Abstract
Severe coronavirus disease 2019 (COVID-19) occurs in approximately 10% of neonates infected with severe acute respiratory syndrome coronavirus 2. Guidelines for optimal management of severe COVID-19 in neonates do not exist. In this report, we describe a late-preterm neonate with severe COVID-19, requiring invasive mechanical ventilation who recovered following treatment with remdesivir and high dose dexamethasone.
Keywords: SARS-CoV-2, respiratory failure, preterm, remdesivir treatment
The majority of neonates hospitalized with coronavirus disease 2019 (COVID-19) recover with supportive management alone.1 In a limited number of reports, corticosteroids or antiviral medications, such as remdesivir, have been administered.2 A prospective surveillance study in the United Kingdom showed a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) incidence of 5.6 per 10,000 live births3; of the 66 neonates with SARS-CoV-2 infection, 42% (n = 28) had severe disease and one-third required respiratory support, suggesting that neonates may be at higher risk of severe disease compared older children and teenagers.
Here we describe the course of a premature neonate with COVID-19-associated respiratory failure who required invasive ventilatory support and who clinically improved following treatment with systemic corticosteroids and remdesivir.
CASE REPORT
Antenatal Presentation and Course
A previously healthy 34-year-old gravida 4 para 0 female at 34 weeks’ gestation presented to a community hospital with preterm rupture of membranes and preterm labor. Before the presentation, the pregnancy had been uncomplicated and antenatal serologies were negative for HIV, hepatitis B virus and syphilis.
The day before admission, the mother developed fever, cough, nausea and vomiting. A nasopharyngeal swab tested positive for SARS-CoV-2 by reverse transcription-polymerase chain reaction (RT-PCR). She received a single intramuscular dose of betamethasone along with intravenous ampicillin and gentamicin for preterm labor. Four hours after admission, she delivered a female neonate via uncomplicated vaginal delivery; delayed cord clamping was carried out for 60 seconds.
Neonatal Course
The APGAR scores at 1 and 5 minutes were both 9 and her birth weight was 2390 g. She was admitted to a level 2 neonatal intensive care unit on room air, and an intravenous dextrose solution was initiated. Laboratory evaluation at birth, including a complete blood count with differential and blood gases, were within reference ranges and blood culture was negative for bacterial growth. Nasopharyngeal swabs were positive for SARS-CoV-2 by RT-PCR at 24 hours of life and remained positive when repeated on the day of life (DOL) 4, 6, 14 and 32.
She was initially stable on room air, with normal oxygen saturations and no respiratory distress. She tolerated enteral feeds until DOL 4 when she developed nasal congestion and tachypnea. A full sepsis workup, including blood, urine and cerebrospinal fluid cultures was performed and she was started on empiric intravenous ampicillin and tobramycin. A mild mixed acidosis on capillary blood gas was detected. Her chest radiograph was normal. The blood, urine and cerebro-spinal fluid culture were negative.
On the following day, her respiratory symptoms worsened with cyanosis requiring respiratory support with continuous positive airway pressure and a FiO2 of 40%. On DOL 6, due to worsening respiratory distress and acidosis, she was intubated and provided ventilatory support. A repeat chest radiograph showed mildly increased peri-hilar markings with bilateral interstitial opacities.
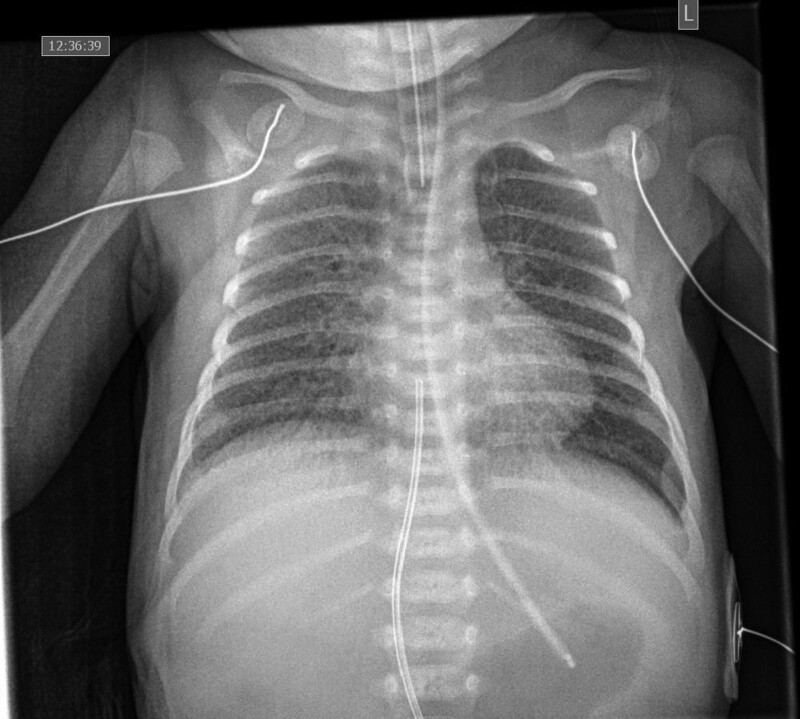
On DOL 6, the infant was then transferred to a tertiary neonatal intensive care unit for continued care of respiratory failure. On admission, she was transitioned to high-frequency oscillatory ventilation, requiring a mean airway pressure of 14 cm H2O and 40%–70% FIO2, and intravenous dexamethasone (0.15mg/kg/day) was started as part of the COVID-19 management protocol. Forty-eight hours after admission, she continued to have progressive oxygen failure, and worsening chest radiograph findings (Fig. 1). Thus, she was sedated with midazolam, morphine and muscle relaxed with rocuronium. Given the severity of the illness, intravenous dexamethasone was increased to 0.5 mg/kg/day, and simultaneously, intravenous remdesivir (2.5 mg/kg loading dose, followed by 1.25 mg/kg daily was started. The highest C-reactive protein was 5.8 (mg/L) on DOL 2 which decreased to 2.8 by DOL 6, at the time of transfer to a tertiary center, and 0.9 by the end of treatment.
FIGURE 1.
Chest radiograph obtained on DOL 9 which illustrates increased peri-hilar markings and diffuse reticular changes. DOL, day of life.
Within 2 days of starting remdesivir and high dose dexamethasone, her respiratory status improved; she was transitioned to conventional ventilation for 3 days, then extubated to continuous positive airway pressure, and 24 hours later was transitioned to low flow oxygen. The dexamethasone dose was reduced to 0.15 mg/kg/day bis in die or twice daily for 48 hours and then to quaque die or once daily; in all, she completed a 10-day course of steroids and 6 days of remdesivir. She was transferred to the pediatric inpatient unit on DOL 19; by DOL 25, she was off all respiratory support and discharged home on DOL 31.
Before discharge, a brain MRI was performed which was remarkable for multiple linear foci of white matter injury and edema with bilateral extra-axial posterior fossa hemorrhages and no sinus venous thrombosis identified. Additionally, placental pathology showed a normal 3-vessel cord with focal hemorrhage and 2 foci of early infarction of the placental disc. Intervillous fibrin deposition and intervillositis with histiocytes and neutrophils were noted. However, no histopathology consistent with chorioamnionitis or deciduitis was seen. The placental swab of fetal and maternal side, tested positive for SARS-CoV-2 by RT-PCR.
DISCUSSION
In this report, we described a neonate who developed severe COVID-19 requiring intensive care, invasive ventilatory support and treatment with dexamethasone and remdesivir. Full recovery was achieved, with initial improvement temporally associated with the initiation of treatment with high dose dexamethasone and remdesivir.
Although the role of corticosteroids or antiviral medications in managing neonates with COVID-19 is uncertain, dexamethasone was given in our case based on the evidence of use in adults.4 Due to severe clinical deterioration, dexamethasone was increased and remdesivir was started. Concurrent with this treatment, significant clinical improvement was noted. Remdesivir is a nucleotide analog prodrug with in vitro efficacy against SARS-CoV-2 that improves clinical outcomes in adults with COVID-19.5 Despite the minimal data for its use in neonates, the American Academy of Pediatrics indicates that it is the preferred antiviral treatment for critically ill neonates with COVID-19.6 We used a single loading dose of 2.5 mg/kg, followed by a maintenance dose of 1.25 mg/kg every 24 hours. The dose was calculated based on a simulation study by Maharaj et al.7 They found that in neonates with body weight <6 kg, a 2 mg/kg loading dose followed by a 1 mg/kg maintenance dose achieved a similar plasma drug concentration as that in adults.7 Despite the rare side effects, close monitoring of liver enzymes and kidney function is recommended both before and during remdesivir treatment.8 In our report, no adverse events occurred, and renal and liver functions remained normal.
The clinical presentation of our case was attributable to SARS-CoV-2 infection. An extensive microbiologic workup did not detect an alternative infectious cause and the clinical presentation was not consistent with noninfectious causes. Specifically, the delayed onset of symptoms and the initial normal chest radiograph were inconsistent with respiratory distress syndrome or transient tachypnea of the newborn as possible etiologies.
Vertical transmission of SARS-CoV-2 may occur in utero via the placenta, intrapartum during the process of delivery, or postnatally through contact with respiratory secretions or potentially through breastmilk.9 In our case, the timing of transmission could not be determined with certainty, as amniotic fluid and fetal blood testing was not performed and initial testing of infant respiratory secretions was performed at approximately 24 hours of life.9,10 However, the detection of SARS-CoV-2 on a placental swab and in a respiratory sample from the infant at approximately 24 hours of life is consistent with possible in utero transmission.9
The white matter findings on brain MRI in our patient were similar to those observed in several previous case reports of neonates with neurological symptoms associated with COVID-19.11 The mechanism of white matter injury in neonates with COVID-19 is uncertain but may be due to vascular inflammation induced by SARS-CoV-2.12
In conclusion, this report suggests that remdesivir in combination with hig- dose dexamethasone can be safely considered in the management of neonates with severe COVID-19 infection.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Alan Daneman for interpreting the chest radiograph and providing the picture.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Informed consent for the use of this patient’s data, including imaging and laboratory data, was obtained prior to the writing of this case report.
REFERENCES
- 1.Trevisanuto D, Cavallin F, Cavicchiolo ME, et al. Coronavirus infection in neonates: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2021;106:330–335. [DOI] [PubMed] [Google Scholar]
- 2.Correia CR, Marçal M, Vieira F, et al. Congenital SARS-CoV-2 infection in a neonate with severe acute respiratory syndrome. Pediatr Infect Dis J. 2020;39:e439–e443. [DOI] [PubMed] [Google Scholar]
- 3.Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021;5:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc. 2020;9:701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maharaj AR, Wu H, Hornik CP, et al.; Best Pharmaceuticals for Children Act–Pediatric Trials Network Steering Committee. Simulated assessment of pharmacokinetically guided dosing for investigational treatments of pediatric patients with coronavirus disease 2019. JAMA Pediatr. 2020;174:e202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yalçin N, Demirkan K. COVID-19 and remdesivir in pediatric patients: the invisible part of the iceberg. Pediatr Res. 2021;89:1326–1327. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Scientific brief. Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2. February 8, 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1. Accessed January 4, 2022.
- 10.Shah PS, Diambomba Y, Acharya G, et al. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz N, Treptow A, Schmidt S, et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J. 2020;39:e212. [DOI] [PubMed] [Google Scholar]