Abstract
Simple Summary
The use of nutraceuticals and phytonutrients in poultry nutrition has been extensively explored over the past decade. The interest in these substances is linked to the search for natural compounds that can be effectively used to prevent and treat some of the main diseases of the chicken. The serious problem of antibiotic resistance and the consequent legislative constraints on their use required the search for alternatives. The purpose of this review is to describe the current status of the effects of some substances, such as probiotics and prebiotics, organic acids, vitamins and phytogenic feed additives, focusing specifically on studies concerning the prevention and treatment of four main gastrointestinal diseases in chicken: salmonellosis, necrotic enteritis (caused by Clostridium perfringens), campylobacteriosis, and coccidiosis. A brief description of these diseases and the effects of the main bioactive principles of the nutraceutical or phytonutrient groups will be provided. Although there are conflicting results, some works show very promising effects, with a reduction in the bacterial or protozoan load following treatment. Further studies are needed to verify the real effectiveness of these compounds and make them applicable in the field.
Abstract
In poultry, severe gastrointestinal diseases are caused by bacteria and coccidia, with important economic losses in the poultry industry and requirement of treatments which, for years, were based on the use of antibiotics and chemotherapies. Furthermore, Salmonella spp., Clostridium perfringens, and Campylobacter jejuni can cause serious foodborne diseases in people, resulting from consumption of poultry meat, eggs, and derived products. With the spread of antibiotic resistance, which affects both animals and humans, the restriction of antibiotic use in livestock production and the identification of a list of “critically important antimicrobials” became necessary. For this reason, researchers focused on natural compounds and effective alternatives to prevent gastrointestinal disease in poultry. This review summarizes the results of several studies published in the last decade, describing the use of different nutraceutical or phytonutrients in poultry industry. The results of the use of these products are not always encouraging. While some of the alternatives have proven to be very promising, further studies will be needed to verify the efficacy and practical applicability of other compounds.
Keywords: poultry, antibiotic alternatives, nutraceuticals, phytonutrients
1. Introduction
For more than 60 years, antibiotics have been used in livestock without any specific control. In the poultry industry, these have been employed for different purposes: treatment of various pathologies (therapy), their prevention (metaphylaxis), and especially as growth promoters [1]. The frequent use of subtherapeutic antibiotic doses, administered to the whole flock in large quantities, has made a great contribution to the development of antibiotic resistance. According to the World Health Organization, it represents “one of the greatest threats to global health, food security and development” today [2], as the connection between the use of sub-therapeutic doses and the development of resistance between different classes of antibiotics has been proven [3]. Even more important, there is clear evidence of the adverse consequences for human health caused by resistant organisms deriving from non-human usage of antibiotics, primarily Salmonella spp. [4]. For this reason, in 2006 the European Union definitively banned the use of antibiotics as growth promoters in animal feed, describing it as “the final step in the phasing out of antibiotics used for non-medicinal purposes” [5,6]. Subsequently, this ban was also approved in the United States in 2017 [7]. However, the use of antibiotics is still allowed for therapeutic purposes, and the appropriate and reasonable use is a duty of both human and veterinary medicine. Guidelines for the choosing of therapeutic action were written by the World Health Organization, highlighting which are the critically important antimicrobials and why [4].
Poultry meat production is always growing, with a new high in the European production of 13.6 million tonnes for the year 2020 [8], giving even more importance to the research of alternatives to the use of antibiotics. This review will focus on the four most important and serious gastrointestinal (GI) diseases of the chicken: salmonellosis, clostridiosis (Necrotic enteritis), campylobacteriosis, and coccidiosis. In all these pathologies, there is an increasing resistance to different classes of antibiotics. Even more important, except to coccidiosis, some species of Salmonella spp., Clostridium spp., and Campylobacter spp. are causes of severe human foodborne diseases. For these reasons, the reduction in circulation of these pathogens in farms and the consequent contamination of meat and eggs it is of fundamental importance. In this paper, the main natural alternatives to antibiotics included in the nutraceutical and phytonutrients groups are reviewed. The potential of these substances to directly reduce the pathogenic load or limit the effects of GI infection in chicken will be described, highlighting the underlying mechanisms of action.
2. Different Classes of Alternative Compounds
Gut health is essential in the pathogenesis of different intestinal disorders. The role of gut microbiota has been well described, showing how dysbiosis can be an important predisposing factor for GI diseases [9]. Different factors are able to influence gut microbiota such as antibiotics, diet, or pathogenic infection. A perfect alternative to antibiotics should be able to prevent various pathogens’ infection or reduce their effect, considering clinical manifestation and organ damages. Ideally, an alternative should have the same mechanism proposed for antibiotic growth promoters in terms of microbiota modulation and immunomodulation [10], while also having a growth-promoting effect. Indeed, growth promotion was one of the main reasons that led to the use of subtherapeutic doses of antibiotics in chicken farming. In the last decade significant work has been contributed for the research of alternatives to antimicrobials, especially referring to substances included in the groups of nutraceuticals and phytonutrients. The term “nutraceutical” was created in 1989 by Stephen DeFelice, combining the terms “nutrition” and “pharmaceutical” [11]. According to DeFelice, nutraceuticals can be defined as “a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease” [12]. Different products, all natural, are utilized as nutraceuticals: dietary fibre, probiotics, prebiotics, organic acids, antioxidants, vitamins, polyphenols, and spices [13]. With the term “phytochemical” instead are described “metabolites from plants, including mostly plant secondary metabolites” [14]. In this term are also enclosed the terms phytonutrient and bioactive, and it can be considered part of nutraceutical group. There is still a big debate on the most appropriate use of these terms and nomenclature, and especially for plant-derived food components there is a lack of standardization in definitions (Table 1) [14]. The purpose of their use in the poultry sector is linked to the possibility of obtaining a regulation of the composition of the intestinal microbiota, improving GI health, the functionality of the intestinal barrier, and the activity of the host’s immune system with an effect also on weight gain and feed conversion ratio [15,16]. Probiotics, prebiotics, organic acids, vitamins, enzyme, phytobiotics, and phytochemicals are included in this group (Table 2). All of these compounds are usually administered by feed, water, or in ovo.
Table 1.
Classification of compounds that can be used in poultry production.
| Compounds | Definition | Origin |
|---|---|---|
| Nutraceutical | A food (or its part) that provides medical or health benefits, including the prevention and/or treatment of a disease [17] |
Plant or animal |
| Phytonutrient | Plant derived compound [18] |
Plant |
| Phytochemical | A variety of plant-derived compounds with therapeutic activities such as anticarcinogenic, antimutagenic, anti-inflammatory, and antioxidant [19] |
Plant |
| Bioactive Compound |
Components in foods or dietary supplements, other than those necessary to the basic nutritional needs, which are responsible for changes in health status [20] |
Plant or animal |
Table 2.
Description of products for the regulation of the intestinal bacteria population in poultry and their principal effects.
| Items | Definition | Mechanism of Action |
|---|---|---|
| Probiotics | Live microorganisms which, when administered in adequate amounts, confer a health benefit on the host [21] |
Competitive exclusion Production of antimicrobial substances Stimulation of immune system Increased intestinal absorption surface Increased growth performance and feed intake Modulation of respiratory and GI microbiota [22,23,24,25,26,27,28,29,30,31] |
| Prebiotics | A nondigestible compound that, through its metabolization by microorganisms in the gut, modulates composition and/or activity of the gut microbiota, thus conferring a beneficial physiological effect on the host [32] |
Nutrient source for the selective growth of beneficial bacteria of the intestinal microbiota Stimulation of short-chain fatty acids production Inhibition of bacterial adhesion to gut lining Change in mucin production Immunity boost Improvement in intestinal health and functionality. [15,33,34,35,36] |
| Vitamins | Vitamins are nutritional elements which are necessary for essential activities such as development, growth, and metabolism of cells [37] |
Antioxidant effect Reduction in free radicals Increase in mucosal immunity Anti-inflammatory effect Immunostimulatory effects Increase in cellular immunity [37,38,39,40,41,42] |
| Phytogenic feed additives (or Phytobiotics) |
Compounds of plant origin incorporated into animal feed to enhance livestock productivity through the improvement of digestibility, nutrient absorption, and elimination of intestinal pathogens [43] |
Increase in growth performance, nutrient digestibility and gut health Introduction into the cell membrane of pathogens and consequent destruction with consequent ions leakage Antioxidant activity Modulation of intestinal microbiota composition [44,45,46,47,48,49] |
| Organic acids | Primarily composed of short-chain fatty acids (SCFA), also commonly referred to as volatile short-chain fatty acids (VSCFA), such as fumaric, propionic, acetic, lactic, butyric, and others. Other organic acids consist of medium-chain fatty acids (MCFA), and long-chain fatty acids (LCFA) [50] |
Lowering pH of GI tract (reduction in acid sensitive bacteria) Potential for incorporation into cell membranes of target cells and promoting the loss of protons or cell ions (such as in Gram-positive bacteria) Promotion of gut health and performance [10,51,52,53,54] |
3. Salmonella spp. Infection
The genus Salmonella is part of the family Enterobacteriaceae and comprises three species: S. enterica, S. bongori, and S. subterranea. The species S. enterica includes six subspecies, but only one (S. enterica subspecies enterica) is associated with the development of disease in warm-blooded animals. In the subspecies S. enterica, the serovars Enteritidis and Typhimurium are most prevalent both in humans and in poultry [55,56]. Salmonella enterica serovar Enteritidis is commonly associated with poultry and derived products, whereas serovar Typhimurium has a wider species range, affecting pigs and cattle as well as poultry [57]. This bacterium has a worldwide distribution and causes big losses in poultry industries. Generally, clinical signs of Salmonella infection are evident only in young chickens that show depression, drooping wings, ruffled feathers, anorexia, emaciation, and watery diarrhoea. The peak of morbidity and mortality is usually around the first 2–3 weeks of life when weight loss or growth retardation are observed, while clinical signs are rare in older birds. [55]. It also has a big relevance for human health considering that, in Europe, S. enterica is the second most common foodborne disease [58]. Multiple epidemiological studies reveal the role of poultry meat and eggs in outbreaks of human salmonellosis [3,59,60,61].
Over the years, antibiotics have been extensively used to treat this pathology, and their improper use has favoured the development of multidrug-resistant strains. The emergence of resistance started at first with antibiotics of older use (ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole) and subsequently involved fluoroquinolones (ciprofloxacin) and extended-spectrum cephalosporins [57]. The problem of resistance concern “critically important antibiotics for human health”, leading to the request of new molecules for therapy (e.g., carbapenems) [57]. Consequently, the spread of Salmonella infection cannot only be treated using antibiotics, as this could promote the development of pathogenic strains as well as led to the presence of antibiotic residues in poultry meat [62]. In order to limit this use as much as possible, there is continued research on sustainable and safe alternatives to administer in poultry.
3.1. Probiotics and Prebiotics
A considerable amount of studies describe the use of different probiotic strains to increase the resistance against salmonellosis in poultry [63,64]. The idea of using probiotics to obtain a microbial control is linked to the concept that a healthy microflora can inhibit pathogens’ colonization through a mechanism of competitive exclusion, where the probiotic can compete for intestinal space, reducing the chance of pathogen colonization [65]. This concept is valid for Salmonella spp. as far as for other pathogens that will be discussed later. However, other mechanisms are also involved in probiotic effects such as reduction in intraluminal pH due to production of short chain fatty acids, production of antimicrobial peptides, optimization of intestinal functionality and activation of immune response. Researchers tested single or multi-strain probiotics, in particular Lactobacillus, Enterococcus, and Bacillus strains, with encouraging results after a Salmonella challenge in chickens, considering parameters such as bacterial count in ceca, faecal bacterial load, growth performance, and immune functionality [56,66,67,68,69,70,71]. The effect of commercially available probiotic supplements such as EarlyBird® (Pacific Vet Group USA Inc., Fayetteville, AR, USA) and FloraMax-B11® (Pacific Vet Group USA Inc., Fayetteville, AR, USA) alone, or with the addition of glycerol, was tested to obtain a protective effect against Salmonella Enteritidis colonization, demonstrating the prevention of intestinal colonization from the pathogen [72,73]. In contrast, Khan and Chousalkar show that the administration of probiotics is not able to reduce shedding and invasion of Salmonella spp. in chickens [74]. The variability of the results is understandable as the works differ for bacteria strains, concentration, period, and duration of administration.
Studies on prebiotics mainly focus on the use of oligosaccharides such as mannanoligosaccharides (MOS), galactooligosaccharides (GOS), fructooligosaccharides (FOS), and inulin. The use of MOS can inhibit the activity of Salmonella spp., reducing the adhesion to intestinal epithelium thanks to the presence of mannose in the lumen [34] and increase the immunity response against S. Enteritidis with higher T lymphocyte infiltration in intestinal mucosa [75]. The commercial product XPC® (Diamond V, Cedar Rapids, IA, USA) reduced faecal Salmonella spp. count, with an increase in butyrate concentration in GI tract [76,77]. Lee et al. [78] evaluated the effect of the commercial prebiotic Biolex® MB40 (Andersen, Barcelona, Spain), registering a not significant reduction in Salmonella spp. counting. Similar inconclusive results are obtained with the use of a prebiotic GOS, however an increase in the gene expression of the cecal tonsils and an influence in the composition of the cecal microbiome were recorded, suggesting the usefulness of this treatment [79].
3.2. Organic Acids
Organic acids have gained attention as a possible alternative to antibiotics. Previous studies showed their multiple effect on GI tract such as increase in growth performances, improved nutrient metabolism, anti-inflammation effects, and reduction in Salmonella spp. colonization [80,81]. Treatments with organic acids, whether they are SCFA, MCFA, or other organic acids, have a different powerful antimicrobial activity, depending on whether they are used individually or in mixtures, and on their concentration [52]. In particular, better result on decreasing Salmonella spp. colonization can be obtained using coated acids [82]. Focusing on propionic and fumaric acids, crop and gizzard are the site in which the greater concentration is obtained after oral administration. Although the crop is an initial site for the settlement of infection, the sites in which there is a greater colonization of Salmonella spp. are the ceca, and it is important that organic acids can reach the low intestinal tracts in order to have a better effect on animal health. Uncoated butyrate has a faster absorption and is therefore not able to reach this site [82]. For this reason, recent studies on the use of butyrate are especially focused on the coating and inclusion technique [83,84]. Feeding broiler with butyrate included in a wax matrix significantly reduces Salmonella spp. colonization in ceca content [81]. Not only butyrate, but also other organic acids, have been added in feed or water. An organic acid blend composed of formic acid and sodium formate mixture (Amasil® NA, BASF, Ludwigshafen, Germany) permits us to obtain a significant effect on reducing S. Thyphymurium infection in broilers [85]. A decrease in Salmonella spp. cecal count can also be achieved by using a feed additive mixture containing organic acids and ß-1,4 mannobiose [86]. Furthermore, it has been shown that adding formic acid to broiler feed appears to prevent Salmonella spp. passing from challenged to non-challenge sheds, without, however, having a reduction in Salmonella spp. counting [87]. Dietary supplementation with a symbiotic and an organic acid can also be used to improve growth performance and reduce carcass Salmonella spp. in broilers [88].
3.3. Vitamins
Another possible way to control Salmonella spp. infection in broilers is the use of vitamins, C and E in particular. Vitamin C can alleviate the effects of multiple stressors in animals and, alone or with other compounds such as curcumin, allows a reduction in Salmonella spp. count [89,90,91] and an improvement of the intestinal health [92]. Feed supplementation with vitamin E results in reduction in oxidative and immune stress that occurs during the infection [93] and its combination with arginine increases resistance against bacterial colonization, although there is no reduction in the concentration of S. Typhimurium in ceca [94].
3.4. Phytogenic Feed Additives (PFAs)
PFAs have gained interest due to their ability to help maintaining a healthy gut environment. It has been reported that essential oils of herbs and spices can play a significant role in bird health and performance by stimulating feed intake, secretion of endogenous enzymes, production of antioxidants, and antibacterial effect [95]. Included in this group are plant extracts and their active ingredients, whose beneficial qualities are linked to some bioactive molecules contained in it such as carvacrol, thymol, capsaicin, cineole, etc. [96]. The comparative effects of antibiotics and different PFAs, such as thymol essential oil, thyme essential oil, anise, and other components, on Salmonella Typhimurium-challenged broilers shows promising results [95]. A PFA containing extract of fennel (Foeniculum vulgarae var. dulce), lemon balm (Melissa officinalis), peppermint (Mentha arvensis), anise (Pimpinella ani-sum), oak (Quercus cortex), cloves (Syzygium aromaticum), and thyme (Thymus vulgaris) was tested in broilers challenged with Salmonella spp., proving that it can be considered as an alternative to improve the growth performances of broilers when exposed to infection [97]. Additionally, the effect of chestnut and quebracho wood was evaluated, showing a reduction in both mortality and Salmonella spp. excretion [98].
4. Campylobacter jejuni Infection
Thermophilic Campylobacter spp., primarily Campylobacter jejuni and C. coli, are colonizers of the intestinal tract of domestic poultry such as chickens and turkeys. These bacteria have a worldwide distribution in poultry flocks, causing little or no clinical symptoms [99] although clinical forms with watery, mucoid, or bloody diarrhoea, damage and inflammation of the mucous membrane, weight loss, and mortality were demonstrated in challenged young chickens [100]. There is a natural faecal–oral transmission of Campylobacter spp., which establishes in the intestinal tract with higher load in caeca [101]. However, it represents a main issue for human health; in fact, in the European Union, it represents the first zoonoses reported in a human in 2020 [58]. For this reason, although Campylobacter spp. is not a main issue for poultry, its control is important for food safety and human health. In human, C. coli and, mainly, C. jejuni, are cause of foodborne gastroenteritis with symptoms such as watery or bloody diarrhoea, fever, abdominal cramps, and possible severe condition in immunocompromised patients and with correlation with Guillain–Barré syndrome (a paralytic autoimmune complication) [99]. These infections are due to carcass contamination during slaughtering and spread to poultry meat and subsequently to consumers [102]. A correlation between the degree of intestine invasion and the level of contamination of the carcass has been demonstrated [103]. Other important elements in the attempt to reduce the spread of Campylobacter spp. are identifying possible sources of contamination and avoiding the persistence in the environment caused by contaminated litter, rodents, flies, other animals, short interruptions in production, inadequate disinfection, and contamination of the water and surrounding environments [104]. Against this pathogen, the intervention strategies are focused on prevention of colonization and/or its reduction. Nutraceuticals can have a great potential in order to avoid the use of antibiotic.
4.1. Probiotics and Prebiotics
The use of probiotics and prebiotics have been investigated to prevent Campylobacter spp. colonization of the GI tract. The genera of probiotic most commonly evaluated against C. jejuni are Lactobacillus spp., Bacillus spp., and Enterococcus spp. [105]. The interest in Lactobacillus spp. is based on the ability to reduce intraluminal pH of GI tract, creating an inhospitable environment for other bacterial species [106,107,108]. The ability of probiotic strains to reduce C. jejuni count is due to a reduction in its adhesion ability to epithelial cells, that prevent the colonization [109,110,111,112,113]. A significant reduction in caecal Campylobacter spp. count is registered after the administration of Butyricicoccus pullicaecorum, a probiotic able to produce butyrate [114]. On the contrary, it has been found that a competitive exclusion mixture could not compete against C. jejuni in challenged broilers [115]. Smialek et al. [105] suggest that a multispecies preparation may have a higher activity against Campylobacter spp. than single one strain probiotic. Few studies also refer to the use of prebiotics, although they are usually in association with probiotics, because they probably cannot be efficacious on their own. A symbiotic product formulated with microencapsulated probiotic Bifidobacterium longum PCB133 and a xylo-oligosaccharide (XOS) showed to be more effective in reducing C. jejuni load in ceca when the product is given over lifelong treatment in comparison to shorter administration [116]. Other similar studies support the use of symbiotics against C. jejuni, also investigating the efficacy of inhibition obtained using Bifidobacterium spp. and Saccharomyces cerevisiae [22,111,117]. An increased antimicrobial activity against Campylobacter spp. is observed using a combination of L. casei and berry pomace phenolic extract (BPPE), because bioactive phenols can stimulate the activity of the probiotic bacteria and its metabolites while inhibiting pathogens growth [118].
Probiotics can also be applied in combination with vaccines used against Campylobacter spp. infection. The use of the probiotic Anaerosporobacter mobilis or L. reuteri in broilers and Leghorn layer chickens is able to increase the immune response to the vaccination [119].
4.2. Organic Acids
Another strategy for the control of C. jejuni spread is the use of organic acid during the broiler rearing phase. The mechanism of action of organic acid is not currently well described, but it is known that, after administration, organic acids enter the cytoplasm, altering the equilibrium of cellular hydrogen that causes an inhibition of essential metabolic cellular reactions and accumulation of toxic anions [120].
Some studies have been conducted to assess the reduction in C. jejuni colonization on chickens during breeding and preslaughter phases, with positive results after experimental challenge [110,121,122]. Recently, Peh et al. showed in vitro synergistic activities of a combination of caprylic, sorbic, and caproic acid against the major Campylobacter species, which could also be promising for an in vivo approach [123]. MCFA added to feed or water can limit C. jejuni colonization [124,125]. However, there are still conflicting results regarding the effects of this administration, because a good efficacy in vitro is not followed by the same effects during in vivo trials [126,127].
4.3. Phytogenic Feed Additives (PFAs)
The use of phytogenic feed additives (PFAs), as essential oils, tannins, and plant extract, is well explored. For compounds of plant origin, most of the studies are initially carried out in vitro, with good results which, however, are often not found after in vivo administration. For example, for cinnamon oil ingredient trans-cinnamaldehyde (CIN) and allicin, a compound extracted from garlic, the efficiency observed in vitro did not allow a reduction in colonization in vivo [128,129]. An in vitro antimicrobial activity has also been described using an extract containing hydrolysable and condensed tannin [130]. Several studies describe the use of essential oils, plant extracts, and secondary plant compounds, without a marked effectiveness against C. jejuni [131,132]. Some good results are described with the use of 0.25% thymol, 2% thymol, 1% carvacrol, and 0.5% thymol and carvacrol [133]. Different essential oils, polyphenol, and terpenoid compounds were tested against C. jejuni, and a strong activity of essential oils and terpenoid compound was reported [134].
5. Clostridium perfringens Infection
Necrotic enteritis (NE) is a disease caused by the toxins produced by pathogenic strains of Clostridium perfringens type A, C, and G, that represent a major cause of losses in the poultry industry. Symptoms of NE are very non-specific, such as depression, diarrhoea, ruffled feathers, anorexia, and dehydration. In acute forms, animals can die without clinical signs. More frequently, subclinical forms of NE causes only a reduction in feed intake and weight. Macroscopic lesions observed during autopsy are characteristic, with the small intestine becoming fragile, hyperaemic, and dilated for the presence of gas. On the mucosal surfaces, light brown pseudo-membranes and occasional bleeding are present [135,136]. C. perfringens is normally found in the GI content of healthy chickens [137], and the development of clinical forms is linked to predisposing factors such as coccidiosis, reduction in feed quality, or presence of other immunosuppressive disease [135]. C. perfringens is also a public health issue due to its ability to produce enterotoxin at the moment of sporulation, causing a foodborne illness in humans, with subtype A that gives diarrhoea and subtype C that causes NE [135]. In the past years, a correlation between outbreaks of subtype A illness in humans and chicken meat consumption has been demonstrated [138]. Traditional strategies to control NE rely on prevention and direct treatment in case of clinical form. Antibiotics, and especially lincomycin, bacitracin, and tylosin, have been widely used. However, the first fundamental element to limit the spread and clinical manifestations of NE lies in obtaining the maximum reduction in predisposing factors, primarily coccidiosis. For this reason, new forms of action against this pathogen are necessary.
5.1. Probiotics and Prebiotics
The importance of the use of probiotics, prebiotics, and symbiotics is aimed not only at achieving a reduction in C. perfringens infection, but also at improving intestinal health, limiting the dysfunctions due to lesions of intestinal tight junction, and consequently alteration of nutrient absorption and/or bacterial translocation. The use of Bacillus subtilis PB6 significantly ameliorates intestinal morphology, increasing villus length and villus length/crypt depth ratio in infected chickens [139]. Multiple strains of Bacillus spp. showed an agonistic activity against C. perfringes thanks to the production of bacteriocins and other antimicrobial peptides, with significant attenuation of C. perfringes symptoms [140,141,142,143,144]. The commercial product FloraMax-B11® (Vetanco, Villa Martelli, Argentina) tested on chickens challenged with E. maxima, S. typhimurium, and C. perfringens, shows a reduction in intestinal lesion and C. perfringens count [145]. Different Lactobacillus strains reduce C. perfringens pathological effects [146,147,148]. The yeast extract NuPro® (Alltech, Nicholasville, KY, USA) administered on chicks challenged with C. perfringens shows a reduction in intestinal lesion score [130]. Similar results are obtained using other commercial yeast additives such as Safmannan® (Phileo by Lesaffre, Marcq-en-Barœul, France) [149] or the yeast cell wall extract from Saccharomyces cerevisiae (Actigen®, Alltech, Nicholasville, KY, USA) [150], with promising results.
5.2. Organic Acids
SCFA (formic, acetic, propionic, and butyric) and MCFA (caproic, caprylic, and capric acids) are described as promising alternatives to antibiotics in C. perfringes infections. These additives provide evidence of reducing the negative effects of pathogen proliferation, such as reduction in weight gain and GI disfunction [151]. Some mixtures of organic acids have been tested, alone or blended with essential oils, showing a potential antimicrobial and protective activity, with a reduction in intestinal lesions [152,153,154,155]. There are still several factors influencing the effects of these products such as their structure, coating, dosage, dietary composition, and environmental condition [153].
5.3. Phytogenic Feed Additives (PFAs)
The study of PFAs is particularly developed with focus on the effect on C. perfringens intestinal burden and intestinal gross lesion. Essential oils (EOs) have a major component in phenolic compounds such as thymol, carvacrol, and eugenol, that showed to have a strong antibacterial activity [153]. The use of essential extracts from Origanum vulgare, Piper nigrum, Syzygium aromaticum, and Thymus vulgaris, and their components (thymol, carvacrol, and eugenol) against C. perfringens has been explored and, although the mechanism of action of this substances is not still well understood, in some cases a direct inhibitory effect on the pathogen or an action against its toxins is described [156]. The effects of thymol and carvacrol essential oils and lysozyme were tested, suggesting that both have positive effects, but that their blend does not improve their effects [157]. Additionally, tannins, and in particular two common sources of tannins, chestnut (Castanea sativa) and quebracho (Schinopsis lorentzii) extracts, have an activity against C. perfringens and its toxins, reducing the severity of intestinal damage and bacterial count and protecting infected intestinal tissues from oxidative damage [158,159,160].
5.4. Vitamins
Vitamins can have a preventive activity in chicken with NE, despite this field being not well explored. In broilers, lesion score due to C. perfringens infection and C. perfringens count in intestine were reduced after treatment with beta-carotene [161].
6. Coccidiosis
Coccidiosis is a poultry disease of universal importance. It is caused by parasites of the genus Eimeria. Poultry are susceptible to seven species of Eimeria (E. acervuline, E. maxima, E. brunetti, E. praecox, E. mitis, E. tenella, and E. necatrix), with most serious condition described consequently to E. tenella and E. necatrix infections [162]. Eimeria spp. causes intestinal damage with impaired digestive process, loss of nutrients absorption capability, dehydration, and increased susceptibility to other pathological agents. Cases of severe outcomes are associated with bloody diarrhoea and very high mortality [162]. With the use of anticoccidial drugs, mainly sulphonamides, there has been a reduction in severe clinical manifestations and now Eimeria spp. infections are mostly associated with subclinical manifestations. However, it still remains the cause of important economic losses for poultry industries. Moreover, Eimeria spp. causes changes in permeability and functionality of the intestinal mucosa, being considered one of the main predisposing factors for bacterial infections [163]. It is a ubiquitous and resistant parasite, so prevention is the most important strategy in poultry farming. Furthermore, as for antibiotics, anticoccidial drugs also saw the development of tolerance with widespread resistance. Vaccination against several Eimeria spp. was very promising, but side effects such as post-vaccination mild infection and reduction in weight gain and feed conversion are discouraging this practice, and the interest towards different nutraceutical and phytochemical remedies with anticoccidial properties is increasing [162].
6.1. Probiotics
Over the past years, many compounds containing one or more bacterial strains such as Bacillus spp., Lactobacillus spp., Enterococcus spp., Pedicoccus spp., and Bifidobacterium were tested with very promising results. Probiotic bacteria prevent Eimeria invasion by adhering to the intestinal mucosa, thus reducing receptor availability during Eimeria spp. infection. This limits the perforation and secretion of sporozoites in the intestinal mucosa, allowing a reduced proliferation and spread of the oocysts [164]. Moreover, probiotic bacteria have an immunomodulating and antioxidant effect, increasing GI microbiota balance and improving intestinal functionality and health [165]. Some commercial probiotics mixture such as PoultryStar® (DSM, Heerlen, The Netherlands) [166,167], Smart ProLive® (Bakın Tarım, Ankara, Turkey) [168], and Primalac® (Star-Labs, Clarksdale, MO, USA) [169], have been able to guarantee a reduction in the intestinal lesion score, higher growth rate, and reduced oocyst shedding.
Probiotics can be administered together with the vaccine in the attempt to eliminate its side effects. Despite vaccines being considered relatively effective for the control of this disease, probiotic addition could indeed enhance animals performance and give a strong protective effect in Eimeria spp. challenged chickens with an improvement of the immune response [167,170]. There are still conflicting results on the effectiveness of administration in reducing infection and symptoms of coccidiosis. A recent study compares the effect of probiotic, prebiotic, salinomycin, and vaccine, suggesting that probiotics and prebiotics are not as effective in controlling coccidiosis and its complications as vaccine or salinomycin [171].
Additionally, some prebiotics have been tested for poultry coccidiosis treatment such as inulin, fructo-oligosaccharides, mannan-oligosaccharides (MOS), and xylo-oligosaccharides [85]. The commercial prebiotic Fermacto® (Pet-Ag, Hampshire, IL, USA), derived from Aspergillus orizae, was evaluated in different Eimeria spp. infections, with very promising results [171]. Furthermore, it has been shown that both the administration of a prebiotic (mannan-oligosaccharides and β-glucans) and a Bacillus subtilis probiotic do not cause negative interactions with the vaccination for coccidiosis and indeed are able to increase the feed conversion ratio [172]. Similarly, the comparison of the effects of MOS and Amprolium (an anticoccidial chemoterapic) administration on performance and GI health of broiler challenged with E. tenella, suggests that MOS are able to improve growth performance and reverse E. tenella lesions [173].
6.2. Organic Acids
The preventive anticoccidial activity of organic acids was mainly attributed to their ability to lower ceca pH and induce protective immunity against Eimeria spp. [174]. Anticoccidial properties of acetic acid against E. tenella were described [85], and its effects compared with Amprolium show almost equivalent results in reducing negative consequence of infection, demonstrating the potential of acetic acid use as an alternative to chemotherapy [175]. Interesting results are also reported for the use of glycerol monolaurate, obtained from lauric acid and glycerol, [176] and for butyrate, clopidol, and their combination [177], with a reduction in coccidian infection and maintenance of the immunity obtained from the first infection. The use of a blend of benzoic acid and essential oil compound is reported in animals challenged with Eimeria spp., resulting in a reduction in intestinal lesions [178].
6.3. Phytogenic Feed Additives (PFAs)
PFAs, together with probiotics, are the most interesting natural alternatives in coccidiosis treatment. Most of the plants and their bioactive compound used against Eimeria spp. infection have been reviewed by El-shall et al. [179]. From this comprehensive review clearly emerges the big interest and potential of phytochemicals in poultry industry, with a huge amount of papers on this topic. In most cases, not only herbs, but also herbal mixtures have been shown to be effective against avian coccidiosis [179]. The mechanism of action is not always known, but some anticoccidial effects were identified: inhibition of different Eimeria spp. growth, prevention of invasion, strengthening of immune response, inhibition of sporulation, prevention of oocysts shedding, and reduction in oocyst score. The herbal extracts and their phenolic compounds react with Eimeria cell membrane causing cell death. Moreover, these extracts increase the intestinal lipid peroxidation, enhance the reparation of injured epithelium, and decrease the permeability of intestinal cells induced by Eimeria spp. with a higher cellular turnover [179].
6.4. Antioxidants
The addition of vitamins in broilers’ diet is a strategy described to limit the consequences of Eimeria spp. infection as it can cause a reduction in cellular content of some antioxidant vitamins in the host cells [174]. Moreover, the limitation of peroxidation is the way of action of some anticoccidial molecules such as toltrazuril and salinomycin [174]. Supplementation with selenium, zinc, vitamin E, copper, and manganese can mitigate the effect of the disease [143,180,181], and the use of a blend of curcumin (Curcuma longa) and microencapsulated phytogenic, containing thymol, cinnamaldehyde, and carvacrol, permits a decrease in coccidian load [182]. Furthermore, feeding vitamin E and arginine to poultry, at higher level than recommended, can improve innate and humoral response against Eimeria spp. challenged animals [183]. Additionally, curcumin and cinnamaldehyde can protect intestinal cells from lipid peroxidation caused by coccidian parasites, increasing antioxidant enzyme levels [184].
7. In Ovo Technique
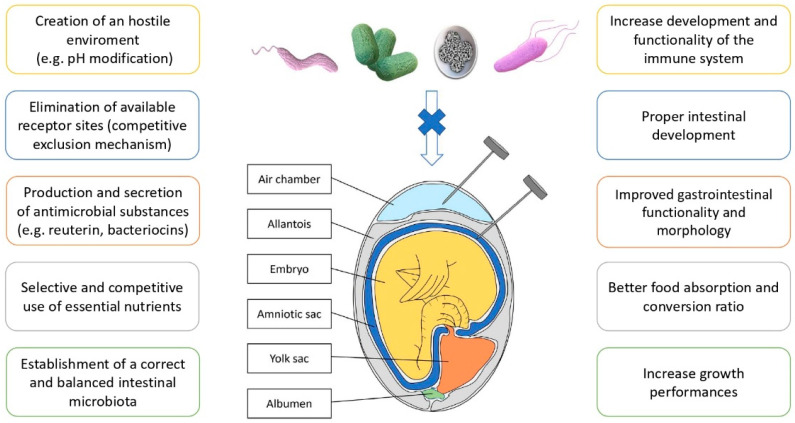
The in ovo inoculation is a method which allows us to administer substances directly to the chicken embryo during the incubation period. It was originally designed for the Marek’s Disease (MD) vaccine to achieve an early and effective immunity [185]. This technique spread rapidly worldwide, especially after the invention of the first automated injection system, the Inovoject® machine, manufactured by Embrex, Inc. (Durham, NC, USA) and initially introduced in the North American poultry industry [186]. The in ovo technique was suddenly explored for the administration of many other different compounds. The concept of in ovo feeding was introduced in 2003 as the administration into the embryonic amnion of nutrients and other natural compounds that can modulate enteric development of the hatchling [187]. Chickens hatch with an incomplete development of the intestinal tract and, consequently, the period from hatching to the first feeding is very critical, so the administration of substances in ovo allows us to obtain important effects on the future animal growth. Injections can be performed in different sites (air chamber, allantoid sac, amniotic sac) and day of incubation (from 12 to 19 day). The preferred site for inoculation is the amniotic sac, where the MD vaccine is also administered, and the inoculated substances are ingested by the chicken before hatching, coming into direct contact with the digestive and respiratory systems [188]. Over the years, this method has been used to deliver various nutrients, vaccines and drugs and it has also been explored as a possible way to obtain an early protection against pathogens, through multiple mechanism (Figure 1), and especially allowing a correct microbiota colonization of the GI tract [189,190] enhancing the development of both GI and immune system [23], allowing an early interaction with the chickens immune system before hatching [191].
Figure 1.
Benefits of in ovo inoculation of nutraceuticals and phytonutrient.
Using in Ovo Inoculation Technique against GI Pathogens
The contact with environmental Salmonella spp. often occurs before the chicken has consumed its first feed. Probiotic bacteria can prevent pathogens’ colonization, with the mechanism of competitive exclusion [192] considering also that a well-developed GI tract can reduce the effect of a mild Salmonella spp. colonization [190]. In this context, in ovo administration of probiotic and prebiotic is able to reduce Salmonella colonization and faecal shedding, with the amniotic sac as preferred site of administration, as the inoculum goes in direct contact with the GI system [193]. The in ovo treatment with Enterococcus faecium resulted in significant effects: after a challenge carried out at 4 days of age, a reduction in the number of chickens positive for S. enteritidis was observed, with an increased effect continuing the administration of the probiotic in diet [190]. Similar results are described after the in ovo administration of the commercial probiotic mixture FloraMax®-B11 (Pacific Vet Group USA Inc., Fayetteville, AR, USA), revealing an increase body weight, higher villi surface area, and decreased S. Enteritidis recovery after challenge at 7 day of age [194]. Administration of probiotics in ovo can also be effective to decrease Salmonella colonization in ceca [195,196].
The use of nutraceuticals and phytonutrients in ovo to prevent Campylobacter jejuni infection is still not explored, but some trials have been performed in relation to Clostridium perfringens infection. Selenium, a non-metallic essential micronutrient, is able to modulate the immune response in chickens challenged first with Eimeria maxima (at 14 day posthatch) and then with C. perfringens (at 18 day posthatch). Treated groups received 10 and 20 μg of Selenium/egg and showed an increased serum antibody levels against C. perfringens α-toxin and NetB toxin and both lower intestinal lesion and oocyst production compared to the non-treated group, suggesting an amelioration of immunity response in the posthatch period [197]. In ovo injection at 12 days of incubation of a raffinose family oligosaccharides (RFO) extracted from Lupinus luteus seeds permitted a 2.5 log reduction in C. perfringens count and an 89% reduction in Eimeria spp. oocysts shedding [198].
In ovo administration of probiotics at day 18 of incubation permits a significant reduction in the severity of macroscopic lesions caused by Eimeria spp. in all intestinal segments and an improvement of the zootechnical performances [169]. Comparing the in ovo administration at day 12 of incubation, of a prebiotic composed by a trans-galactooligosaccharides (Bi2tos), to an antibiotic, given individually or together, it emerges that prebiotic, with or without the antibiotic supplementation, can reduce intestinal lesion and oocyst shedding in natural infected chickens [199]. After the in ovo injection of vitamin D3 and 25-hydroxyvitamin D3 (25OHD3) in chickens challenged at 14 days, it was observed that, in the group treated with the 25OHD3, compared to D3 and the control group, there was no reduction in performances that had previously been observed in the other groups [200].
8. Discussion and Conclusions
Nutraceuticals and phytonutrients have gained great interest in recent years, being presented as a possible alternative to antimicrobials and answering the high demand of antibiotic-free poultry products. A valid substitute for antibiotics must have similar properties in increasing growth performances, optimizing feed conversion and limiting infections from pathogens. Several natural compounds are currently available on the market as valid substitutes to antibiotics, and they are also able to stimulate the immune system, making the animals more resistant to infections.
In the last 10 years much research has been conducted on the use of nutraceuticals such as probiotics, prebiotics, vitamins, phytogenic extracts, and organic acids to verify their efficacy and safety against the most common GI pathogens of poultry. The results presented are sometimes conflicting; in fact, despite some very favourable effects, not all these natural substances have an efficacy in prevention or treatment of the disease. It is difficult to compare the various products, as dosages, methods of administration, age of the birds, and duration of administration are different in each trial. In any case, even when not able to directly inhibit the infection, the use of nutraceuticals and phytonutrients has proven to be of fundamental importance for GI health, minimizing pathogens’ effects. Most of the losses are due to subclinical form, and the enhancement in intestinal immunity is essential, allowing a reduction in morbidity and periods of non-weight gain of the animals. The reduction in the bacterial load and faecal elimination also represents an important element to reduce meat contamination during slaughter, and thus the spread of clinical forms in the final consumer. The in ovo technique is very promising, allowing the product to explain its effect before the chicken enters into contact with environmental pathogens, having a positive influence on influencing the development of GI and immune system, and thus leading to a further form of resistance to infections.
Regardless of the administration route, it is evident that, although not always able to be directly effective in the prevention of infections, nutraceuticals and phytonutrients allow a reduction in symptoms with an increase in the activity of the immune system and growth performances. Moreover, the diversity of the results presented in the various trials highlights the complexity of the effects deriving from the use of these compounds, and further studies will be necessary to highlight the mechanisms of action to make their use even more effective in poultry production.
Author Contributions
Conceptualization, L.B. and G.R.; writing—original draft preparation, L.B. and L.G.; writing—review and editing, A.R., A.-R.A. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia Coli: A Global Overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antibiotic Resistance. [(accessed on 17 December 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
- 3.Cosby D.E., Cox N.A., Harrison M.A., Wilson J.L., Buhr R.J., Fedorka-Cray P.J. Salmonella and Antimicrobial Resistance in Broilers: A Review. J. Appl. Poult. Res. 2015;24:408–426. doi: 10.3382/japr/pfv038. [DOI] [Google Scholar]
- 4.World Health Organization . Critically Important Antimicrobials for Human Medicine. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 5.Castanon J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 6.EU R. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. [(accessed on 17 December 2021)];Bruss. Eur. 2005 Available online: https://agenceurope.eu/aewebsite_dev/en/bulletin/article/9099/22. [Google Scholar]
- 7.Editors A. US Bans Antibiotics Use for Enhancing Growth in Livestock. 2017. [(accessed on 17 December 2021)]. Available online: [DOI]
- 8.Agricultural Production—Livestock and Meat. [(accessed on 17 December 2021)]. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_livestock_and_meat.
- 9.Shehata A.A., Yalçın S., Latorre J.D., Basiouni S., Attia Y.A., Abd El-Wahab A., Visscher C., El-Seedi H.R., Huber C., Hafez H.M., et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms. 2022;10:395. doi: 10.3390/microorganisms10020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huyghebaert G., Ducatelle R., Immerseel F.V. An Update on Alternatives to Antimicrobial Growth Promoters for Broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Alagawany M., Elnesr S.S., Farag M.R., Abd El-Hack M.E., Barkat R.A., Gabr A.A., Foda M.A., Noreldin A.E., Khafaga A.F., El-Sabrout K. Potential Role of Important Nutraceuticals in Poultry Performance and Health-A Comprehensive Review. Res. Vet. Sci. 2021;137:9–29. doi: 10.1016/j.rvsc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Brower V. Nutraceuticals: Poised for a Healthy Slice of the Healthcare Market? Nat. Biotechnol. 1998;16:728–731. doi: 10.1038/nbt0898-728. [DOI] [PubMed] [Google Scholar]
- 13.Das L., Bhaumik E., Raychaudhuri U., Chakraborty R. Role of Nutraceuticals in Human Health. J. Food Sci. Technol. 2012;49:173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank J., Fukagawa N.K., Bilia A.R., Johnson E.J., Kwon O., Prakash V., Miyazawa T., Clifford M.N., Kay C.D., Crozier A. Terms and Nomenclature Used for Plant-Derived Components in Nutrition and Related Research: Efforts toward Harmonization. Nutr. Rev. 2020;78:451–458. doi: 10.1093/nutrit/nuz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiharto S. Role of Nutraceuticals in Gut Health and Growth Performance of Poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. doi: 10.1016/j.jssas.2014.06.001. [DOI] [Google Scholar]
- 16.Swaggerty C.L., Bortoluzzi C., Lee A., Eyng C., Pont G.D., Kogut M.H. Potential Replacements for Antibiotic Growth Promoters in Poultry: Interactions at the Gut Level and Their Impact on Host Immunity. In: Wu G., editor. Recent Advances in Animal Nutrition and Metabolism. Springer International Publishing; Cham, Switzerland: 2022. pp. 145–159. Advances in Experimental Medicine and Biology. [DOI] [PubMed] [Google Scholar]
- 17.Chauhan B., Kumar G., Kalam N., Ansari S.H. Current Concepts and Prospects of Herbal Nutraceutical: A Review. J. Adv. Pharm. Technol. Res. 2013;4:4–8. doi: 10.4103/2231-4040.107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan L., An S., Lv Z., Wang Z., Wu Y., Zhu Y., Zhao M., Sun C., Lv M., Zhu Z., et al. Effects of Phytonutrients on Growth Performance, Antioxidative Status, and Energy Utilization of Broilers Fed Low Energy Diets. Anim. Nutr. 2019;5:270–277. doi: 10.1016/j.aninu.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sherbiny I.M., El-Baz N.M., Hefnawy A. Potential of Nanotechnology in Nutraceuticals Delivery for the Prevention and Treatment of Cancer. In: Grumezescu A.M., editor. Nutraceuticals. Academic Press; Cambridge, MA, USA: 2016. pp. 117–152. Nanotechnology in the Agri-Food Industry. [Google Scholar]
- 20.Solicitation of Written Comments on Proposed Definition of Bioactive Food Components. [(accessed on 3 January 2022)]; Available online: https://www.federalregister.gov/documents/2004/09/16/04-20892/solicitation-of-written-comments-on-proposed-definition-of-bioactive-food-components.
- 21.Nutrition Division . Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation—Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. FAO/WHO; Rome, Italy: 2006. FAO Food and Nutrition Paper. [Google Scholar]
- 22.Bratz K., Gölz G., Janczyk P., Nöckler K., Alter T. Analysis of in Vitro and in Vivo Effects of Probiotics against Campylobacter spp. Berl. Munch. Tierarztl. Wochenschr. 2015;128:155–162. [PubMed] [Google Scholar]
- 23.Cox C.M., Dalloul R.A. Immunomodulatory Role of Probiotics in Poultry and Potential in Ovo Application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- 24.Kemgang T.S., Kapila S., Shanmugam V.P., Kapila R. Cross-talk between Probiotic Lactobacilli and Host Immune System. J. Appl. Microbiol. 2014;117:303–319. doi: 10.1111/jam.12521. [DOI] [PubMed] [Google Scholar]
- 25.Travers M.-A., Florent I., Kohl L., Grellier P. Probiotics for the Control of Parasites: An Overview. J. Parasitol. Res. 2011;2011:610769. doi: 10.1155/2011/610769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., Chio J.S. Effects of Probiotic-Supplemented Diets on Growth Performance and Intestinal Immune Characteristics of Broiler Chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- 27.Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K. Effect of Supplementation of Bacillus subtilis LS 1-2 to Broiler Diets on Growth Performance, Nutrient Retention, Caecal Microbiology and Small Intestinal Morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a Probiotic Mixture on Intestinal Microflora, Morphology, and Barrier Integrity of Broilers Subjected to Heat Stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Sun J., Zhong H., Li N., Xu H., Zhu Q., Liu Y. Effect of Probiotics on the Meat Flavour and Gut Microbiota of Chicken. Sci. Rep. 2017;7:6400. doi: 10.1038/s41598-017-06677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav S., Jha R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z.F., Kim I.H. Effects of Multistrain Probiotics on Growth Performance, Apparent Ileal Nutrient Digestibility, Blood Characteristics, Cecal Microbial Shedding, and Excreta Odor Contents in Broilers. Poult. Sci. 2014;93:364–370. doi: 10.3382/ps.2013-03314. [DOI] [PubMed] [Google Scholar]
- 32.Pourabedin M., Zhao X. Prebiotics and Gut Microbiota in Chickens. FEMS Microbiol. Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- 33.Alloui N., Szczurek W., Swiatkiewicz S. The Usefulness of Prebiotics and Probiotics in Modern Poultry Nutrition: A Review. Ann. Anim. Sci. 2013;13:17–32. doi: 10.2478/v10220-012-0055-x. [DOI] [Google Scholar]
- 34.Micciche A.C., Foley S.L., Pavlidis H.O., McIntyre D.R., Ricke S.C. A Review of Prebiotics against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications. Front. Vet. Sci. 2018;5:191. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricke S.C. Potential of Fructooligosaccharide Prebiotics in Alternative and Nonconventional Poultry Production Systems. Poult. Sci. 2015;94:1411–1418. doi: 10.3382/ps/pev049. [DOI] [PubMed] [Google Scholar]
- 36.Ricke S.C. Focus: Nutrition and Food Science: Impact of Prebiotics on Poultry Production and Food Safety. Yale J. Biol. Med. 2018;91:151. [PMC free article] [PubMed] [Google Scholar]
- 37.Shojadoost B., Yitbarek A., Alizadeh M., Kulkarni R.R., Astill J., Boodhoo N., Sharif S. Centennial Review: Effects of Vitamins A, D, E, and C on the Chicken Immune System. Poult. Sci. 2021;100:100930. doi: 10.1016/j.psj.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elwinger K., Fisher C., Jeroch H., Sauveur B., Tiller H., Whitehead C.C. A Brief History of Poultry Nutrition over the Last Hundred Years. Worlds Poult. Sci. J. 2016;72:701–720. doi: 10.1017/S004393391600074X. [DOI] [Google Scholar]
- 39.El-Senousey H.K., Chen B., Wang J.Y., Atta A.M., Mohamed F.R., Nie Q.H. Effects of Dietary Vitamin C, Vitamin E, and Alpha-Lipoic Acid Supplementation on the Antioxidant Defense System and Immune-Related Gene Expression in Broilers Exposed to Oxidative Stress by Dexamethasone. Poult. Sci. 2018;97:30–38. doi: 10.3382/ps/pex298. [DOI] [PubMed] [Google Scholar]
- 40.Khan R.U., Rahman Z.U., Nikousefat Z., Javdani M., Tufarelli V., Dario C., Selvaggi M., Laudadio V. Immunomodulating Effects of Vitamin E in Broilers. Worlds Poult. Sci. J. 2012;68:31–40. doi: 10.1017/S0043933912000049. [DOI] [Google Scholar]
- 41.Lucas A., Morales J., Velando A. Differential Effects of Specific Carotenoids on Oxidative Damage and Immune Response of Gull Chicks. J. Exp. Biol. 2014;217:1253–1262. doi: 10.1242/jeb.098004. [DOI] [PubMed] [Google Scholar]
- 42.Yuan J., Roshdy A.R., Guo Y., Wang Y., Guo S. Effect of Dietary Vitamin A on Reproductive Performance and Immune Response of Broiler Breeders. PLoS ONE. 2014;9:e105677. doi: 10.1371/journal.pone.0105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kholif A.E., Olafadehan O.A. Essential Oils and Phytogenic Feed Additives in Ruminant Diet: Chemistry, Ruminal Microbiota and Fermentation, Feed Utilization and Productive Performance. Phytochem. Rev. 2021;20:1087–1108. doi: 10.1007/s11101-021-09739-3. [DOI] [Google Scholar]
- 44.Abdelli N., Solà-Oriol D., Pérez J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals. 2021;11:3471. doi: 10.3390/ani11123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenes A., Roura E. Essential Oils in Poultry Nutrition: Main Effects and Modes of Action. Anim. Feed Sci. Technol. 2010;158:1–14. doi: 10.1016/j.anifeedsci.2010.03.007. [DOI] [Google Scholar]
- 46.Cherian G., Orr A., Burke I.C., Pan W. Feeding Artemisia Annua Alters Digesta PH and Muscle Lipid Oxidation Products in Broiler Chickens. Poult. Sci. 2013;92:1085–1090. doi: 10.3382/ps.2012-02752. [DOI] [PubMed] [Google Scholar]
- 47.Mohammadi Gheisar M., Kim I.H. Phytobiotics in Poultry and Swine Nutrition—A Review. Ital. J. Anim. Sci. 2018;17:92–99. doi: 10.1080/1828051X.2017.1350120. [DOI] [Google Scholar]
- 48.Yakhkeshi S., Rahimi S., Gharib Naseri K. The Effects of Comparison of Herbal Extracts, Antibiotic, Probiotic and Organic Acid on Serum Lipids, Immune Response, GIT Microbial Population, Intestinal Morphology and Performance of Broilers. J. Med. Plants. 2011;10:80–95. [Google Scholar]
- 49.Yang C., Chowdhury M.A.K., Huo Y., Gong J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dittoe D.K., Ricke S.C., Kiess A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018;5:216. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassan H.M.A., Mohamed M.A., Youssef A.W., Hassan E.R. Effect of Using Organic Acids to Substitute Antibiotic Growth Promoters on Performance and Intestinal Microflora of Broilers. Asian-Australas. J. Anim. Sci. 2010;23:1348–1353. doi: 10.5713/ajas.2010.10085. [DOI] [Google Scholar]
- 52.Khan S.H., Iqbal J. Recent Advances in the Role of Organic Acids in Poultry Nutrition. J. Appl. Anim. Res. 2016;44:359–369. doi: 10.1080/09712119.2015.1079527. [DOI] [Google Scholar]
- 53.Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a Protected Inclusion of Organic Acids and Essential Oils as Antibiotic Growth Promoter Alternative on Growth Performance, Intestinal Morphology and Gut Microflora in Broilers. Anim. Sci. J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- 54.Saki A.A., Harcini R.N., Rahmatnejad E., Salary J. Herbal Additives and Organic Acids as Antibiotic Alternatives in Broiler Chickens Diet for Organic Production. Afr. J. Biotechnol. 2012;11:2139–2145. [Google Scholar]
- 55.Gast R.K., Porter R.E., Jr. Diseases of Poultry. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2020. Salmonella Infections; pp. 717–753. [Google Scholar]
- 56.Kowalska J.D., Nowak A., Śliżewska K., Stańczyk M., Łukasiak M., Dastych J. Anti-Salmonella Potential of New Lactobacillus Strains with the Application in the Poultry Industry. Pol. J. Microbiol. 2020;69:5. doi: 10.33073/pjm-2020-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 58.European Food Safety Authority and European Centre for Disease Prevention and Control The European Union One Health 2019 Zoonoses Report. EFSA J. 2021;19:e06406. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foley S.L., Nayak R., Hanning I.B., Johnson T.J., Han J., Ricke S.C. Population Dynamics of Salmonella enterica Serotypes in Commercial Egg and Poultry Production. Appl. Environ. Microbiol. 2011;77:4273–4279. doi: 10.1128/AEM.00598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrow P.A., Jones M.A., Smith A.L., Wigley P. The Long View: Salmonella–the Last Forty Years. Avian Pathol. 2012;41:413–420. doi: 10.1080/03079457.2012.718071. [DOI] [PubMed] [Google Scholar]
- 61.Velasquez C.G., Macklin K.S., Kumar S., Bailey M., Ebner P.E., Oliver H.F., Martin-Gonzalez F.S., Singh M. Prevalence and Antimicrobial Resistance Patterns of Salmonella Isolated from Poultry Farms in Southeastern United States. Poult. Sci. 2018;97:2144–2152. doi: 10.3382/ps/pex449. [DOI] [PubMed] [Google Scholar]
- 62.Muhammad J., Khan S., Su J.Q., Hesham A.E.-L., Ditta A., Nawab J., Ali A. Antibiotics in Poultry Manure and Their Associated Health Issues: A Systematic Review. J. Soils Sediments. 2020;20:486–497. doi: 10.1007/s11368-019-02360-0. [DOI] [Google Scholar]
- 63.El-Shall N.A., Awad A.M., El-Hack M.E.A., Naiel M.A., Othman S.I., Allam A.A., Sedeik M.E. The Simultaneous Administration of a Probiotic or Prebiotic with Live Salmonella Vaccine Improves Growth Performance and Reduces Fecal Shedding of the Bacterium in Salmonella-Challenged Broilers. Animals. 2020;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Sharkawy H., Tahoun A., Rizk A.M., Suzuki T., Elmonir W., Nassef E., Shukry M., Germoush M.O., Farrag F., Bin-Jumah M. Evaluation of Bifidobacteria and Lactobacillus Probiotics as Alternative Therapy for Salmonella Typhimurium Infection in Broiler Chickens. Animals. 2020;10:1023. doi: 10.3390/ani10061023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuller R. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 66.Nakphaichit M., Sobanbua S., Siemuang S., Vongsangnak W., Nakayama J., Nitisinprasert S. Protective Effect of Lactobacillus Reuteri KUB-AC5 against Salmonella Enteritidis Challenge in Chickens. Benef. Microbes. 2019;10:43–54. doi: 10.3920/BM2018.0034. [DOI] [PubMed] [Google Scholar]
- 67.Prado-Rebolledo O.F., de Jesus Delgado-Machuca J., Macedo-Barragan R.J., Garcia-Márquez L.J., Morales-Barrera J.E., Latorre J.D., Hernandez-Velasco X., Tellez G. Evaluation of a Selected Lactic Acid Bacteria-Based Probiotic on Salmonella enterica Serovar Enteritidis Colonization and Intestinal Permeability in Broiler Chickens. Avian Pathol. 2017;46:90–94. doi: 10.1080/03079457.2016.1222808. [DOI] [PubMed] [Google Scholar]
- 68.Carter A., Adams M., La Ragione R.M., Woodward M.J. Colonisation of Poultry by Salmonella Enteritidis S1400 Is Reduced by Combined Administration of Lactobacillus Salivarius 59 and Enterococcus faecium PXN-33. Vet. Microbiol. 2017;199:100–107. doi: 10.1016/j.vetmic.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 69.Bae D., Kim D.-H., Chon J.-W., Song K.-Y., Seo K.-H. Synergistic Effects of the Early Administration of Lactobacillus Kefiranofaciens DN1 and Kluyveromyces Marxianus KU140723-05 on the Inhibition of Salmonella Enteritidis Colonization in Young Chickens. Poult. Sci. 2020;99:5999–6006. doi: 10.1016/j.psj.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khochamit N., Siripornadulsil S., Sukon P., Siripornadulsil W. Bacillus subtilis and Lactic Acid Bacteria Improve the Growth Performance and Blood Parameters and Reduce Salmonella Infection in Broilers. Vet. World. 2020;13:2663–2672. doi: 10.14202/vetworld.2020.2663-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neveling D.P., van Emmenes L., Ahire J.J., Pieterse E., Smith C., Dicks L.M.T. Effect of a Multi-Species Probiotic on the Colonisation of Salmonella in Broilers. Probiotics Antimicrob. Proteins. 2020;12:896–905. doi: 10.1007/s12602-019-09593-y. [DOI] [PubMed] [Google Scholar]
- 72.Delgado R., Latorre J.D., Vicuña E., Hernandez-Velasco X., Vicente J.L., Menconi A., Kallapura G., Layton S., Hargis B.M., Tellez G. Glycerol Supplementation Enhances the Protective Effect of Dietary FloraMax-B11 against Salmonella Enteritidis Colonization in Neonate Broiler Chickens. Poult. Sci. 2014;93:2363–2369. doi: 10.3382/ps.2014-03927. [DOI] [PubMed] [Google Scholar]
- 73.Biloni A., Quintana C.F., Menconi A., Kallapura G., Latorre J., Pixley C., Layton S., Dalmagro M., Hernandez-Velasco X., Wolfenden A. Evaluation of Effects of EarlyBird Associated with FloraMax-B11 on Salmonella Enteritidis, Intestinal Morphology, and Performance of Broiler Chickens. Poult. Sci. 2013;92:2337–2346. doi: 10.3382/ps.2013-03279. [DOI] [PubMed] [Google Scholar]
- 74.Khan S., Chousalkar K.K. Short-Term Feeding of Probiotics and Synbiotics Modulates Caecal Microbiota during Salmonella Typhimurium Infection but Does Not Reduce Shedding and Invasion in Chickens. Appl. Microbiol. Biotechnol. 2020;104:319–334. doi: 10.1007/s00253-019-10220-7. [DOI] [PubMed] [Google Scholar]
- 75.Lourenço M.C., Kuritza L.N., Hayashi R.M., Miglino L.B., Durau J.F., Pickler L., Santin E. Effect of a Mannanoligosaccharide-Supplemented Diet on Intestinal Mucosa T Lymphocyte Populations in Chickens Challenged WithSalmonella Enteritidis. J. Appl. Poult. Res. 2015;24:15–22. doi: 10.3382/japr/pfu002. [DOI] [Google Scholar]
- 76.Park S.H., Kim S.A., Lee S.I., Rubinelli P.M., Roto S.M., Pavlidis H.O., McIntyre D.R., Ricke S.C. Original XPCTM Effect on Salmonella Typhimurium and Cecal Microbiota from Three Different Ages of Broiler Chickens When Incubated in an Anaerobic In Vitro Culture System. Front. Microbiol. 2017;8:1070. doi: 10.3389/fmicb.2017.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feye K.M., Anderson K.L., Scott M.F., McIntyre D.R., Carlson S.A. Inhibition of the Virulence, Antibiotic Resistance, and Fecal Shedding of Multiple Antibiotic-Resistant Salmonella Typhimurium in Broilers Fed Original XPCTM. Poult. Sci. 2016;95:2902–2910. doi: 10.3382/ps/pew254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee S.I., Park S.H., Ricke S.C. Assessment of Cecal Microbiota, Integron Occurrence, Fermentation Responses, and Salmonella Frequency in Conventionally Raised Broilers Fed a Commercial Yeast-Based Prebiotic Compound. Poult. Sci. 2016;95:144–153. doi: 10.3382/ps/pev322. [DOI] [PubMed] [Google Scholar]
- 79.Hughes R.-A., Ali R.A., Mendoza M.A., Hassan H.M., Koci M.D. Impact of Dietary Galacto-Oligosaccharide (GOS) on Chicken’s Gut Microbiota, Mucosal Gene Expression, and Salmonella Colonization. Front. Vet. Sci. 2017;4:192. doi: 10.3389/fvets.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 81.Onrust L., Baeyen S., Haesebrouck F., Ducatelle R., Van Immerseel F. Effect of in Feed Administration of Different Butyrate Formulations on Salmonella Enteritidis Colonization and Cecal Microbiota in Broilers. Vet. Res. 2020;51:56. doi: 10.1186/s13567-020-00780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bedford A., Gong J. Implications of Butyrate and Its Derivatives for Gut Health and Animal Production. Anim. Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moquet P.C.A., Salami S.A., Onrust L., Hendriks W.H., Kwakkel R.P. Butyrate Presence in Distinct Gastrointestinal Tract Segments Modifies Differentially Digestive Processes and Amino Acid Bioavailability in Young Broiler Chickens. Poult. Sci. 2018;97:167–176. doi: 10.3382/ps/pex279. [DOI] [PubMed] [Google Scholar]
- 84.van den Borne J.J.G.C., Heetkamp M.J.W., Buyse J., Niewold T.A. Fat Coating of Ca Butyrate Results in Extended Butyrate Release in the Gastrointestinal Tract of Broilers. Livest. Sci. 2015;175:96–100. doi: 10.1016/j.livsci.2015.02.016. [DOI] [Google Scholar]
- 85.Adhikari P., Kiess A., Adhikari R., Jha R. An Approach to Alternative Strategies to Control Avian Coccidiosis and Necrotic Enteritis. J. Appl. Poult. Res. 2020;29:515–534. doi: 10.1016/j.japr.2019.11.005. [DOI] [Google Scholar]
- 86.van Kuijk S.J.A., Han Y. Efficacy of a Synergistic Blend of Organic Acids and SS-1,4 Mannobiose on Cecal Salmonella Counts and Growth Performance in Salmonella Challenged Broiler Chickens: A Meta-Analysis. Animals. 2021;11:2988. doi: 10.3390/ani11102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bourassa D.V., Wilson K.M., Ritz C.R., Kiepper B.K., Buhr R.J. Evaluation of the Addition of Organic Acids in the Feed and/or Water for Broilers and the Subsequent Recovery of Salmonella Typhimurium from Litter and Ceca. Poult. Sci. 2018;97:64–73. doi: 10.3382/ps/pex289. [DOI] [PubMed] [Google Scholar]
- 88.Sobotik E.B., Ramirez S., Roth N., Tacconi A., Pender C., Murugesan R., Archer G.S. Evaluating the Effects of a Dietary Synbiotic or Synbiotic plus Enhanced Organic Acid on Broiler Performance and Cecal and Carcass Salmonella Load. Poult. Sci. 2021;100:101508. doi: 10.1016/j.psj.2021.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh T., Srivastava S.K., Gaurav A., Kumar A., Kumar P., Yadav A.S., Pathania R., Navani N.K. A Combination of Linalool, Vitamin C, and Copper Synergistically Triggers Reactive Oxygen Species and DNA Damage and Inhibits Salmonella enterica subsp. enterica Serovar Typhi and Vibrio fluvialis. Appl. Environ. Microbiol. 2019;85:e02487-18. doi: 10.1128/AEM.02487-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hernandez-Patlan D., Solis-Cruz B., Méndez-Albores A., Latorre J.D., Hernandez-Velasco X., Tellez G., López-Arellano R. Comparison of PrestoBlue® and Plating Method to Evaluate Antimicrobial Activity of Ascorbic Acid, Boric Acid and Curcumin in an in Vitro Gastrointestinal Model. J. Appl. Microbiol. 2018;124:423–430. doi: 10.1111/jam.13659. [DOI] [PubMed] [Google Scholar]
- 91.Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Latorre J.D., Hernandez-Velasco X., Merino-Guzman R., Mendez-Albores A., Hargis B.M., Lopez-Arellano R., Tellez-Isaias G. Evaluation of Ascorbic Acid or Curcumin Formulated in a Solid Dispersion on Salmonella Enteritidis Infection and Intestinal Integrity in Broiler Chickens. Pathogens. 2019;8:229. doi: 10.3390/pathogens8040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gan L., Fan H., Mahmood T., Guo Y. Dietary Supplementation with Vitamin C Ameliorates the Adverse Effects of Salmonella Enteritidis-Challenge in Broilers by Shaping Intestinal Microbiota. Poult. Sci. 2020;99:3663–3674. doi: 10.1016/j.psj.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y.J., Zhao L.H., Mosenthin R., Zhang J.Y., Ji C., Ma Q.G. Protective Effect of Vitamin E on Laying Performance, Antioxidant Capacity, and Immunity in Laying Hens Challenged with Salmonella Enteritidis. Poult. Sci. 2019;98:5847–5854. doi: 10.3382/ps/pez227. [DOI] [PubMed] [Google Scholar]
- 94.Liu X., Byrd J.A., Farnell M., Ruiz-Feria C.A. Arginine and Vitamin E Improve the Immune Response after a Salmonella Challenge in Broiler Chicks. Poult. Sci. 2014;93:882–890. doi: 10.3382/ps.2013-03723. [DOI] [PubMed] [Google Scholar]
- 95.Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The Effect of Phytogenic Feed Additives to Substitute In-Feed Antibiotics on Growth Traits and Blood Biochemical Parameters in Broiler Chicks Challenged with Salmonella Typhimurium. Environ. Sci. Pollut. Res. 2016;23:24151–24157. doi: 10.1007/s11356-016-7665-2. [DOI] [PubMed] [Google Scholar]
- 96.Murugesan G.R., Syed B., Haldar S., Pender C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015;2:21. doi: 10.3389/fvets.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wati T., Ghosh T.K., Syed B., Haldar S. Comparative Efficacy of a Phytogenic Feed Additive and an Antibiotic Growth Promoter on Production Performance, Caecal Microbial Population and Humoral Immune Response of Broiler Chickens Inoculated with Enteric Pathogens. Anim. Nutr. 2015;1:213–219. doi: 10.1016/j.aninu.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casanova N.A., Redondo L.M., Redondo E.A., Joaquim P.E., Dominguez J.E., Fernández-Miyakawa M.E., Chacana P.A. Efficacy of Chestnut and Quebracho Wood Extracts to Control Salmonella in Poultry. J. Appl. Microbiol. 2021;131:135–145. doi: 10.1111/jam.14948. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Q., Sahin O. Diseases of Poultry. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2020. Campylobacteriosis; pp. 754–769. [Google Scholar]
- 100.Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. Campylobacter jejuni Is Not Merely a Commensal in Commercial Broiler Chickens and Affects Bird Welfare. mBio. 2014;5:e01364-14. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Awad W.A., Hess C., Hess M. Re-Thinking the Chicken-Campylobacter Jejuni Interaction: A Review. Avian Pathol. J. WVPA. 2018;47:352–363. doi: 10.1080/03079457.2018.1475724. [DOI] [PubMed] [Google Scholar]
- 102.García-Sánchez L., Melero B., Jaime I., Hänninen M.-L., Rossi M., Rovira J. Campylobacter jejuni Survival in a Poultry Processing Plant Environment. Food Microbiol. 2017;65:185–192. doi: 10.1016/j.fm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 103.Hue O., Allain V., Laisney M.-J., Le Bouquin S., Lalande F., Petetin I., Rouxel S., Quesne S., Gloaguen P.-Y., Picherot M., et al. Campylobacter Contamination of Broiler Caeca and Carcasses at the Slaughterhouse and Correlation with Salmonella Contamination. Food Microbiol. 2011;28:862–868. doi: 10.1016/j.fm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Agunos A., Waddell L., Léger D., Taboada E. A Systematic Review Characterizing On-Farm Sources of Campylobacter Spp. for Broiler Chickens. PLoS ONE. 2014;9:e104905. doi: 10.1371/journal.pone.0104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Śmiałek M., Kowalczyk J., Koncicki A. The Use of Probiotics in the Reduction of Campylobacter Spp. Prevalence in Poultry. Animals. 2021;11:1355. doi: 10.3390/ani11051355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kemmett K. Probiotics and Enzymes: A Good Combination. AFMA Matrix. 2015;24:35–37. [Google Scholar]
- 107.Wang X., Zhang X., Dong X., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wooten J., Liu X., Miller M.J. Draft Genome Sequence of Lactobacillus crispatus JCM5810, Which Can Reduce Campylobacter jejuni Colonization in Chicken Intestine. Genome Announc. 2016;4:e00255-16. doi: 10.1128/genomeA.00255-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arsi K., Donoghue A.M., Woo-Ming A., Blore P.J., Donoghue D.J. The Efficacy of Selected Probiotic and Prebiotic Combinations in Reducing Campylobacter Colonization in Broiler Chickens. J. Appl. Poult. Res. 2015;24:327–334. doi: 10.3382/japr/pfv032. [DOI] [Google Scholar]
- 110.Ghareeb K., Awad W.A., Mohnl M., Porta R., Biarnés M., Böhm J., Schatzmayr G. Evaluating the Efficacy of an Avian-Specific Probiotic to Reduce the Colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2012;91:1825–1832. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- 111.Guyard-Nicodème M., Keita A., Quesne S., Amelot M., Poezevara T., Le Berre B., Sánchez J., Vesseur P., Martín Á., Medel P., et al. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period. Poult. Sci. 2016;95:298–305. doi: 10.3382/ps/pev303. [DOI] [PubMed] [Google Scholar]
- 112.Saint-Cyr M.J., Haddad N., Taminiau B., Poezevara T., Quesne S., Amelot M., Daube G., Chemaly M., Dousset X., Guyard-Nicodème M. Use of the Potential Probiotic Strain Lactobacillus Salivarius SMXD51 to Control Campylobacter jejuni in Broilers. Int. J. Food Microbiol. 2017;247:9–17. doi: 10.1016/j.ijfoodmicro.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 113.Šikić Pogačar M., Langerholc T., Mičetić-Turk D., Možina S.S., Klančnik A. Effect of Lactobacillus Spp. on Adhesion, Invasion, and Translocation of Campylobacter jejuni in Chicken and Pig Small-Intestinal Epithelial Cell Lines. BMC Vet. Res. 2020;16:34. doi: 10.1186/s12917-020-2238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van Immerseel F. The Probiotic Butyricicoccus Pullicaecorum Reduces Feed Conversion and Protects from Potentially Harmful Intestinal Microorganisms and Necrotic Enteritis in Broilers. Front. Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomrongsuwannakij T., Chuanchuen R., Chansiripornchai N. Identification of Competitive Exclusion and Its Ability to Protect Against Campylobacter jejuni in Broilers. Thai Vet. Med. 2016;46:279–286. [Google Scholar]
- 116.Baffoni L., Gaggìa F., Garofolo G., Di Serafino G., Buglione E., Di Giannatale E., Di Gioia D. Evidence of Campylobacter jejuni Reduction in Broilers with Early Synbiotic Administration. Int. J. Food Microbiol. 2017;251:41–47. doi: 10.1016/j.ijfoodmicro.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 117.Peng M., Reichmann G., Biswas D. Lactobacillus Casei and Its Byproducts Alter the Virulence Factors of Foodborne Bacterial Pathogens. J. Funct. Foods. 2015;15:418–428. doi: 10.1016/j.jff.2015.03.055. [DOI] [Google Scholar]
- 118.Tabashsum Z., Peng M., Kahan E., Rahaman S.O., Biswas D. Effect of Conjugated Linoleic Acid Overproducing Lactobacillus with Berry Pomace Phenolic Extracts on Campylobacter jejuni Pathogenesis. Food Funct. 2019;10:296–303. doi: 10.1039/C8FO01863D. [DOI] [PubMed] [Google Scholar]
- 119.Nothaft H., Perez-Muñoz M.E., Gouveia G.J., Duar R.M., Wanford J.J., Lango-Scholey L., Panagos C.G., Srithayakumar V., Plastow G.S., Coros C. Coadministration of the Campylobacter Jejuni N-Glycan-Based Vaccine with Probiotics Improves Vaccine Performance in Broiler Chickens. Appl. Environ. Microbiol. 2017;83:e01523-17. doi: 10.1128/AEM.01523-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beier R.C., Byrd J.A., Caldwell D., Andrews K., Crippen T.L., Anderson R.C., Nisbet D.J. Inhibition and Interactions of Campylobacter Jejuni from Broiler Chicken Houses with Organic Acids. Microorganisms. 2019;7:223. doi: 10.3390/microorganisms7080223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skånseng B., Kaldhusdal M., Moen B., Gjevre A.-G., Johannessen G.S., Sekelja M., Trosvik P., Rudi K. Prevention of Intestinal Campylobacter Jejuni Colonization in Broilers by Combinations of In-Feed Organic Acids. J. Appl. Microbiol. 2010;109:1265–1273. doi: 10.1111/j.1365-2672.2010.04766.x. [DOI] [PubMed] [Google Scholar]
- 122.Hankel J., Popp J., Meemken D., Zeiger K., Beyerbach M., Taube V., Klein G., Visscher C. Influence of Lauric Acid on the Susceptibility of Chickens to an Experimental Campylobacter jejuni Colonisation. PLoS ONE. 2018;13:e0204483. doi: 10.1371/journal.pone.0204483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peh E., Kittler S., Reich F., Kehrenberg C. Antimicrobial Activity of Organic Acids against Campylobacter Spp. and Development of Combinations—A Synergistic Effect? PLoS ONE. 2020;15:e0239312. doi: 10.1371/journal.pone.0239312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gracia M.I., Millán C., Sánchez J., Guyard-Nicodème M., Mayot J., Carre Y., Csorbai A., Chemaly M., Medel P. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period: Part B. Poult. Sci. 2016;95:886–892. doi: 10.3382/ps/pev346. [DOI] [PubMed] [Google Scholar]
- 125.Hermans D., Martel A., Garmyn A., Verlinden M., Heyndrickx M., Gantois I., Haesebrouck F., Pasmans F. Application of Medium-Chain Fatty Acids in Drinking Water Increases Campylobacter jejuni Colonization Threshold in Broiler Chicks. Poult. Sci. 2012;91:1733–1738. doi: 10.3382/ps.2011-02106. [DOI] [PubMed] [Google Scholar]
- 126.Greene G., Koolman L., Whyte P., Lynch H., Coffey A., Lucey B., Egan J., O’Connor L., Bolton D. The Efficacy of Organic Acid, Medium Chain Fatty Acid and Essential Oil Based Broiler Treatments; in Vitro Anti-Campylobacter jejuni Activity and the Effect of These Chemical-Based Treatments on Broiler Performance. J. Appl. Microbiol. 2021;132:687–695. doi: 10.1111/jam.15204. [DOI] [PubMed] [Google Scholar]
- 127.Haughton P.N., Lyng J., Fanning S., Whyte P. Potential of a Commercially Available Water Acidification Product for Reducing Campylobacter in Broilers Prior to Slaughter. Br. Poult. Sci. 2013;54:319–324. doi: 10.1080/00071668.2013.786806. [DOI] [PubMed] [Google Scholar]
- 128.Hermans D., Martel A., van Deun K., van Immerseel F., Heyndrickx M., Haesebrouck F., Pasmans F. The Cinnamon-Oil Ingredient Trans-Cinnamaldehyde Fails to Target Campylobacter jejuni Strain KC 40 in the Broiler Chicken Cecum despite Marked in Vitro Activity. J. Food Prot. 2011;74:1729–1734. doi: 10.4315/0362-028X.JFP-10-487. [DOI] [PubMed] [Google Scholar]
- 129.Robyn J., Rasschaert G., Hermans D., Pasmans F., Heyndrickx M. Is Allicin Able to Reduce Campylobacter jejuni Colonization in Broilers When Added to Drinking Water? Poult. Sci. 2013;92:1408–1418. doi: 10.3382/ps.2012-02863. [DOI] [PubMed] [Google Scholar]
- 130.Anderson R.C., Vodovnik M., Min B.R., Pinchak W.E., Krueger N.A., Harvey R.B., Nisbet D.J. Bactericidal Effect of Hydrolysable and Condensed Tannin Extracts on Campylobacter jejuni in Vitro. Folia Microbiol. 2012;57:253–258. doi: 10.1007/s12223-012-0119-4. [DOI] [PubMed] [Google Scholar]
- 131.Epps S.V.R., Harvey R.B., Byrd J.A., Petrujkić B.T., Sedej I., Beier R.C., Phillips T.D., Hume M.E., Anderson R.C., Nisbet D.J. Comparative Effect of Thymol or Its Glucose Conjugate, Thymol-β-D-Glucopyranoside, on Campylobacter in Avian Gut Contents. J. Environ. Sci. Health B. 2015;50:55–61. doi: 10.1080/03601234.2015.965634. [DOI] [PubMed] [Google Scholar]
- 132.Kurekci C., Al Jassim R., Hassan E., Bishop-Hurley S.L., Padmanabha J., McSweeney C.S. Effects of Feeding Plant-Derived Agents on the Colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2014;93:2337–2346. doi: 10.3382/ps.2014-03950. [DOI] [PubMed] [Google Scholar]
- 133.Arsi K., Donoghue A.M., Venkitanarayanan K., Kollanoor-Johny A., Fanatico A.C., Blore P.J., Donoghue D.J. The Efficacy of the Natural Plant Extracts, Thymol and Carvacrol against Campylobacter Colonization in Broiler Chickens. J. Food Saf. 2014;34:321–325. doi: 10.1111/jfs.12129. [DOI] [Google Scholar]
- 134.Kurekci C., Padmanabha J., Bishop-Hurley S.L., Hassan E., Al Jassim R.A.M., McSweeney C.S. Antimicrobial Activity of Essential Oils and Five Terpenoid Compounds against Campylobacter jejuni in Pure and Mixed Culture Experiments. Int. J. Food Microbiol. 2013;166:450–457. doi: 10.1016/j.ijfoodmicro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 135.Boulianne M., Uzal F.A., Opengart K. Diseases of Poultry. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2020. Clostridial Diseases; pp. 966–994. [Google Scholar]
- 136.M’Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the Control of Necrotic Enteritis in Broiler Chickens with In-Feed Antibiotics Phasing-out Worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Caly D.L., D’Inca R., Auclair E., Drider D. Alternatives to Antibiotics to Prevent Necrotic Enteritis in Broiler Chickens: A Microbiologist’s Perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in Poultry: An Emerging Threat for Animal and Public Health. Avian Pathol. J. WVPA. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- 139.Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 Improves Intestinal Health of Broiler Chickens Challenged with Clostridium perfringens-Induced Necrotic Enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- 140.Mongkolthanaruk W. Classification of Bacillus Beneficial Substances Related to Plants, Humans and Animals. J. Microbiol. Biotechnol. 2012;22:1597–1604. doi: 10.4014/jmb.1204.04013. [DOI] [PubMed] [Google Scholar]
- 141.Abudabos A. Bacillus subtilis PB6 Based-Probiotic (CloSTATTM) Improves Intestinal Morphological and Microbiological Status of Broiler Chickens under Clostridium perfringens Challenge. Int. J. Agric. Biol. 2013;15:978–982. [Google Scholar]
- 142.Ramlucken U., Ramchuran S.O., Moonsamy G., Lalloo R., Thantsha M.S., Jansen van Rensburg C. A Novel Bacillus Based Multi-Strain Probiotic Improves Growth Performance and Intestinal Properties of Clostridium perfringens Challenged Broilers. Poult. Sci. 2020;99:331–341. doi: 10.3382/ps/pez496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bortoluzzi C., Vieira B.S., Applegate T.J. Influence of Dietary Zinc, Copper, and Manganese on the Intestinal Health of Broilers under Eimeria Challenge. Front. Vet. Sci. 2020;7:13. doi: 10.3389/fvets.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hussein E.O.S., Ahmed S.H., Abudabos A.M., Suliman G.M., Abd El-Hack M.E., Swelum A.A., Alowaimer A.N. Ameliorative Effects of Antibiotic-, Probiotic- and Phytobiotic-Supplemented Diets on the Performance, Intestinal Health, Carcass Traits, and Meat Quality of Clostridium perfringens-Infected Broilers. Animals. 2020;10:669. doi: 10.3390/ani10040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Layton S.L., Hernandez-Velasco X., Chaitanya S., Xavier J., Menconi A., Latorre J.D., Kallapura G., Kuttappan V.A., Wolfenden R.E., Andreatti Filho R.L., et al. The Effect of a Lactobacillus-Based Probiotic for the Control of Necrotic Enteritis in Broilers. Food Nutr. Sci. 2013;4:1–7. [Google Scholar]
- 146.Guo S., Liu D., Zhang B., Li Z., Li Y., Ding B., Guo Y. Two Lactobacillus Species Inhibit the Growth and α-Toxin Production of Clostridium perfringens and Induced Proinflammatory Factors in Chicken Intestinal Epithelial Cells in Vitro. Front. Microbiol. 2017;8:2081. doi: 10.3389/fmicb.2017.02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qing X., Zeng D., Wang H., Ni X., Liu L., Lai J., Khalique A., Pan K., Jing B. Preventing Subclinical Necrotic Enteritis through Lactobacillus Johnsonii BS15 by Ameliorating Lipid Metabolism and Intestinal Microflora in Broiler Chickens. AMB Express. 2017;7:139. doi: 10.1186/s13568-017-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang H., Ni X., Liu L., Zeng D., Lai J., Qing X., Li G., Pan K., Jing B. Controlling of Growth Performance, Lipid Deposits and Fatty Acid Composition of Chicken Meat through a Probiotic, Lactobacillus Johnsonii during Subclinical Clostridium perfringens Infection. Lipids Health Dis. 2017;16:38. doi: 10.1186/s12944-017-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abudabos A.M., Yehia H.M. Effect of Dietary Mannan Oligosaccharide from Saccharomyces Cerevisiae on Live Performance of Broilers Under Clostridium perfringens Challenge. Ital. J. Anim. Sci. 2013;12:e38. doi: 10.4081/ijas.2013.e38. [DOI] [Google Scholar]
- 150.M’Sadeq S.A., Wu S.-B., Choct M., Forder R., Swick R.A. Use of Yeast Cell Wall Extract as a Tool to Reduce the Impact of Necrotic Enteritis in Broilers. Poult. Sci. 2015;94:898–905. doi: 10.3382/ps/pev035. [DOI] [PubMed] [Google Scholar]
- 151.Abdelli N., Pérez J.F., Vilarrasa E., Cabeza Luna I., Melo-Duran D., D’Angelo M., Solà-Oriol D. Targeted-Release Organic Acids and Essential Oils Improve Performance and Digestive Function in Broilers under a Necrotic Enteritis Challenge. Animals. 2020;10:259. doi: 10.3390/ani10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar A., Toghyani M., Kheravii S., Pineda L., Han Y., Swick R., Wu S.-B. Potential of Blended Organic Acids to Improve Performance and Health of Broilers Infected with Necrotic Enteritis. Anim. Nutr. 2021;7:440–449. doi: 10.1016/j.aninu.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pham V.H., Kan L., Huang J., Geng Y., Zhen W., Guo Y., Abbas W., Wang Z. Dietary Encapsulated Essential Oils and Organic Acids Mixture Improves Gut Health in Broiler Chickens Challenged with Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2020;11:18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pham V.H., Abbas W., Huang J., He Q., Zhen W., Guo Y., Wang Z. Effect of Blending Encapsulated Essential Oils and Organic Acids as an Antibiotic Growth Promoter Alternative on Growth Performance and Intestinal Health in Broilers with Necrotic Enteritis. Poult. Sci. 2022;101:101563. doi: 10.1016/j.psj.2021.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun Y., Ni A., Jiang Y., Li Y., Huang Z., Shi L., Xu H., Chen C., Li D., Han Y., et al. Effects of Replacing In-Feed Antibiotics with Synergistic Organic Acids on Growth Performance, Health, Carcass, and Immune and Oxidative Statuses of Broiler Chickens Under Clostridium perfringens Type A Challenge. Avian Dis. 2020;64:393–400. doi: 10.1637/aviandiseases-D-19-00101. [DOI] [PubMed] [Google Scholar]
- 156.Diaz Carrasco J.M., Redondo L.M., Redondo E.A., Dominguez J.E., Chacana A.P., Fernandez Miyakawa M.E. Use of Plant Extracts as an Effective Manner to Control Clostridium perfringens Induced Necrotic Enteritis in Poultry. BioMed Res. Int. 2016;2016:3278359. doi: 10.1155/2016/3278359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Du E., Guo Y. Dietary Supplementation of Essential Oils and Lysozyme Reduces Mortality and Improves Intestinal Integrity of Broiler Chickens with Necrotic Enteritis. Anim. Sci. J. Nihon Chikusan Gakkaiho. 2021;92:e13499. doi: 10.1111/asj.13499. [DOI] [PubMed] [Google Scholar]
- 158.Elizondo A.M., Mercado E.C., Rabinovitz B.C., Fernandez-Miyakawa M.E. Effect of Tannins on the in Vitro Growth of Clostridium perfringens. Vet. Microbiol. 2010;145:308–314. doi: 10.1016/j.vetmic.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 159.Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of Dietary Polyphenol-Rich Grape Products on Intestinal Microflora and Gut Morphology in Broiler Chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- 160.Tosi G., Massi P., Antongiovanni M., Buccioni A., Minieri S., Marenchino L., Mele M. Efficacy Test of a Hydrolysable Tannin Extract Against Necrotic Enteritis in Challenged Broiler Chickens. Ital. J. Anim. Sci. 2013;12:e62. doi: 10.4081/ijas.2013.e62. [DOI] [Google Scholar]
- 161.Kang M., Oh J.-Y., Cha S.-Y., Kim W.-I., Cho H.-S., Jang H.-K. Efficacy of Polymers from Spontaneous Carotenoid Oxidation in Reducing Necrotic Enteritis in Broilers. Poult. Sci. 2018;97:3058–3062. doi: 10.3382/ps/pey180. [DOI] [PubMed] [Google Scholar]
- 162.McDougald L.R., Cervantes H.M., Jenkins M.C., Hess M., Beckstead R. Diseases of Poultry. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2020. Protozoal Infections; pp. 1192–1254. [Google Scholar]
- 163.Williams R.B. Intercurrent Coccidiosis and Necrotic Enteritis of Chickens: Rational, Integrated Disease Management by Maintenance of Gut Integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- 164.Madlala T., Okpeku M., Adeleke M.A. Understanding the Interactions between Eimeria Infection and Gut Microbiota, towards the Control of Chicken Coccidiosis: A Review. Parasite. 2021;28:48. doi: 10.1051/parasite/2021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mohsin M., Abbas R.Z., Yin G., Sindhu Z.-U.-D., Abbas A., Huang Z., Aleem M.T., Saeed Z., Afzal M.Z., Ejaz A. Probiotics as Therapeutic, Antioxidant and Immunomodulatory Agents against Poultry Coccidiosis. Worlds Poult. Sci. J. 2021;77:331–345. doi: 10.1080/00439339.2021.1883412. [DOI] [Google Scholar]
- 166.Abdelrahman W., Mohnl M., Teichmann K., Doupovec B., Schatzmayr G., Lumpkins B., Mathis G. Comparative Evaluation of Probiotic and Salinomycin Effects on Performance and Coccidiosis Control in Broiler Chickens. Poult. Sci. 2014;93:3002–3008. doi: 10.3382/ps.2014-04212. [DOI] [PubMed] [Google Scholar]
- 167.Ritzi M.M., Abdelrahman W., van-Heerden K., Mohnl M., Barrett N.W., Dalloul R.A. Combination of Probiotics and Coccidiosis Vaccine Enhances Protection against an Eimeria Challenge. Vet. Res. 2016;47:111. doi: 10.1186/s13567-016-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Erdoğmuş S.Z., Gülmez N., Findik A., Hüseyin Ş., Gülmez M. Efficacy of Probiotics on Health Status and Growth Performance of Eimeria Tenella Infected Broiler Chickens. Kafkas Üniversitesi Vet. Fakültesi Derg. 2019;25:311–320. [Google Scholar]
- 169.Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. Effects of in Ovo Supplementation of Probiotics on Performance and Immunocompetence of Broiler Chicks to an Eimeria Challenge. Benef. Microbes. 2016;7:699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- 170.Stringfellow K., Caldwell D., Lee J., Mohnl M., Beltran R., Schatzmayr G., Fitz-Coy S., Broussard C., Farnell M. Evaluation of Probiotic Administration on the Immune Response of Coccidiosis-Vaccinated Broilers. Poult. Sci. 2011;90:1652–1658. doi: 10.3382/ps.2010-01026. [DOI] [PubMed] [Google Scholar]
- 171.Behnamifar A.R., Rahimi S., Kiaei M.M., Fayazi H. Comparison of the Effect of Probiotic, Prebiotic, Salinomycin and Vaccine in Control of Coccidiosis in Broiler Chickens. Iran. J. Vet. Res. 2019;20:51. [PMC free article] [PubMed] [Google Scholar]
- 172.Wang X., Peebles E.D., Kiess A.S., Wamsley K.G.S., Zhai W. Effects of Coccidial Vaccination and Dietary Antimicrobial Alternatives on the Growth Performance, Internal Organ Development, and Intestinal Morphology of Eimeria-Challenged Male Broilers. Poult. Sci. 2019;98:2054–2065. doi: 10.3382/ps/pey552. [DOI] [PubMed] [Google Scholar]
- 173.Chand N., Faheem H., Khan R.U., Qureshi M.S., Alhidary I.A., Abudabos A.M. Anticoccidial Effect of Mananoligosacharide against Experimentally Induced Coccidiosis in Broiler. Environ. Sci. Pollut. Res. Int. 2016;23:14414–14421. doi: 10.1007/s11356-016-6600-x. [DOI] [PubMed] [Google Scholar]
- 174.Khater H.F., Ziam H., Abbas A., Abbas R.Z., Raza M.A., Hussain K., Younis E.Z., Radwan I.T., Selim A. Avian Coccidiosis: Recent Advances in Alternative Control Strategies and Vaccine Development. Agrobiol. Rec. 2020;1:11–25. doi: 10.47278/journal.abr/2020.004. [DOI] [Google Scholar]
- 175.Abbas R.Z., Munawar S.H., Manzoor Z., Iqbal Z., Khan M.N., Saleemi M.K., Zia M.A., Yousaf A. Anticoccidial Effects of Acetic Acid on Performance and Pathogenic Parameters in Broiler Chickens Challenged with Eimeria Tenella. Pesqui. Veterinária Bras. 2011;31:99–103. doi: 10.1590/S0100-736X2011000200001. [DOI] [Google Scholar]
- 176.Fortuoso B.F., Dos Reis J.H., Gebert R.R., Barreta M., Griss L.G., Casagrande R.A., de Cristo T.G., Santiani F., Campigotto G., Rampazzo L. Glycerol Monolaurate in the Diet of Broiler Chickens Replacing Conventional Antimicrobials: Impact on Health, Performance and Meat Quality. Microb. Pathog. 2019;129:161–167. doi: 10.1016/j.micpath.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 177.Ali A.M., Seddiek S.A., Khater H.F. Effect of Butyrate, Clopidol and Their Combination on the Performance of Broilers Infected with Eimeria maxima. Br. Poult. Sci. 2014;55:474–482. doi: 10.1080/00071668.2014.920488. [DOI] [PubMed] [Google Scholar]
- 178.Aristimunha P.C., Rosa A.P., Boemo L.S., Garcez D.C., Rosa D.P., Londero A., Scher A., Forgiarini J. A Blend of Benzoic Acid and Essential Oil Compounds as an Alternative to Antibiotic Growth Promoters in Broiler Diets. J. Appl. Poult. Res. 2016;25:455–463. doi: 10.3382/japr/pfw015. [DOI] [Google Scholar]
- 179.El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022;101:101542. doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Georgieva N.V., Gabrashanska M., Koinarski V., Ermidou-Pollet S. Antioxidant Status in Eimeria Acervulina Infected Chickens after Dietary Selenium Treatment. Trace Elem. Electrolytes. 2011;28:42. doi: 10.5414/TEP28042. [DOI] [Google Scholar]
- 181.Georgieva N.V., Gabrashanska M., Koinarski V., Yaneva Z. Zinc Supplementation against Eimeria Acervulina-Induced Oxidative Damage in Broiler Chickens. Vet. Med. Int. 2011;2011:647124. doi: 10.4061/2011/647124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Galli G.M., Gerbet R.R., Griss L.G., Fortuoso B.F., Petrolli T.G., Boiago M.M., Souza C.F., Baldissera M.D., Mesadri J., Wagner R. Combination of Herbal Components (Curcumin, Carvacrol, Thymol, Cinnamaldehyde) in Broiler Chicken Feed: Impacts on Response Parameters, Performance, Fatty Acid Profiles, Meat Quality and Control of Coccidia and Bacteria. Microb. Pathog. 2020;139:103916. doi: 10.1016/j.micpath.2019.103916. [DOI] [PubMed] [Google Scholar]
- 183.Perez-Carbajal C., Caldwell D., Farnell M., Stringfellow K., Pohl S., Casco G., Pro-Martinez A., Ruiz-Feria C.A. Immune Response of Broiler Chickens Fed Different Levels of Arginine and Vitamin E to a Coccidiosis Vaccine and Eimeria Challenge. Poult. Sci. 2010;89:1870–1877. doi: 10.3382/ps.2010-00753. [DOI] [PubMed] [Google Scholar]
- 184.Khan R.U., Naz S., Javdani M., Nikousefat Z., Selvaggi M., Tufarelli V., Laudadio V. The Use of Turmeric (Curcuma longa) in Poultry Feed. Worlds Poult. Sci. J. 2012;68:97–103. doi: 10.1017/S0043933912000104. [DOI] [Google Scholar]
- 185.Sharma J.M., Burmester B.R. Disease Control in Avian Species by Embryonal Vaccination. 4,458,630. U.S. Patent. 1984 July 10;
- 186.Peebles E.D. In Ovo Applications in Poultry: A Review. Poult. Sci. 2018;97:2322–2338. doi: 10.3382/ps/pey081. [DOI] [PubMed] [Google Scholar]
- 187.Uni Z., Ferket P.R. Enhancement of Development of Oviparous Species by in Ovo Feeding. 6,592,878 B2. U.S. Patent. 2003 July 15;
- 188.Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early Nutrition Programming (in Ovo and Post-Hatch Feeding) as a Strategy to Modulate Gut Health of Poultry. Front. Vet. Sci. 2019;6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tako E., Glahn R.P., Knez M., Stangoulis J.C. The Effect of Wheat Prebiotics on the Gut Bacterial Population and Iron Status of Iron Deficient Broiler Chickens. Nutr. J. 2014;13:1–10. doi: 10.1186/1475-2891-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De Oliveira J.E., Van der Hoeven-Hangoor E., Van de Linde I.B., Montijn R.C., Van der Vossen J. In Ovo Inoculation of Chicken Embryos with Probiotic Bacteria and Its Effect on Posthatch Salmonella Susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- 191.Madej J.P., Stefaniak T., Bednarczyk M. Effect of in Ovo-Delivered Prebiotics and Synbiotics on Lymphoid-Organs’ Morphology in Chickens. Poult. Sci. 2015;94:1209–1219. doi: 10.3382/ps/pev076. [DOI] [PubMed] [Google Scholar]
- 192.Nurmi E., Rantala M. New Aspects of Salmonella Infection in Broiler Production. Nature. 1973;241:210–211. doi: 10.1038/241210a0. [DOI] [PubMed] [Google Scholar]
- 193.Hosseini-Mansoub N., Vahdatpour T., Arjomandi M., Vahdatpour S. Comparison of Different Methods of Probiotic Prescription against Salmonella Infection in Hatchery Broiler Chickens. Adv. Envrion. Biol. 2011;5:1857–1860. [Google Scholar]
- 194.Teague K.D., Graham L.E., Dunn J.R., Cheng H.H., Anthony N., Latorre J.D., Menconi A., Wolfenden R.E., Wolfenden A.D., Mahaffey B.D. In Ovo Evaluation of FloraMax®-B11 on Marek’s Disease HVT Vaccine Protective Efficacy, Hatchability, Microbiota Composition, Morphometric Analysis, and Salmonella Enteritidis Infection in Broiler Chickens. Poult. Sci. 2017;96:2074–2082. doi: 10.3382/ps/pew494. [DOI] [PubMed] [Google Scholar]
- 195.Silva I.G.O., Vellano I.H.B., Moraes A.C., Lee I.M., Alvarenga B., Milbradt E.L., Hataka A., Okamoto A.S., Andreatti R.L. Evaluation of a Probiotic and a Competitive Exclusion Product Inoculated in Ovo on Broiler Chickens Challenged with Salmonella Heidelberg. Braz. J. Poult. Sci. 2017;19:19–26. doi: 10.1590/1806-9061-2016-0409. [DOI] [Google Scholar]
- 196.Badri F.B.A., Amin S.A. Influence of in ovo inoculation of bifidobacteria on productive performance, antioxidant and immune status and gut microfloraof broiler chickens. Egypt. J. Nutr. Feed. 2017;20:267–276. doi: 10.21608/ejnf.2017.75184. [DOI] [Google Scholar]
- 197.Lee S.H., Lillehoj H.S., Jang S.I., Jeong M.S., Xu S.Z., Kim J.B., Park H.J., Kim H.R., Lillehoj E.P., Bravo D.M. Effects of in Ovo Injection with Selenium on Immune and Antioxidant Responses during Experimental Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2014;93:1113–1121. doi: 10.3382/ps.2013-03770. [DOI] [PubMed] [Google Scholar]
- 198.Stadnicka K., Bogucka J., Stanek M., Graczyk R., Krajewski K., Maiorano G., Bednarczyk M. Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens. Animals. 2020;10:592. doi: 10.3390/ani10040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Angwech H., Tavaniello S., Ongwech A., Kaaya A.N., Maiorano G. Efficacy of In Ovo Delivered Prebiotics on Growth Performance, Meat Quality and Gut Health of Kuroiler Chickens in the Face of a Natural Coccidiosis Challenge. Animals. 2019;9:876. doi: 10.3390/ani9110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fatemi S.A., Elliott K.E.C., Bello A., Peebles E.D. Effects of the in Ovo Injection of Vitamin D3 and 25-Hydroxyvitamin D3 in Ross 708 Broilers Subsequently Challenged with Coccidiosis. I. Performance, Meat Yield and Intestinal Lesion Incidence1,2,3. Poult. Sci. 2021;100:101382. doi: 10.1016/j.psj.2021.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.