSN responses to mixtures versus their components have revealed diverse interactions, such as hypo-addition, partial addition, hyper-addition (see Glossary), inducing inhibition or enhancement effects reconcilable with perception in a variety of species (e.g. Ache et al., 1988; Chaput et al., 2012; El Mountassir et al., 2016), including insects (e.g. Akers and Getz, 1993; Ochieng et al., 2002; Su et al., 2011; Deisig et al., 2012). Single sensory cell responses (Xu et al., 2020; Pfister et al., 2020; Rospars et al., 2008; Singh et al., 2019) highlight that interactions occurring at the OR level (Box 1, interaction 1) can account for interactions at the OSN level, and contribute to receptor-mediated computation of mixture information in the olfactory epithelium prior to transmission to the olfactory bulb. This would enhance the capacity of the olfactory system to discriminate between some mixtures and their components (Kurian et al., 2021).
In newborn rabbits, recent recordings of electro-olfactograms (EOG; see Glossary) in olfactory turbinates suggest that the configural AB mixture is differently processed by ORs compared with the elemental A′B′ and AC mixtures (Duchamp-Viret et al., 2021). Direct mixture-related interactions at the OR are presumably related to the physicochemical features of the odorants (Sanz et al., 2008); therefore, diverse species sharing homologous ORs or presenting similar response spectra may show similar mixture interaction patterns at the olfactory periphery and thus similar constraints on mixture perception. Although this explanation could theoretically apply for insects, insect ORs belong to a completely different receptor family (Benton, 2006). Therefore, if this hypothesis holds for both mammals and insects, evolutionary convergence in the response ranges of their ORs responding to the AB mixture should be invoked.
In addition, interactions between peripheral neurons, in particular OSNs housed within the same sensillum, exist in insects (Box 1, interaction 2). For instance, inhibition between adjacent OSNs, without the use of chemical synapses, has been observed in Drosophila (Su et al., 2012). Thus, unique encoding of mixtures similar to the ligand–ligand interactions evoked above may take place within an insect sensillum. Interactions between mammalian OSNs can also occur through ephaptic interactions between axons as they pass to the olfactory bulb (Blinder et al., 2003), which could serve a similar function.
More centrally, input from different ORs converges, directly or indirectly, which may be critical for odour mixture processing (Wilson and Sullivan, 2011). Within the olfactory bulb (OB)/antennal lobe (AL) of mammals/insects (Box 1, interaction 3), lateral inhibition modifies the information projected to higher-order areas and therefore contributes to mixture representation (Dulac, 2006; Deisig et al., 2010; Szyszka and Stierle, 2014). The ratio of configural mixtures is also coded in the AL in some insects (Lei et al., 2013). In mammals, mitral/tufted OB cells respond to odorants alone or in mixtures, but the firing rates evoked by mixtures (compared with elements) are partially suppressed (Kadohisa and Wilson, 2006; Davison and Katz, 2007). Inhibition may result from overlapping activation patterns favoured by the physicochemical similarity between mixed odorants (Grossman et al., 2008). Moreover, perceptual responses rely not only on which specific ensemble of cells is activated but also on the relative temporal sequence of cell activation (Chong et al., 2020). In line with configural processing, computational modelling suggests that mixing odorants with similar glomerular patterns results in lateral inhibition, leading to information loss about the odorants which could favour specific bulbar activation and coding for the mixture by itself (Linster and Cleland, 2004). Such phenomena may explain why, in pseudo-conditioned rabbit pups, the AB and A′B′ mixtures induce similar activation in the OB glomerular layer, although AB induces higher activation than A′B′ in the granular layer (Schneider et al., 2016). Moreover, in pups conditioned to B, AB induces higher activation than A′B′ at the glomerular level, and the opposite at the granular level (Schneider et al., 2016). In the AL of honeybees and fruit flies, processing by local neurons changes mixture glomerular activity maps so that they are more different from those of the components at its output (projection neurons) than at its input (Deisig et al., 2006, 2010; Silbering and Galizia, 2007).
Mixture-specific representations can be found in higher-order centres (Box 1, interaction 4); for instance, in the olfactory cortex (e.g. Litaudon et al., 1997; Wilson, 2003; Barnes et al., 2008; Bekkers and Suzuki, 2013). Piriform cortical neurons can discriminate a mixture from its components in adult mammals, while the OB still computes the mixture as the sum of odorants (rats: Wilson, 1998, 2000). More precisely, the aPC and posterior piriform cortex (pPC) can respectively encode odorant identity and similarity/quality (rats: Kadohisa and Wilson, 2006; humans: Gottfried, 2009, 2010; Howard et al., 2009). The ensemble single-unit data from rodents suggest that the unique quality of the configural mixture is differentially encoded in the aPC. Similarly, in newborn rabbits, the piriform cortex is distinctively activated by the configural/elemental AB/A′B′ mixtures, although the configural/elemental distinction occurs in both aPC and pPC (Schneider et al., 2016). In humans, recent functional magnetic resonance imaging data obtained using the configural AB mixture suggest the specific involvement of the left orbital part of the inferior frontal gyrus in configural processes (Sinding et al., 2021). In insects, the mushroom bodies, which house neurons (Kenyon cells) that are only activated by concomitant patterns of second-order neurons from the AL, may present configural units activated only by a mixture and not by its components (Sandoz, 2011), supporting successful performance in configural learning tasks (see also Devaud et al., 2015). Such units could be especially numerous in the case of the AB mixture, allowing high discrimination performance.
Conclusions
How odour mixtures are perceived, and whether there are general rules across species regarding elemental and configural mixture processing, is poorly understood despite the biological relevance of these questions. The comparative approach reviewed here has identified that the same AB mixture triggers the perception of a particular odour quality different from the qualities of its elements in various phyla: lagomorphs, rodents, primates and insects. The results are expressed regardless of the behavioural assay. Moreover, the AB mixture does not have any known intrinsic biological value, except in humans, where it was designed to generate the perception of pineapple. These findings support convergence in configural perception in both vertebrates and invertebrates, at least in the species described here, and lead us to hypothesize that physicochemical properties of some odorants (e.g. chemical family, concentration, volatility) and component ratios promote configural processing at the receptor and/or central circuit level, whereas other odorants do not.
More generally, we propose that organisms from different phyla, exposed to similar constraints during development at the individual level, and during evolution at the species level, may have conserved important neurophysiological mechanisms to efficiently process odour mixtures. Such mechanisms would support the decrease of perceptual complexity of the chemosensory environment and enhance the ability of organisms to behave selectively toward social and non-social odours contributing critically to their adaptation. This may explain why different species present converging traits in the way they perceive the same mixtures of odorants. Further experiments are required to precisely understand this processing of odour mixtures in the animal kingdom, and the diverse fascinating ways species, including humans, represent by smell the world in which they are living.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by Agence Nationale de la Recherche MEMOLAP (ANR-2010-JCJC-1410-1)/Burgundy Region/FEDER grants to G.C. and T.T.-D., Agence Nationale de la Recherche Bee-o-CHOC grant (ANR-17-CE20-003) to J.-C.S., CNRS PICS CONFODOUR grant to G.C. and D.A.W., and National Institutes of Health grant R01-AG037693 to D.A.W. Deposited in PMC for release after 12 months.
References
- Ache, B. W., Gleeson, R. A. and Thompson, H. A. (1988). Mechanisms for mixture suppression in olfactory receptors of the spiny lobster. Chem. Senses 13, 425-434. 10.1093/chemse/13.3.425 [DOI] [Google Scholar]
- Akers, R. P. and Getz, W. M. (1993). Response of olfactory receptor neurons in honeybees to odorants and their binary mixtures. J. Comp. Physiol. A 173, 169-185. 10.1007/BF00192976 [DOI] [Google Scholar]
- Barkat, S., Le Berre, E., Coureaud, G., Sicard, G. and Thomas-Danguin, T. (2012). Perceptual blending in odor mixtures depends on the nature of odorants and human olfactory expertise. Chem. Senses 37, 159-166. 10.1093/chemse/bjr086 [DOI] [PubMed] [Google Scholar]
- Barnes, D. C., Hofacer, R. D., Zaman, A. R., Rennaker, R. L. and Wilson, D. A. (2008). Olfactory perceptual stability and discrimination. Nat. Neurosci 11, 1378-1380. 10.1038/nn.2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers, J. M. and Suzuki, N. (2013). Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci. 36, 429-438. 10.1016/j.tins.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Benton, R. (2006). On the Origin of smell: odorant receptors in insects. Cell. Mol. Life Sci. 63, 1579-1585. 10.1007/s00018-006-6130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman, M. E., Menzel, R., Fietz, A. and Schäfer, S. (1983). Classical conditioning of proboscis extension in honeybees. J. Comp. Psychol. 97, 107-119. 10.1037/0735-7036.97.2.107 [DOI] [PubMed] [Google Scholar]
- Blinder, K. J., Pumplin, D. W., Paul, D. L. and Keller, A. (2003). Intercellular interactions in the mammalian olfactory nerve. J. Comp. Neurol. 466, 230-239. 10.1002/cne.10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, M. J., Allen, D., Aleman, D., Azmitia, E. C. and Quartermain, D. (1984). Age differences in within-session habituation of exploratory behavior: effects of stimulus complexity. Behav. Neural. Biol 42, 61-72. 10.1016/S0163-1047(84)90436-9 [DOI] [PubMed] [Google Scholar]
- Byers, K. J., Bradshaw, H. D., Jr and Riffell, J. A. (2014). Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). J. Exp. Biol. 217, 614-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso, N. and Chaput, M. A. (1990). Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. J. Neurophysiol. 63, 447-454. 10.1152/jn.1990.63.3.447 [DOI] [PubMed] [Google Scholar]
- Caron, R. F. and Caron, A. J. (1968). The effects of repeated exposure and stimulus complexity on visual fixation in infants. Psychon. Sci. 10, 207-208. 10.3758/BF03331483 [DOI] [Google Scholar]
- Caron, R. F. and Caron, A. J. (1969). Degree of stimulus complexity and habituation of visual fixation in infants. Psychon. Sci. 14, 78-79. 10.3758/BF03336439 [DOI] [Google Scholar]
- Chapuis, J. and Wilson, D. A. (2011). Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat. Neurosci. 15, 155-161. 10.1038/nn.2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput, M. A., El Mountassir, F., Atanasova, B., Thomas-Danguin, T., Le Bon, A. M., Perrut, A., Ferry, B. and Duchamp-Viret, P. (2012). Interactions of odorants with olfactory receptors and receptor neurons match the perceptual dynamics observed for woody and fruity odorant mixtures. Eur. J. Neurosci. 35, 584-597. 10.1111/j.1460-9568.2011.07976.x [DOI] [PubMed] [Google Scholar]
- Chen, J. Y., Marachlian, E., Assisi, C., Huerta, R., Smith, B. H., Locatelli, F. and Bazhenov, M. (2015). Learning modifies odor mixture processing to improve detection of relevant components. J. Neurosci 35, 179-197. 10.1523/JNEUROSCI.2345-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, E., Moroni, M., Wilson, C., Shoham, S., Panzeri, S. and Rinberg, D. (2020). Manipulating synthetic optogenetic odors reveals the coding logic of olfactory perception. Science 368, eaba2357. 10.1126/science.aba2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland, T. A., Morse, A., Yue, E. L. and Linster, C. (2002). Behavioral models of odor similarity. Behav. Neurosci. 116, 222-231. 10.1037/0735-7044.116.2.222 [DOI] [PubMed] [Google Scholar]
- Clifford, M. R. and Riffell, J. A. (2013). Mixture and odorant processing in the olfactory systems of insects: a comparative perspective. J. Comp. Physiol. A 199, 911-928. 10.1007/s00359-013-0818-6 [DOI] [PubMed] [Google Scholar]
- Cohen, L. B., DeLoache, J. S. and Rissman, M. W. (1975). The effect of stimulus complexity on infant visual attention and habituation. Child Dev. 46, 611-617. 10.2307/1128557 [DOI] [PubMed] [Google Scholar]
- Coureaud, G. (2001). Olfactory regulation of sucking in newborn rabbit: Ethological and chemical characterization of a pheromonal signal. PhD Thesis, University Paris 13, Paris. [Google Scholar]
- Coureaud, G. and Wilson, D. A. (2019). Cortical processing of configurally perceived odor mixtures. Chem. Senses 45, 119-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureaud, G., Moncomble, A. S., Montigny, D., Dewas, M., Perrier, G. and Schaal, B. (2006). A pheromone that rapidly promotes learning in the newborn. Curr. Biol. 16, 1956-1961. 10.1016/j.cub.2006.08.030 [DOI] [PubMed] [Google Scholar]
- Coureaud, G., Thomas-Danguin, T., Le Berre, E. and Schaal, B. (2008). Perception of odor blending mixtures in the newborn rabbit. Physiol. Behav. 95, 194-199. 10.1016/j.physbeh.2008.05.018 [DOI] [PubMed] [Google Scholar]
- Coureaud, G., Hamdani, Y., Schaal, B. and Thomas-Danguin, T. (2009). Elemental and configural processing of odour mixtures in the newborn rabbit. J. Exp. Biol. 212, 2525-2531. 10.1242/jeb.032235 [DOI] [PubMed] [Google Scholar]
- Coureaud, G., Charra, R., Datiche, F., Sinding, C., Thomas-Danguin, T., Languille, S., Hars, B. and Schaal, B. (2010). A pheromone to behave, a pheromone to learn: the rabbit mammary pheromone. J. Comp. Physiol. A 196, 779-790. 10.1007/s00359-010-0548-y [DOI] [PubMed] [Google Scholar]
- Coureaud, G., Gibaud, D., Le Berre, E., Schaal, B. and Thomas-Danguin, T. (2011). Proportion of odorants impacts the configural versus elemental perception of a blending mixture in newborn rabbits. Chem. Senses 36, 693-700. 10.1093/chemse/bjr049 [DOI] [PubMed] [Google Scholar]
- Coureaud, G., Thomas-Danguin, T., Wilson, D. A. and Ferreira, G. (2014a). Neonatal representation of odour objects: distinct memories of the whole and its parts. Proc. R. Soc. B 281, 20133319. 10.1098/rspb.2013.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureaud, G., Thomas-Danguin, T., Datiche, F., Wilson, D. A. and Ferreira, G. (2014b). Differential memory persistence of odour mixture and components in newborn rabbits: Competition between the whole and its parts. Front. Behav. Neurosci. 8, 211. 10.3389/fnbeh.2014.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureaud, G., Letagneaux, C., Thomas-Danguin, T. and Romagny, S. (2020). Developmental changes in elemental and configural perception of odor mixtures in young rabbits. Dev. Psychobiol 62, 471-483. 10.1002/dev.21929 [DOI] [PubMed] [Google Scholar]
- Czerny, M., Mayer, F. and Grosch, W. (1999). Sensory study on the character impact odorants of roasted arabica coffee. J. Agric. Food Chem 47, 695-699. 10.1021/jf980759i [DOI] [PubMed] [Google Scholar]
- Davison, I. G. and Katz, L. C. (2007). Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J. Neurosci. 27, 2091-2101. 10.1523/JNEUROSCI.3779-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, J. H., Semin, G. R. and Smeets, M. A. (2017). On the communicative function of body odors: a theoretical integration and review. Perspect. Psychol. Sci. 12, 306-324. 10.1177/1745691616676599 [DOI] [PubMed] [Google Scholar]
- Deisig, N., Lachnit, H., Giurfa, M. and Hellstern, F. (2001). Configural olfactory learning in honeybees: Negative and positive patterning discrimination. Learn. Mem. 8, 70-78. 10.1101/lm.38301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisig, N., Lachnit, H. and Giurfa, M. (2002). The effect of similarity between elemental stimuli and compounds in olfactory patterning discriminations. Learn. Mem. 9, 112-121. 10.1101/lm.41002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisig, N., Lachnit, H., Sandoz, J. C., Lober, K. and Giurfa, M. (2003). A modified version of the unique cue theory accounts for olfactory compound processing in honeybees. Learn. Mem. 10, 199-208. 10.1101/lm.55803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisig, N., Giurfa, M., Lachnit, H. and Sandoz, J. C. (2006). Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur. J. Neurosci. 24, 1161-1174. 10.1111/j.1460-9568.2006.04959.x [DOI] [PubMed] [Google Scholar]
- Deisig, N., Giurfa, M. and Sandoz, J. C. (2010). Antennal lobe processing increases separability of odor mixture representations in the honeybee. J. Neurophysiol. 103, 2185-2194. 10.1152/jn.00342.2009 [DOI] [PubMed] [Google Scholar]
- Deisig, N., Kropf, J., Vitecek, S., Pevergne, D., Rouyar, A., Sandoz, J.-C., Lucas, P., Gadenne, C., Anton, S. and Barrozo, R. (2012). Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE 7, e33159. 10.1371/journal.pone.0033159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud, J. M., Papouin, T., Carcaud, J., Sandoz, J. C., Grünewald, B. and Giurfa, M. (2015). Neural substrate for higher-order learning in an insect: Mushroom bodies are necessary for configural discriminations. Proc. Natl. Acad. Sci. USA 112, E5854-E5862. 10.1073/pnas.1508422112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret, P., Duchamp, A. and Chaput, M. A. (2000). Peripheral odor coding in the rat and frog: quality and intensity specification. J. Neurosci. 20, 2383-2390. 10.1523/JNEUROSCI.20-06-02383.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret, P., Boyer, J., Lavilla, F. and Coureaud, G. (2021). Brief olfactory learning drives perceptive sensitivity in newborn rabbits: new insights in peripheral processing of odor mixtures and induction. Physiol. Behav. 229, 113217. 10.1016/j.physbeh.2020.113217 [DOI] [PubMed] [Google Scholar]
- Dulac, C. (2006). Sparse encoding of natural scents. Neuron 50, 816-818. 10.1016/j.neuron.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Eisthen, H. L. (2002). Why are olfactory systems of different animals so similar? Brain Behav. Evol. 59, 273-293. 10.1159/000063564 [DOI] [PubMed] [Google Scholar]
- El Mountassir, F., Belloir, C., Briand, L., Thomas-Danguin, T. and Le Bon, A. M. (2016). Encoding odorant mixtures by human olfactory receptors. Flav. Frag. J. 31, 400-407. 10.1002/ffj.3331 [DOI] [Google Scholar]
- Faber, T., Joerges, J. and Menzel, R. (1999). Associative learning modifies neural representations of odors in the insect brain. Nat. Neurosci. 2, 74-78. 10.1038/4576 [DOI] [PubMed] [Google Scholar]
- Fernandez, P. C., Locatelli, F. F., Person-Rennell, N., Deleo, G. and Smith, B. H. (2009). Associative conditioning tunes transient dynamics of early olfactory processing. J. Neurosci. 29, 10191-10202. 10.1523/JNEUROSCI.1874-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine-Levy, J. B., Daniel, P. C., Girardot, M. N. and Derby, C. D. (1989). Behavioral resolution of quality of odorant mixtures by spiny lobsters: differential aversive conditioning of olfactory responses. Chem. Senses 14, 503-524. 10.1093/chemse/14.4.503 [DOI] [Google Scholar]
- Fletcher, M. and Wilson, D. A. (2001). Ontogeny of odor discrimination: a method to assess novel odor discrimination in neonatal rats. Physiol. Behav. 74, 589-593. 10.1016/S0031-9384(01)00602-3 [DOI] [PubMed] [Google Scholar]
- Friedrich, R. W. and Korsching, S. I. (1997). Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 18, 737-752. 10.1016/S0896-6273(00)80314-1 [DOI] [PubMed] [Google Scholar]
- Gerber, B., Eschbach, C., Vogt, K. and Schmuker, M. (2011). The similarity between odors and their binary mixtures in Drosophila. Chem. Senses 36, 613-621. 10.1093/chemse/bjr016 [DOI] [PubMed] [Google Scholar]
- Giurfa, M. and Sandoz, J. C. (2012). Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54-66. 10.1101/lm.024711.111 [DOI] [PubMed] [Google Scholar]
- Gottfried, J. A. (2009). Function follows form: ecological constraints on odor codes and olfactory percepts. Curr. Opin. Neurobiol. 19, 422-429. 10.1016/j.conb.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried, J. A. (2010). Central mechanisms of odour object perception. Nat. Rev. Neurosci. 11, 628-641. 10.1038/nrn2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyert, H. F., Frank, M. E., Gent, J. F. and Hettinger, T. P. (2007). Characteristic component odors emerge from mixtures after selective adaptation. Brain Res. Bull. 72, 1-9. 10.1016/j.brainresbull.2006.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, K. J., Mallik, A. K., Ross, J., Kay, L. M. and Issa, N. P. (2008). Glomerular activation patterns and the perception of odor mixtures. Eur. J. Neurosci. 27, 2676-2685. 10.1111/j.1460-9568.2008.06213.x [DOI] [PubMed] [Google Scholar]
- Guerrieri, F., Schubert, M., Sandoz, J. C. and Giurfa, M. (2005). Perceptual and neural olfactory similarity in honeybees. PLoS. Biol. 3, e60. 10.1371/journal.pbio.0030060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. A. (2006). Elemental representations of stimuli in associative learning. Psychol. Rev. 113, 584-605. 10.1037/0033-295X.113.3.584 [DOI] [PubMed] [Google Scholar]
- Hildebrand, J. G. and Shepherd, G. M. (1997). Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595-631. 10.1146/annurev.neuro.20.1.595 [DOI] [PubMed] [Google Scholar]
- Hopfield, J. F. and Gelperin, A. (1989). Differential conditioning to a compound stimulus and its components in the terrestrial mollusc Limax maximus. Behav. Neurosci. 103, 329-333. 10.1037/0735-7044.103.2.329 [DOI] [Google Scholar]
- Howard, J. D., Plailly, J., Grueschow, M., Haynes, J. D. and Gottfried, J. A. (2009). Odor quality coding and categorization in human posterior piriform cortex. Nat. Neurosci. 12, 932-939. 10.1038/nn.2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. and Distel, H. (1982). The pattern of behaviour of rabbit pups in the nest. Behaviour 79, 255-271. 10.1163/156853982X00292 [DOI] [Google Scholar]
- Hunter, M. A., Ross, H. S. and Ames, E. W. (1982). Preferences for familiar or novel toys: Effects of familiarization time in one-year olds. Dev. Psychol. 18, 519-529. 10.1037/0012-1649.18.4.519 [DOI] [Google Scholar]
- Jefferis, G. S., Potter, C. J., Chan, A. M., Marin, E. C., Rohlfing, T., Maurer, C. R., Jr and Luo, L. (2007). Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128, 1187-1203. 10.1016/j.cell.2007.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerges, J., Küttner, A., Galizia, C. G. and Menzel, R. (1997). Representations of odours and odour mixtures visualized in the honeybee brain. Nature 387, 285-288. 10.1038/387285a0 [DOI] [Google Scholar]
- Johnson, B. A. and Leon, M. (2007). Chemotopic odorant coding in a mammalian olfactory system. J. Comp. Neurol. 503, 1-34. 10.1002/cne.21396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourjine, N. and Hoekstra, H. E. (2021). Expanding evolutionary neuroscience: insights from comparing variation in behavior. Neuron 109, 1084-1099. 10.1016/j.neuron.2021.02.002 [DOI] [PubMed] [Google Scholar]
- Kadohisa, M. and Wilson, D. A. (2006). Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc. Natl. Acad. Sci. USA 103, 15206-15211. 10.1073/pnas.0604313103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, L. M., Lowry, C. A. and Jacobs, H. A. (2003). Receptor contributions to configural and elemental odor mixture perception. Behav. Neurosci. 117, 1108-1114. 10.1037/0735-7044.117.5.1108 [DOI] [PubMed] [Google Scholar]
- Kay, L. M., Crk, T. and Thorngate, J. (2005). A redefinition of odor mixture quality. Behav. Neurosci. 119, 726-733. 10.1037/0735-7044.119.3.726 [DOI] [PubMed] [Google Scholar]
- Komischke, B., Sandoz, J. C., Malun, D. and Giurfa, M. (2005). Partial unilateral lesions of the mushroom bodies affect olfactory learning in honeybees Apis mellifera L. Eur. J. Neurosci. 21, 477-485. 10.1111/j.1460-9568.2005.03879.x [DOI] [PubMed] [Google Scholar]
- Kundu, S., Ganguly, A., Chakraborty, T. S., Kumar, A. and Siddiqi, O. (2016). Synergism and combinatorial coding for binary odor mixture perception in Drosophila. eNeuro 3, e0056-e0014. 10.1523/ENEURO.0056-14.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian, S. M., Naressi, R. G., Manoel, D., Barwich, A.-S., Malnic, M. and Saraiva, L. R. (2021). Odor coding in the mammalian olfactory epithelium. Cell Tissue Res. 383, 445-456. 10.1007/s00441-020-03327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachnit, H., Giurfa, M. and Menzel, R. (2004). Odor processing in honeybees: is the whole equal to, more than, or different from the sum of its parts? Adv. Stud. Behav. 34, 241-264. 10.1016/S0065-3454(04)34006-4 [DOI] [Google Scholar]
- Laloi, D., Bailez, O., Blight, M. M., Roger, B., Pham-Delègue, M. H. and Wadhams, L. J. (2000). Recognition of complex odors by restrained and free-flying honeybees, Apis mellifera. J. Chem. Ecol. 26, 2307-2319. 10.1023/A:1005522826673 [DOI] [Google Scholar]
- Laska, M. and Hudson, R. (1993). Discriminating parts from the whole: determinants of odor mixture perception in squirrel monkeys, Saimiri sciureus. J. Comp. Physiol. A 173, 249-256. 10.1007/BF00192984 [DOI] [PubMed] [Google Scholar]
- Laska, M. and Shepherd, G. M. (2007). Olfactory discrimination ability of CD-1 mice for a large array of enantiomers. Neuroscience 144, 295-301. 10.1016/j.neuroscience.2006.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski, L. and Dorman, D. C. (2013). Explosives detection by military working dogs: Olfactory generalization from components to mixtures. Appl. Anim. Behav. Sci. 151, 84-93. 10.1016/j.applanim.2013.11.010 [DOI] [Google Scholar]
- Le Berre, E., Béno, N., Ishii, A., Chabanet, C., Etiévant, P. and Thomas-Danguin, T. (2008a). Just noticeable differences in component concentrations modify the odor quality of a blending mixture. Chem. Senses 33, 389-395. 10.1093/chemse/bjn006 [DOI] [PubMed] [Google Scholar]
- Le Berre, E., Thomas-Danguin, T., Béno, N., Coureaud, G., Etiévant, P. and Prescott, J. (2008b). Perceptual processing strategy and exposure influence the perception of odor mixtures. Chem. Senses 33, 193-199. 10.1093/chemse/bjm080 [DOI] [PubMed] [Google Scholar]
- Le Berre, E., Jarmuzek, E., Béno, N., Etiévant, P., Prescott, J. and Thomas-Danguin, T. (2010). Learning influences the perception of odor mixtures. Chem. Percept 3, 156-166. 10.1007/s12078-010-9076-y [DOI] [Google Scholar]
- Lei, H. and Vickers, N. (2008). Central processing of natural odor mixtures in insects. J. Chem. Ecol. 34, 915-927. 10.1007/s10886-008-9487-2 [DOI] [PubMed] [Google Scholar]
- Lei, H., Chiu, H. Y. and Hildebrand, J. G. (2013). Responses of protocerebral neurons in Manduca sexta to sex-pheromone mixtures. J. Comp. Physiol. A 199, 997-1014. 10.1007/s00359-013-0844-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster, C. and Smith, B. H. (1999). Generalization between binary odor mixtures and their components in the rat. Physiol. Behav. 66, 701-707. 10.1016/S0031-9384(99)00007-4 [DOI] [PubMed] [Google Scholar]
- Linster, C. and Cleland, T. A. (2004). Configurational and elemental odor mixture perception can arise from local inhibition. J. Comput. Neurosci. 16, 39-47. 10.1023/B:JCNS.0000004840.87570.2e [DOI] [PubMed] [Google Scholar]
- Litaudon, P., Datiche, F. and Cattarelli, M. (1997). Optical recording of the rat piriform cortex activity. Prog. Neurobiol. 52, 485-510. 10.1016/S0301-0082(97)00027-0 [DOI] [PubMed] [Google Scholar]
- Livermore, A. and Laing, D. G. (1998). The influence of chemical complexity on the perception of multicomponent odor mixtures. Percept. Psychophys. 60, 650-661. 10.3758/BF03206052 [DOI] [PubMed] [Google Scholar]
- Locatelli, F. F., Fernandez, P. C. and Smith, B. H. (2016). Learning about natural variation of odor mixtures enhances categorization in early olfactory processing. J. Exp. Biol. 219, 2752-2762. 10.1242/jeb.141465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic, B., Hirono, J., Sato, T. and Buck, L. B. (1999). Combinatorial receptor codes for odors. Cell 96, 713-723. 10.1016/S0092-8674(00)80581-4 [DOI] [PubMed] [Google Scholar]
- Menzel, R. (1999). Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323-340. 10.1007/s003590050392 [DOI] [Google Scholar]
- Mobbs, P. G. (1982). The brain of the honeybee Apis mellifera. I. The connections and spatial organization of the mushroom bodies. Phil. Trans. R. Soc. B 298, 309-354. [Google Scholar]
- Morawo, T. and Fadamiro, H. (2016). Identification of key plant-associated volatiles emitted by Heliothis virescens larvae that attract the parasitoid, Microplitis croceipes: Implications for parasitoid perception of odor blends. J. Chem. Ecol. 42, 1112-1121. 10.1007/s10886-016-0779-7 [DOI] [PubMed] [Google Scholar]
- Mori, K. and Sakano, H. (2011). How is the olfactory map formed and interpreted in the mammalian brain? Ann. Rev. Neurosci. 34, 467-499. 10.1146/annurev-neuro-112210-112917 [DOI] [PubMed] [Google Scholar]
- Ochieng, S. A., Park, K. C. and Baker, T. C. (2002). Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J. Comp. Physiol. A 188, 325-333. 10.1007/s00359-002-0308-8 [DOI] [PubMed] [Google Scholar]
- Olsson, M. J. (1998). An integrated model of intensity and quality of odor mixtures. Ann. N. Y. Acad. Sci. 855, 837-840. 10.1111/j.1749-6632.1998.tb10672.x [DOI] [PubMed] [Google Scholar]
- Pearce, J. M. (1994). Similarity and discrimination: a selective review and a connectionist model. Psychol. Rev 101, 587-607. 10.1037/0033-295X.101.4.587 [DOI] [PubMed] [Google Scholar]
- Perez-Orive, J., Mazor, O., Turner, G. C., Cassenaer, S., Wilson, R. I. and Laurent, G. (2002). Oscillations and sparsening of odor representations in the mushroom body. Science 297, 359-365. 10.1126/science.1070502 [DOI] [PubMed] [Google Scholar]
- Pfister, P., Smith, B. C., Evans, B. J., Brann, J. H., Trimmer, C., Sheikh, M., Arroyave, R., Reddy, G., Jeong, H.-J., Raps, D. A.et al. (2020). Odorant receptor inhibition is fundamental to odor encoding. Curr. Biol. 30, 2574-2587. 10.1016/j.cub.2020.04.086 [DOI] [PubMed] [Google Scholar]
- Prescott, J. (2015). Multisensory processes in flavour perception and their influence on food choice. Curr. Opin. Food Sci. 3, 47-52. 10.1016/j.cofs.2015.02.007 [DOI] [Google Scholar]
- Rabin, M. D. (1988). Experience facilitates olfactory quality discrimination. Percept. Psychophys 44, 532-540. 10.3758/BF03207487 [DOI] [PubMed] [Google Scholar]
- Reinhard, J., Sinclair, M., Srinivasan, M. V. and Claudianos, C. (2010). Honeybees learn odour mixtures via a selection of key odorants. PLoS ONE 5, e9110. 10.1371/journal.pone.0009110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou, M., Party, V., Rouyar, A. and Anton, S. (2015). Olfactory signal coding in an odor background. Biosystems 136, 35-45. 10.1016/j.biosystems.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Rescorla, R. A., Gruau, J. W. and Durlach, P. J. (1985). Analysis of the unique cue in configural discriminations. J. Exp. Psychol. Anim. Behav. Process 11, 356-366. 10.1037/0097-7403.11.3.356 [DOI] [PubMed] [Google Scholar]
- Riffell, J. A., Lei, H., Christensen, T. A. and Hildebrand, J. G. (2009). Characterization and coding of behaviorally significant odor mixtures. Curr. Biol. 19, 335-340. 10.1016/j.cub.2009.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle, M. M., Prévost, G. J. and Acree, T. E. (2018). Computing odor images. J. Agric. Food Chem. 66, 2219-2225. 10.1021/acs.jafc.6b05573 [DOI] [PubMed] [Google Scholar]
- Rojas-Líbano, D. and Kay, L. M. (2012). Interplay between sniffing and odorant sorptive properties in the rat. J. Neurosci. 32, 15577-15589. 10.1523/JNEUROSCI.1464-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagny, S., Thomas-Danguin, T. and Coureaud, G. (2014). Newborn rabbit perception of 6-odorant mixtures depends on configural processing and number of familiar elements. PLoS ONE 9, e107560. 10.1371/journal.pone.0107560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagny, S., Coureaud, G. and Thomas-Danguin, T. (2018). Key odorants or key associations? Insights into elemental and configural odour processing in humans. Flav. Frag. J. 33, 97-105. 10.1002/ffj.3429 [DOI] [Google Scholar]
- Rospars, J. P., Lansky, P., Chaput, M. and Duchamp-Viret, P. (2008). Competitive and noncompetitive odorant interactions in the early neural coding of odorant mixtures. J. Neurosci. 28, 2659-2666. 10.1523/JNEUROSCI.4670-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz, J. C. (2011). Behavioral and neurophysiological study of olfactory perception and learning in honeybees. Front. Syst. Neurosci. 5, 98. 10.3389/fnsys.2011.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz, J. C., Galizia, C. G. and Menzel, R. (2003). Side-specific olfactory conditioning leads to more specific odor representation between sides but not within sides in the honeybee antennal lobes. Neurosci. 120, 1137-1148. 10.1016/S0306-4522(03)00384-1 [DOI] [PubMed] [Google Scholar]
- Sanz, G., Thomas-Danguin, T., Hamdani, E. H., Le Poupon, C., Briand, L., Pernollet, J.-C. and Tromelin, A. (2008). Relationships between molecular structure and perceived odor quality of ligands for a human olfactory receptor. Chem. Senses 33, 639-653. 10.1093/chemse/bjn032 [DOI] [PubMed] [Google Scholar]
- Schaal, B., Coureaud, G., Langlois, D., Giniès, C., Sémon, E. and Perrier, G. (2003). Chemical and behavioural characterization of the rabbit mammary pheromone. Nature 424, 68-72. 10.1038/nature01739 [DOI] [PubMed] [Google Scholar]
- Schneider, N. Y., Datiche, F., Wilson, D. A., Gigot, V., Thomas-Danguin, T., Ferreira, G. and Coureaud, G. (2016). Brain processing of a configural vs elemental odor mixture in the newborn rabbit. Brain Struct. Funct. 221, 2527-2539. 10.1007/s00429-015-1055-2 [DOI] [PubMed] [Google Scholar]
- Schubert, M., Sandoz, J. C., Galizia, G. and Giurfa, M. (2015). Odourant dominance in olfactory mixture processing: what makes a strong odourant? Proc. R. Soc. B 282, 20142562. 10.1098/rspb.2014.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbering, A. F. and Galizia, C. G. (2007). Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J. Neurosci. 27, 11966-11977. 10.1523/JNEUROSCI.3099-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinding, C., Thomas-Danguin, T., Crepeaux, G., Schaal, B. and Coureaud, G. (2011). Experience influences elemental and configural perception of certain binary odour mixtures in newborn rabbits. J. Exp. Biol. 214, 4171-4178. 10.1242/jeb.063610 [DOI] [PubMed] [Google Scholar]
- Sinding, C., Thomas-Danguin, T., Chambault, A., Béno, N., Dosne, T., Chabanet, C., Schaal, B. and Coureaud, G. (2013). Rabbit neonates smell as well as human adults ... and even better elementally. PLoS ONE 8, e53534. 10.1371/journal.pone.0053534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinding, C., Coureaud, G., Bervialle, B., Martin, C., Schaal, B. and Thomas-Danguin, T. (2015). Experience-induced modulation of blending and non-blending odour mixture perception. Atten. Percept. Psychophys 77, 1794-1806. 10.3758/s13414-015-0883-8 [DOI] [PubMed] [Google Scholar]
- Sinding, C., Hummel, T., Béno, N., Prescott, J., Bensafi, M., Coureaud, G. and Thomas-Danguin, T. (2021). Configural memory of a blending aromatic mixture reflected in activation of the left orbital part of the inferior frontal gyrus. Behav. Brain Res. 402, 113088. 10.1016/j.bbr.2020.113088 [DOI] [PubMed] [Google Scholar]
- Singh, V., Murphy, N. R., Balasubramanian, V. and Mainland, J. D. (2019). Competitive binding predicts nonlinear responses of olfactory receptors to complex mixtures. Proc. Natl. Acad. Sci. USA 116, 9598-9603. 10.1073/pnas.1813230116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, R. J. and Wilson, D. A. (2007). Odour perception: an object-recognition approach. Perception 36, 1821-1833. 10.1068/p5563 [DOI] [PubMed] [Google Scholar]
- Su, C. Y., Martelli, C., Emonet, T. and Carlson, J. R. (2011). Temporal coding of odor mixtures in an olfactory receptor neuron. Proc. Natl. Acad. Sci. USA 108, 5075-5080. 10.1073/pnas.1100369108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, C. Y., Menuz, K., Reisert, J. and Carlson, J. R. (2012). Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492, 66-71. 10.1038/nature11712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka, P. and Stierle, J. S. (2014). Mixture processing and odor-object segregation in insects. Prog. Brain Res. 208, 63-85. 10.1016/B978-0-444-63350-7.00003-6 [DOI] [PubMed] [Google Scholar]
- Thomas-Danguin, T., Sinding, C., Romagny, S., El Mountassir, F., Barkat, S., Atanasova, B., Le Berre, E., Le Bon, A. M. and Coureaud, G. (2014). The perception of odor objects in everyday life: A review on the processing of odor mixtures. Front. Psychol. 5, 504. 10.3389/fpsyg.2014.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, N. and Mainen, Z. F. (2008). Odor concentration invariance by chemical ratio coding. Front. Syst. Neurosci 1, 3. 10.3389/neuro.06.003.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentincic, T., Kralj, J., Stenovec, M., Koce, A. and Caprio, J. (2000). The behavioral detection of binary mixtures of amino acids and their individual components by catfish. J. Exp. Biol 203, 3307-3317. 10.1242/jeb.203.21.3307 [DOI] [PubMed] [Google Scholar]
- Vosshall, L. B., Wong, A. M. and Axel, R. (2000). An olfactory sensory map in the fly brain. Cell 102, 147-159. 10.1016/S0092-8674(00)00021-0 [DOI] [PubMed] [Google Scholar]
- Weiss, T., Snitz, K., Yablonka, A., Khan, R. M., Gafsou, D., Schneidman, E. and Sobel, N. (2012). Perceptual convergence of multi-component mixtures in olfaction implies an olfactory white. Proc. Natl. Acad. Sci. USA 109, 19959-19964. 10.1073/pnas.1208110109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D. A. (1998). Habituation of odor responses in the rat anterior piriform cortex. J. Neurophysiol. 79, 1425-1440. 10.1152/jn.1998.79.3.1425 [DOI] [PubMed] [Google Scholar]
- Wilson, D. A. (2000). Odor specificity of habituation in the rat anterior piriform cortex. J. Neurophysiol. 83, 139-145. 10.1152/jn.2000.83.1.139 [DOI] [PubMed] [Google Scholar]
- Wilson, D. A. (2003). Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J. Neurophysiol. 90, 65-72. 10.1152/jn.00133.2003 [DOI] [PubMed] [Google Scholar]
- Wilson, D. A. and Stevenson, R. J. (2003). Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci. Biobehav. Rev. 27, 307-328. 10.1016/S0149-7634(03)00050-2 [DOI] [PubMed] [Google Scholar]
- Wilson, D. A. and Sullivan, R. M. (2011). Cortical processing of odor objects. Neuron 72, 506-519. 10.1016/j.neuron.2011.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D. A., Fleming, G., Vervoordt, S. and Coureaud, G. (2020). Cortical processing of configurally perceived odor mixtures. Brain Res. 1729, 146617. 10.1016/j.brainres.2019.146617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, G. A., Skinner, B. D. and Smith, B. H. (2002). Ability of honeybee, Apis mellifera, to detect and discriminate odors of varieties of canola (Brassica rapa and Brassica napus) and snapdragon flowers (Antirrhinum majus). J. Chem. Ecol. 28, 721-740. 10.1023/A:1015232608858 [DOI] [PubMed] [Google Scholar]
- Wyatt, T. D. (2015). The search for human pheromones: the lost decades and the necessity of returning to first principles. Proc. R. Soc. B 282, 20142994. 10.1098/rspb.2014.2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycke, A. M., Coureaud, G., Thomas-Danguin, T. and Sandoz, J. C. (2020). Configural perception of a binary olfactory mixture in honeybees as in humans, rodents and newborn rabbits. J. Exp. Biol. 223, jeb227611. 10.1242/jeb.227611 [DOI] [PubMed] [Google Scholar]
- Xu, L., Li, W., Voleti, V., Zou, D.-J., Hillman, E. M. C. and Firestein, S. (2020). Widespread receptor-driven modulation in peripheral olfactory coding. Science 368, eaaz5390. 10.1126/science.aaz5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrow, M. X., Denenberg, V. H. and Anderson, C. O. (1965). Rabbit: frequency of suckling in the pup. Science 150, 1835-1836. 10.1126/science.150.3705.1835 [DOI] [PubMed] [Google Scholar]