Abstract
BACKGROUND:
Low socioeconomic status (SES) has been associated with a higher risk of aggressive breast cancer (BC) subtypes, but few studies have examined the independent effects of both neighborhood-level socioeconomic status (nSES) and individual-level SES measures.
METHODS:
This study included 5547 women from the Pathways and Life After Cancer Epidemiology cohorts who were diagnosed with invasive BC. Generalized estimating equation models were used to examine associations of nSES (a composite score based on income, poverty, education, occupation, employment, rent, and house value) and individual-level SES (income and education) with BC subtypes: luminal B (LumB), Her2-enriched (Her2-e), and triple-negative breast cancer (TNBC) relative to luminal A (LumA). Models controlled for age, race, nativity, stage, days from diagnosis to survey, and study cohort and simultaneously for nSES and individual-level SES.
RESULTS:
In fully adjusted models, low nSES was significantly associated with the LumB (odds ratio for quartile 1 vs quartile 4 [OR Q1vQ4], 1.31; 95% confidence interval [CI], 1.11–1.54; P for trend = .005) and TNBC subtypes (ORQ1vQ4, 1.32; 95% CI, 1.02–1.71; P for trend = .037) relative to LumA. Conversely, individual education was significantly associated with only the Her2-e subtype (odds ratio for high school degree or less vs postgraduate, 1.68; 95% CI, 1.03–2.75; P for trend = .030) relative to LumA. Individual income was not significantly associated with any BC subtype.
CONCLUSIONS:
nSES and individual-level SES are independently associated with different BC subtypes; specifically, low nSES and individual-level education are independent predictors of more aggressive BC subtypes relative to LumA.
Keywords: breast neoplasms, California, estrogen receptors, progesterone receptor, social class, women
INTRODUCTION
Breast cancer (BC) is often classified into 4 intrinsic subtypes with unique gene expression profiles, which are often approximated by the expression of Her2 and the status of 2 hormone receptors (HRs), estrogen receptor (ER) and progesterone receptor (PR).1–3 The use of immunohistochemistry (IHC) markers plus Ki-67 or a surrogate marker of cell proliferation such as the tumor grade also improves the accuracy of approximating BC subtypes and reduces misclassification of the luminal A (LumA) and luminal (LumB) subtypes.3,4 The 4 BC subtypes from least to most aggressive are LumA (ER+/PR+/Her2− and well/moderately differentiated), LumB (ER+/Her2+ or ER+/Her2− and PR− or poorly differentiated/undifferentiated), Her2-enriched (Her2-e; ER−/PR−/Her2+), and triple-negative breast cancer (TNBC; ER−/PR−/Her2−).5
Women of lower socioeconomic status (SES) have a higher incidence of HR− subtypes than women of higher SES.6–8 Most prior work has focused on the effects of neighborhood-level socioeconomic status (nSES) on BC subtypes; women of higher nSES have had lower odds of HR− BC, whereas women of lower nSES have had higher odds.2,6–12 Commonly used nSES measures based on census data reflect both contextual (ie, neighborhood-level) and compositional (ie, individual-level) effects. Both nSES and individual-level SES must be simultaneously examined to understand their independent effects; however, few prior studies have taken this approach. Sineshaw et al13 created a composite SES variable based on neighborhood-level income and individual-level insurance, and they found that women with low SES had higher odds of HR− subtypes; however, independent effects of nSES and individual-level SES could not be ascertained from this study. Qin et al14 investigated the independent effect of nSES by adjusting for individual education, poverty level, and type of insurance, and they found that Black women of lower SES had a higher risk for TNBC relative to LumA.
Despite the heterogeneity of SES measures, sample sizes, and data sources, the data suggest that women living in areas characterized as lower SES are more likely to be diagnosed with more aggressive BC subtypes.2,7–13 To our knowledge, no studies have examined the independent effects of nSES and individual-level SES on all major BC subtypes. Therefore, we investigated associations between multilevel SES and BC subtypes relative to LumA by combining data from 2 studies for a total of 5547 racially diverse patients with BC recruited from the Kaiser Permanente Northern California (KPNC) population.
MATERIALS AND METHODS
Study Population
This study included BC survivors from the Pathways and Life After Cancer Epidemiology (LACE) cohorts. The Pathways cohort included 4505 women recruited from KPNC between 2006 and 2013 who were at least 21 years old; were diagnosed between 2005 and 2013 with invasive BC; had no history of cancer; spoke English, Spanish, Cantonese, or Mandarin; and lived within 65 miles of a field interviewer.15 The LACE cohort included 2263 women recruited primarily from KPNC (82%) between 2000 and 2002 who were 18 to 79 years old at diagnosis, were diagnosed between 1997 and 2000 with early-stage BC (stage I, II, or IIIA) within 39 months of enrollment, had no history of other cancer 5 years before enrollment, completed BC treatment, and had no evidence of recurrence at enrollment.16 The Pathways survey was administered by an interviewer at an in-person interview. The LACE participants responded to a mailed, self-administered survey. In each cohort, women responded to questions on individual-level education and income. Additional details of the Pathways and LACE cohorts have been reported previously.15,16 The University of California Irvine did not access human subjects data at their site and received a ‘not human subjects’ determination from their institutional review board (IRB). The University of California San Francisco ceded scientific review to the Kaiser Permanente Northern California IRB which approved the study prior to beginning research.
For women from Pathways, the current address at the baseline was identified from census data, which were already linked to 2010 geocodes. For women from LACE, the address at the baseline was obtained from the electronic health record (EHR), with missing addresses obtained from the following sources (listed in order of preference): study database, paper survey, EHR within 2 years after the baseline, cancer registry within 2 years after the baseline, EHR within 2 years before the baseline, and cancer registry within 2 years before the baseline. The LACE addresses were geocoded in ArcGIS at the point address or street address levels, and the resulting coordinates were assigned to Census 2000 block groups and census tracts.
Women from the LACE study who were not recruited at KPNC were excluded because of a lack of access to medical records (n = 389). Women were excluded from this analysis in a stepwise process if they had missing or incomplete IHC information (n = 511) or were missing data on individual education (n = 12), nSES (n = 155), zip code (n = 117), or other covariates (n = 37). The final study cohort included 4079 women from the Pathways cohort and 1468 women from the LACE cohort for a total of 5547 women.
Study Variables
BC tissue markers and subtypes
Information on Her2 expression, HR status (ER and PR), and tumor grade was obtained from the KPNC Cancer Registry or through medical record review. We used 2013 St. Gallen BC subtyping with the grade in lieu of Ki-67 to categorize BC into 4 subtypes: LumA (ER+/PR+/Her2− and well/moderately differentiated), LumB (ER+/Her2+ or ER+/Her2− and PR− or poorly differentiated/undifferentiated), Her2-e (ER−/PR−/Her2+), and TNBC (ER−/PR−/Her2−).
Socioeconomic status
Individual-level SES
Individual-level education and income were used to measure individual-level SES.
In Pathways, women indicated their highest level of education from the following options: less than 8th grade, 8th to 11th grade, high school graduate or equivalent (GED), vocational or trade school, some college, college graduate, and postgraduate. In LACE, women indicated how many years of school they had completed from the following options: 1 to 11 years, 12 years (high school graduate), 13 to 15 years (some college or technical school), 16 years (college graduate), and some graduate school or advanced degree. For our analyses, we generated a 4-level individual education variable with the following categories: high school degree or less, some college, college degree, and postgraduate. Women in the postgraduate category composed the reference group.
In Pathways, women indicated their total household income at the time of study enrollment by using the following options: less than $15,000, $15,000 to $19,999, $20,000 to $24,999, $25,000 to $34,999, $35,000 to $49,999, $50,000 to $69,999, $70,000 to $89,999, and $90,000 or more. In LACE, baseline income data were not available; however, income data were available from a follow-up questionnaire collected on average 5.2 years after the baseline. Women indicated their household income in the last year by using the following options: less than $20,000, $20,000 to $39,999, $40,000 to $59,999, $60,000 to $79,000, $80,000 to $99,999, and $100,000 or more. Given the differences in the response options and in the years of income ascertainment, we created cohort-specific income tertiles to enable harmonization of this variable. Women in the highest tertile (highest income) composed the reference group.
nSES
nSES at the block group level was operationalized with a previously described and widely used composite nSES score derived from principal components analysis, which used data from the 2000 census (for cases enrolled before 2006) and 2006–2010 American Community Survey data (for cases enrolled in 2006 and onward).17 The score was based on the following 7 components: income, poverty, education, occupation, employment, rent, and house value. Because of differences in census years in our data set, a continuous measure of nSES could not be evaluated in this study. Therefore, study-specific, score-based quartiles were created and then pooled in the final data set (“pooled quartiles”). As a secondary measure, nSES quartiles by census year were created on the basis of the statewide distribution of nSES scores in California (“state-based quartiles”).
A heatmap of nSES in the KPNC service area was created on the basis of the state-based quartiles of nSES (Fig. 1). The map was created with KP Maps, an online geographic information system hosted by Kaiser Permanente.
Figure 1.
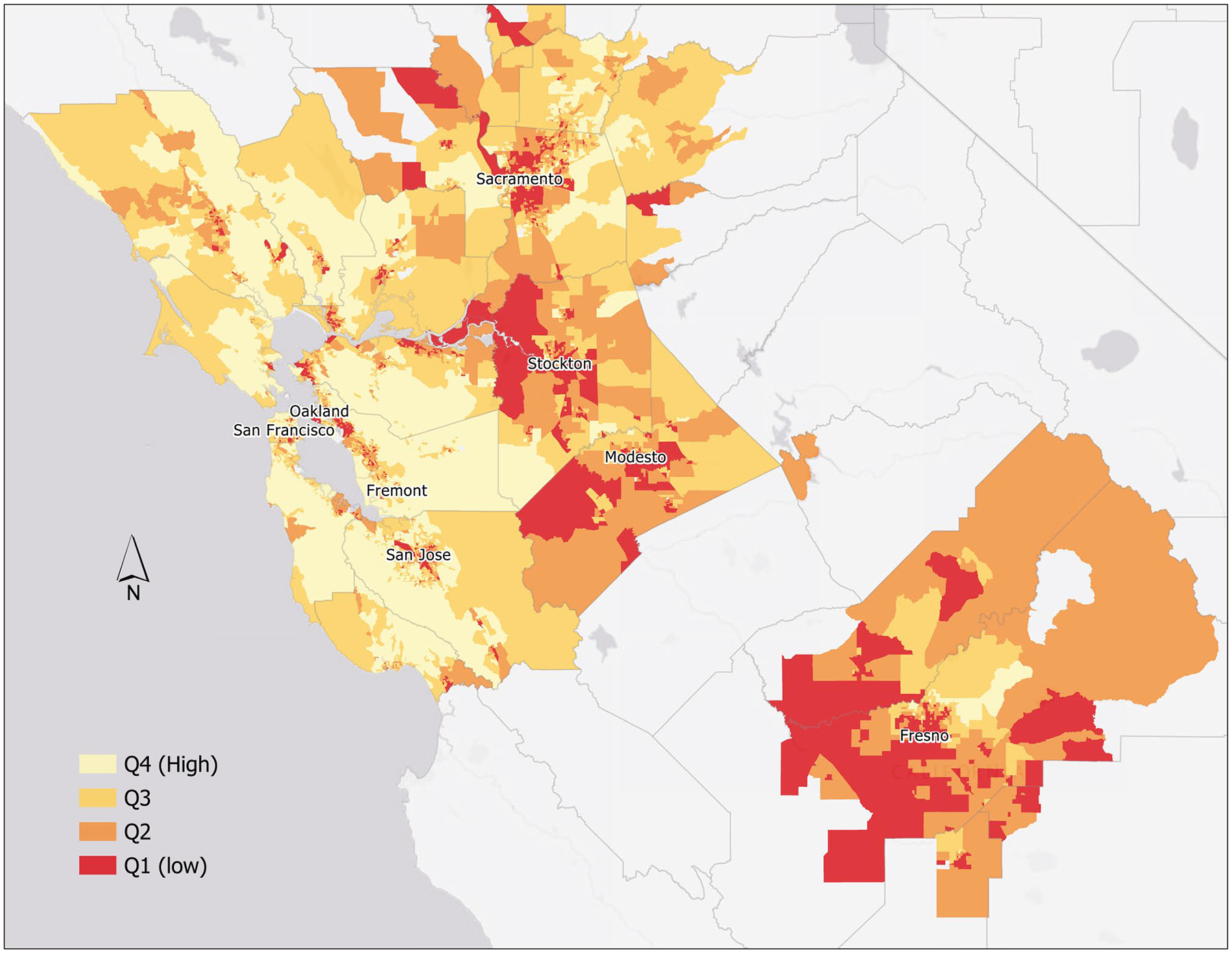
State-based neighborhood-level socioeconomic status quartiles based on 2010 US Census data in the Kaiser Permanente Northern California service area. Q1 indicates quartile 1; Q2, quartile 2; Q3, quartile 3; Q4, quartile 4.
Other covariates
Information on age, race, and nativity were self-reported at the baseline. Consistent with prior studies, covariates included the following: age at diagnosis, race (non-Latina White [NLW], Black, Latina, Asian, or other race), nativity (foreign-born or US-born), study (Pathways or LACE), days from diagnosis to study enrollment, and stage at diagnosis (stages I-IV). Age, race, nativity, and stage were selected a priori as potential confounding variables; we adjusted for study and days from diagnosis to study enrollment to address potential sampling differences between cohorts.
Statistical Analysis
We used generalized estimating equation models to compute adjusted odds ratios (ORs) for each BC subtype (relative to LumA) while accounting for clustering by zip code with an exchangeable correlation structure for the working covariance. We considered alternative clustering models (generalized linear mixed models with a logit link) with clustering at the census block group or tract level; however, 85% of block groups and 52% of tracts in this study contained only 1 to 2 women. We chose zip codes to account for clustering because 25% of zip codes in our study contained 1 to 2 women, with 75% of zip codes containing >2 women. Generalized estimating equation models were used because parameter estimates are robust to any underlying neighborhood correlation structure that may be present.18 In age-adjusted models, we separately examined bivariate associations between nSES and individual-level SES variables and BC subtypes with adjustments for age at diagnosis. In model 1, we additionally adjusted for race, nativity, study, days from diagnosis to study enrollment, and stage at diagnosis. Model 2 additionally included either nSES or individual education and income. Model 3 is included only in Table 4 (associations for individual education), which is additionally adjusted for individual income. Pairwise correlations for nSES and individual-level SES predictors revealed weak correlations (r < 0.25), and this allowed for simultaneous inclusion in the final models.
TABLE 4.
ORs for the Associations of Individual Education With Breast Cancer Subtypes Relative to LumA (n = 5554)
| Individual-Level Education | |||||
|---|---|---|---|---|---|
| Postgraduate | College Degree | Some College | High School Degree or Less | P for Trend | |
| LumA (n = 2723) | 583 | 662 | 994 | 484 | |
| LumB (n = 1856) | 423 | 361 | 624 | 448 | |
| OR, age-adjusteda | 1.00 | 0.91 | 0.88 | 1.08 | .754 |
| 95% CI | 0.77–1.09 | 0.76–1.03 | 0.9–1.3 | ||
| OR, model 1b | 1.00 | 0.93 | 0.87 | 1.02 | .699 |
| 95% CI | 0.78–1.11 | 0.74–1.02 | 0.84–1.23 | ||
| OR, model 2c | 1.00 | 0.91 | 0.83 | 0.96 | .303 |
| 95% CI | 0.76–1.09 | 0.71–0.98 | 0.78–1.16 | ||
| OR, model 3d | 1.00 | 0.90 | 0.82 | 0.93 | .211 |
| 95% CI | 0.75–1.08 | 0.69–0.96 | 0.77–1.14 | ||
| Q statistice | 4.64 | ||||
| P | .031 | ||||
| LACE (n = 517): model 3d,f | 1.00 | 0.91 | 1.10 | 1.15 | .301 |
| 95% CI | 0.61–1.36 | 0.81–1.49 | 0.82–1.63 | ||
| Pathways (n = 1339): model 3d,f | 1.00 | 0.89 | 0.73 | 0.85 | .022 |
| 95% CI | 0.72–1.09 | 0.6–0.87 | 0.66–1.09 | ||
| Her2-e (n = 267) | 36 | 51 | 104 | 76 | |
| OR, age-adjusteda | 1.00 | 1.69 | 1.74 | 1.83 | .006 |
| 95% CI | 1.11–2.56 | 1.16–2.62 | 1.18–2.84 | ||
| OR, model 1b | 1.00 | 1.59 | 1.77 | 1.80 | .007 |
| 95% CI | 1.03–2.46 | 1.16–2.7 | 1.13–2.86 | ||
| OR, model 2c | 1.00 | 1.56 | 1.70 | 1.71 | .023 |
| 95% CI | 1–2.43 | 1.1–2.63 | 1.05–2.77 | ||
| OR, model 3d | 1.00 | 1.55 | 1.70 | 1.68 | .030 |
| 95% CI | 0.99–2.43 | 1.09–2.63 | 1.03–2.75 | ||
| Q statistice | 3.78 | ||||
| P | .052 | ||||
| LACE (n = 65): model 3d,f | 1.00 | 1.27 | 1.41 | 3.07 | .009 |
| 95% CI | 0.47–3.48 | 0.59–3.35 | 1.32–7.13 | ||
| Pathways (n = 203): model 3d,f | 1.00 | 1.52 | 1.72 | 1.10 | .452 |
| 95% CI | 0.95–2.45 | 1.05–2.81 | 0.59–2.05 | ||
| TNBC (n = 701) | 137 | 154 | 264 | 146 | |
| OR, age-adjusteda | 1.00 | 0.88 | 1.13 | 1.42 | .002 |
| 95% CI | 0.68–1.14 | 0.91–1.41 | 1.1–1.84 | ||
| OR, model 1b | 1.00 | 0.91 | 1.04 | 1.34 | .036 |
| 95% CI | 0.7–1.18 | 0.83–1.31 | 1.01–1.76 | ||
| OR, model 2c | 1.00 | 0.89 | 1.00 | 1.25 | .125 |
| 95% CI | 0.68–1.16 | 0.79–1.26 | 0.94–1.67 | ||
| OR, model 3d | 1.00 | 0.89 | 0.99 | 1.22 | .171 |
| 95% CI | 0.68–1.16 | 0.78–1.26 | 0.92–1.63 | ||
| Q statistice | 0.29 | ||||
| P | .586 | ||||
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; Her2-e, Her2-enriched; LACE, Life After Cancer Epidemiology; LumA, luminal A; LumB, luminal B; nSES, neighborhood-level socioeconomic status; OR, odds ratio; SES, socioeconomic status; TNBC, triple-negative breast cancer.
Adjusted for age at diagnosis.
Model 1 adjusted for possible confounders, including age at diagnosis (continuous), race (non-Latina White, Black, Latina, Asian, or other), nativity (US-born or foreign-born), AJCC stage (I, II, III, or IV), days from diagnosis to baseline measures (continuous), and study (LACE or Pathways).
Model 2 adjusted for variables in model 1 plus pooled nSES.
Model 3 adjusted for variables in model 2 plus individual income.
The Q statistic was calculated for model 2 with a continuous variable for days from diagnosis to study enrollment and a recategorized stage variable in which stages II, III, and IV were collapsed into 1 level (given differences in study recruitment).
Study-specific associations are shown for model 3 because of evidence of study heterogeneity.
To assess heterogeneity by study cohort, we computed the Q statistic comparing the study-specific effects for the fully adjusted models. When the Q statistic indicated statistically significant heterogeneity, we reported study-specific findings.
To determine whether reproductive factors might help to explain associations of nSES (pooled quartiles) with BC subtypes, we additionally evaluated models adjusted for parity (continuous) and breastfeeding (ever/never), which were available for 99.8% of the women in our data set. Models included covariates from model 2 plus parity and breastfeeding.
RESULTS
In pooled data, nSES differed by age, race/ethnicity, stage, state-based nSES quartile, individual education, and individual income (Table 1). The highest nSES quartile included higher proportions of NLW (76%) and Asian women (13%) and lower proportions of Black (2%) and Latina women (7%) in comparison with the lowest nSES quartile (NLWs, 58%; Asians, 9%; Blacks, 15%; and Latinas, 15%). Women from higher nSES areas had higher levels of individual education and income, and this was consistent with the notion that nSES is in part determined by the composition of its inhabitants.
TABLE 1.
Baseline Characteristics by Pooled Quartile of nSES (n = 5547)
| nSES by Pooled Quartilea,d | |||||
|---|---|---|---|---|---|
| Q4: High nSES | Q3: Mid-High nSES | Q2: Mid-Low nSES | Q1: Low nSES | P b | |
| No. | 1387 | 1387 | 1387 | 1386 | |
| Cohort variables | |||||
| Study, % | |||||
| LACE | 26.5 | 26.5 | 26.5 | 26.5 | |
| Pathways | 73.5 | 73.5 | 73.5 | 73.5 | |
| Time from diagnosis to baseline, % | |||||
| <52 d | 24.7 | 27.2 | 28.1 | 27.0 | .598 |
| 52–75 d | 28.3 | 29.5 | 27.3 | 27.1 | |
| 76–519 d | 27.7 | 26.0 | 26.2 | 27.3 | |
| ≥520 d | 19.3 | 17.4 | 18.5 | 18.6 | |
| Demographic variables | |||||
| Age at diagnosis, % | |||||
| <50 y | 20.0 | 25.7 | 21.7 | 22.3 | .009 |
| 50–64 y | 47.4 | 41.9 | 46.9 | 44.6 | |
| ≥65 y | 32.6 | 32.4 | 31.4 | 33.1 | |
| Race/ethnicity, % | |||||
| Non-Latina White | 76.1 | 69.4 | 67.9 | 57.7 | <.001 |
| Black | 2.3 | 4.8 | 8.1 | 14.9 | |
| Latina | 6.5 | 9.0 | 11.3 | 15.4 | |
| Asian | 12.9 | 13.8 | 9.5 | 9.4 | |
| Other | 2.2 | 3.0 | 3.2 | 2.6 | |
| Foreign-born, % | 19.7 | 20.6 | 18.2 | 18.3 | .310 |
| Tumor characteristics | |||||
| AJCC stage, % | |||||
| I | 54.0 | 52.6 | 50.5 | 48.9 | .049 |
| II | 38.1 | 37.6 | 40.5 | 42.1 | |
| III | 6.9 | 8.4 | 8.4 | 7.6 | |
| IV | 1.0 | 1.4 | 0.7 | 1.4 | |
| BC subtype, % | |||||
| LumA | 54.2 | 49.6 | 48.4 | 44.2 | <.001 |
| LumB | 31.2 | 34.0 | 33.5 | 35.1 | |
| Her2-e | 4.1 | 4.9 | 5.2 | 5.1 | |
| TNBC | 10.6 | 11.5 | 12.9 | 15.6 | |
| nSES | |||||
| State-based quartiles, % | |||||
| Q1 (low nSES) | 0.0 | 0.0 | 0.0 | 27.9 | <.001 |
| Q2 (mid-low nSES) | 0.0 | 0.0 | 6.9 | 69.5 | |
| Q3 (mid-high nSES) | 0.0 | 31.7 | 93.2 | 2.7 | |
| Q4 (high nSES) | 100.0 | 68.4 | 0.0 | 0.0 | |
| Individual-level SES | |||||
| Education, % | |||||
| High school or less | 10.7 | 16.7 | 21.1 | 27.3 | <.001 |
| Some college | 28.2 | 34.8 | 37.9 | 42.4 | |
| College degree | 28.3 | 26.5 | 23.4 | 17.8 | |
| Postgraduate | 32.8 | 22.1 | 17.7 | 12.4 | |
| Income, %c | |||||
| Tertile 1 (<$49,000) | 16.8 | 24.2 | 29.0 | 39.5 | <.001 |
| Tertile 2 ($40,000-$89,000) | 25.6 | 28.3 | 30.5 | 27.9 | |
| Tertile 3 (≥$80,000) | 42.5 | 32.5 | 23.9 | 14.7 | |
| Missing | 15.1 | 14.9 | 16.6 | 17.9 | |
Abbreviations: AJCC, American Joint Committee on Cancer; BC, breast cancer; Her2-e, Her2-enriched; LACE, Life After Cancer Epidemiology; LumA, luminal A; LumB, luminal B; nSES, neighborhood-level socioeconomic status; Q1, quartile 1; Q2, quartile 2; Q3, quartile 3; Q4, quartile 4; SES, socioeconomic status; TNBC, triple-negative breast cancer.
Study-specific nSES quartiles were created and then pooled.
P values for ordinal variables were generated from the Mantel-Haenszel χ2 test; P values for nonordinal variables were generated from the Pearson χ2 test.
Individual income tertiles were created for each study separately and then pooled; this resulted in overlapping ranges.
Columns may not add to 100% due to rounding.
Study cohorts differed by days from diagnosis to baseline, race/ethnicity, nativity, BC subtype, state-based nSES quartile, individual education, and individual income (Supporting Table 1). The LACE study enrolled higher proportions of NLW women (78.2% vs 64.0% in Pathways) and women residing in the highest state-based nSES quartile (46.9% vs 40.4%). The Pathways study enrolled higher proportions of foreign-born women (20.9% vs 14.2% in LACE), women with higher educational attainment (college degree or higher; 49.3% vs 34.1% in LACE), and women in the highest individual income tertile (31.3% vs 20.6% in LACE).
In the map of state-based nSES quartiles in the KPNC region (Fig. 1), areas of higher nSES (lighter shade) tended to occur in more urbanized areas (San Francisco, Oakland, and San Jose) and in areas located on the coast. Areas of lower nSES (darker shade) were typically more inland and in relatively rural areas (Fresno, Modesto, and Stockton).
Lower nSES as defined by the pooled quartile was associated with higher odds of all 3 BC subtypes relative to LumA in age-adjusted models (Table 2). Adjustment for potential confounders (model 1) attenuated effects for all 3 subtypes, and the association for Her2-e was no longer statistically significant. Adjustment for individual-level SES (model 2) further attenuated effect estimates for Her2-e and slightly attenuated them for TNBC. In the fully adjusted model, associations remained significant for LumB (odds ratio for quartile 1 vs quartile 4 [ORQ1vQ4], 1.31, 95% confidence interval [CI], 1.11–1.54; P for trend = .005) and TNBC (ORQ1vQ4, 1.32; 95% CI, 1.02–1.71; P for trend = .037). In analyses adjusted additionally for history of breastfeeding and parity, neither parity nor breastfeeding was associated with LumB, but parity was associated with Her2-e (OR, 1.19; 95% CI, 1.09–1.30; P for continuous < .001), and both parity (OR, 1.10; 95% CI, 1.03–1.18; P for continuous = .006) and never breastfeeding (OR, 1.32; 95% CI, 1.08–1.62; P = .007) were associated with TNBC. Nevertheless, adjusting additionally for breastfeeding and parity did not qualitatively alter the ORs for the associations of nSES with BC subtypes (data not shown). We found no evidence for heterogeneity by study cohort in these analyses.
TABLE 2.
ORs of Pooled nSES Quartiles and Breast Cancer Subtypes Relative to LumA (n = 5547)
| Pooled, Study-Based nSES Quartilea | |||||
|---|---|---|---|---|---|
| Q4 (Reference) | Q3 | Q2 | Q1 | P for Trend | |
| LumA (n = 2723) | 751 | 688 | 671 | 613 | |
| LumB (n = 1856) | 432 | 472 | 465 | 487 | |
| OR, age-adjustedb | 1.00 | 1.18 | 1.20 | 1.38 | <.001 |
| 95% CI | 1.01–1.38 | 1.02–1.42 | 1.19–1.60 | ||
| OR, model 1c | 1.00 | 1.15 | 1.15 | 1.29 | .004 |
| 95% CI | 0.98–1.34 | 0.97–1.37 | 1.11–1.50 | ||
| OR, model 2d | 1.00 | 1.16 | 1.16 | 1.31 | .005 |
| 95% CI | 0.99–1.36 | 0.97–1.39 | 1 .11–1.54 | ||
| Q statistice | 0.61 | ||||
| P | .431 | ||||
| Her2-e (n = 267) | 57 | 68 | 72 | 70 | |
| OR, age-adjustedb | 1.00 | 1.27 | 1.40 | 1.52 | .016 |
| 95% CI | 0.91–1.77 | 1.00–1.96 | 1.08–2.15 | ||
| OR, model 1c | 1.00 | 1.26 | 1.36 | 1.41 | .069 |
| 95% CI | 0.89–1.77 | 0.96–1.92 | 0.97–2.05 | ||
| OR, model 2d | 1.00 | 1.18 | 1.27 | 1.28 | .230 |
| 95% CI | 0.84–1.68 | 0.89–1.82 | 0.85–1.92 | ||
| Q statistice | 0.14 | ||||
| P | .705 | ||||
| TNBC (n = 701) | 147 | 159 | 179 | 216 | |
| OR, age-adjustedb | 1.00 | 1.15 | 1.34 | 1.77 | <.001 |
| 95% CI | 0.91–1.44 | 1.06–1.70 | 1.40–2.25 | ||
| OR, model 1c | 1.00 | 1.05 | 1.15 | 1.39 | .007 |
| 95% CI | 0.84–1.33 | 0.90–1.48 | 1.09–1.78 | ||
| OR, model 2d | 1.00 | 1.04 | 1.13 | 1.32 | .037 |
| 95% CI | 0.83–1.31 | 0.87–1.46 | 1.02–1.71 | ||
| Q statistice | 0.58 | ||||
| P | .446 | ||||
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; Her2-e, Her2-enriched; LACE, Life After Cancer Epidemiology; LumA, luminal A; LumB, luminal B; nSES, neighborhood-level socioeconomic status; OR, odds ratio; Q1, quartile 1; Q2, quartile 2; Q3, quartile 3; Q4, quartile 4; SES, socioeconomic status; TNBC, triple-negative breast cancer.
Study-specific nSES quartiles were created and then pooled.
Adjusted for age at diagnosis.
Model 1 adjusted for possible confounders, including age at diagnosis (continuous), race (non-Latina White, Black, Latina, Asian, or other), nativity (US-born or foreign-born), AJCC stage (I, II, III, or IV), days from diagnosis to baseline measures (<52, 52–75, 76–519, or ≥520), and study (LACE or Pathways).
Model 2 adjusted for variables in model 1 plus individual education and individual income.
The Q statistic was calculated for model 2 with a continuous variable for days from diagnosis to study enrollment and a recategorized stage variable in which stages II, III, and IV were collapsed into 1 level (given differences in study recruitment).
Similarly, lower nSES as defined by the state-based quartile was associated with higher odds of all 3 BC subtypes relative to LumA in age-adjusted models (Table 3). Adjustments for potential confounders (model 1) and individual-level SES (model 2) attenuated effect estimates for the Her2-e and TNBC subtypes. In model 2, although effects were highest in Q2, we noted a statistically significant association between nSES and LumB (P for trend = .031). Again, we found no evidence for heterogeneity by study cohort.
TABLE 3.
ORs for the Associations of State-Based nSES Quartiles With Breast Cancer Subtypes Relative to LumA (n = 5547)
| State-Based nSES Quartilea | |||||
|---|---|---|---|---|---|
| Q4 (Reference) | Q3 | Q2 | Q1 | P for Trend | |
| LumA (2723) | 1229 | 849 | 464 | 181 | |
| LumB (n = 1856) | 743 | 610 | 384 | 119 | |
| OR, age-adjustedb | 1.00 | 1.19 | 1.37 | 1.09 | .002 |
| 95% CI | 1.03–1.38 | 1.17–1.61 | 0.83–1.43 | ||
| OR, model 1c | 1.00 | 1.17 | 1.30 | 1.02 | .024 |
| 95% CI | 1.01–1.36 | 1.11–1.53 | 0.78–1.35 | ||
| OR, model 2d | 1.00 | 1.17 | 1.31 | 1.03 | .031 |
| 95% CI | 1.01–1.37 | 1.11–1.55 | 0.77–1.37 | ||
| Q statistice | 0.28 | ||||
| P | .597 | ||||
| Her2-e (n = 267) | 102 | 95 | 47 | 23 | |
| OR, age-adjustedb | 1.00 | 1.35 | 1.24 | 1.59 | .032 |
| 95% CI | 1.02–1.78 | 0.87–1.77 | 0.99–2.54 | ||
| OR, model 1c | 1.00 | 1.33 | 1.16 | 1.39 | .168 |
| 95% CI | 1–1.77 | 0.8–1.68 | 0.82–2.38 | ||
| OR, model 2d | 1.00 | 1.27 | 1.08 | 1.27 | .405 |
| 95% CI | 0.95–1.71 | 0.73–1.61 | 0.74–2.21 | ||
| Q statistice | 1.06 | ||||
| P | .303 | ||||
| TNBC (n = 701) | 261 | 214 | 163 | 63 | |
| OR, age-adjustedb | 1.00 | 1.17 | 1.65 | 1.63 | <.001 |
| 95% CI | 0.96–1.42 | 1.32–2.05 | 1.13–2.34 | ||
| OR, model 1c | 1.00 | 1.07 | 1.41 | 1.18 | .017 |
| 95% CI | 0.88–1.31 | 1.13–1.76 | 0.83–1.68 | ||
| OR, model 2d | 1.00 | 1.05 | 1.35 | 1.09 | .087 |
| 95% CI | 0.86–1.29 | 1.06–1.71 | 0.76–1.56 | ||
| Q statistice | 0.16 | ||||
| P | .684 | ||||
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; Her2-e, Her2-enriched; LACE, Life After Cancer Epidemiology; LumA, luminal A; LumB, luminal B; nSES, neighborhood-level socioeconomic status; OR, odds ratio; Q1, quartile 1; Q2, quartile 2; Q3, quartile 3; Q4, quartile 4; SES, socioeconomic status; TNBC, triple-negative breast cancer.
For each censal year, quartiles were created on the basis of the state distribution of nSES at the census block group level and then were applied to the addresses of the women in the cohort.
Adjusted for age at diagnosis.
Model 1 adjusted for possible confounders, including age at diagnosis (continuous), race (non-Latina White, Black, Latina, Asian, or other), nativity (US-born or foreign-born), AJCC stage (I, II, III, or IV), days from diagnosis to baseline measures (<52, 52–75, 76–519, or ≥520), and study (LACE or Pathways).
Model 2 adjusted for variables in model 1 plus individual education and individual income.
The Q statistic was calculated for model 2 with a continuous variable for days from diagnosis to study enrollment and a recategorized stage variable in which stages II, III, and IV were collapsed into 1 level (given differences in study recruitment).
Lower individual education was associated with higher odds of the Her2-e and TNBC subtypes relative to LumA in age-and multivariate-adjusted models (Table 4). Adjustment for nSES (model 2) slightly attenuated the association with the Her2-e and TNBC subtypes. Additional adjustment for individual income (model 3) did not further attenuate associations; however, the association remained significant for only Her2-e (odds ratio for high school degree or less vs postgraduate, 1.68; 95% CI, 1.03–2.75; P for trend = .030). The Q statistics for associations with LumB and Her2-e suggested study heterogeneity; therefore, study-specific associations are presented.
Lower individual income was not significantly associated with any BC subtype in covariate-adjusted analyses (data not shown).
DISCUSSION
In a large cohort of BC survivors in Northern California, we evaluated the associations of nSES and individual-level SES with BC subtypes. In models simultaneously adjusted for individual-level SES and nSES, pooled SES was associated with LumB and TNBC, state-based nSES was associated only with LumB, and individual education was associated only with Her2-e. Individual income was not associated with BC subtype in any fully adjusted model. Our findings suggest that both lower nSES and individual education are important predictors of aggressive BC subtypes relative to the least aggressive LumA subtype, and this reflects either a lower incidence of LumA BC or a higher incidence of HR− BC. To our knowledge, our study is the first study of multilevel SES and all BC subtypes.
Previous studies evaluating the relationship between SES and BC subtypes have indicated a positive association between low nSES and more aggressive BC subtypes. Most prior studies used cancer registry data and were not able to account for individual-level SES. In studies using California Cancer Registry data, women who lived in areas of higher median household income had lower odds of TNBC,6 whereas women of lower nSES (measured as a composite index score) were overrepresented for Her2-e BC and TNBC,7,9 and Hispanic women of lower nSES had an increased risk of TNBC and Her2-e BC.10 In studies using Surveillance, Epidemiology, and End Results (SEER) data, women diagnosed with BC subtypes other than HR+/Her2− were more likely to live in impoverished counties,2 whereas women living in high-poverty areas had higher odds of TNBC,12 women living in a medium- or high-poverty county had a higher risk of HR− BC,8 and women of higher nSES had a higher risk of HR+ BC subtypes.11 Despite the heterogeneity in methods, including SES measures, sample sizes, and data sources, most studies show that women living in areas characterized by lower SES are more likely to be diagnosed with more aggressive BC subtypes relative to LumA.2,7–13 Our findings are consistent with prior work but provide further evidence of contextual influences independent of the individual influences of income and education.
Mechanisms purported to explain the association between SES and BC subtypes include reproductive and environmental factors and chronic stress. Reproductive factors such as parity, breast feeding, time from menarche to first pregnancy, age at first birth, and oral contraceptive use have each been shown to be differentially associated with BC subtypes.19–22 Over the past several decades, the availability of hormonal contraceptives and an increasing number of women entering the workforce have delayed age at first birth, particularly among women with greater educational attainment.23–25 A 2014 literature review found moderate to strong evidence that nulliparity or low parity, a long interval between menarche and first pregnancy, and a higher age at first birth were all associated with a higher risk of HR+ BC.19 Other studies found that parous women who never breast-fed had an elevated risk of HR− BC,20,21 and breast feeding was associated with a lower risk of TNBC.22 As women with higher levels of education tend to delay childbearing and have lower parity, their risk increases for HR+ BC.19 Conversely, women of lower SES and with less education are less likely to use contraception, are more likely to be younger at first birth, and have higher parity with lower rates of breastfeeding, all of which increase the risk of HR− BC.19,23 Consistent with individual SES trends, low nSES is also predictive of increased adolescent pregnancies and birthrates, decreased contraceptive use,26 and lower rates of breast feeding.27 Low nSES can affect reproductive behaviors, especially in adolescent women, through implicit social norms shaping attitudes around reproductive behavior and through the perception of few opportunities for upward social mobility resulting in lower perception of the costs associated with an unplanned pregnancy.28 Nonetheless, in multivariable models additionally adjusted for history of breastfeeding (ever/never) and parity, ORs for the associations of nSES with BC subtype were unchanged (data not shown).
Environmental exposures may also contribute to findings. Areas of lower SES and higher concentrations of ethnic minorities tend to have higher exposure to air pollutants, hazardous jobs, and deteriorating housing.29 Previous studies have linked environmental exposures to BC,30 and a recent study has shown that heavy metal air pollutants can increase the risk of some BC subtypes.31
The psychological environment may also differentially influence BC subtypes.32 Women of low SES tend to be exposed to chronic stress related to financial insecurity, discrimination, and a lack of safety. Chronic stress may suppress production of estrogen, and this may increase the risk of aggressive BC types.32 Chronic stress may also lead to unhealthy behavioral coping through poorer diet and reductions in physical activity leading to obesity, which is a risk factor for HR− BC in premenopausal women.32,33
The strengths of this study include the ability to adjust simultaneously for nSES and individual-level SES variables, the large study size, and the data on receptor variables and grade that allowed for the careful development of BC subtypes. Unlike registry-based studies, our study cohort affords a unique opportunity to adjust simultaneously for nSES and individual-level SES. Most previous work has evaluated BC subtypes with only IHC markers; however, the use of IHC markers plus Ki-67 or a surrogate marker of cell proliferation such as tumor grade improves the accuracy of approximating BC subtypes and reduces misclassification of the LumA and LumB subtypes.3,4
Inferences about the associations between nSES and BC subtypes should be made cautiously. Women in this study were geocoded on the basis of their residence at the time of study enrollment, and we could not take into account how long women were living in their current residence or any early-life neighborhood exposures. Nonetheless, people tend to move to socioeconomically similar neighborhoods, so this may not be a major concern.34 The women in our cohort tended to live in areas of higher nSES, and this resulted in a skewed distribution and limited power in some analyses when state-based quartiles were being used. To address this imbalance, we pooled study-specific nSES quartiles to ensure an even distribution of women. As a result, the pooled high-nSES reference group represents areas of higher nSES in comparison with the state-based high-nSES reference group. Results for both categorizations of nSES have been presented for comparison. As Her2-e BC is less common, our cohort included relatively few women with the Her2-e subtype, and this resulted in limited power to examine associations. Lastly, our study population included only women diagnosed with BC in Northern California, and this raises concerns about generalizability. Nevertheless, our results are consistent with previous studies conducted with data from national samples (SEER 178 and SEER 182) and with data from a different locale (an Atlanta-based population12), and this allays our concerns.
In summary, we have found that nSES and individual-level SES are independently associated with different BC subtypes relative to LumA. Our results show that low nSES and individual-level education are independent predictors of more aggressive BC subtypes relative to LumA, even after adjustments for covariates and simultaneous adjustments for nSES and individual-level SES. Further study is needed to determine the exact mechanisms by which nSES and individual-level SES affect the risk of BC subtypes.
Supplementary Material
FUNDING SUPPORT
Candyce H. Kroenke reports grants from the National Cancer Institute (R01 CA230440) and American Cancer Society (RSG-16-167-01-CPPB). Lawrence H. Kushi also reports grants from the National Cancer Institute (R01 CA105274 and U01 CA195565), and Jacqueline M. Torres reports grants from the National Institute on Aging (K01 AG056602 and R01 AG069090).
CONFLICT OF INTEREST DISCLOSURES
Scarlett Lin Gomez reports honoraria from Grail and Merck. Lawrence H. Kushi is on the external advisory boards of several National Cancer Institute–funded cancer epidemiology research projects. The other authors made no disclosures.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn H-J. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:619–626. doi: 10.1158/1055-9965.EPI-17-0627 [DOI] [PubMed] [Google Scholar]
- 6.Linnenbringer E, Geronimus AT, Davis KL, Bound J, Ellis L, Gomez SL. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among Black and White women. Breast Cancer Res Treat. 2020;180:437–447. doi: 10.1007/s10549-020-05545-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)–negative, progesterone receptor (PR)–negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618 [DOI] [PubMed] [Google Scholar]
- 8.Andaya AA, Enewold L, Horner M-J, Jatoi I, Shriver CD, Zhu K. Socioeconomic disparities and breast cancer hormone receptor status. Cancer Causes Control. 2012;23:951–958. doi: 10.1007/s10552-012-9966-1 [DOI] [PubMed] [Google Scholar]
- 9.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x [DOI] [PubMed] [Google Scholar]
- 10.Banegas MP, Tao L, Altekruse S, et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat. 2014;144:625–634. doi: 10.1007/s10549-014-2882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinyemiju TF, Pisu M, Waterbor JW, Altekruse SF. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springerplus. 2015;4:508. doi: 10.1186/s40064-015-1282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund MJ, Butler EN, Hair BY, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes. Cancer. 2010;116:2549–2559. doi: 10.1002/cncr.25016 [DOI] [PubMed] [Google Scholar]
- 13.Sineshaw HM, Gaudet M, Ward EM, et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010–2011). Breast Cancer Res Treat. 2014;145:753–763. doi: 10.1007/s10549-014-2976-9 [DOI] [PubMed] [Google Scholar]
- 14.Qin B, Babel RA, Plascak JJ, et al. Neighborhood social environmental factors and breast cancer subtypes among Black women. Cancer Epidemiol Biomarkers Prev. 2021;30:344–350. doi: 10.1158/1055-9965.EPI-20-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan ML, Ambrosone CB, Lee MM, et al. The Pathways study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–1076. doi: 10.1007/s10552-008-9170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) study: a cohort of early stage breast cancer survivors (United States). Cancer Causes Control. 2005;16:545–556. doi: 10.1007/s10552-004-8340-3 [DOI] [PubMed] [Google Scholar]
- 17.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 18.Diggle P, Diggle PJ, Heagerty P, Liang K-Y, Heagerty PJ, Zeger S. Analysis of Longitudinal Data. Oxford University Press; 2002. [Google Scholar]
- 19.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144:1–10. doi: 10.1007/s10549-014-2852-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John EM, Hines LM, Phipps AI, et al. Reproductive history, breastfeeding and risk of triple negative breast cancer: the Breast Cancer Etiology in Minorities (BEM) study. Int J Cancer. 2018;142:2273–2285. doi: 10.1002/ijc.31258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20:1883–1891. doi: 10.1158/1055-9965.EPI-11-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambertini M, Santoro L, Del Mastro L, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76. doi: 10.1016/j.ctrv.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 23.Lyons S, Arcara J, Deardorff J, Gomez AM. Financial strain and contraceptive use among women in the United States: differential effects by age. Womens Health Issues. 2019;29:153–160. doi: 10.1016/j.whi.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Simoni MK, Mu L, Collins SC. Women’s career priority is associated with attitudes towards family planning and ethical acceptance of reproductive technologies. Hum Reprod. 2017;32:2069–2075. doi: 10.1093/humrep/dex275 [DOI] [PubMed] [Google Scholar]
- 25.Livingston G. They’re waiting longer, but U.S. women today more likely to have children than a decade ago Pew Research Center. Published January 18, 2018. Accessed February 16, 2021. https://www.pewresearch.org/social-trends/2018/01/18/theyre-waiting-longer-but-u-s-women-today-more-likely-to-have-children-than-a-decade-ago/ [Google Scholar]
- 26.Decker MJ, Isquick S, Tilley L, et al. Neighborhoods matter. A systematic review of neighborhood characteristics and adolescent reproductive health outcomes. Health Place. 2018;54:178–190. doi: 10.1016/j.healthplace.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 27.Yourkavitch J, Kane JB, Miles G. Neighborhood disadvantage and neighborhood affluence: associations with breastfeeding practices in urban areas. Matern Child Health J. 2018;22:546–555. doi: 10.1007/s10995-017-2423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akers AY, Muhammad MR, Corbie-Smith G. “When you got nothing to do, you do somebody”: a community’s perceptions of neighborhood effects on adolescent sexual behaviors. Soc Sci Med. 2011;72:91–99. doi: 10.1016/j.socscimed.2010.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gochfeld M, Burger J. Disproportionate exposures in environmental justice and other populations: the importance of outliers. Am J Public Health. 2011;101(suppl 1):S53–S63. doi: 10.2105/AJPH.2011.300121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: an updated review of epidemiological literature informed by biological mechanisms. Environ Res. 2018;160:152–182. doi: 10.1016/j.envres.2017.08.045 [DOI] [PubMed] [Google Scholar]
- 31.Kresovich JK, Erdal S, Chen HY, Gann PH, Argos M, Rauscher GH. Metallic air pollutants and breast cancer heterogeneity. Environ Res. 2019;177:108639. doi: 10.1016/j.envres.2019.108639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnenbringer E, Gehlert S, Geronimus AT. Black-White disparities in breast cancer subtype: the intersection of socially patterned stress and genetic expression. AIMS Public Health. 2017;4:526–556. doi: 10.3934/publichealth.2017.5.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–397. doi: 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley SE, Reynolds P, Goldberg DE, et al. Residential mobility in the California Teachers Study: implications for geographic differences in disease rates. Soc Sci Med. 2005;60:1547–1555. doi: 10.1016/j.socscimed.2004.07.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


