INTRODUCTION
Alcohol drinking has been popular in many cultures, and fermented beverages were brewed as early as the seventh millennium bc.1 According to the recent global status report on alcohol and health by the World Health Organization (WHO), approximately 2.3 billion people are current drinkers. Alcohol is consumed by more than half of the population in the Americas, Europe, and western Pacific.2 Alcohol drinking is also a major problem in adolescents between 15 and 19 years old, and more than a quarter of the population (– 155 million) in this age group are current drinkers.2 Total per capita alcohol consumption in the world’s population more than 15 years of age increased from 5.5 L of pure alcohol in 2005 to 6.4 L in 2016; the highest levels of per capita alcohol consumption are observed in WHO European region.2 Taken together, alcohol is the most widely abused psychoactive substance, despite the knowledge of its potential adverse health and social outcomes.3
ALCOHOL CONSUMPTION AND ALCOHOL-ASSOCIATED LIVER DISEASE
Globally, alcohol use was the seventh leading risk factor for both deaths and disability-adjusted life-years (DALYs) in 2016, accounting for 2.2% of age-standardized female deaths and 6.8% of age-standardized male deaths.4 For the population aged 15 to 49 years, female attributable DALYs were 2.3% and male attributable DALYs were 8.9%.4 In 2016, of all deaths attributable to alcohol consumption worldwide, 28.7% were caused by injuries, 21.3% were caused by digestive diseases, 19% were caused by cardiovascular diseases, 12.9% were caused by infectious diseases, and 12.6% were caused by cancers. In contrast, about 49% of alcohol-attributable DALYs are caused by noncommunicable and mental health conditions, and about 40% are caused by injuries.2
Drinking becomes excessive when it causes or increases the risk for alcohol-related problems or complicates the management of other health problems. A standard drink is a measure of alcohol consumption in each type of alcoholic beverage, which contains a specific amount of pure alcohol. In the United States, 1 standard drink contains approximately 14 g of pure alcohol, which is equivalent to 355 mL (12 ounces) of regular beer, 150 mL (5 ounces) of wine, or 45 mL (1.5 ounces) of distilled spirits.5 Accord-ing to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), excessive drinking is defined as men drinking more than 4 standard drinks in a day (or more than 14 per week) and women drinking more than 3 standard drinks in a day (or more than 7 per week).6
There is a direct relationship between the amount of alcohol consumed and the risk for alcohol-associated liver disease (ALD).7,8 Early studies in France suggested that alcohol consumption of more than 80 g/d in men and 20 g/d in women significantly increased the risk of alcoholic cirrhosis.9,10 The association between daily alcohol intake, type of alcoholic beverage consumed, drinking patterns, and ALD has been examined in 6534 subjects from 2 communities in northern Italy.11 The risk threshold for developing either noncirrhotic alcohol-induced liver injury or alcoholic cirrhosis in both sexes was consuming 30 g of alcohol per day, and an increased risk of ALD was also found with increasing alcohol intake.11 The relationship between self-reported alcohol intake and the risk of ALD was studied in 13,285 subjects with 12 years of follow-up. No liver injury was reported when the weekly alcohol intake was between 1 and 6 beverages; however, the risk was significantly increased when alcohol consumption was beyond this level: 7 to 13 beverages weekly for women and 14 to 27 beverages weekly for men.12 The level of risky drinking for ALD differs from each study primarily because of the retrospective study designs with broad assumptions and estimation of alcohol consumed.13
There is a gender difference influencing the development of ALD. The greater vulnerability of women, and lower safe limits for consumption, have been recognized.14 At any given level of alcohol intake, women had a significantly higher relative risk of developing ALD than men. In a large Danish population-based prospective study of 6152 subjects with a history of alcohol abuse, the alcoholic cirrhosis mortality was increased 27-fold and 35-fold in men and women, respectively, compared with that of the general population.15 However, men had an overall higher incidence of alcohol-induced cirrhosis (0.2% annually) compared with women (0.03% annually).10
The types and patterns of alcohol intake also influence the development of ALD, independently of the level of alcohol consumption. For instance, the consumption of red wine may have a lower risk of ALD compared with other types of alcoholic beverages.16 Drinking alcohol outside the mealtime and drinking multiple different alcoholic beverages both increase the risk of developing ALD.11
SPECTRUM OF ALCOHOL-ASSOCIATED LIVER DISEASE
ALD comprises a spectrum of histopathologic changes in patients with excessive alcohol use, ranging from simple alcoholic steatosis to steatohepatitis, alcoholic hepatitis (AH), fibrosis, and cirrhosis.17-19 Alcoholic steatosis occurs in most of the excessive drinkers. A classic study reported that consumption of alcohol in sufficient quantities in the range of 120 to 150 g/d for a few weeks leads to the development of steatosis. The condition is reversible, with 4 weeks of abstinence leading to its resolution.20 AH is a clinical syndrome and is characterized by an abrupt increase in serum bilirubin levels, fever, coagulopathy, and liver-related complications.18,21 It occurs in a subset of patients with excessive alcohol use and is associated with significant morbidity and mortality.18,22-24 Approximately 15% to 20% of excessive drinkers develop cirrhosis in their lifetimes.25 Patients with alcoholic cirrhosis, in general, have clinical features similar to those with other chronic liver diseases. The liver test abnormalities in alcoholic cirrhosis are less pronounced than those in AH. The liver tests are nearly normal in patients with the compensated state. Complications from portal hypertension, such as ascites, hepatic encephalopathy, and esophageal varices, are common in decompensated patients.
EPIDEMIOLOGY OF ALCOHOL-ASSOCIATED LIVER DISEASE
Epidemiology of Alcoholic Steatosis
Although alcoholic steatosis is common among excessive drinkers, its prevalence in the general population is difficult to determine because most patients are asymptomatic and do not seek any medical attention. The estimated prevalence also depends on the diagnostic modalities being used to screen for steatosis. Ultrasonography is an acceptable initial screening tool for alcoholic steatosis because it is noninvasive, inexpensive, and widely available.26 Steatosis normally appears as a diffuse hyperechogenicity caused by increased parenchymal reflectivity. Ultrasonography has a sensitivity around 60% to 90% and a specificity of approximately 90% to 95%, depending on the severity or degree of steatosis.27 Magnetic resonance spectroscopy allows noninvasive studies into the molecular composition of tissues in vivo with high accuracy for measuring hepatic fat.26,28 The controlled attenuation parameter is a noninvasive tool to assess alcoholic steatosis. The software is incorporated as part of liver stiffness measurement to enable it to measure the liver steatosis and fibrosis simultaneously.29 Using the national health examination survey data from questionnaires, abdominal ultrasonography, and laboratory tests on US patients, the estimated prevalence of alcoholic steatosis was calculated.30 Alcoholic steatosis was defined by alcohol consumption greater than 28 g/d in women and greater than 42 g/d in men, alanine aminotransferase (ALT) level greater than 25 U/L in women and greater than 35 U/L in men, and total bilirubin level less than 3 mg/dL, in the absence of viral hepatitis and metabolic syndrome. Hepatic fibrosis was determined with aspartate aminotransferase/platelet ratio and Fibrosis-4 score. Among approximately 34,000 respondents, 4.3% of the US population were identified with alcoholic steatosis. Over the 14-year study period, the prevalence of alcoholic steatosis remained stable at 4.3%; alcoholic steatosis with fibrosis stage 2 increased from 0.6% to 1.5% and with fibrosis stage 3 remained stable at 0.1% to 0.2%.30 Although thought to be a benign condition previously, hepatic steatosis may trigger the development of advanced liver disease, with approximately 18% of patients progressing into fibrosis or cirrhosis in a decade.31 This finding is supported by a recent meta-analysis finding that the annualized rate of progression from alcoholic steatosis to cirrhosis is approximately 3%, with annualized mortality around 6%. Of importance, patients with alcoholic steatosis also have higher nonliver (4% per year) mortality compared with liver-specific (1% per year) mortality.32
Prevalence of Alcoholic Hepatitis
The precise incidence and prevalence of AH are difficult to estimate because patients with AH may be completely asymptomatic and undiagnosed clinically.21 In a nationwide population-based estimate of AH incidence in Denmark between 1999 and 2008, the annual incidence increased from 37 million to 46 million in men and 24 million to 34 million in women, with a significant increase among middle-aged women.33 The epidemiology of AH can also be examined by analyzing the hospitalization data, notably for patients with severe disease.19,34 The authors reported an increase in total cases of AH-related hospitalization from 249,884 (0.66% of total admission in 2002) to 326,403 (0.83% of total admission in 2010) in the United States.19 Hospitalized patients had a high prevalence of comorbidities, such as sepsis, acute renal insufficiency, and gastrointestinal bleeding.19
AH poses a significant financial burden in the health care system because of the high readmission rate, ranging from 20% to 25% at 30 days and around 37% at 90 days after discharge.19,35-39 The data from a health care claims analysis of more than 15,000 commercially insured hospitalized patients with AH showed nearly 40,000 rehospitalizations, with more than 50% of the survivors rehospitalized within a year and nearly 75% through the second year, with the total health care cost of $2.2 billion over the 5-year study period.37 The data from the National Readmissions Database showed the annual cost burden of $164 million and $321 million for 30-day and 90-day AH-related readmission, respectively.39 The annualized rate of progression of AH to cirrhosis is around 10%, with annualized mortality from 5% to 15%.32 Several clinical scoring systems predict patients with AH who are at high risk for mortality. The Maddrey discriminant function is used widely to predict 30-day mortality and identify a subset of patients who may benefit from treatment with corticosteroids.40 The model for end-stage liver disease (MELD), using serum creatinine, serum bilirubin, and international normalized ratio, can accurately predict 30-day and 90-day mortality in patients with AH.41,42 In addition, the Lille model, including an evolution of the bilirubin level in addition to the 3 baseline variables albumin, prothrombin time, and creatinine, can also predict mortality in AH.43 A Lille score of more than 0.45 after 1 week of corticosteroid therapy is associated with 75% mortality in 6 months.43 The combination of MELD and Lille score can better predict outcomes of patients with AH, compared with either model alone.44
Prevalence of Alcoholic Cirrhosis
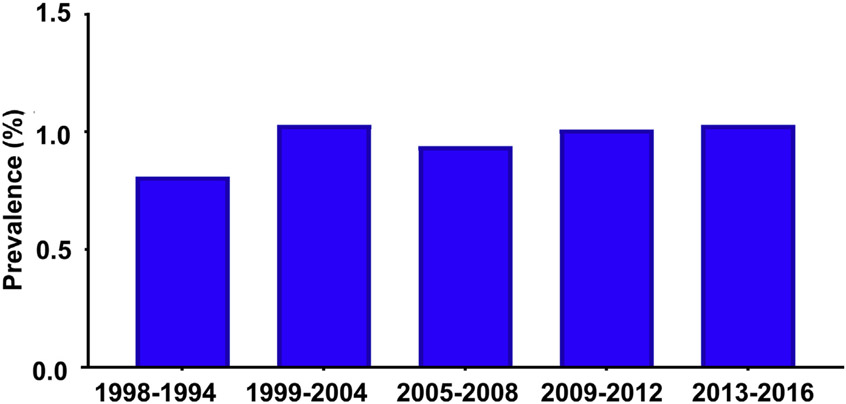
Globally, ALD caused 23.6 million and 2.46 million cases of compensated and decompensated cirrhosis in 2017.45 The age-standardized prevalence of decompensated alcoholic cirrhosis increased from 25.3 per 100,000 in 1990 to 30 per 100,000 in 2017, whereas the age-standardized prevalence of compensated alcoholic cirrhosis was stable from 290 per 100,000 to 288.1 per 100,000 in 1990 and 2017, respectively.45 The overall prevalence of ALD in the US population is stable around 0.8% to 1% from 1988 to 201646 (Fig. 1). However, patients with stage 3 to 4 fibrosis increased from 2.2% in 2001 to 2002 to 6.6% in 2015 to 2016.30 The number of hospitalizations among patients with alcoholic cirrhosis per 1000 increased by 32.8%, with the annualized mortality around 8%.32,47 Patients aged 25 to 34 years experience the highest average annual percentage change in mortality from cirrhosis of any cause: 8.9% in 2009 to 12.2% in 2016. Increased death rates in this age group are driven primarily by alcoholic cirrhosis, with an average annual percentage change of 10.5% from 2009 to 2016.48 During this period, the total number of patients with alcoholic cirrhosis listed for liver transplant increased by 63.4%.47
Fig. 1.
The prevalence of alcohol-associated liver cirrhosis in the US population from 1998 to 2016 based on the National Health and Nutrition Examination Survey.46
FACTORS INFLUENCING THE NATURAL HISTORY AND PROGRESSION OF ALCOHOL-ASSOCIATED LIVER DISEASE
In addition to alcohol consumption, multiple factors may influence the natural history and progression of ALD.
Obesity and Nonalcoholic Fatty Liver Disease
The obesity epidemic has led to an increase in the incidence of nonalcoholic fatty liver disease (NAFLD).49,50 There seems to be a synergistic interaction between alcohol consumption and obesity and the development of liver disease.51 The Dionysos study reveals that obese individuals with excessive drinking, defined as greater than 100 kg of alcohol consumed over a lifetime or greater than 60 g of alcohol daily, have a significantly higher prevalence of hepatic steatosis.52 The data from a population-based study in the United States show a higher prevalence of abnormal ALT activity in overweight and obese individuals who consume alcohol compared with overweight and obese individuals with no alcohol consumption history.53 Body mass index is an independent predictor for AH severity.18 In our multicenter case-control study of 1293 patients with alcoholic cirrhosis compared with 754 heavy-drinking controls without liver disease, we found a significantly higher prevalence of diabetes (20.5% vs 6.5%) and body mass index (26.3 vs 24.2 kg/m2) in patients with alcoholic cirrhosis compared with that in controls.54 The specific mechanisms of liver injury caused by the combination of obesity and alcohol are not well understood; however, it is plausible that both conditions may augment hepatic oxidative stress through cytochrome P450 2E1 and proinflammatory cytokines leading to worsening liver injury from either condition alone.51,55
Genetic Factors
As previously mentioned, only a subset of excessive drinkers develop AH or alcoholic cirrhosis. Excessive drinkers with the gene variants in the patatinlike phospholipase domain–containing protein 3 (PNPLA3), transmembrane 6 superfamily 2 (TM6SF2), and membrane bound O-acyltransferase domain-containing 7 (MBOAT7) are susceptible to alcoholic cirrhosis.56 The variants in hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) and Fas-associated Factor family member 2 (FAF2) are associated with a reduced risk of ALD.57,58
Coffee or Tea Consumption
The authors found that regular coffee consumption is associated with a lower risk of AH and alcoholic cirrhosis in heavy drinkers.36,54,59 In our recent case-control study, we found that heavy drinkers without underlying liver disease are more likely to have been a coffee drinker during the period of excessive drinking and to have drunk more coffee per day compared with those with alcoholic cirrhosis.54 We also found marginally significant protective effects of tea consumption when both tea and coffee were considered in the analysis.54
PERSPECTIVES AND CONCLUSION
The public health impact of ALD is growing, partly because of the increase in the incidence of AH and alcoholic cirrhosis.17,60,61 In parallel, mortality and liver transplant rates for persons with ALD have increased substantially.48,60,62 A recent modeling study using the Markov model showed a grim projection in the prevalence and mortality caused by ALD in the next 2 decades.63 In a large study of more than 3000 patients with chronic liver diseases worldwide, only 3.8% of patients with ALD were seen at the early stages, defined as those without any signs of chronic liver disease or complications of portal hypertension, compared with ~17% to 30% for those with NAFLD or viral hepatitis.64 Early detection of ALD among excessive drinkers is important because of the adverse impact on the survival of late diagnosis of ALD.65 At the individual level, behavioral and pharmacologic interventions have been proved to reduce the harm of excessive alcohol use.66 However, ALD is a population-level problem that requires broad policy-based solutions. Increased alcohol taxes may result in decreased alcohol sales and consumption and then reduced incidence of ALD.67-69 One key measure of ALD’s population-level burden is the number of patients evaluated and waitlisted for a liver transplant. The effect of alcohol tax on alcohol consumption and its influences on liver transplant listing for patients with ALD have been reported.69 Future studies focusing on the efforts and strategies to decrease excessive alcohol use at the individual and population levels are urgently required to prevent adverse outcomes from ALD.
KEY POINTS.
The public health impact of alcohol-associated liver disease (ALD) is growing, partly because of the increase in the incidence of alcoholic hepatitis and alcoholic liver cirrhosis.
There is a direct relationship between the risk for ALD and the amount of alcohol consumed. However, other factors, such as obesity and underlying genetic variants, may also influence the progression of ALD.
The mortality and liver transplant rates for persons with ALD have increased substantially, with a grim projection for the disease burden over the next 2 decades.
The efforts to reduce excessive alcohol use at the individual and population levels are urgently required to prevent adverse outcomes from ALD.
Sources of funding:
Z.Y. is supported by NIH K01AA26385, Indiana University School of Medicine Research Support Fund Grant (IU RSFG), and the Ralph W. and Grace M. Showalter Research Trust, Indiana University School of Medicine; S.L. is supported in part by R01 DK107682, R01 AA025208, U01 AA026917, UH2 AA026903, VA Merit Award 1I01CX000361, and Dean’s Scholar in Medical Research Indiana University School of Medicine.
Footnotes
Conflict of interest: None of the authors have any conflicts of interest relevant to this work.
REFERENCES
- 1.McGovern PE, Zhang J, Tang J, et al. Fermented beverages of pre- and protohistoric China. Proc Natl Acad Sci U S A 2004;101:17593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf?ua=1. Accessed March 12, 2021.
- 3.Balakrishnan M, Pappas SC. Alcohol and the Law. Clin Liver Dis 2019;23:25–38. [DOI] [PubMed] [Google Scholar]
- 4.Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gmel G, Rehm J. Harmful alcohol use. National Institute on Alcohol Abuse and Alcoholism, National Institute of Health; 2003. Available at: http://pubs.niaaa.nih.gov/publications/arh27-1/52-62.htm. [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JP, Litten RZ. Recommendations on use of biomarkers in alcoholism treatment trials. Alcohol Clin Exp Res 2003;27:1667–70. [DOI] [PubMed] [Google Scholar]
- 7.Ramstedt M Per capita alcohol consumption and liver cirrhosis mortality in 14 European countries. Addiction 2001;96(Suppl 1):S19–33. [DOI] [PubMed] [Google Scholar]
- 8.Cutright P, Fernquist RM. Predictors of per capita alcohol consumption and gender-specific liver cirrhosis mortality rates: thirteen European countries, circa 1970-1984 and 1995-2007. Omega (Westport) 2010;62:269–83. [DOI] [PubMed] [Google Scholar]
- 9.Tuyns AJ, Pequignot G. Greater risk of ascitic cirrhosis in females in relation to alcohol consumption. Int J Epidemiol 1984;13:53–7. [DOI] [PubMed] [Google Scholar]
- 10.Corrao G, Bagnardi V, Zambon A, et al. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction 1999;94:1551–73. [DOI] [PubMed] [Google Scholar]
- 11.Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997;41:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025–9. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis 2012;16:659–66. [DOI] [PubMed] [Google Scholar]
- 14.Frezza M, di PC, Pozzato G, et al. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 1990;322:95–9. [DOI] [PubMed] [Google Scholar]
- 15.Kamper-Jorgensen M, Gronbaek M, Tolstrup J, et al. Alcohol and cirrhosis: dose–response or threshold effect? J Hepatol 2004;41:25–30. [DOI] [PubMed] [Google Scholar]
- 16.Becker U, Gronbaek M, Johansen D, et al. Lower risk for alcohol-induced cirrhosis in wine drinkers. Hepatology 2002;35:868–75. [DOI] [PubMed] [Google Scholar]
- 17.Liangpunsakul S, Haber P, McCaughan GW. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016;150:1786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liangpunsakul S, Puri P, Shah VH, et al. Effects of age, sex, body weight, and quantity of alcohol consumption on occurrence and severity of alcoholic hepatitis. Clin Gastroenterol Hepatol 2016;14:1831–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinjuvadia R, Liangpunsakul S. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2015;49:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber CS, Jones DP, Decarli LM. Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest 1965;44:1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology 2016;150:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chayanupatkul M, Liangpunsakul S. Alcoholic hepatitis: a comprehensive review of pathogenesis and treatment. World J Gastroenterol 2014;20:6279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandrekar P, Bataller R, Tsukamoto H, et al. Alcoholic hepatitis: translational approaches to develop targeted therapies. Hepatology 2016;64:1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 25.Mills SJ, Harrison SA. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr Gastroenterol Rep 2005;7:32–6. [DOI] [PubMed] [Google Scholar]
- 26.Moreno C, Mueller S, Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol 2019;70:273–83. [DOI] [PubMed] [Google Scholar]
- 27.Lupsor-Platon M, Stefanescu H, Muresan D, et al. Noninvasive assessment of liver steatosis using ultrasound methods. Med Ultrason 2014;16:236–45. [DOI] [PubMed] [Google Scholar]
- 28.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiele M, Rausch V, Fluhr G, et al. Controlled attenuation parameter and alcoholic hepatic steatosis: diagnostic accuracy and role of alcohol detoxification. J Hepatol 2018;68:1025–32. [DOI] [PubMed] [Google Scholar]
- 30.Wong T, Dang K, Ladhani S, et al. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001-2016. JAMA 2019;321:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teli MR, Day CP, Burt AD, et al. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995;346:987–90. [DOI] [PubMed] [Google Scholar]
- 32.Parker R, Aithal GP, Becker U, et al. Natural history of histologically proven alcohol-related liver disease: a systematic review. J Hepatol 2019;71:586–93. [DOI] [PubMed] [Google Scholar]
- 33.Sandahl TD, Jepsen P, Thomsen KL, et al. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol 2011;54:760–4. [DOI] [PubMed] [Google Scholar]
- 34.Liangpunsakul S Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol 2011;45:714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comerford M, Lourens S, Liangpunsakul S, et al. Challenges in patient enrollment and retention in clinical studies for alcoholic hepatitis: experience of the TREAT consortium. Alcohol Clin Exp Res 2017;41:2000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lourens S, Sunjaya DB, Singal A, et al. Acute alcoholic hepatitis: natural history and predictors of mortality using a multicenter prospective study. Mayo Clin Proc Innov Qual Outcomes 2017;1:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson JA, Martinson N, Martinson M. Mortality and costs associated with alcoholic hepatitis: a claims analysis of a commercially insured population. Alcohol 2018;71:57–63. [DOI] [PubMed] [Google Scholar]
- 38.Peeraphatdit TB, Kamath PS, Karpyak VM, et al. Alcohol rehabilitation within 30 Days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Clin Gastroenterol Hepatol 2020;18:477–85.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adejumo AC, Cholankeril G, Iqbal U, et al. Readmission rates and associated outcomes for alcoholic hepatitis: a nationwide cohort study. Dig Dis Sci 2020;65:990–1002. [DOI] [PubMed] [Google Scholar]
- 40.Maddrey WC, Boitnott JK, Bedine MS, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–9. [PubMed] [Google Scholar]
- 41.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005;41:353–8. [DOI] [PubMed] [Google Scholar]
- 42.Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–54. [DOI] [PubMed] [Google Scholar]
- 44.Louvet A, Labreuche J, Artru F, et al. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology 2015;149:398–406 e8 [quiz e16-7]. [DOI] [PubMed] [Google Scholar]
- 45.Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–8. [DOI] [PubMed] [Google Scholar]
- 47.Dang K, Hirode G, Singal AK, et al. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol 2020;115:96–104. [DOI] [PubMed] [Google Scholar]
- 48.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liangpunsakul S, Chalasani N. Lipid mediators of liver injury in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 2019;316:G75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Alazawi W. Non-alcoholic fatty liver disease. Clin Med (Lond) 2020;20:509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Idalsoaga F, Kulkarni AV, Mousa OY, et al. Non-alcoholic fatty liver disease and alcohol-related liver disease: two Intertwined entities. Front Med (Lausanne) 2020;7:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000;132:112–7. [DOI] [PubMed] [Google Scholar]
- 53.Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol 2005;3:1260–8. [DOI] [PubMed] [Google Scholar]
- 54.Whitfield JB, Masson S, Liangpunsakul S, et al. Obesity, diabetes, coffee, tea, and cannabis Use Alter risk for alcohol-related cirrhosis in 2 large cohorts of high-risk drinkers. Am J Gastroenterol 2021;116:106–15. [DOI] [PubMed] [Google Scholar]
- 55.Liangpunsakul S, Kolwankar D, Pinto A, et al. Activity of CYP2E1 and CYP3A enzymes in adults with moderate alcohol consumption: a comparison with nonalcoholics. Hepatology 2005;41:1144–50. [DOI] [PubMed] [Google Scholar]
- 56.Buch S, Stickel F, Trepo E, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet 2015;47:1443–8. [DOI] [PubMed] [Google Scholar]
- 57.Abul-Husn NS, Cheng X, Li AH, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med 2018;378:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwantes-An TH, Darlay R, Mathurin P, et al. Genome-wide association study and meta-analysis on alcohol-related liver cirrhosis identifies novel genetic risk factors. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 59.Liangpunsakul S, Beaudoin JJ, Shah VH, et al. Interaction between the patatinlike phospholipase domain-containing protein 3 genotype and coffee drinking and the risk for acute alcoholic hepatitis. Hepatol Commun 2018;2:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018;68:872–82. [DOI] [PubMed] [Google Scholar]
- 61.Flemming JA, Dewit Y, Mah JM, et al. Incidence of cirrhosis in young birth cohorts in Canada from 1997 to 2016: a retrospective population-based study. Lancet Gastroenterol Hepatol 2019;4:217–26. [DOI] [PubMed] [Google Scholar]
- 62.Lee BP, Vittinghoff E, Dodge JL, et al. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med 2019;179:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Julien J, Ayer T, Bethea ED, et al. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public Health 2020;5:e316–23. [DOI] [PubMed] [Google Scholar]
- 64.Shah ND, Ventura-Cots M, Abraldes JG, et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol 2019;17:2320–9.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Innes H, Morling JR, Aspinall EA, et al. Late diagnosis of chronic liver disease in a community cohort (UK biobank): determinants and impact on subsequent survival. Public Health 2020;187:165–71. [DOI] [PubMed] [Google Scholar]
- 66.Peng JL, Patel MP, McGee B, et al. Management of alcohol misuse in patients with liver diseases. J Investig Med 2017;65:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elder RW, Lawrence B, Ferguson A, et al. The effectiveness of tax policy interventions for reducing excessive alcohol consumption and related harms. Am J Prev Med 2010;38:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aslam S, Buggs J, Melo S, et al. The association between alcoholic liver disease and alcohol tax. Am Surg 2021;87:92–6. [DOI] [PubMed] [Google Scholar]
- 69.Shen NT, Bray J, Wahid NA, et al. Evaluation of alcohol taxes as a public health opportunity to reduce liver transplant listings for alcohol-related liver disease. Alcohol Clin Exp Res 2020;44:2307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]