Abstract
Simple Summary
Young, Mexican women are more susceptible to breast cancer compared to other populations. However, studies on germline young, Mexican women are scarce and limited to a panel of 143 genes. This is partially due to the lack of gene annotation tools and difficulties in determining the causal genes in understudied populations. Here, we used whole exome sequencing combined with a powerful annotation tool to analyze 862 cancer genes in 115 young, Mexican women diagnosed with breast cancer. Our results showed well-known genes and many barely reported variants in our population. Moreover, we were able to assign candidate causal genes to 34% of patients, surpassing previous studies. These results suggest that deeper bioinformatic analyses could inform medical decisions to improve diagnosis, treatment, and life expectancy in young women with breast cancer.
Abstract
Breast cancer (BC) is one of the most frequent cancer types in women worldwide. About 7% is diagnosed in young women (YBC) less than 40 years old. In Mexico, however, YBC reaches 15% suggesting a higher genetic susceptibility. There have been some reports of germline variants in YBC across the world. However, there is only one report from a Mexican population, which is not restricted by age and limited to a panel of 143 genes resulting in 15% of patients carrying putatively pathogenic variants. Nevertheless, expanding the analysis to whole exome involves using more complex tools to determine which genes and variants could be pathogenic. We used germline whole exome sequencing combined with the PeCanPie tool to analyze exome variants in 115 YBC patients. Our results showed that we were able to identify 49 high likely pathogenic variants involving 40 genes on 34% of patients. We noted many genes already reported in BC and YBC worldwide, such as BRCA1, BRCA2, ATM, CHEK2, PALB2, and POLQ, but also others not commonly reported in YBC in Latin America, such as CLTCL1, DDX3X, ERCC6, FANCE, and NFKBIE. We show further supporting and controversial evidence for some of these genes. We conclude that exome sequencing combined with robust annotation tools and further analysis, can identify more genes and more patients affected by germline mutations in cancer.
Keywords: breast cancer, variant annotation, variant pathogenicity, cancer predisposition, bioinformatic pipeline, cancer genes
1. Introduction
Breast cancer (BC) is one of the most frequent types of cancer among women; 2.3 million cases were reported in 2020, causing more than 680,000 deaths globally [1]. The BC incidence increases with age. The majority of the diagnosed patients are 40 years and older, reaching 89%: 4% between 39 and 35, 5% less than 35, and 1.75% in women under 30 (https://gco.iarc.fr/ (accessed on 1 December 2021), [1]). In Mexico, BC is the first cause of death among women diagnosed with a malignant neoplasia [1], with an estimate of 26% of cancer-related deaths. Notably, the incidence of breast cancer in young women (YBC) in Mexico reached 15% in 2020, which is considerably higher than worldwide [1,2].
It has been observed that clinical outcomes and tumor biology in young patients are different from older women [3,4]. Tumors in young women are more likely to be of higher histological grade and are usually classified as estrogen receptor (ER) and progesterone negative receptors [5]. These patients typically present local recurrences to be diagnosed at an advanced stage and have inferior 5-year survival compared to the older premenopausal counterparts [5]. Moreover, YBC tends to present a more aggressive subtype such as triple-negative or HER2+ [6].
In some populations, up to 24% of the hereditary BC is linked to germline mutations in BRCA1/2 specific genes [7], and the prevalence may reach 14% in patients not showing familial history [7]. Mutations in these genes have a lifetime risk of developing BC up to 65% [8]. Other genes with germline mutations had been reported to confer moderate cancer risk, such as ATM, CHECK2, and PALB2, which are also associated with other cancers [8,9,10].
In addition to BRCA1, BRCA2, ATM, CHEK2, and PALB2, germline mutations in Latin America have been reported for CDH1, NBN, NF1, TP53, MLH1, BRIP1, MSH2, MSH6, and PMS2 in populations from Chile, Brazil, Colombia, and México [11]. In Mexico, a study in hereditary breast and ovarian cancer, which used a panel of 143 genes, found 21 germline mutations in BRCA1 and BRCA2, while another 19 genes showed 1 or 2 mutations, including the FANC(I/B/C/L/M) gene family accounting for 6 mutations [12]. Importantly, the above study found only 15% of patients (46 of 300) showing a germline mutation in at least one of the 143 panel genes. The low detection rate highlights the need to interrogate more genes in the population known to carry familial susceptibility.
The sequencing of gene panels has been a cost-efficient tool to determine the prevalence of specific genes among cancer populations [13,14,15,16]. However, more genes need to be studied to determine pathogenicity in most patients, for example, by whole exome sequencing. One issue is that pathogenicity is challenging to assess in not well-known or reported genes without further functional assays. Fortunately, extensive sequencing efforts, such as the 1000 genomes project [17] and the gnomAD (accessed on 1 December 2021) [18], provide databases of human variation among a relatively healthy population that help remove many common variants. In addition, other databases, such as ClinVar (accessed on 1 December 2021), help to mark pathogenic variants [19]. Still, massive sequencing generates several unseen variants increasing the need to explore methodologies to identify possible causal variants. In this context, Pediatric Cancer Variant Pathogenicity Information Exchange (PeCanPIE, accessed on 1 December 2021) is a web-based tool that integrates many sources of information supported by the guidelines from the American College of Medical Genetics and Genomics (ACMG) that are useful to identify interesting candidate variants quickly [20]. PeCanPIE uses a variant categorization for putative pathogenicity based on a “medal ceremony” concept of four levels. The Gold category is assigned for highly likely pathogenic truncating or splicing variants, whose genes are already found in pathogenic databases and whose variants are rare among healthy populations. Silver variants are in-frame, indels, and truncations in non-cancer genes or predicted to be damaging and matching pathogenic databases. Bronze variants are those whose effects are predicted to be tolerated. An unknown label is assigned otherwise.
To determine the possible etiology of BC in young women, in this study, we performed whole exome sequencing followed by the PeCanPie bioinformatic analysis of 115 young patients diagnosed with BC and focused on known 862 cancer genes where pathology has already been associated with diseases. Briefly, we obtained 49 variants classified as Gold resulting in more than 20 genes that have not been reported previously in LA BC. In addition, 106 variants were classified as Silver.
2. Materials and Methods
2.1. Patients
We included 115 patients from the National Cancer Institute of Mexico (INCAN, Instituto Nacional de Cancerología) diagnosed at 40 years old or younger with histopathological confirmation of BC. Patients were recruited between September 2015 and December 2017. Medical records and electronic files with detailed clinical and sociodemographic information were obtained from the INCAN. Trained nurses obtained blood samples after patients received genetic counseling and signed the informed consent.
2.2. Ethical Considerations
Regulatory approval was obtained from the INCAN Research and Ethics Committee (Approval ID CEI/1123/16). Genetic counseling and information about potential germline findings were provided to patients in addition to assurance of patient confidentiality and relevant information concerning the project, sample management, and DNA shipment for analysis to the National Cancer Institute (NCI), USA. Based on the recommendations provided by the American College of Medical Genetics, hereditary cancer diagnostic variants in their DNA were reported to the patients.
2.3. Sample and Panel Library Preparation for Sequencing
The DNA was extracted from peripheral leukocytes using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA), following the manufacturer’s instructions. The resulting DNA was purified using Agencourt AMPure XP reagent (Beckman Coulter Inc., Brea, CA, USA) following the manufacturer’s protocol. In addition, an adapter-ligated library was prepared using the KAPA HyperPlus Kit (KAPA Biosystems, Wilmington, MA, USA) with NEXTflexTM DNA Barcoded Adapters (Perkin Elmer Waltham, MA, USA), according to the KAPA-provided protocol.
2.4. Sequencing
The deep whole exome sequencing was performed at the Laboratory of Translational Genomics, Division of Cancer Epidemiology and Genetics (NCI, Rockville, MD, USA). The GRCh37 (hg19) genome assembly was used for genome mapping reference. The DNA sequencing was performed using the Illumina HiSeq 2000 sequencer for 2 × 100-pb paired-end cartridge (Illumina, San Diego, CA, USA). The sequencing included regulatory, splicing, and 3′ and 5′ UTR regions.
2.5. Variant Calling
The reads were aligned using Novoalign software (v3.00.05, Novocraft Technologies Sdn Bhd, Petaling Jaya Selangor, Malaysia). Duplicate reads were removed using MarkDuplicates from Picard Software (v1.126, Broad Institute, Cambridge, MA, USA). For variant calling, the pipeline involved RealignerTargetCreator, IndelReligner, BaseRecalibrator, UnifiedGenotyper, and HaplotyperCaller tools from GATK (v4.1.3.0, Broad Institute, Cambridge, MA, USA). Variants that failed to pass the pipeline control metrics (CScorefilter) had a read depth minor to 10, ABHet (reference to alternate reads ratio) <0.2 or >0.8 were excluded for the analysis. In addition, all variants were filtered using popmaxfreq <0.01.
Before variant categorization, variants were filtered to remove those that do not show at least 10 reads in the alternate allele or that the minor allelic fraction was lower than 0.25.
2.6. Variant Categorization
The obtained variant calling files were analyzed using PeCanPIE, which classified the variants into three categories: Gold, Silver, and Bronze [20]. The variants were assigned as Gold if it is a truncating variant, including splicing, or a loss-of-function associated with a reported disease documented in ClinVar with at least two starts, and at least one of the following databases: IARC: Tp53, ASU: TERT, ARUP: Ret, BIC, PCFP, and COSMIC. The variants assigned as Silver are considered as in-frame indels, truncation events in non-tumor suppressor genes. Variants had to be reported in at least one of the following databases: UMD, LOVD, RB1, or ALSoD [20]. The variant is assigned as Bronze if predicted to be tolerant by in silico algorithms.
For PeCanPie, we used the union of their internal set of cancer predisposition genes and the Cancer Gene Census v94 from COSMIC [21] containing 712 and 723 genes, respectively, generating a list of 862 genes.
Only gold and silver variants were considered and studied separately for further analysis and filtering. The variants with fewer than 20 total reads or variant allele frequency (VAF) less than 0.25 were discarded for further analysis.
2.7. Statistical Analysis
We performed a descriptive analysis to calculate central tendency and dispersion measures for quantitative variables and absolute/relative frequencies for categorical variables. We also constructed logistic regression models to analyze the predictors of Gold variants. The final model included age < 30 years at diagnosis, family history of cancer, immunohistochemical subtype, advanced stage (IIB–IV), and continuous BMI. To evaluate disease-free and overall survival, we performed a Kaplan–Meier analysis, stratified by Gold variant, and considered time (in months) from diagnosis to the date of first recurrence or death. To evaluate differences by mutational status, we performed a Log-rank test. For all tests, we assumed a p-value < 0.05 to determine statistical significance. We used STATA 14® software to perform all statistical analysis tests.
3. Results
3.1. Clinical Population
A summary of our cohort characteristics, including 115 patients, is shown in Table 1 (raw data in Table S1). The median age was 33.9 years at diagnosis, and 18.3% were younger than 30. Patients diagnosed at advanced clinical stages (IIB–IV) corresponded to 65% of the cohort. Ductal histology (85%) and Luminal-B (50%) subtype were the most common in this group. A family history of cancer was present in 32.2% of these young women. All participants were premenopausal. The mean age at menarche was 12.34 years, 28.7% of participants were nulliparous, and the average number of children among parous was 2.1. Breastfeeding was practiced in 68.7% of parous women. Overweight and obesity was a condition present in 61.7% of the participants.
Table 1.
Clinical data of 115 BC young patients.
| Clinical Data | n | Frequency (%) | |
|---|---|---|---|
| Patients | n | 115 | |
| Age | Mean | 33.9 | |
| Interquartile range | 31–38 | ||
| Age at menarche | Mean | 12.34 | |
| Interquartile range | 11–13 | ||
| Parity | Nulliparous | 33 | 28.7 |
| 1 child | 27 | 23.2 | |
| 2 children | 32 | 23.5 | |
| >3 children | 23 | 20.0 | |
| Breastfeeding | No | 37 | 32.2 |
| Yes | 78 | 67.8 | |
| Luminal B (Her2-positive) | 19 | 13.2 | |
| Luminal B (Her2-negative) | 31 | 28.3 | |
| Her2-positive (non-luminal) | 8 | 6.6 | |
| Triple Negative | 30 | 28.3 | |
| Histology | Ductal | 98 | 85.2 |
| Lobular | 6 | 5.2 | |
| Mixed | 9 | 7.8 | |
| Others | 2 | 1.7 | |
| Clinical Stage | I | 15 | 13.0 |
| II | 45 | 39.1 | |
| III | 49 | 42.6 | |
| IV | 6 | 5.2 | |
| Consumption of Hormonal contraceptives | No | 50 | 43.5 |
| Yes | 65 | 56.55 | |
| First-grade family history of cancer | No | 90 | 78.3 |
| Yes | 25 | 21.7 | |
| Second-grade family history of cancer | No | 78 | 67.8 |
| Yes | 37 | 32.2 | |
| Body Mass Index | Normal (BMI < 25) | 44 | 38.3 |
| Overweight | 45 | 39.1 | |
| Obesity (BMI > 30) | 26 | 22.6 |
3.2. Variant Categorization
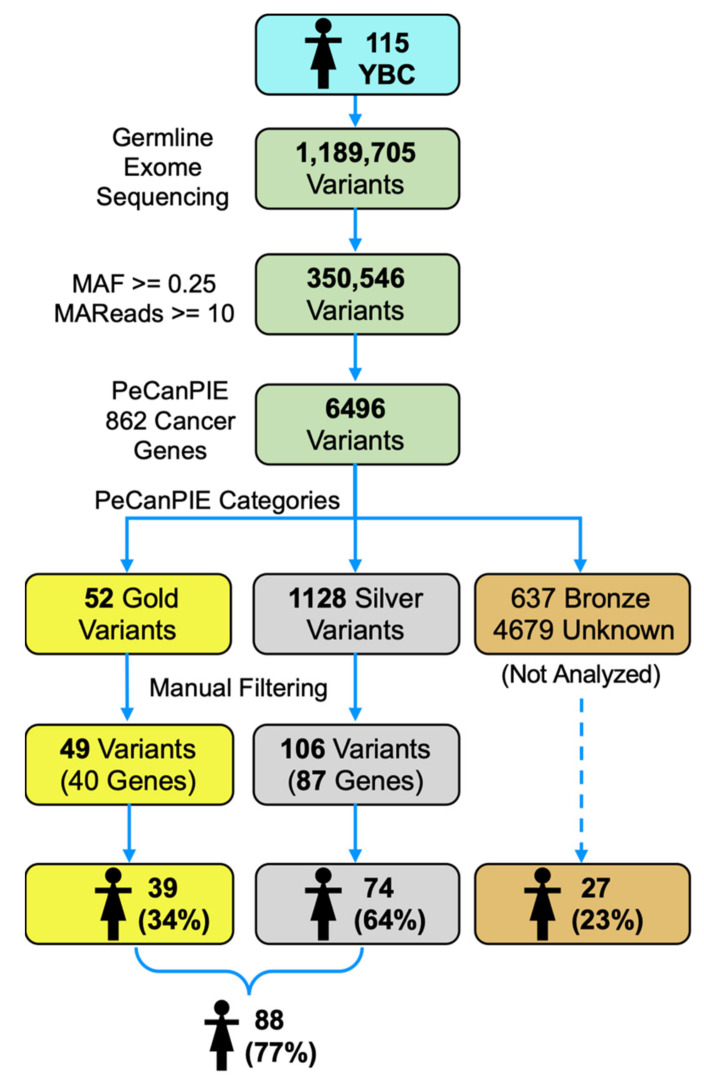
From the 1,189,705 raw variants, quality filtering generated 350,546 variants used for PeCanPIE annotation. PeCanPIE detected 6496 variants within the 862 cancer genes selected. After further filtering (for the number of reads described in the Methods section), 49 were categorized as Gold and 106 as Silver, corresponding to 40 and 87 genes, respectively (Figure 1). Only 39 patients (34%) showed high confidence Gold variants, while 74 (64%) showed Silver variants. Overall, 88 patients showed Gold or Silver variants.
Figure 1.
The diagram summarizes all the obtained variants with their exclusion criteria. YBC young Mexican breast cancer patients.
3.3. High Confident Germline Variants in Cancer Genes
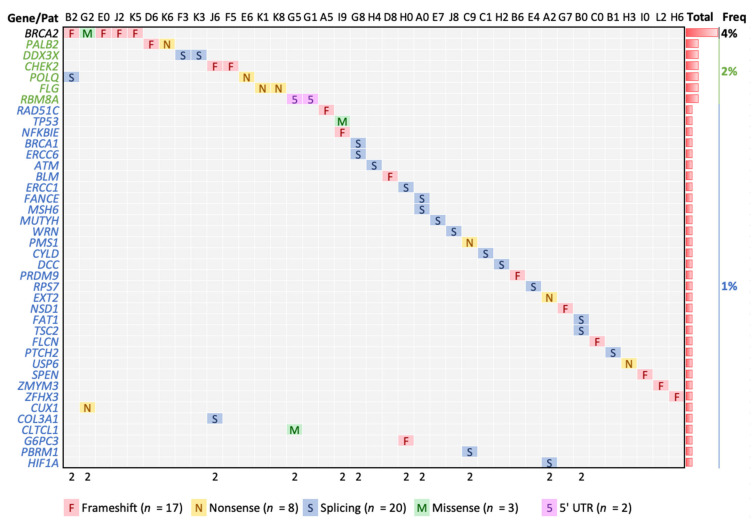
The criteria for Gold variants involve known pathogenic variants, a strong alteration variant (nonsense, frameshift, truncation) in a known pathogenic gene, and low allele frequency in public non-cancer databases [20]. From the 52 Gold variants obtained before manual filtering, two were removed (chr19, positions 34945343 and 34945354, UBA2 gene) because six patients showed alternative and varied genotypes at those positions, always with fewer reads in the alternate genotype suggesting mapping and sequencing artifacts. Additionally, one variant in (chr16, position 72991715, ZFHX3 gene) was also removed because it was present in all patients suggesting a common variant. Thus, 49 variants involving 40 genes and 39 patients were finally designated as Gold (Figure 2 and Table 2). All variants were heterozygous. Except for one variant present in two patients (RBM8A), all Gold variants were observed only in one patient. Of these variants, we noted 20 splicing, 17 frameshift, eight nonsense, three missense, and one ‘5-UTR. Most patients showed only one Gold variant, but 11 patients of 39 (28%) showed two. The well-known BRCA2 gene was the most recurrently altered, observed in five patients, showing four frameshifts and one missense. The following most frequent alterations were observed in CHEK2, PALB2, POLQ, DDX3X, and FLG affecting two patients. From these genes observed in two or more patients, the variants observed in BRCA2, PALB2, and CHEK2 were also found in ClinVar with documented association to BC (Table S2). However, variants in POLQ, DDX3X, FLG, and RBM8A are barely reported in BC and will be further described.
Figure 2.
Gold variants detected. The top seven genes show two or more patients affected (black and green), while the rest show one patient only (blue). The 11 patients (in columns) marked with “2” show two Gold variants.
Table 2.
Gold variants.
| Chr | Position | Ref * | Alt * | Depths | Gene | LA + | Type | AA Chg | Pat | pLI | AF Lat § | ProtSize |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 108175579 | G | A | 21,19 | ATM | Splice | E1892_E37 | H4 | 0 | 1 × 10−4 | 3056 | |
| 15 | 91341429 | A | AG | 15,25 | BLM | Frameshift | T1074fs | D8 | 0 | - | 1417 | |
| 17 | 41258472 | C | A | 54,35 | BRCA1 | Splice | R71_E4 | G8 | 0 | 3 × 10−5 | 1863 | |
| 13 | 32914033 | CA | C | 18,20 | BRCA2 | Frameshift | S1848fs | E0 | 0 | 3 × 10−5 | 3418 | |
| 13 | 32914122 | AC | A | 52,30 | BRCA2 | Frameshift | N1877fs | J2 | 0 | 3 × 10−5 | 3418 | |
| 13 | 32930653 | C | CA | 15,17 | BRCA2 | Frameshift | S2509fs | B2 | 0 | - | 3418 | |
| 13 | 32937507 | A | G | 44,30 | BRCA2 | Missense | D2723G | G2 | 0 | A:C 0 | 3418 | |
| 13 | 32954260 | CG | C | 23,17 | BRCA2 | Frameshift | V3079fs | K5 | 0 | 2 × 10−4 | 3418 | |
| 22 | 29091718 | TA | T | 29,23 | CHEK2 | Frameshift | L413fs | J6 | 0 | - | 543 | |
| 22 | 29115401 | A | ATGAT | 28,16 | CHEK2 | Frameshift | M222fs | F5 | 0 | 1 × 10−4 | 543 | |
| 22 | 19222211 | C | T | 33,36 | CLTCL1 | No | Missense | E330K | G5 | 0 | 9 × 10−4 | 1640 |
| 2 | 189868190 | T | A | 35,26 | COL3A1 | No | Splice | P869P | J6 | 1 | 9 × 10−5 | 1466 |
| 7 | 101926377 | C | T | 57,41 | CUX1 | No | Nonsense | Q678 * | G2 | 1 | 3 × 10−3 | 678 |
| 16 | 50818362 | T | A | 30,39 | CYLD | No | Splice | I650_E13 | C1 | 1 | 3 × 10−5 | 956 |
| 18 | 50734187 | G | A | 28,23 | DCC | No | Splice | V621_E11 | H2 | 0.99 | 2 × 10−4 | 1447 |
| X | 41206109 | T | C | 46,39 | DDX3X | No | Splice | G539_E15 | K3 | 1 | 4 × 10−5 | 662 |
| X | 41206562 | T | C | 44,41 | DDX3X | No | Splice | S590_E16 | F3 | 1 | 2 × 10−3 | 662 |
| 19 | 45917294 | T | C | 21,22 | ERCC1 | Splice | V235_E7 | H0 | 0 | 6 × 10−5 | 297 | |
| 10 | 50680422 | C | T | 16,25 | ERCC6 | No | Splice | R975_E16 | G8 | 0 | C:A 3 × 10−5 | 1493 |
| 11 | 44228353 | G | A | 33,34 | EXT2 | No | Nonsense | W535 * | A2 | 0 | 1 × 10−4 | 718 |
| 6 | 35425330 | C | T | 25,34 | FANCE | No | Splice | D286_E3 | A0 | 0 | 3 × 10−4 | 536 |
| 4 | 187530955 | C | G | 34,21 | FAT1 | No | Splice | T3356T | B0 | 0 | 0 | 4588 |
| 17 | 17117000 | CG | C | 45,40 | FLCN | No | Frameshift | R570fs | C0 | 0.79 | C:T 3 × 10−5 | 579 |
| 1 | 152285000 | G | A | 46,43 | FLG | No | Nonsense | R788 * | K8 | 0 | 6 × 10−4 | 4061 |
| 1 | 152285861 | G | A | 45,34 | FLG | No | Nonsense | R501 * | K1 | 0 | 4 × 10−3 | 4061 |
| 17 | 42148542 | TC | T | 11,14 | G6PC3 | No | Frameshift | I70fs | H0 | 0 | 8 × 10−4 | 346 |
| 14 | 62203827 | G | A | 20,20 | HIF1A | No | Splice | D417_E9 | A2 | 0 | 2 × 10−4 | 826 |
| 2 | 48033791 | GT(26) | G | 15,12 | MSH6 | Splice | R1334_E9 | A0 | 0 | 1 × 10−4 & | 1360 | |
| 1 | 45797228 | C | T | 25,23 | MUTYH | Splice | G396_E13 | E7 | 0 | 3 × 10−3 | 546 | |
| 6 | 44233331 | G | GC | 21,12 | NFKBIE | No | Frameshift | A57fs | I9 | 0.77 | 3 × 10−4 | 500 |
| 5 | 176722446 | TC(6) | T | 27,25 | NSD1 | No | Frameshift | S2424fs | G7 | 1 | - | 2696 |
| 16 | 23641062 | CAG | C | 25,39 | PALB2 | Frameshift | S804fs | D6 | 0 | 1 × 10−4 | 1186 | |
| 16 | 23641139 | G | C | 24,36 | PALB2 | Nonsense | S779 * | K6 | 0 | 9 × 10−5 | 1186 | |
| 3 | 52620706 | TG | T | 15,12 | PBRM1 | No | Splice | E1017_E21 | C9 | 1 | 0 & | 1689 |
| 2 | 190728500 | C | T | 26,29 | PMS1 | No | Nonsense | R630 * | C9 | 0 | 1 × 10−4 | 932 |
| 3 | 121168273 | T | C | 19,22 | POLQ | Splice | I2385V | B2 | 0 | - | 2590 | |
| 3 | 121207489 | A | T | 20,15 | POLQ | Nonsense | L1430 * | E6 | 0 | 9 × 10−5 | 2590 | |
| 5 | 23527845 | CA | C | 31,42 | PRDM9 | No | Frameshift | T883fs | B6 | 0 | 6 × 10−5 | 894 |
| 1 | 45294985 | C | T | 14,13 | PTCH2 | No | Splice | L406_E10 | B1 | 0 | 3 × 10−5 | 1203 |
| 17 | 56774167 | C | CT | 47,59 | RAD51C | Frameshift | A173fs | A5 | 0 | - | 376 | |
| 1 | 145507646 | G | A | 15,27 | RBM8A | No | UTR_5 | E1_UTR_5 | G1 | 0.57 | 1 × 10−2 | 174 |
| 1 | 145507646 | G | A | 18,24 | RBM8A | No | UTR_5 | E1_UTR_5 | G5 | 0.57 | 1 × 10−2 | 174 |
| 2 | 3623181 | G | A | 51,58 | RPS7 | No | Splice | E4 | 0.95 | - | 194 | |
| 1 | 16262459 | G | GC(27) | 27,17 | SPEN | No | Frameshift | A3242fs | I0 | 1 | 9 × 10−4 & | 3664 |
| 17 | 7578406 | C | T | 22,29 | TP53 | Missense | R175H | I9 | 0.53 | 0 | 393 | |
| 16 | 2124201 | C | T | 44,31 | TSC2 | No | Splice | R786C | B0 | 1 | 0 | 1807 |
| 17 | 5074956 | T | A | 83,62 | USP6 | No | Nonsense | Y1343 * | H3 | 0 | 9 × 10−5 | 1406 |
| 8 | 31014882 | A | G | 13,13 | WRN | Splice | K1274_E33 | J8 | 0 | 1 × 10−4 | 1432 | |
| 16 | 72991713 | C | CC(9) | 20,14 | ZFHX3 | No | Frameshift | A778fs | H6 | 1 | 0 | 3703 |
| X | 70466308 | GTGGT | G | 28,11 | ZMYM3 | No | Frameshift | P821fs | L2 | 1 | - | 1370 |
* Numbers in parenthesis represent the total length. + Represent whether the gene has been reported in Latin-American BC patients in the Urbina-Lara et al. analysis [11]. § Allele frequency in Latino population from GnomAD website (https://gnomad.broadinstitute.org/, accessed on 1 December 2021). Variants in GnomAD slightly different to those found are explicitly indicated or marked with &. A total of 50 variants is shown, 49 unique (RBM8A is present in two patients). Genomic positions correspond to hg19. Ref and Alt refer to reference and alternate alleles respectively. Depths refers to Ref and Alt alleles respectively. AA Chg refers to aminoacid change. Pat refers to patient. pLI refers to the probability of LoF intolerance. ProtSize refers to canonical transcript protein size in aminoacids. Bold genes mark those found more than once. AF Lat = Allele Frequency in Latin Americans.
DDX3X, located in Xp11.4, encodes for an RNA helicase linked to somatic mutations in medulloblastoma [22]. It is also X chromosome inactivated in ovarian cancer [23]. Germline mutations have been reported in female brain development and disability, whose variants were observed on the Helicase ATP-binding and the Helicase C-terminal domains [24,25]. The two observed T→C heterozygous variants in our cohort affect the exon-intron splicing sites located in the Helicase C-terminal region (G539 and S590) responsible for the interaction with the nuclear mRNA export receptor TAP [26]. Moreover, DDX3X plays a role in DNA damage response [27]. There is no evidence in the clinical record for mental disability in these patients. These pieces of evidence suggest that DDX3X is potentially a predisposing gene in young BC patients.
POLQ encodes a polymerase involved in DNA repair [28,29]. Germline mutations in BC have been reported mainly in non BRCA1/2 carriers [30,31]. In our data, we observed one splicing and one nonsense variant in I2385V and L1430*, respectively. Consistent to the above studies, these POLQ positive patients were not carriers of BRCA1/2 or TP53 variants.
FLG encodes for filaggrin that aggregates keratin intermediate filaments in the mammalian epidermis. FLG germline variants have been recently reported in around 16% of Taiwanese BC patients [28] and 17% of hepatocellular carcinomas from Thailand [29]. In addition, it has been found somatically mutated in 10% of ER + BC patients [30]. We observed two G→A nonsense mutations (R788* and R501*), each one affecting a single patient. One of these patients carrying a FLG germline variant had a second primary contralateral breast cancer (K1).
We observed a promoter variant in RBM8A affecting two patients. According to ClinVar (ID 30464), this recessive variant causes a decrease in gene expression [31], which is crucial when combined with another severe variant affecting patients with Radial aplasia-thrombocytopenia syndrome. RBM8A differential expression has been noted in several cancers types but observed more expressed in the tumor than in the normal tissue [32], which seems counterintuitive. The allelic fraction reported in gnomAD is 1.8%, similar to the 1.7% observed in our sample, suggesting that promoter variants may be random. Like reports in ClinVar, it may need to be combined with another unknown alteration. Overall, the evidence is unclear, suggesting that the promoter variant in RBM8A is a variant of unknown significance (VUS).
From the genes present in only one patient, 12 genes have strong support shown by their reports in ClinVar for pathogenic or likely pathogenic variants in ATM, BLM, BRCA1, CLTCL1, ERCC6, FANCE, G6PC3, MSH6, MUTYH, TP53, and TSC2. Moreover, some of these genes also show germline mutations in BC patients. For example, ERCC6 has also been reported in Brazilian YBC patients [33] and Lebanon familial BC [34]; BLM in Russian YBC patients [35] and USA patients [36]; TSC2 in Italian patients [37]; and ATM is also well known in BC [11].
The remaining Gold variants genes are, by definition in PeCanPIE, associated with diseases in databases; inquiringly, they do not show clear evidence of pathogenicity, specifically in ClinVar. All these variants carry protein truncations in genes where the loss of function (LoF) mutations is a known mechanism of disease (BLM, COL3A1, CUX1, CYLD, ERCC1, EXT2, FAT1, FLCN, NFKBIE, NSD1, PBRM1, PMS1, PRDM9, PTCH2, RAD51C, RPS7, USP6, WRN, and ZFHX3). Many of these genes already show evidence of variants in BC in other populations such as BLM in Slavic [38] and Brazilians [39]; ERCC1 in Brazilians [40]; PMS1 in men [41]; WRN in Latins [42], or in other cancers such as CYLD in head and neck [43]; EXT2 in osteochondromas and chondrosarcomas [44,45]; FAT1 in retinoblastomas [46]; NSD1 and PBRM1 in renal cell carcinoma [47,48]; PRDM9 in acute lymphoblastic leukemia [49]; PTCH2 in rhabdomyosarcoma [50]; RAD51C also in breast cancer [51]; RPS7 in hypocellular bone marrow failure [52]; SPEN, USP6, and ZFHX3 in pancreatic adenosquamous carcinoma [53]; TICAM1 in thyroid cancer [54]; ZFHX3 also in endometrial cancer [55]; and DCC in gallbladder cancer [56].
We also noted seven genes marked with “caution” in PeCanPIE showing the truncation close to the C-terminal (CUX1, FLCN, FLG, MSH6, NSD1, PRDM9, and USP6), questioning its functional effects in the cancer context. To provide additional support for these variants, we considered the probability of LoF intolerance (pLI) provided by gnomAD [18]. Natural selection purifies highly deleterious variants, therefore, genes showing fewer than expected LoF variants in a large healthy population are seen as highly LoF intolerant, proposing association to disease when observed in an individual. Thus, pLI close to 0.9 and up to 1 are a strong indicator of LoF intolerance as recommended by gnomAD. We noted pLI = 1 in NSD1, strongly suggesting some contribution to disease consistent with previous evidence [47]. We also noted pLI = 1 for CUX1, but the stop codon gain is shown at the last protein amino acid (Q678), marked in gnomAD as ‘low confidence loss of function’. In between, we observed pLI = 0.79 in FLCN and pLI = 0.77 for NFKBIE. Contrary, we noted pLI = 0 for PRDM9 and USP6; thus, although categorized as Gold, these variants are less likely to be pathogenic. We also noted pLI > 0.9 for COL3A1, DCC, DDX3X, PBRM1, RPS7, SPEN, TSC2, ZFHX3, and ZMYM3, in which a considerable proportion of the protein is altered by a frameshift, splice, or nonsense variant. We noted that one of the patients carrying TP53 and NFKBIE presented a second primary glioblastoma (I9).
We observed that the variant allelic fraction in Latino populations, is in general, low (Table 2), validating the PeCanPie filtering. Nevertheless, few variants in the order or few per a thousand (10−3) could indicate a random finding due to our sample size.
We explored associations between patients carrying Gold variants and those not, along with clinical co-variables. The adjusted model showed an association of Gold variants with first- and second-family history of cancer (OR 3.21; CI 95% 1.15–8.95) and age < 30 years (OR 3.74; CI 95% 1.20–11.70). None of the tumor subtypes was associated with carrying a Golden variant.
We also observed that an increase in one unit of continuous BMI raises the odds of detecting a Gold variant in young women with breast cancer (OR 1.19; CI 95% 1.07–1.34), suggesting that BMI might be a modifier in women with Gold germline variants, and might reduce the age of breast cancer presentation. This phenomenon has been described previously for BRCA1/2 carriers, but our results suggest BMI could be a modifier for other genes as well [57,58,59].
We noticed that women with a Gold variant were diagnosed in advanced stages: IIB-IV (OR 3.21; CI 95% 1.21–8.98), suggesting that a Gold variant might increase breast cancer aggressiveness. It has been described that BRCA1/2 carriers have a higher risk of lymph node involvement at diagnosis [60].
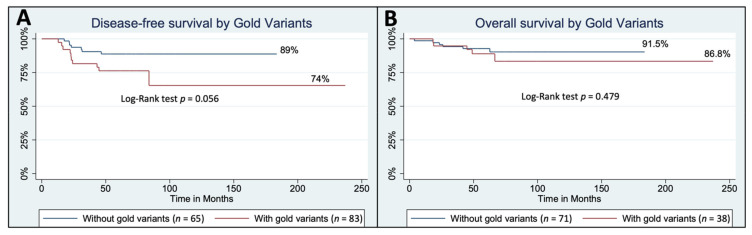
The Median follow-up of this cohort was 62 months (48–73). Although not significant at p < 0.05 using a Log-Rank test probably due to the small sample size, we found that disease-free survival could be higher in women without Gold variants (89%) versus women with Gold variants (74%) (Figure 3A) after adjusting for clinical prognostic factors. We observed fewer differences for overall survival (OS), with 91.5% of OS in women without carrying a Gold variant and 86.8% OS in women with a Gold variant (Log-Rank test p = 0.48, Figure 3B). These results are consistent with many previous studies involving hereditary breast cancer and survival [61,62].
Figure 3.
Survival analyses of YBC patients carrying Gold variants. (A) Disease free survival. (B) Overall survival.
3.4. Modest Confidence Germline Variants
The criteria for Silver are in-frame indels, truncation events in non-tumor suppressor genes but associated to diseases, variants predicted to be damaging by in silico algorithms, and matches to databases such as ClinVar with fewer than two stars, BRCA Share, ALSoD, LOVD, and a locus-specific database for APC, MSH2, and RB1. In addition, we filtered for the 862 genes associated with cancer. Under these conditions, PeCanPIE detected 1128 Silver variants. To further filter Silver variants to choose those more likely pathogenic, we reasoned that if a variant is the same as a somatic mutation found in a cancer patient, specifically in the tumor biopsy, it could indicate a higher degree of confidence. Thus, to further explore potential pathogenic variants, we only considered Silver variants categorized as Gold or Silver in the PeCanPIE somatic category. This additional category considers somatic databases such as COSMIC and PCGP [63].
Thus, 106 Silver variants were obtained distributed in 87 genes (Table S2). Of 115 patients, 74 (64%) presented one or more silver variants, all heterozygous. Of these, 18 were missense, four splice site, one frameshift, one nonsense, six protein deletions, eight protein insertions, and 78 splice regions. Of these genes, the most frequent were AKAP9 and ATM in four patients, followed by KMT2D, MGA, COL3A1, NCOR2, ERBB2, and MLLT3 in three patients each. We noted the following gene families: BRCA1/2, CDK 4/N2A, ERBB 2/3, ERCC 1/2/3, FANC A/D2/E/M, MRE 11/11A, NOTCH 1/2, and SMARC A4/B1/E1. Interestingly, we detected the same missense mutation in ERBB2 (R896H) in two patients. The gene position has been reported to activate HER2 function (R896C) [64], suggesting that R896H could affect normal function and potentially contribute to tumorigenesis.
4. Discussion
In the current work, we report an exome analysis of 115 young Mexican BC patients using the pipeline PeCanPIE focused on well-defined evidence of pathogenicity following ACMG guidelines. To our knowledge, this is the first effort for Mexican patients covering the germline whole exome. Previous efforts in Mexico and Latin America have focused on gene panels from 20 to 140 genes irrespective of the age of diagnosis [12,65]. Similar approaches showed a prevalence of 10.2% of pathogenic variants in BC in the USA [66]. Nevertheless, age at diagnosis is important because it may indicate an accelerated tumorigenesis process supported by recent reports showing an increase in BC incidence in young women [1,2,67,68,69,70]. Therefore, we focused on extreme phenotypes patients, where BC was diagnosed at 40 years old or less. We found that 39 patients (34%) showed a likely pathogenic Gold variant in 40 genes. This finding is higher than recent prevalence estimations in Latin American countries (13–25%) [42], which is likely due to our increased analysis in over the more than 800 genes by whole exome, and a higher genetic risk background of the younger population.
Comparing our Gold variants results with those of Quezada-Urban et al. in Mexican’s BC, where more than 53% were older than 40 years old and using a panel of 143 genes [12], only six genes were overlapping (BRCA1/2, ATM, WRN, RAD51C, and CHEK2). We did not find variants in 15 of the 21 genes reported (MSR1, ERCC3 [1 Silver], LIG4, PDE11A, ATR, FANC(I/B/C/L/M), RECQL4, SDHB, MLH1, NBN, and PTEN), and we found 36 other genes which were not present in the Quezada-Urban et al. study. Some genes, however, showed similar gene families, such as ERCC1/6 and FANCE. We also noted that many genes were not reported in BC for Latin-American countries (Table 2).
In our sample, we noted that 58% of the patients were overweight at the time of diagnosis, which is consistent with the 60% reported by Quezada-Urban et al. [12] and other reports in Mexico [2]. Weight loss in the young woman has been associated with lower cancer risk in BRCA1 carriers [71]. Thus, we explored possible associations of known variants (Gold) to BMI. We observed a small but significant increase in BMI among Gold variant carriers. These results should be confirmed in larger cohorts.
We noted many Gold variants in genes not previously reported in Latin American cohorts but reported in BC in the USA, Europe, Asia, or other cancer types and gene families. For instance, Fanconi anemia and excision repair genes (FANC and ERCC genes) have been reported in Latin American BC cases [11]. These findings highlight the use of a broader set of genes combined with powerful analysis tools, to expand the results.
PeCanPIE uses a pipeline considering the observed variant frequency among ‘healthy’ populations deposited in databases such as ExAC, which is primarily based on Caucasian populations [72]. In the Silver category, we noted few variants present in many individuals in our cohort even with low allelic fraction in ExAC, confirming that estimations of Latin America variations are needed to identify common variants in this population. Although PeCanPIE was initially conceived for pediatric cancers, they included several gene databases from other cancers reaching 712 genes. To complement this, we added 723 genes from COSMIC v94. Thus, our analysis was limited to the unified 862 genes from these two sources. If we extend the analysis to the whole exome and focus on Gold variants, besides the two common variants that would need to be removed, six genes are added (C8B, DMD, HBB, IRF8, KCNQ1, and MYBPC3, of which three are splices, two frameshifts, and one nonsense). Nevertheless, these were not considered in our analysis, since we focus on more likely genes with a stronger background in the cancer context.
We focused on Gold variants because pathogenicity is theoretically the highest provided by PeCanPIE. Nevertheless, Silver variants may also show interesting results, such as that mentioned for HER2 (ERBB2 R896H) and other gene families such as CDK, BRCA1/2, ERBB, ERCC, and FANC. However, more careful revision is needed for Silver variants. For example, we noted a missense mutation in five patients in WRN (R834C) that has been shown to abolish important WRN function [73]. Although the variant was filtered out because of quality criteria (fewer than 10 reads), this polymorphism is frequent in the Mexican and is also unlikely to be pathogenic [74]. Thus, Silver variants should be handled more thoroughly. This evidence also highlights that annotation tools are crucial to facilitate interpretation but that results must be revised, and tools should be continuously updated.
Overall, our study provides candidate pathogenic variants in Mexican YBC, a barely studied population. Some variants need more careful analyses; for example, those regarding splice site variants and those in RBM8A. In addition, recent evidence rises questions even for well-known breast cancer genes [75]. Thus, confirmatory information may be needed either by specific experimental assays or analyses of large cohorts to potentially translate our findings into clinical practice.
5. Conclusions
We conclude that using whole exome sequencing to analyze an extended set of cancer genes, and a rigorous bioinformatic pipeline that includes PeCanPIE, we were able to identify candidate pathogenic genes for a more extensive set in young, Mexican breast cancer patients.
Acknowledgments
The authors would like to express our sincere gratitude to Rosa María Álvarez-Gómez, Yulianna Sánchez, and Paulina Álvarez and Talia Wegman, from the Hereditary Cancer Clinic at the National Cancer Institute in Mexico, for providing the facilities to recruit the patients for this study. The authors thank Michael Dean for facilitating whole exome sequencing at the NCI. Verónica Guzmán for her support in administering this project.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14071647/s1, Table S1: Clinical Data, Table S2: Gold Variants.
Author Contributions
Conceptualization, L.G.-F.-R. and A.M.; methodology, L.G.-F.-R., A.M., M.T.-M., L.G., R.O.-L. and V.T.; software, V.T.; validation, L.G.-F.-R.; formal analysis, L.G.-F.-R., A.L.B.-A., M.T.-M. and V.T.; investigation, L.G.-F.-R. and V.T.; data curation, A.L.B.-A., L.G., R.O.-L. and V.T.; writing—original draft preparation, A.L.B.-A. and V.T.; writing—review and editing, L.G.-F.-R., A.L.B.-A. and V.T.; visualization, A.L.B.-A., V.T. and M.T.-M.; supervision, A.M. and V.T.; project administration, L.G.-F.-R. and A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Mexican National Council of Science and Technology (CONACyT, grant number S0008-2016-1/272823) and supported by the program of Cátedras-CONACYT and the Intramural Program of the NIH. A.L.B.-A. has a CONACyT fellowship 662328. The APC was funded by Tecnologico de Monterrey.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of National Cancer Institute of Mexico (INCAN, Instituto Nacional de Cancerología) (protocol code CEI/1123/16 approved in 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study may be available on request from the corresponding author. The data are not publicly available due to embargo period.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Villarreal-Garza C., Platas A., Miaja M., Fonseca A., Mesa-Chavez F., Garcia-Garcia M., Chapman J.A., Lopez-Martinez E.A., Pineda C., Mohar A., et al. Young women with breast cancer in Mexico: Results of the pilot phase of the Joven & Fuerte prospective cohort. J. Glob. Oncol. 2020;6:395–406. doi: 10.1200/JGO.19.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gómez-Flores-ramos L., Castro-Sánchez A., Peña-Curiel O., Mohar-Betancourt A. Molecular biology in young women with breast cancer: From tumor gene Expression to DNA mutations. Rev. Investig. Clin. 2017;69:181–192. doi: 10.24875/RIC.17002225. [DOI] [PubMed] [Google Scholar]
- 4.Kudela E., Samec M., Kubatka P., Nachajova M., Laucekova Z., Liskova A., Dokus K., Biringer K., Simova D., Gabonova E., et al. Breast cancer in young women: Status quo and advanced disease management by a predictive, preventive, and personalized approach. Cancers. 2019;11:1791. doi: 10.3390/cancers11111791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabriel C.A., Domchek S.M. Breast cancer in young women. Breast Cancer Res. 2010;12:212. doi: 10.1186/bcr2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assi H., Khoury K., Dbouk H., Khalil L., Mouhieddine T., El Saghir N. Epidemiology and prognosis of breast cancer in young women. J. Thorac. Dis. 2013;5((Suppl. S1)):S2–S8. doi: 10.3978/J.ISSN.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kast K., Rhiem K., Wappenschmidt B., Hahnen E., Hauke J., Bluemcke B., Zarghooni V., Herold N., Ditsch N., Kiechle M., et al. Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J. Med. Genet. 2016;53:465–471. doi: 10.1136/jmedgenet-2015-103672. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen F.C., Van Overeem Hansen T., Sørensen C.S. Hereditary breast and ovarian cancer: New genes in confined pathways. Nat. Rev. Cancer. 2016;16:599–612. doi: 10.1038/nrc.2016.72. [DOI] [PubMed] [Google Scholar]
- 9.Wendt C., Margolin S. Identifying breast cancer susceptibility genes—A review of the genetic background in familial breast cancer. Acta Oncol. 2019;58:135–146. doi: 10.1080/0284186X.2018.1529428. [DOI] [PubMed] [Google Scholar]
- 10.Economopoulou P., Dimitriadis G., Psyrri A. Beyond BRCA: New hereditary breast cancer susceptibility genes. Cancer Treat. Rev. 2015;41:1–8. doi: 10.1016/j.ctrv.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Urbina-Jara L.K., Rojas-Martinez A., Martinez-Ledesma E., Aguilar D., Villarreal-Garza C., Ortiz-Lopez R. Landscape of germline mutations in dna repair genes for breast cancer in latin america: Opportunities for parp-like inhibitors and immunotherapy. Genes. 2019;10:786. doi: 10.3390/genes10100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urban R.Q., Velásquez C.E.D., Gitler R., Castillo M.P.R., Toporek M.S., Morales A.F., García O.M., Esquivel L.G., Mejía G.T., Dean M., et al. Comprehensive Analysis of Germline Variants in Mexican Patients with Hereditary Breast and Ovarian Cancer Susceptibility. Cancers. 2018;10:361. doi: 10.3390/cancers10100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fountzilas C., Kaklamani V.G. Multi-gene Panel Testing in Breast Cancer Management. Optim. Breast Cancer Manag. 2018;173:121–140. doi: 10.1007/978-3-319-70197-4_8. [DOI] [PubMed] [Google Scholar]
- 14.Spurdle A.B., Bowman M.A., Shamsani J., Kirk J. Endometrial cancer gene panels: Clinical diagnostic vs research germline DNA testing. Mod. Pathol. 2017;30:1048–1068. doi: 10.1038/modpathol.2017.20. [DOI] [PubMed] [Google Scholar]
- 15.Smolle M.A., Kashofer K., Riedl J.M., Stotz M., Gerger A. Genetic Analysis Using a Gene Panel in 87 Caucasian Patients With Colorectal Cancer: Own Results and Review of Literature. Anticancer Res. 2019;39:847–852. doi: 10.21873/anticanres.13184. [DOI] [PubMed] [Google Scholar]
- 16.Yatabe Y., Sunami K., Goto K., Nishio K., Aragane N., Ikeda S., Inoue A., Kinoshita I., Kimura H., Sakamoto T., et al. Multiplex gene-panel testing for lung cancer patients. Pathol. Int. 2020;70:921–931. doi: 10.1111/pin.13023. [DOI] [PubMed] [Google Scholar]
- 17.Auton A., Abecasis G.R., Altshuler D.M., Durbin R.M., Bentley D.R., Chakravarti A., Clark A.G., Donnelly P., Eichler E.E., Flicek P., et al. A global reference for human genetic variation. Nature. 2015;68:526. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landrum M.J., Chitipiralla S., Brown G.R., Chen C., Gu B., Hart J., Hoffman D., Jang W., Kaur K., Liu C., et al. ClinVar: Improvements to accessing data. Nucleic Acids Res. 2020;48:D835–D844. doi: 10.1093/nar/gkz972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmonson M.N., Patel A.N., Hedges D.J., Wang Z., Rampersaud E., Kesserwan C.A., Zhou X., Liu Y., Newman S., Rusch M.C., et al. Pediatric Cancer Variant Pathogenicity Information Exchange (PeCanPIE): A cloud-based platform for curating and classifying germline variants. Genome Res. 2019;29:1555–1565. doi: 10.1101/gr.250357.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sondka Z., Bamford S., Cole C.G., Ward S.A., Dunham I., Forbes S.A. The COSMIC Cancer Gene Census: Describing genetic dysfunction across all human cancers. Nat. Rev. Cancer. 2018;18:696–705. doi: 10.1038/s41568-018-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson G., Parker M., Kranenburg T.A., Lu C., Chen X., Ding L., Phoenix T.N., Hedlund E., Wei L., Zhu X., et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winham S.J., Larson N.B., Armasu S.M., Fogarty Z.C., Larson M.C., McCauley B.M., Wang C., Lawrenson K., Gayther S., Cunningham J.M., et al. Molecular signatures of X chromosome inactivation and associations with clinical outcomes in epithelial ovarian cancer. Hum. Mol. Genet. 2019;28:1331–1342. doi: 10.1093/hmg/ddy444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scala M., Torella A., Severino M., Morana G., Castello R., Accogli A., Verrico A., Vari M.S., Cappuccio G., Pinelli M., et al. Three de novo DDX3X variants associated with distinctive brain developmental abnormalities and brain tumor in intellectually disabled females. Eur. J. Hum. Genet. 2019;27:1254–1259. doi: 10.1038/s41431-019-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blok L.S., Madsen E., Juusola J., Gilissen C., Baralle D., Reijnders M.R.F., Venselaar H., Helsmoortel C., Cho M.T., Hoischen A., et al. Mutations in DDX3X Are a Common Cause of Unexplained Intellectual Disability with Gender-Specific Effects on Wnt Signaling. Am. J. Hum. Genet. 2015;97:343–352. doi: 10.1016/j.ajhg.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai M.-C., Lee Y.-H.W., Tarn W.-Y. The DEAD-Box RNA Helicase DDX3 Associates with Export Messenger Ribonucleoproteins as well asTip-associated Protein and Participates in Translational Control. Mol. Biol. Cell. 2008;19:3847–3858. doi: 10.1091/mbc.e07-12-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cargill M.J., Morales A., Ravishankar S., Warren E.H. RNA helicase, DDX3X, is actively recruited to sites of DNA damage in live cells. DNA Repair. 2021;103:103137. doi: 10.1016/j.dnarep.2021.103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Midha M.K., Huang Y.-F., Yang H.-H., Fan T.-C., Chang N.-C., Chen T.-H., Wang Y.-T., Kuo W.-H., Chang K.-J., Shen C.-Y., et al. Comprehensive Cohort Analysis of Mutational Spectrum in Early Onset Breast Cancer Patients. Cancers. 2020;12:2089. doi: 10.3390/cancers12082089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunadirek P., Chuaypen N., Jenjaroenpun P., Wongsurawat T., Pinjaroen N., Sirichindakul P., Nookaew I., Tangkijvanich P. Cell-Free DNA Analysis by Whole-Exome Sequencing for Hepatocellular Carcinoma: A Pilot Study in Thailand. Cancers. 2021;13:2229. doi: 10.3390/cancers13092229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gellert P., Segal C.V., Gao Q., López-Knowles E., Martin L.A., Dodson A., Li T., Miller C.A., Lu C., Mardis E.R., et al. Impact of mutational profiles on response of primary oestrogen receptor-positive breast cancers to oestrogen deprivation. Nat. Commun. 2016;7:13294. doi: 10.1038/ncomms13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albers C.A., Paul D.S., Schulze H., Freson K., Stephens J.C., Smethurst P.A., Jolley J.D., Cvejic A., Kostadima M., Bertone P., et al. Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome. Nat. Genet. 2012;44:435–439. doi: 10.1038/ng.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei N., Chen H., Zhao N., Yi Y., Li C. A Comprehensive Pan-Cancer Analysis of RBM8A Based on Data Mining. J. Oncol. 2021;2021:9983354. doi: 10.1155/2021/9983354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Encinas G., Sabelnykova V.Y., De Lyra E.C., Katayama M.L.H., Maistro S., Valle P.W.M.D.V., Pereira G.F.D.L., Rodrigues L.M., Serio P.A.D.M.P., De Gouvêa A.C.R.C., et al. Somatic mutations in early onset luminal breast cancer. Oncotarget. 2018;9:22460–22479. doi: 10.18632/oncotarget.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jalkh N., Chouery E., Haidar Z., Khater C., Atallah D., Ali H., Marafie M.J., Al-Mulla M.R., Al-Mulla F., Megarbane A. Next-generation sequencing in familial breast cancer patients from Lebanon. BMC Med. Genom. 2017;10:8. doi: 10.1186/s12920-017-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokolenko A.P., Preobrazhenskaya E.V., Aleksakhina S.N., Iyevleva A.G., Mitiushkina N.V., Zaitseva O.A., Yatsuk O.S., Tiurin V.I., Strelkova T.N., Togo A.V., et al. Candidate gene analysis of BRCA1/2 mutation-negative high-risk Russian breast cancer patients. Cancer Lett. 2015;359:259–261. doi: 10.1016/j.canlet.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Ellingson M.S., Hart S.N., Kalari K.R., Suman V., Schahl K.A., Dockter T.J., Felten S.J., Sinnwell J.P., Thompson K.J., Tang X., et al. Exome sequencing reveals frequent deleterious germline variants in cancer susceptibility genes in women with invasive breast cancer undergoing neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2015;153:435–443. doi: 10.1007/s10549-015-3545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tedaldi G., Tebaldi M., Zampiga V., Danesi R., Arcangeli V., Ravegnani M., Cangini I., Pirini F., Petracci E., Rocca A., et al. Multiple-gene panel analysis in a case series of 255 women with hereditary breast and ovarian cancer. Oncotarget. 2017;8:47064–47075. doi: 10.18632/oncotarget.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson E.R., Doyle M.A., Ryland G.L., Rowley S.M., Choong D.Y.H., Tothill R.W., Thorne H., Barnes D.R., Li J., Ellul J., et al. Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes FANCC and BLM as Potential Breast Cancer Susceptibility Alleles. PLoS Genet. 2012;8:e1002894. doi: 10.1371/journal.pgen.1002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokolenko A.P., Bogdanova N., Kluzniak W., Preobrazhenskaya E.V., Kuligina E.S., Iyevleva A.G., Aleksakhina S.N., Mitiushkina N.V., Gorodnova T.V., Bessonov A.A., et al. Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res. Treat. 2014;145:553–562. doi: 10.1007/s10549-014-2971-1. [DOI] [PubMed] [Google Scholar]
- 40.Torrezan G.T., de Almeida F.G.d.S.R., Figueiredo M.C.P., Barros B.D.d.F., de Paula C.A.A., Valieris R., de Souza J.E.S., Ramalho R.F., da Silva F.C.C., Ferreira E.N., et al. Complex Landscape of Germline Variants in Brazilian Patients With Hereditary and Early Onset Breast Cancer. Front. Genet. 2018;9:161. doi: 10.3389/fgene.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarpitta R., Zanna I., Aretini P., Gambino G., Scatena C., Mei B., Ghilli M., Rossetti E., Roncella M., Congregati C., et al. Germline investigation in male breast cancer of DNA repair genes by next-generation sequencing. Breast Cancer Res. Treat. 2019;178:557–564. doi: 10.1007/s10549-019-05429-z. [DOI] [PubMed] [Google Scholar]
- 42.Oliver J., Quezada Urban R., Franco Cortés C.A., Díaz Velásquez C.E., Montealegre Paez A.L., Pacheco-Orozco R.A., Castro Rojas C., García-Robles R., López Rivera J.J., Gaitán Chaparro S., et al. Latin American Study of Hereditary Breast and Ovarian Cancer LACAM: A Genomic Epidemiology Approach. Front. Oncol. 2019;9:1429. doi: 10.3389/fonc.2019.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhoeft K.R., Ngan H.L., Lui V.W.Y. The cylindromatosis (CYLD) gene and head and neck tumorigenesis. Cancers Head Neck. 2016;1:10. doi: 10.1186/s41199-016-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heddar A., Fermey P., Coutant S., Angot E., Sabourin J.-C., Michelin P., Parodi N., Charbonnier F., Vezain M., Bougeard G., et al. Familial solitary chondrosarcoma resulting from germline EXT2 mutation. Genes Chromosom. Cancer. 2017;56:128–134. doi: 10.1002/gcc.22419. [DOI] [PubMed] [Google Scholar]
- 45.Santos S.C.L., Rizzo I.M.P.O., Takata R.I., Speck-Martins C.E., Brum J.M., Sollaci C. Analysis of mutations in EXT1 and EXT2 in Brazilian patients with multiple osteochondromas. Mol. Genet. Genom. Med. 2018;6:382–392. doi: 10.1002/mgg3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afshar A.R., Pekmezci M., Bloomer M.M., Cadenas N.J., Stevers M., Banerjee A., Roy R., Olshen A.B., Van Ziffle J., Onodera C., et al. Next-Generation Sequencing of Retinoblastoma Identifies Pathogenic Alterations beyond RB1 Inactivation That Correlate with Aggressive Histopathologic Features. Ophthalmology. 2020;127:804–813. doi: 10.1016/j.ophtha.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S., Supek F., Lehner B. Systematic discovery of germline cancer predisposition genes through the identification of somatic second hits. Nat. Commun. 2018;9:2601. doi: 10.1038/s41467-018-04900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benusiglio P.R., Couvé S., Gilbert-Dussardier B., Deveaux S., Le Jeune H., Da Costa M., Fromont G., Memeteau F., Yacoub M., Coupier I., et al. A germline mutation in PBRM1 predisposes to renal cell carcinoma. J. Med. Genet. 2015;52:426–430. doi: 10.1136/jmedgenet-2014-102912. [DOI] [PubMed] [Google Scholar]
- 49.Spinella J.-F., Healy J., Saillour V., Richer C., Cassart P., Ouimet M., Sinnett D. Whole-exome sequencing of a rare case of familial childhood acute lymphoblastic leukemia reveals putative predisposing mutations in Fanconi anemia genes. BMC Cancer. 2015;15:539. doi: 10.1186/s12885-015-1549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taeubner J., Brozou T., Qin N., Bartl J., Ginzel S., Schaper J., Felsberg J., Fulda S., Vokuhl C., Borkhardt A., et al. Congenital embryonal rhabdomyosarcoma caused by heterozygous concomitant PTCH1 and PTCH2 germline mutations. Eur. J. Hum. Genet. 2018;26:137–142. doi: 10.1038/s41431-017-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li N., McInerny S., Zethoven M., Cheasley D., Lim B.W.X., Rowley S.M., Devereux L., Grewal N., Ahmadloo S., Byrne D., et al. Combined Tumor Sequencing and Case-Control Analyses of RAD51C in Breast Cancer. J. Natl. Cancer Inst. 2019;111:1332–1338. doi: 10.1093/jnci/djz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blombery P., Fox L.C., Ryland G.L., Thompson E.R., Lickiss J., McBean M., Yerneni S., Hughes D., Greenway A., Mechinaud F., et al. Utility of clinical comprehensive genomic characterization for diagnostic categorization in patients presenting with hypocellular bone marrow failure syndromes. Haematologica. 2021;106:64–73. doi: 10.3324/haematol.2019.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H., Song B., Guo S., Li G., Jin G. Identification of germline and somatic mutations in pancreatic adenosquamous carcinoma using whole exome sequencing. Cancer Biomark. 2020;27:389–397. doi: 10.3233/CBM-190236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigurdson A.J., Brenner A.V., Roach J.A., Goudeva L., Müller J.A., Nerlich K., Reiners C., Schwab R., Pfeiffer L., Waldenberger M., et al. Selected single-nucleotide polymorphisms in FOXE1, SERPINA5, FTO, EVPL, TICAM1 and SCARB1 are associated with papillary and follicular thyroid cancer risk: Replication study in a German population. Carcinogenesis. 2016;37:677–684. doi: 10.1093/carcin/bgw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker C.J., Miranda M.A., O’Hern M.J., McElroy J.P., Coombes K.R., Bundschuh R., Cohn D.E., Mutch D.G., Goodfellow P.J. Patterns of CTCF and ZFHX3 Mutation and Associated Outcomes in Endometrial Cancer. J. Natl. Cancer Inst. 2015;107:11. doi: 10.1093/jnci/djv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rai R., Sharma K.L., Tiwari S., Misra S., Kumar A., Mittal B. DCC (deleted in colorectal carcinoma) gene variants confer increased susceptibility to gallbladder cancer (Ref. No.: Gene-D-12-01446) Gene. 2013;518:303–309. doi: 10.1016/j.gene.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.J., Lubinski J., Huzarski T., Møller P., Armel S., Karlan B.Y., Senter L., Eisen A., Foulkes W.D., Singer C.F., et al. Weight Gain and the Risk of Ovarian Cancer in BRCA1 and BRCA2 Mutation Carriers. Cancer Epidemiol. Biomark. Prev. 2021;30:2038–2043. doi: 10.1158/1055-9965.EPI-21-0296. [DOI] [PubMed] [Google Scholar]
- 58.Qian F., Rookus M.A., Leslie G., Risch H.A., Greene M.H., Aalfs C.M., Adank M.A., Adlard J., Agnarsson B.A., Ahmed M., et al. Mendelian randomisation study of height and body mass index as modifiers of ovarian cancer risk in 22,588 BRCA1 and BRCA2 mutation carriers. Br. J. Cancer. 2019;121:180–192. doi: 10.1038/s41416-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian F., Wang S., Mitchell J., McGuffog L., Barrowdale D., Leslie G., Oosterwijk J.C., Chung W.K., Evans D.G., Engel C., et al. Height and Body Mass Index as Modifiers of Breast Cancer Risk in BRCA1/2 Mutation Carriers: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2019;111:350–364. doi: 10.1093/jnci/djy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y.A., Jian J.-W., Hung C.-F., Peng H.-P., Yang C.-F., Cheng H.-C.S., Yang A.-S. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315. doi: 10.1186/s12885-018-4229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y., Wu J., Zhang C., Sun S., Zhang J., Liu W., Zhang Z., Huang J. BRCA mutations and survival in breast cancer: An updated systematic review and meta-analysis. Oncotarget. 2016;7:70113–70127. doi: 10.18632/oncotarget.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verhoog L.C., Brekelmans C.T.M., Seynaeve C., Dahmen G., Van Geel A.N., Bartels C.C.M., Tilanus-Linthorst M.M.A., Wagner A., Devilee P., Halley D.J.J., et al. Survival in Hereditary Breast Cancer Associated with Germline Mutations of BRCA2. J. Clin. Oncol. 1999;17:3396–3402. doi: 10.1200/JCO.1999.17.11.3396. [DOI] [PubMed] [Google Scholar]
- 63.Downing J.R., Wilson R.K., Zhang J., Mardis E.R., Pui C.H., Ding L., Ley T.J., Evans W.E. The pediatric cancer genome project. Nat. Genet. 2012;44:619–622. doi: 10.1038/ng.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bose R., Kavuri S.M., Searleman A.C., Shen W., Shen D., Koboldt D.C., Monsey J., Goel N., Aronson A.B., Li S., et al. Activating HER2 Mutations in HER2 Gene Amplification Negative Breast Cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Da Costa E Silva Carvalho S., Cury N.M., Brotto D.B., De Araujo L.F., Rosa R.C.A., Texeira L.A., Plaça J.R., Marques A.A., Peronni K.C., Ruy P.D.C., et al. Germline variants in DNA repair genes associated with hereditary breast and ovarian cancer syndrome: Analysis of a 21 gene panel in the Brazilian population. BMC Med. Genom. 2020;13:21. doi: 10.1186/s12920-019-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Couch F.J., Shimelis H., Hu C., Hart S.N., Polley E.C., Na J., Hallberg E., Moore R., Thomas A., Lilyquist J., et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017;3:1190–1196. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fazel A., Hasanpour-Heidari S., Salamat F., Rajaie S., Kazeminezhad V., Naeimi-Tabiei M., Jahangirrad A., Sedaghat S.M., Hosseinpoor R., Ghasemi-Kebria F., et al. Marked increase in breast cancer incidence in young women: A 10-year study from Northern Iran, 2004–2013. Cancer Epidemiol. 2019;62:101573. doi: 10.1016/j.canep.2019.101573. [DOI] [PubMed] [Google Scholar]
- 68.Dimitrova N., Znaor A., Agius D., Eser S., Sekerija M., Ryzhov A., Primic-Žakelj M., Coebergh J.W., Agius D., Coza D., et al. Breast cancer in South-Eastern European countries since 2000: Rising incidence and decreasing mortality at young and middle ages. Eur. J. Cancer. 2017;83:43–55. doi: 10.1016/j.ejca.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Rocha-Brischiliari S.C., De Oliveira R.R., Andrade L., Brischiliari A., Gravena A.A.F., Carvalho M.D.D.B., Pelloso S.M. The Rise in Mortality from Breast Cancer in Young Women: Trend Analysis in Brazil. PLoS ONE. 2017;12:e0168950. doi: 10.1371/journal.pone.0168950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colonna M., Delafosse P., Uhry Z., Poncet F., Arveux P., Molinie F., Cherie-Challine L., Grosclaude P. Is breast cancer incidence increasing among young women? An analysis of the trend in France for the period 1983–2002. Breast. 2008;17:289–292. doi: 10.1016/j.breast.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Friebel T.M., Domchek S.M., Rebbeck T.R. Modifiers of Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014;106:dju091. doi: 10.1093/jnci/dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lek M., Karczewski K., Minikel E., Samocha K., Banks E., Fennell T., O’Donnell-Luria A., Ware J., Hill A., Cummings B., et al. Analysis of protein-coding genetic variation in 60,706 humans. bioRxiv. 2015;536:030338. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamath-Loeb A.S., Welcsh P., Waite M., Adman E.T., Loeb L.A. The Enzymatic Activities of the Werner Syndrome Protein Are Disabled by the Amino Acid Polymorphism R834C. J. Biol. Chem. 2004;279:55499–55505. doi: 10.1074/jbc.M407128200. [DOI] [PubMed] [Google Scholar]
- 74.Kamath-Loeb A.S., Zavala-van Rankin D.G., Flores-Morales J., Emond M.J., Sidorova J.M., Carnevale A., Del Carmen Cárdenas-Cortés M., Norwood T.H., Monnat R.J., Loeb L.A., et al. Homozygosity for the WRN Helicase-Inactivating Variant, R834C, does not confer a Werner syndrome clinical phenotype. Sci. Rep. 2017;7:srep44081. doi: 10.1038/srep44081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dorling L., Carvalho S., Allen J., González-Neira A., Luccarini C., Wahlström C., Pooley K.A., Parsons M.T., Fortuno C. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021;384:428–439. doi: 10.1056/nejmoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study may be available on request from the corresponding author. The data are not publicly available due to embargo period.