Abstract
Background
Negative symptoms are diagnostic characteristics of schizophrenia. They can result from primary (i.e., idiopathic) or secondary (i.e., due to other factors such as depression, anxiety, psychosis, disorganization, medication effects) features of the illness. Although secondary sources of negative symptoms are prevalent among individuals meeting criteria for clinical high-risk syndromes that are due to high rates of comorbidity, the extent to which secondary sources account for variance in negative symptom domains is unknown. Addressing this gap is an important step in informing vulnerability models and treatments for negative symptoms. This study aimed to investigate secondary sources of negative symptoms in those meeting criteria for a clinical high-risk syndrome (N = 192).
Methods
Simultaneous regression and hierarchical partitioning methods were used to determine the proportion of variance explained by selective serotonin reuptake inhibitor use, anxiety, depression, unusual thought content, and disorganized communication in predicting severity of five negative symptom domains (avolition, anhedonia, asociality, blunted affect, and alogia).
Results
Findings revealed that depression explained the largest proportion of variance in avolition, asociality, and anhedonia. Anxiety was the most predictive of blunted affect, and selective serotonin reuptake inhibitor use explained the most variance in alogia. Analyses within male and female samples revealed that in males, depression explained a large proportion of variance in several negative symptom domains, while in females, selective serotonin reuptake inhibitor use explained variance in alogia.
Conclusions
Results highlight heterogeneity in variance explained by secondary sources of negative symptoms. These findings guide treatment development for secondary sources of negative symptoms. Furthermore, results inform etiologic models of psychosis and negative symptom conceptualizations.
Keywords: Attenuated psychosis syndrome, Negative symptoms, Prevention, Prodrome, Secondary negative symptoms, Ultra high-risk
Negative symptoms have long been considered a core component of psychopathology in individuals with schizophrenia (1,2). Early factor analytic studies in schizophrenia have supported a two-factor negative symptom structure consisting of diminished expression and motivation and pleasure (3, 4, 5). However, more recent confirmatory factor analytic and network analysis studies that directly tested the latent structure of the construct suggest that the two-factor model offers a poor fit; rather, a five-factor model, corresponding to the five domains identified in the National Institute of Mental Health consensus conference on negative symptoms (6), provides an excellent fit. These findings have been replicated across contemporary negative symptom scales (Brief Negative Symptom Scale, Clinical Assessment Interview for Negative Symptoms, Scale for the Assessment of Negative Symptoms), across multiple cultures, using multiple mathematical techniques (confirmatory factor analysis, network analysis), and across chronic, first episode, and clinical high-risk (CHR) phases of illness (10, 11, 12, 13, 7, 8, 9). In addition, negative symptoms can result from primary (i.e., idiopathic) or secondary (i.e., due to depression, anxiety, hallucinations, delusions, disorganization, medication effects) sources of influences (14, 15, 16), and this notion can complicate conceptualization and treatment. Essentially, two individuals can have the exact same score on a negative symptom rating scale but for very different reasons (i.e., equifinality). Importantly, when secondary sources of negative symptoms are identified and appropriately targeted, they are typically effectively treated (16), whereas primary negative symptoms remain largely resistant to current pharmacological and psychosocial interventions (17).
Historically, primary and secondary negative symptoms have been distinguished clinically using the Schedule for the Deficit Syndrome, which requires clinicians to judge whether negative symptoms are of clinically significant severity and whether they likely result from secondary sources (e.g., anxiety, depression, positive symptoms, extrapyramidal symptoms); in the absence of clear secondary sources deemed to drive negative symptoms, they are rated as primary. Cases where negative symptoms are considered of sufficient severity, to be driven by primary (rather than secondary) factors, and to be persistent (rather than transient) are deemed to meet criteria for the deficit syndrome (a putative schizophrenia subgroup with distinct etiologic factors). Of note, secondary effects of positive symptoms on negative symptoms have been identified in the literature (14,16). For example, an individual may experience persecutory thoughts and withdraw from social interactions (16). Other work has found influences of extrapyramidal side effects on blunted affect (18) and relationships between negative symptoms and depression and anxiety (19,20). In addition, disorganization is associated with blunted affect (e.g., facial and vocal flattening due to limited available cognitive resources) (21) as well as motivational impairments (22).
Individuals meeting criteria for a CHR syndrome also display clinically significant negative symptoms (23, 24, 25, 26, 27). These impairments have been found to emerge before attenuated positive symptoms, are linked to poor functional outcome, and are predictive of transition to a psychotic disorder (26,28,29). However, the extent to which negative symptoms are driven by secondary sources in this group is unclear. Currently, there are measures such as the Negative Symptom Inventory-Psychosis Risk (NSI-PR) (25,27) that are geared toward isolating primary negative symptoms. However, distinct categorizations (i.e., nondeficit and deficit syndrome) observed in schizophrenia are not yet possible to implement in CHR groups. Investigating the potential influences of secondary sources (i.e., the variance explained) can provide foundation for this future work. One might expect secondary sources to account for some of the proportion of variance in negative symptom scores, given elevated rates of comorbid mood and anxiety disorders, high rates of psychotropic medications prescription, and the presence of attenuated positive and disorganized symptoms (30,31). Similar to schizophrenia (13), negative symptoms exhibit a five-factor structure in those meeting criteria for a CHR syndrome (7), and it will be critical to determine the extent to which these individual domains are predicted by various secondary sources. Determining the proportion of variance explained by common secondary factors can inform early intervention and prevention efforts, given that negative symptoms are known to remit in some individuals with schizophrenia when the appropriate secondary source is effectively targeted (16).
The aim of this study was to investigate sources of secondary negative symptoms in a sample of individuals meeting criteria for a CHR syndrome. Specifically, we assessed the proportion of variance in five negative symptom domains (avolition, asociality, anhedonia, blunted affect, alogia), in line with current findings suggesting that a five-factor structure of negative symptoms is optimal in CHR groups (7), accounted for by anxiety, depression, unusual thought content, disorganization, and psychotropic medication prescription. To achieve these aims, regression analyses and hierarchical partitioning were used. Given the research suggesting relationships between mood symptoms and both anhedonia and asociality in schizophrenia (19,20) (e.g., bidirectional relationships between feeling depressed and anxious and reduced enjoyment in activities or wanting to interact with others), we predicted that mood symptoms (i.e., anxiety, depression) would explain the largest variance in anhedonia and asociality. Furthermore, given the evidence of the role of extrapyramidal side effects on blunted affect in schizophrenia (18), medication use would explain most of the variance in blunted affect and alogia. Finally, given the findings of relationships between disorganization and both blunted affect and avolition in schizophrenia (21,22), we predicted that disorganization would explain a large portion of the variance in blunted affect and avolition. It is also possible that anxiety and unusual thought content would explain some variance across all negative symptom domains because these facets of psychopathology have evidenced relationships with negative symptoms broadly defined.
Methods and Materials
Participants
In this cross-sectional design, participants were 192 individuals meeting criteria for a CHR syndrome, aged 13–30 (mean = 19.90, SD = 2.21) years. The sample included participants recruited to the Adolescent Development and Preventive Treatment Program at University of Colorado Boulder and Northwestern University (principal investigator: VAM) and the Multisite Assessment of Psychosis-Risk study (32). Participant data were collected during baseline assessments across sites. The Structured Interview for Psychosis Risk Syndromes (SIPS) (33) was administered to assess the presence of psychosis-risk symptoms. The SIPS is designed to detect a CHR syndrome by assessing positive symptom domains (e.g., unusual thought content/delusion ideas, suspiciousness/persecutory ideas). Participants received a CHR status by receiving a score ranging from 3 (moderate) to 5 (severe but not psychotic) on any SIPS positive symptom domain, a standardized approach in the field (33). Furthermore, participants were considered as meeting the criteria for a CHR syndrome if they met the criteria for schizotypal personality disorder or had a family member with a schizophrenia spectrum and other psychotic disorder diagnosis, with a decline in functioning (7% of the sample). The Structured Clinical Interview for the DSM (34) was used to assess for comorbid diagnoses.
Measures
Negative Symptoms
The NSI-PR (25,27) was administered to assess negative symptom domains. Items were averaged for each negative symptom subscale to create five domain scores (avolition, asociality, anhedonia, blunted affect, alogia). All raters went through a gold standard training program for reliability purposes. See the Supplement for more details regarding this interview.
Information was also obtained regarding internal experience (e.g., wanting to spend time with friends, thinking about goals) and behavior (e.g., amount of time spent with friends, engaging in goal-directed activities) for avolition and asociality specifically, in line with negative symptom studies in schizophrenia (5). Discrepancies between internal experience and behavior (i.e., higher difference scores) are meaningful in separating phenomenology from behavior. Furthermore, higher difference scores could be reflective of secondary sources of negative symptoms. While internal experience and behavioral analyses as well as differences scores are not central to the study, we do include findings in the supplemental analyses (see more information below).
Measures Assessing Sources of Secondary Negative Symptoms
Selective serotonin reuptake inhibitor (SSRI): Participants provided information regarding whether they were currently prescribed an SSRI medication during the background section of the assessment. The variable of interest was dichotomized (yes/no) to assess current use.
Anxiety: Anxiety scores were a combination of self-report measures available—the State-Trait Anxiety Inventory (STAI), Trait Version (35), Anxiety subscale (35) (n = 85) and the Beck Anxiety Inventory (36) (n = 102). The STAI questionnaire measures symptoms of generalized anxiety, excluding items loading on a depression factor. Final variables were sums of all item responses, and both scales were z scored to combine into a broader anxiety domain and limit missing data in analyses. Analyses with just the STAI (and not Beck Anxiety Inventory) are included in the Supplement.
Depression: Depression was scored using a combination of self-report measures available as well—Center for Epidemiological Studies, Depression Scale (CES-D) (37) (n = 83) and the Beck Depression Inventory (38) (n = 101). The CES-D is a shortened version of the original CES-D scale, which includes 14 items assessing the severity of depressive symptoms in the past week. Final variables were sum scores of all item responses, and both scales were z scored to combine into a broader depression domain and limit missing data in analyses. Analyses with just the CES-D (and not Beck Depression Inventory) are included in the Supplement.
Unusual thought content/delusional ideas: As mentioned, the SIPS was used to identify a risk syndrome and includes several questions intended to assess the presence and severity of unusual thought content/delusional ideas. There are a total of five questions assessing perplexity and delusion mood, six for first-rank symptoms, five for overvalued beliefs, three for other unusual thoughts, and two for nonpersecutory ideas of reference. See the Supplement for example questions. A rating is given on a 0 (absent) to 6 (severe and psychotic) scale based on question responses.
Disorganized communication: The disorganized communication rating from the SIPS was also used in analyses. This variable probes for difficulties in thinking reflected in speech. In addition to question responses, behavioral observations during the clinical interview (e.g., coherence during the interview) are taken into account in the final rating.
Data Analysis
Levine’s test of homogeneity of variance indicated that SIPS-attenuated positive symptoms were not different across sites, so the samples were combined. To begin with, site differences were examined in negative symptom domains. Where negative symptom scores were significantly different across sites, site was controlled for in analyses, and a note was made as to whether this was a significant predictor. Regression analyses included both simultaneous linear regression and hierarchical partitioning, and a false discovery rate was used to account for multiple comparisons in central analyses. Linear regression models examined overall model fit and unique effects of each predictor variable (SSRI medications, anxiety scores, depression scores, unusual thought content, disorganized communication). If the overall regression model was significant or at trend level, hierarchical partitioning (39) was used to investigate contributions of predictors independent of all other predictors. Hierarchical partitioning averages across all possible permutations of a regression model to determine the proportion of predictive value contributed by each independent variable in a statistical model. In contrast to traditional regression, hierarchical partitioning can target each predictor’s relative explanatory weight without being influenced by multicollinearity and dependencies. Hierarchical partitioning was used using the R statistical software (Version 3.5.1) package hier.part to examine the proportion of variance in each of the five negative symptom domains (avolition, asociality, anhedonia, blunted affect, alogia) accounted for by common secondary negative symptoms (SSRI use, anxiety, depression, unusual thought content, disorganized communication). In addition, we included analyses assessing motor abnormalities from the Sensorimotor and Activity Psychosis-Risk scale to investigate the contributions of this possible secondary source on negative symptoms (see the Supplement) (40). We did not evaluate antipsychotic medications owing to limited sample size reporting current use.
Ethical Standards
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
Demographics
The sample consisted of individuals with an average age of 20 years (mean [± SD], 19.90 [2.21]) and included 45% males and 54% females. The average parental education was an Associate’s degree or 2 years in college. In terms of race and ethnicity, the sample was diverse (Asian = 15%, Black = 15%, White = 58%, American Indian = 3%, Native Hawaiian or Pacific Islander = 1%, and Hispanic = 19%). Participants endorsed attenuated positive symptoms with an average sum score of 11 (3.99) and exhibited mild-moderate levels of disorganized communication (1.65 [1.25]) and unusual thought content (3.05 [1.13]). In addition, approximately 15% of the sample were taking SSRI medications. Approximately 26% of the sample met DSM criteria for substance use–related disorders, 36% met the criteria for depressive disorders, and 50% met the criteria for anxiety disorders. Participants reported negative symptoms that ranged from absent to severe (anhedonia, 1.42 [1.09]; avolition, 1.55 [1.18]; asociality, 1.54 [1.22]; alogia, 0.83 [1.19]; blunted affect, 1.32 [1.25]). In addition, participants reported a range of depression and anxiety scores as well in the mild to severe range (STAI, 18.39 [5.24]; Beck Anxiety Inventory, 18.61 [12.63]; CES-D, 19.87 [5.62]; Beck Depression Inventory, 16.57 [11.59]). There were site differences in avolition (F188 = 3.93, p = .02), blunted affect (F186 = 3.43, p = .03), and alogia (F185 = 5.65, p = .004) scores. Given these site differences, site was a covariate in subsequent analyses.
Proportion of Variance in Five Negative Symptom Domains Accounted for by Common Secondary Sources
Avolition
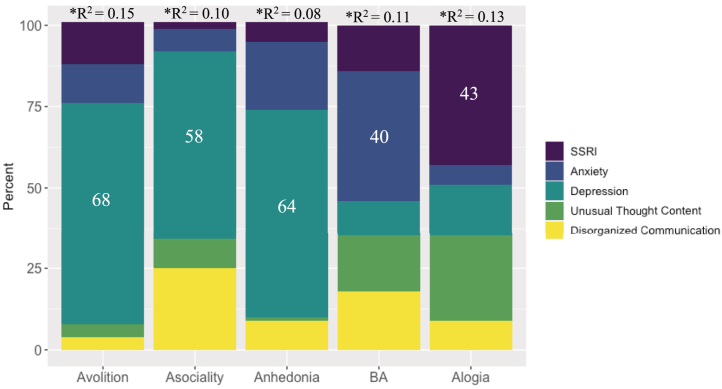
The overall regression model, when controlling for site, was significant (R2 = 0.14, F6,169 = 4.52, pcorrected = .001). Regression analyses indicated that depression explained the largest portion of variance in the model (β = 0.40, p = .0002). Hierarchical partitioning analyses confirmed findings (see Figure 1).
Figure 1.
Proportion of variance in negative symptom domains accounted for by common secondary sources in a clinical high-risk sample (N = 192). Effects ≥30% are labeled. Negative symptom domains (i.e., avolition, asociality, anhedonia, blunted affect, alogia) represent domain scores from the Negative Symptom Inventory-Psychosis Risk. ∗Indicates that the regression model was significant, p < .05. Selective serotonin reuptake inhibitor (SSRI) scores are dichotomous (yes/no). Anxiety scores were collected using the State-Trait Anxiety Inventory, Trait Version, Anxiety Subscale, and Beck Anxiety Inventory. Depression scores are sum scores from the Center for Epidemiological Studies, Depression Scale and Beck Depression Inventory. Unusual thought content and disorganized communication are domain scores from the Structured Interview for Psychosis-Risk Syndromes. BA, blunted affect.
Asociality
Regression analyses indicated a significant overall model fit (R2 = 0.10, F5,168 = 3.78, pcorrected = .001) and suggested that depression (β = 0.35, p = .002) explained a large portion of the variance in asociality, and disorganized communication was at trend level (β = 0.14, p = .08). Similarly, hierarchical partitioning confirmed these findings, as shown in Figure 1.
See Table S1 for findings showing overall regression model fit in avolition behavior and internal experience (n = 134) and in asociality behavior (n = 132) and internal experience (n = 131) in a sample with available data. In addition, see Table S2, which displays the proportion of variance accounted for by common secondary sources in avolition behavior and asociality internal experience (i.e., negative symptom regression models that were significant from Table S1). Findings from regression analyses and hierarchical partitioning indicated that anxiety explained the largest proportion of variance in avolition behavior, while no findings were observed with avolition internal experience. Furthermore, depression explained most of the variance in asociality internal experience, but no findings were observed with asociality behavior. See the Supplement for correlations between behavior-internal experience difference scores and secondary sources investigated (no significant findings) (Table S3).
Anhedonia
The overall regression model was significant (R2 = 0.08, F5,170 = 2.81, pcorrected = .02). Regression findings revealed that depression explained most of the variance in anhedonia (β = 0.26, p = .01). Hierarchical partitioning confirmed these analyses.
Blunted Affect
There was a significant overall model fit (R2 = 0.11, F6,167 = 3.27, pcorrected = .002). Regression analyses indicated that anxiety explained a large portion of variance in blunted affect (β = −0.30, p = .008). It is also important to note that site was also a significant predictor (p = .02). Hierarchical partitioning confirmed regression findings.
Exploratory analyses were conducted unpacking blunted affect to investigate the proportion of variance in each of the three blunted affect items (body gestures, blunted facial affect, blunted vocal affect) accounted for by common secondary negative symptoms (see Table 1). Linear regression revealed a significant overall model fit for blunted facial affect (F5,170 = 4.59, p = .0006, R2 = 0.12), suggesting that anxiety (β = −0.49, p = .0001) and depression (β = 0.44, p = .0004) explained a large portion of the variance in the model. Hierarchical partitioning confirmed these findings as well. There was also a significant model fit for reductions in body gestures (F7,166 = 3.00, p = .005, R2 = 0.11), with disorganized communication explaining a portion of the variance at trend level (β = 0.15, p = .12) and anxiety at trend level (β = −0.23, p = .09). Site was also a significant predictor in the model (p = .01) and was controlled for in this analysis. Hierarchical partitioning confirmed these findings. Finally, the linear regression model was not statistically significant for blunted vocal affect (F5,168 = 1.95, p = .09, R2 = 0.05), and thus, hierarchical partitioning was not used.
Table 1.
Proportion (%) of Variance in Blunted Facial Expressions and Body Gestures Accounted for by Common Secondary Negative Symptoms in a Clinical High-Risk Sample
| Secondary Negative Symptoms | Blunted Facial Expressions | Blunted Body Gesturing |
|---|---|---|
| SSRI | 0 | 17 |
| Anxiety | 45 | 28 |
| Depression | 36 | 7 |
| Unusual Thought Content | 8 | 14 |
| Disorganized Communication | 10 | 34 |
Blunted affect specific negative symptom items (i.e., blunted facial expressions and body gesture) from the Negative Symptom Inventory-Psychosis Risk. Blunted vocal affect was not included, given that the regression analyses were not significant, so hierarchical partitioning was not used. SSRI scores are dichotomous (yes/no). Anxiety scores were collected using the State-Trait Anxiety Inventory, Trait Version, Anxiety Subscale, and Beck Anxiety Inventory. Depression scores are sum scores from the Center for Epidemiological Studies, Depression Scale and the Beck Depression Inventory. Unusual thought content and disorganized symptoms are domain scores from the Structured Interview for Psychosis-Risk Syndromes.
SSRI, selective serotonin reuptake inhibitor.
Alogia
When controlling for site, the overall regression model was significant (R2 = 0.13, F7,167 = 3.51, pcorrected = .002), suggesting that SSRI medication use (β = 0.54, p = .03) and depression (β = 0.23, p = .04) explained the largest proportion of variance in alogia. Site was also a significant predictor (p = .002). Hierarchical partitioning showed a similar pattern.
Longitudinal Analyses
See Table S5 for longitudinal analyses conducted with available 12-month follow-up data (baseline secondary sources predicting follow-up negative symptoms, controlling for baseline negative symptom scores). Analyses suggested that SSRI use and disorganized communication significantly predicted anhedonia (n = 55), unusual thought content and SSRI use significantly predicted blunted affect (n = 56), and unusual thought content significantly predicted alogia (n = 57) at follow-up.
Differences in Secondary Sources of Negative Symptoms Between Males and Females
Given the differences in the distribution of biological sex, supplemental analyses were conducted to investigate interactions between sex and secondary sources in predicting negative symptoms. There were significant interactions for avolition (F11,163 = 2.91, p = .002), asociality (F11,161 = 2.97, p = .001), anhedonia (F11,163 = 1.98, p = .03), and alogia (F11,162 = 2.24, p = .01).
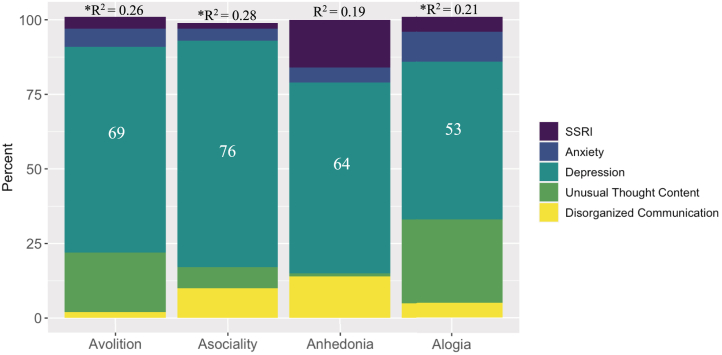
When unpacking significant interaction effects (and controlling for site in analyses with avolition and alogia) in the female group, secondary sources did not predict avolition, asociality, or anhedonia (see Table 2). However, SSRI use did explain some of the variance in alogia (20% were on SSRIs). In the male group, depression explained a large portion of the variance in avolition, asociality, anhedonia, and alogia (see Figure 2). Please note that in the sample of males, 25% met the criteria for depression, 29% met the criteria for anxiety, and 8% were on SSRIs.
Table 2.
Regression Findings Reflecting the Proportion of Variance in Negative Symptom Domains Accounted for by Common Secondary Sources by Biological Sex in a Clinical High-Risk Sample
| Domain | R2 | F (df) | p Value | Significant Secondary Source Predictor | β | p Value |
|---|---|---|---|---|---|---|
| Females (54%) | ||||||
| Avolition | 0.10 | 1.74 (6,93) | .12 | N/A | N/A | N/A |
| Asociality | 0.05 | 1.08 (5,94) | .38 | N/A | N/A | N/A |
| Anhedonia | 0.05 | 0.92 (5,94) | .47 | N/A | N/A | N/A |
| Alogia | 0.16 |
2.85 (6,93) |
.01 |
SSRI |
0.72 |
.006 |
| Males (45%) | ||||||
| Avolition | 0.26 | 3.39 (7,68) | .004 | Depression, UTC | 0.54, 0.30 | .0004, .05 |
| Asociality | 0.28 | 5.21 (5,68) | .004 | Depression | 0.68 | <.001 |
| Anhedonia | 0.19 | 3.33 (5,70) | .009 | Depression | 0.48 | .002 |
| Alogia | 0.21 | 2.61 (7,67) | .02 | Depression | 0.51 | .003 |
Negative symptom domains are taken from the Negative Symptom Inventory-Psychosis Risk. Anxiety scores were collected using the State-Trait Anxiety Inventory, Trait Version, Anxiety Subscale, and Beck Anxiety Inventory. Depression scores are sum scores from the Center for Epidemiological Studies, Depression Scale and the Beck Depression Inventory.
NA, not applicable; SSRI, selective serotonin reuptake inhibitor; UTC, unusual thought content.
Figure 2.
Proportion of variance in negative symptom domains accounted for by common secondary sources in male clinical high-risk sample (45% of the whole sample). Effects ≥30% are labeled. Negative symptom domains that had significant interactions are included (i.e., asociality, anhedonia, blunted affect, alogia) and represent domain scores from the Negative Symptom Inventory-Psychosis Risk. ∗Indicates regression model was significant, p < .05. Selective serotonin reuptake inhibitor (SSRI) scores are dichotomous (yes/no). Anxiety scores were collected using the State-Trait Anxiety Inventory, Trait Version, Anxiety Subscale, and Beck Anxiety Inventory. Depression scores are sum scores from the Center for Epidemiological Studies, Depression Scale and Beck Depression Inventory. Unusual thought content and disorganized communication are domain scores from the Structured Interview for Psychosis-Risk Syndromes.
Discussion
To our knowledge, this is the first study to examine the proportion of variance in negative symptom domains accounted for by common secondary sources in a sample of individuals meeting criteria for a CHR syndrome. These findings revealed differences in secondary source contributions in negative symptom domains, suggesting heterogeneity across these symptoms in their potential influences on specific negative symptoms. To ensure unbiased estimates of each predictor’s effects, we conducted hierarchical partitioning analyses to partition the explained variance while adjusting for possible multicollinearity. The hierarchical partitioning results indicate the proportion of the explained variance accounted for by each predictor. Overall, proportions of variance by these models were in the small-medium range (41). The central takeaway of these findings is that secondary sources contribute a substantial portion of variance in negative symptom severity scores (suggestive of driving negative symptoms, although future research is warranted) in negative symptoms, and results provide information regarding which secondary sources may be particularly relevant for specific negative symptoms. Together, these data suggest that considering secondary sources in vulnerability models, the prevention of psychosis, and treatment development for negative symptoms are critical. While this study did not systematically assess for primary and secondary symptoms (i.e., using tools and interviews designed for this), these data also provide foundation for additional work for this area.
A consistent finding in this study across analyses with negative symptom domains was that mood symptoms contributed to large portions of variance in negative symptoms. Specifically, results indicated that depression explained the largest proportion of variance avolition (68%), asociality (58%), and anhedonia (64%). In addition, anxiety explained a large portion of variance in blunted affect (40%), particularly blunted facial affect. Together, these data indicate that depression and anxiety may be useful treatment targets for individuals presenting with clinical elevations in these negative symptom domains. Depression and anxiety are common comorbid diagnoses in this group (30,42, 43, 44, 45) and are large contributors to seeking treatment initially (45). Along these lines, depression and anxiety are suggested to predict transition to psychosis and are correlated with negative symptoms in studies with CHR groups (31). While findings with depression were not surprising, results suggesting that anxiety predicts blunted affect (particularly blunted facial affect) were not what we expected. It is possible that in social situations, individuals may be threat activated, which in turn contributes to reduced cognitive resources and variability in displays of facial expressions. In contrast, when thinking about the other direction of findings, it is possible that blunted affect may lead to negative social evaluation and responses (e.g., rejection), which in turn could contribute to anxiety. While all speculative, these data do highlight the interconnected nature of anxiety and blunted facial affect that may be a topic of further inquiry in future work.
In analyses with alogia, SSRI medication use and depression explained the largest proportion of variance. Given the findings suggesting overlaps between depression and negative symptom severity in schizophrenia (46) and CHR groups (24,47), it is expected that SSRI use would explain some of the variance in negative symptoms. While speculative, it is possible that SSRI use (e.g., medication side effect) may induce alogia. Alternatively, increased alogia may cause clinicians to prescribe SSRIs more often.
Furthermore, disorganized communication explained some of the proportion of variance in asociality and reduction in body gestures. Disorganized communication from the SIPS (33) reflects disorganized thinking or thought disorder, observed through speech (e.g., tangential, circumstantial speech). From a cognitive perspective, it is possible that disorganized communication may be contributing to negative symptoms, such as blunted affect, through a cognitive pathway in which processing and coordinating information becomes overloaded (48). It is also important to note that site was a significant predictor in analyses with blunted affect, particularly blunted body gestures (as well as alogia). While this is the limitation of this study, it also may suggest that blunted affect and alogia may vary depending on geographic location, community, and cultural context. However, further research investigating this is needed.
Our findings also indicated that males in our sample endorsed secondary sources that explained large amounts of the variance in the five negative symptom domains. Specifically, in the male group, depression explained a large portion of the variance in negative symptoms. Furthermore, these findings are in contrast to results with female subjects in whom no significant findings were observed except with alogia. Specifically, SSRI medication use explained a portion of the variance in alogia. Together, these data suggest that it would be valuable to consider biological sex in the treatment of secondary sources and negative symptoms. However, additional research is warranted.
As noted, these data have important clinical implications for vulnerability models, the prevention of psychosis, and treatment development. First, these data provide support for the possibility of staging models to be updated to include earlier phases that focus on negative symptoms specifically, perhaps with a critical role for general symptoms (e.g., anxiety) that are common secondary sources. At present, these models do not fully incorporate negative symptoms. This is a gap in these heuristics because negative symptoms often develop years before attenuated positive symptoms and predict conversion more strongly when they occur before age 18 (49). Critically, future research is needed to determine whether those with primary or secondary negative symptoms are more likely to convert; however, CHR clinics would benefit from assessing negative symptoms and common secondary sources concurrently to capture both possibilities. Second, relatedly, findings have implications for conversion, suggesting that the combination of negative, positive, disorganized, and general symptoms variables into the same predictive models may be beneficial. If secondary factors are driving negative symptoms, the combination of negative symptoms along with these secondary sources may enhance risk calculators; however, this has not yet become practice in current risk prediction algorithms. Incorporating next-generation measures, such as the NSI-PR, that are capable of assessing negative symptoms per modern conceptualizations should maximize predictive potential. Third, findings have implications for understanding the mechanisms underlying negative symptoms. It may be beneficial to examine the presence of equifinality and determine whether, much like schizophrenia (50), CHR cases can develop the same negative symptom profiles due to different underlying mechanisms. The greater preponderance of secondary negative symptoms in CHR groups makes this especially likely, particularly given the wide range of secondary sources driving heterogeneity in negative symptoms. Identifying the mechanisms underlying these given domains will be necessary for developing effective treatments for each distinct target.
A potential treatment avenue might include a greater focus on adequately treating secondary sources. For example, given the prevalence of depression and anxiety in CHR groups, psychotherapies designed to target these comorbid conditions, such as cognitive behavioral therapy, are particularly relevant and implicated. Other treatment examples for secondary sources might include specific interventions to target communication deficits resulting from medication side effects, such as noninvasive brain stimulation or psychotherapy or both, as these communicative deficits are critical for social cognitive functions. In schizophrenia, secondary sources are themselves responsive to treatments and, once effectively targeted, lead to a concurrent improvement in negative symptoms (16). Adequate assessment will be a critical step in this process. Commonly used scales for this group, such as the SIPS, have significant limitations in their negative symptom items (27). However, scales based on modern conceptualizations, such as the NSI-PR, may isolate the constructs with greater precision and allow for adequate delineation of the contribution of secondary sources when used in combination with additional measures assessing these factors.
While there are many strengths to the current study, there are important limitations to discuss. In our study, we did not examine all potential sources of secondary negative symptoms (e.g., neurodevelopmental problems) (48); additional research should consider assessing other types of secondary sources as well. Further research is also needed to tease apart mechanistic pathways that may be underlying secondary sources of negative symptoms. Another critical direction involves assessing different types of anxiety and depressive symptoms as well as using instruments assessing mood symptoms that also capture resulting blunted affect and alogia; given that we did not see depression accounting for any of the variance in blunted affect or alogia, it is possible that this may be due to overlap in measurement content across constructs. In our study, most participants met inclusion criteria for attenuated positive symptom syndrome; future work assessing these research questions in different subgroups (e.g., genetic) is needed. There were also site differences in negative symptom domains, and it is possible that geographic (e.g., urban), contextual, cultural, and other environment-related factors may play a role in the expression and manifestation of these symptoms; future research on these topics is warranted. Importantly, we did not attempt to isolate a primary negative symptom subgroup, given that the tools to make the deficit syndrome categorization have not been developed or validated in CHR. Future work should consider developing instruments and tools to specifically isolate primary and enduring negative symptoms in CHR, similar to what has been done in schizophrenia. Considering both current and past medications (e.g., past SSRI use) and other medications (e.g., serotonin-norepinephrine reuptake inhibitors, antipsychotics) is also a critical future direction. Along these lines, additional investigation of motor abnormalities, which are prevalent in early psychosis even outside of medication use, is critical, as these may have an impact on negative symptoms.
Acknowledgments and Disclosures
The research reported in this article was supported by the National Institute of Mental Health (Grant Nos. F31MH121018-01A1 [to TG], RO1MH112545 [to VAM], R01MH112613 [to LME], and R01MH112612 to [JS]).
TG, VAM, and GPS developed the study concept and design in consultation with JS, LME, HRC, and AP-B. TG, VAM, JS, LME, HRC, and AP-B contributed to data collection. TG in consultation with HRC, VAM, GPS, JS, LME, and AP-B conducted data analyses. TG interpreted findings under the supervision of VAM and GPS and in consultation with JS, LME, HRC, and AP-B. TG drafted the manuscript, and all authors contributed to revisions. All authors approved this version of the manuscript before submission.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.05.008.
Supplementary Material
References
- 1.Bleuler E. International Universities Press; New York: 1950. Dementia Praecox, or the Group of Schizophrenias. [Google Scholar]
- 2.Kraepelin E. Robert E. Kreiger Publishing Co., Inc.; New York: 1919. Dementia Praecox and Paraphrenia. [Google Scholar]
- 3.Blanchard J.J., Cohen A.S. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horan W.P., Kring A.M., Gur R.E., Reise S.P., Blanchard J.J. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophr Res. 2011;132:140–145. doi: 10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss G.P., Hong L.E., Gold J.M., Buchanan R.W., McMahon R.P., Keller W.R., et al. Factor structure of the brief negative symptom scale. Schizophr Res. 2012;142:96–98. doi: 10.1016/j.schres.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick B., Fenton W.S., Carpenter W.T., Marder S.R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang W.C., Strauss G.P., Ahmed A.O., Wong S.C.Y., Chan J.K.N., Lee E.H.M., et al. The latent structure of negative symptoms in individuals with attenuated psychosis syndromes and early psychosis: Support for the 5 consensus domains. Schizophr Bull. 2021;47:386–394. doi: 10.1093/schbul/sbaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A.O., Kirkpatrick B., Galderisi S., Mucci A., Rossi A., Bertolino A., et al. Cross-cultural validation of the 5-factor structure of negative symptoms in schizophrenia. Schizophr Bull. 2019;45:305–314. doi: 10.1093/schbul/sby050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss G.P., Nuñez A., Ahmed A.O., Barchard K.A., Granholm E., Kirkpatrick B., et al. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry. 2018;75:1303. doi: 10.1001/jamapsychiatry.2018.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauss G.P., Esfahlani F.Z., Galderisi S., Mucci A., Rossi A., Bucci P., et al. Network analysis reveals the latent structure of negative symptoms in schizophrenia. Schizophr Bull. 2019;45:1033–1041. doi: 10.1093/schbul/sby133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucci A., Vignapiano A., Bitter I., Austin S.F., Delouche C., Dollfus S., et al. A large European, multicenter, multinational validation study of the Brief Negative Symptom Scale. Eur Neuropsychopharmacol. 2019;29:947–959. doi: 10.1016/j.euroneuro.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Ang M.S., Rekhi G., Lee J. Validation of the Brief Negative Symptom Scale and its association with functioning. Schizophr Res. 2019;208:97–104. doi: 10.1016/j.schres.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Strauss G.P., Ahmed A.O., Young J.W., Kirkpatrick B. Reconsidering the latent structure of negative symptoms in schizophrenia: A review of evidence supporting the 5 consensus domains. Schizophr Bull. 2019;45:725–729. doi: 10.1093/schbul/sby169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter W.T., Jr., Heinrichs D.W., Wagman A.M. Deficit and nondeficit forms of schizophrenia: The concept. Am J Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick B., Buchanan R.W., Ross D.E., Carpenter W.T. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 16.Kirschner M., Aleman A., Kaiser S. Secondary negative symptoms — A review of mechanisms, assessment and treatment. Schizophr Res. 2017;186:29–38. doi: 10.1016/j.schres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Fusar-Poli P., Papanastasiou E., Stahl D., Rocchetti M., Carpenter W., Shergill S., McGuire P. Treatments of negative symptoms in schizophrenia: Meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley M.E., van Kammen D.P., Allen D.N. Empirical validation of primary negative symptoms: Independence from effects of medication and psychosis. Am J Psychiatry. 1999;156:406–411. doi: 10.1176/ajp.156.3.406. [DOI] [PubMed] [Google Scholar]
- 19.Kulhara P., Avasthi A., Chadda R., Chandiramani K., Mattoo S.K., Kota S.K., Joseph S. Negative and depressive symptoms in schizophrenia. Br J Psychiatry. 1989;154:207–211. doi: 10.1192/bjp.154.2.207. [DOI] [PubMed] [Google Scholar]
- 20.Lysaker P.H., Salyers M.P. Anxiety symptoms in schizophrenia spectrum disorders: Associations with social function, positive and negative symptoms, hope and trauma history. Acta Psychiatr Scand. 2007;116:290–298. doi: 10.1111/j.1600-0447.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen A.S., McGovern J.E., Dinzeo T.J., Covington M.A. Speech deficits in serious mental illness: A cognitive resource issue? Schizophr Res. 2014;160:173–179. doi: 10.1016/j.schres.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss G.P., Horan W.P., Kirkpatrick B., Fischer B.A., Keller W.R., Miski P., et al. Deconstructing negative symptoms of schizophrenia: Avolition–apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusar-Poli P., Borgwardt S., Bechdolf A., Addington J., Riecher-Rössler A., Schultze-Lutter F., et al. The psychosis high-risk state: A comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta T., Cowan H.R., Strauss G.P., Walker E.F., Mittal V.A. Deconstructing negative symptoms in individuals at clinical high-risk for psychosis: Evidence for volitional and diminished emotionality subgroups that predict clinical presentation and functional outcome. Schizophr Bull. 2021;47:54–63. doi: 10.1093/schbul/sbaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelletier-Baldelli A., Strauss G.P., Visser K.H., Mittal V.A. Initial development and preliminary psychometric properties of the Prodromal Inventory of Negative Symptoms (PINS) Schizophr Res. 2017;189:43–49. doi: 10.1016/j.schres.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piskulic D., Addington J., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Heinssen R., et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196:220–224. doi: 10.1016/j.psychres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss G.P., Pelletier-Baldelli A., Visser K.F., Walker E.F., Mittal V.A. A review of negative symptom assessment strategies in youth at clinical high-risk for psychosis. Schizophr Res. 2020;222:104–112. doi: 10.1016/j.schres.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusar-Poli P., Bonoldi I., Yung A.R., Borgwardt S., Kempton M.J., Valmaggia L., et al. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 29.Gupta T., Haase C.M., Strauss G.P., Cohen A.S., Mittal V.A. Alterations in facial expressivity in youth at clinical high-risk for psychosis. J Abnorm Psychol. 2019;128:341–351. doi: 10.1037/abn0000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addington J., Piskulic D., Liu L., Lockwood J., Cadenhead K.S., Cannon T.D., et al. Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr Res. 2017;190:90–95. doi: 10.1016/j.schres.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fusar-Poli P., Nelson B., Valmaggia L., Yung A.R., McGuire P.K. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: Impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. doi: 10.1093/schbul/sbs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellman L.M., Schiffman J., Mittal V.A. Community psychosis-risk screening: An instrument development investigation. J Psychiatr Brain Sci. 2020;5 doi: 10.20900/jpbs.20200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGlashan T.H., Miller T.J., Woods S.W., Rosen J.L., Hoffman R.E., Davidson L. Yale School of Medicine; New Haven, Connecticut: 2001. Structured interview for prodromal syndromes: PRIME Research Clinic. [Google Scholar]
- 34.First M.B. Structured clinical interview for the DSM (SCID) Encycl Clin Psychol. 2014;1–6 [Google Scholar]
- 35.Bieling P.J., Antony M.M., Swinson R.P. The State-Trait Anxiety Inventory, Trait version: Structure and content re-examined. Behav Res Ther. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 36.Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 37.Kohout F.J., Berkman L.F., Evans D.A., Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 38.Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 39.Chevan A., Sutherland M. Hierarchical partitioning. Am Stat. 1991;45:90–96. [Google Scholar]
- 40.Damme K.S.F., Schiffman J., Ellman L.M., Mittal V.A. Sensorimotor and activity psychosis-risk (SMAP-R) scale: An exploration of scale structure with replication and validation. Schizophr Bull. 2021;47:332–343. doi: 10.1093/schbul/sbaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J. 2nd ed. L. Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Science. [Google Scholar]
- 42.McAusland L., Buchy L., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Heinssen R., et al. Anxiety in youth at clinical high risk for psychosis. Early Interv Psychiatry. 2017;11:480–487. doi: 10.1111/eip.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yung A.R., Buckby J.A., Cosgrave E.M., Killackey E.J., Baker K., Cotton S.M., McGorry P.D. Association between psychotic experiences and depression in a clinical sample over 6 months. Schizophr Res. 2007;91:246–253. doi: 10.1016/j.schres.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Azar M., Pruessner M., Baer L.H., Iyer S., Malla A.K., Lepage M. A study on negative and depressive symptom prevalence in individuals at ultra-high risk for psychosis. Early Interv Psychiatry. 2018;12:900–906. doi: 10.1111/eip.12386. [DOI] [PubMed] [Google Scholar]
- 45.Stowkowy J., Colijn M.A., Addington J. Pathways to care for those at clinical high risk of developing psychosis. Early Interv Psychiatry. 2013;7:80–83. doi: 10.1111/j.1751-7893.2012.00368.x. [DOI] [PubMed] [Google Scholar]
- 46.Krynicki C.R., Upthegrove R., Deakin J.F.W., Barnes T.R.E. The relationship between negative symptoms and depression in schizophrenia: A systematic review. Acta Psychiatr Scand. 2018;137:380–390. doi: 10.1111/acps.12873. [DOI] [PubMed] [Google Scholar]
- 47.Vargas T., Ahmed A.O., Strauss G.P., Brandes C.M., Walker E.F., Buchanan R.W., et al. The latent structure of depressive symptoms across clinical high risk and chronic phases of psychotic illness. Transl Psychiatry. 2019;9:229. doi: 10.1038/s41398-019-0563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strauss G.P., Cohen A.S. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43:712–719. doi: 10.1093/schbul/sbx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang T., Xu L., Tang Y., Li H., Tang X., Cui H., et al. Prediction of psychosis in prodrome: Development and validation of a simple, personalized risk calculator. Psychol Med. 2019;49:1990–1998. doi: 10.1017/S0033291718002738. [DOI] [PubMed] [Google Scholar]
- 50.Strauss G.P., Waltz J.A., Gold J.M. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40:S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.