Abstract
The ketolides HMR 3004 and HMR 3647 (telithromycin) are a new class of macrolides that have a potential clinical efficacy against intracellular pathogens. The objectives of this study were to investigate the MIC, minimum bactericidal concentration, and time-dependent killing of two Chlamydia pneumoniae strains of the two ketolides. The killing effect was also studied with a newly developed intracellular in vitro kinetic model. Furthermore, HMR 3647 was studied for the effect of a subinhibitory concentration of 0.5 times the MIC after a preexposure of 10 times the MIC during 12 h. The MICs for both strains were 0.0039 and 0.0156 mg/liter for HMR 3004 and HMR 3647, respectively. Killing with 10 times the MIC was time dependent, increasing from a 1-log-unit decrease in the number of inclusions per well at 48 h to a maximal effect of 2.8-log-unit decrease after 96 h. A preexposure of 10 times the MIC of HMR 3647 for 12 h followed by a subinhibitory concentration of 0.5 times the MIC increased the killing effect to a 1.2-log-unit reduction in inclusions per well. An exposure for 12 h gave poor reduction of inclusions, while a static dose of 10 times the MIC for 72 h showed a 2.2-log-unit reduction in inclusions per well. In the kinetic model, a small number of inclusions were detected after 72 h by one exposure of 10 times the MIC. Regrowth could not be detected after 120 h. The ketolides HMR 3004 and HMR 3647 have bactericidal activity and show a significant sub-MIC effect on the intracellular pathogen C. pneumoniae.
Chlamydia pneumoniae is an obligate intracellular bacterium with a growth cycle of 72 h. It is a widespread human pathogen causing a variety of community-acquired respiratory tract infections in adults and children. Several reports have indicated that chronic C. pneumoniae infections may be associated with coronary heart disease, atherosclerosis, and asthma (4, 9, 12, 17). Although the acute infection is usually self-limiting, prolonged respiratory symptoms are sometimes a feature, and in such cases C. pneumoniae has been difficult to eradicate with antibiotics (6, 7). The optimal choice of antibiotic treatment and dosing regimen still needs to be defined.
Pharmacodynamic studies are important tools for the determination of optimal dosing regimens of antimicrobial agents. The ketolides, a new class of macrolides characterized by a 3-keto function, which gives a strong acid stability (3), act by inhibiting bacterial protein synthesis. The ketolides have shown in vitro activity against a broad range of respiratory tract pathogens (1, 2, 15; I. Odenholt, E. Löwdin, and O. Cars, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 540, 1999). Although macrolide antibiotics show long postantibiotic effects, their antibacterial activity is not concentration dependent. The major determinant of efficacy is the time that free drug concentrations are greater than the MIC (5). The pharmacodynamics of the ketolides is less well known. However, significant postantibiotic effect and postantibiotic sub-MIC effect have been demonstrated for the ketolides (Odenholt et al., 39th ICAAC). They have been shown to accumulate in phagocytes (19, 20) and have potential clinical efficacy against intracellular pathogens, e.g., Legionella spp. (18) and C. pneumoniae (16).
We have investigated the MICs, minimum bactericidal concentrations (MBCs) and pharmacodynamics for two C. pneumoniae strains of the two ketolides HMR 3004 and HMR 3647 with static antibiotic concentrations. HMR 3647 was also studied with respect to the effect of a subinhibitory concentration following a preexposure to 10 times the MIC during 12 h. Furthermore, we developed an intracellular kinetic model where the killing could be monitored for up to 5 days.
MATERIALS AND METHODS
Cell culture.
Human epithelial cell line HEp-2 (ATCC CCL 23) was grown in medium consisting of RPMI 1640 (Gibco BRL, Life Technologies Ltd., Paisly, United Kingdom), 10% fetal calf serum, 20 mM HEPES, 2 mM glutamine, and 0.05% NaHCO3 at 35°C in 5% CO2. The cells were treated with trypsin and distributed in 48-well cell culture plates at a density of 105 to 2 × 105 cells/ml and grown for 24 h to a monolayer. In the kinetic model, Falcon cell culture inserts (pore size, 0.4 μm) were used instead of cell culture plates. During the experiments 5% fetal calf serum was used, and the medium was supplemented with 0.03 M glucose and 1 μg of cycloheximide (Sigma, St. Louis, Mo.)/ml. The pH was 7.2 ± 0.2 throughout the experiments.
Preparation of antibiotics.
HMR 3004 and HMR 3647 with known potencies were kindly provided by Hoechst-Marion-Roussel, Romainville, France. A fresh stock solution was made before each experiment by dissolving 10.2 mg of the substance in 10 ml of sterile distilled water supplemented with 2 drops of glacial acetic acid.
Bacteria.
C. pneumoniae CWL 029 (ATCC VR 1310) and a clinical strain of C. pneumoniae (G 954) derived from a patient at the University Hospital, Uppsala, Sweden, were used. The strains were stored at −70°C. The inoculum was adjusted before the experiments to give 5 × 103 to 104 inclusion-forming units per ml. Unexposed controls were included in all experiments to verify the inoculum size.
Infection of cells.
The monolayer of the HEp-2 cells was inoculated with 0.5 ml of bacterial dilution in cell culture medium. The plates were centrifuged for 1 h at 3,600 rpm at 30°C and incubated for 2 h at 37°C. For this process the inserts were placed in six-well cell culture plates. The medium was changed to a fresh medium with 5% fetal calf serum, 1 μg of cycloheximide/ml, 0.03 M glucose, and the antibiotics in different concentrations (see below).
Determination of MIC.
Twofold serial dilutions from 0.125 to 0.00195 mg/liter were prepared in cell culture medium. Infected cells were exposed to the different concentrations and incubated at 35°C for 72 h. The cultures were fixed in methanol for 10 min and stained with monoclonal antibodies for Chlamydia spp. (Pathfinder; Kallestad Diagnostics, Chaska, Minn.). The results were assessed with an inverted fluorescence microscope (Ziess Diavert). MIC was defined as the lowest concentration with a minimum of 95% reduction of inclusions compared with the controls. The determination of MIC was made in triplicate.
Determination of MBCs.
The two strains were exposed to 5, 10, 20, 40, 80, 160, and 320 times the MIC of the antibiotics. After 72 h of incubation at 35°C, the cultures were washed three times with phosphate-buffered saline (PBS), pH 7.2, scraped with a transfer pipette, and passed onto a new monolayer of HEp-2 cells. After centrifugation for 1 h at 2,500 × g at 30°C and penetration for 2 h, the medium was changed to a fresh medium with 5% fetal calf serum, 1 μg of cycloheximide/ml, 0.03 M glucose, and 20 mg of gentamicin/liter. The plates were thereafter incubated for another 72 h before staining and evaluation. MBC was defined as the lowest antibiotic concentration at which no inclusions were observed. The determination of MBC was made in triplicate.
Determination of antibiotic concentration.
Samples from the inserts were collected during the kinetic experiments. A microbiological agar diffusion method was used with tryptone-glucose agar, pH 7.4. Plates were seeded with a standardized inoculum of Sarcina lutea. Antibiotic standards and samples from the inserts were placed into agar wells at a volume of 0.03 ml. All assays were made in triplicate, and the plates were incubated overnight at 37°C. The limit of detection was 0.03 mg/liter, and the correlation coefficient for the standard curves was always >0.99.
Pharmacodynamics after exposure with 10 times the MIC.
Infected cells in a 48-well cell culture plate were exposed to 10 times the MIC of the respective antibiotic in medium. Controls without antibiotics were included. Duplicates were made for direct staining and passage to new cells (cf. the MBC method) after 24, 48, 72, and 96 h. The new plates were incubated for 72 h. The experiments were made in triplicate.
The effect of a subinhibitory concentration of HMR 3647 after previous exposure to 10 times the MIC.
Cell cultures were infected as described above and exposed to 10 times the MIC of HMR 3647. After 12 h the medium was aspirated and the cultures were washed three times with PBS and reexposed to a subinhibitory concentration of 0.5 times the MIC. For comparison three sets of cultures were included: (i) cultures exposed to 10 times the MIC during 12 h, washed three times with PBS, and covered with fresh medium for regrowth; (ii) cultures exposed to 10 times the MIC during 72 h; and (iii) unexposed controls. After 72 h of incubation at 35°C, all cultures were stained for examination as well as washed and passaged as described above. The experiments were made in triplicate.
In vitro kinetic model.
The model has previously been described (11). It consists of a glass chamber with two exits and a metal rack fitting Falcon cell culture inserts (Becton Dickinson, Franklin Lake, N.J.) (Fig. 1). The inserts incorporated membranes with 0.45-μm pores. The glass chamber was connected to a pump (C6-T; Alitea AB, Stockholm, Sweden) by Santoprene tubes (Alipren; Alitea AB). A magnet was placed in the bottom of the glass chamber to ensure homogenous mixing of the antibiotic. The device was placed on a magnetic stirrer in a thermostat at 35°C with 5% CO2.
FIG. 1.
The pump is connected to the glass chamber with the cell culture inserts.
HEp-2 cells were prepared in cell culture inserts and infected with the C. pneumoniae strains as mentioned above. The medium in the inserts was changed to 0.4 ml of cell culture medium with the antibiotic at a concentration of 10 times the MIC. The inserts were transferred into the glass chamber that contained the same medium. Dilution of the antibiotic concentration was achieved by a constant flow of fresh medium supplied by a pump. Controls were performed in accordance with the kinetic model and yielded the same number of inclusions as controls cultured in the companion plate for inserts. In the experiments the latter controls were used. For HMR 3647, the half-life inside the inserts was adjusted to 10 h. The experiment continued for 72 h, after which two inserts of each strain were fixed and stained. The remaining two inserts were passaged as described above for detection of viable bacteria. The controls were treated in the same manner. The experiments were made in triplicate.
The HMR 3004 experiments were designed in a slightly different way. HEp-2 cells were infected as described above. The medium in the inserts was changed to 0.4 ml of cell culture medium with 10 times the MIC of HMR 3004 and transferred into the glass chamber that contained the same medium. The pump was adjusted to give a half-life of approximately 5 h inside the inserts. A constant flow of fresh medium without antibiotics continued for 48 h. Then, the inserts were removed to a Falcon companion TC plate without antibiotics, and the experiment continued in a static way for 72 h, for a total of 120 h. However, the medium was changed daily. Controls without antibiotics were grown simultaneously with inserts placed in a companion plate. Inserts were fixed and stained for detection of C. pneumoniae after 24, 72, and 120 h. The experiments were made in triplicate.
RESULTS
MIC and MBC.
The MICs and MBCs are presented in Table 1.
TABLE 1.
MICs and MBCsa
| C. pneumoniae strain | HMR 3004
|
HMR 3647
|
||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| CWL 029 | 0.0039 | 0.624–1.25 | 0.0156 | 2.5–5 |
| G 954 | 0.0039 | 0.312–0.624 | 0.0156 | 0.312–2.5 |
Units for all values are milligrams per liter.
Pharmacodynamics after static exposure with 10 times the MIC.
In the exposed cultures that were stained directly after 24, 48, 72, and 96 h, inclusions could not be detected. The results from the passaged cultures are presented in Fig. 2. At 24 h, the mean reduction in number of inclusions per well was only 0.5 log units, compared with the initial inoculum. The killing effect significantly increased with the time. After 96 h the reduction was 2.8 log inclusions/well. There was no difference between the two ketolides. In the unexposed controls that were passaged, almost every cell in the monolayer contained an inclusion body and the increase in inclusion-forming units was by a factor of 10 to 15.
FIG. 2.
Pharmacodynamics with 10 times the MIC. ⧫, CWL 029; ■, G 954; ▴, control CWL 029; ×, control G 954. Shown are means of three experiments ± standard deviations with HMR 3004 (a) and HMR 3647 (b). Incl, inclusions.
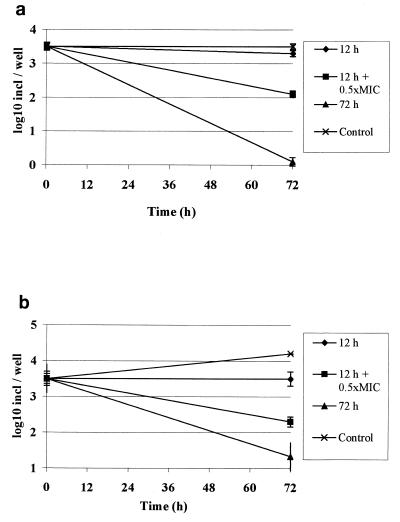
Effect of reexposure to a subinhibitory concentration with HMR 3647.
The results are exhibited in Fig. 3, where the prominent effect of a subinhibitory concentration is obvious. Since there was no difference between the strains, both are included in the figure.
FIG. 3.
Effect of reexposure to a subinhibitory concentration with HMR 3647, i.e., an initial exposure of 10 times the MIC during 12 h followed by 0.5 times the MIC. As controls, cultures were exposed during 12 and 72 h and one culture was unexposed. Shown are means of six experiments ± standard deviations. (a) Direct staining after 72 h. (b) Passaged cultures after 72 h. Incl, inclusions.
Pharmacodynamics in the in vitro kinetic model.
The results for the passaged cultures with HMR 3647 are given in Table 2. The mean initial antibiotic concentration was 0.15 ± 0.013 mg/liter and fell below MIC after 33 h. The initial number of inclusions was 2,500 ± 800 per well. In the samples stained after 72 h, no inclusions could be detected. In the passaged cultures, a reduction of 2 log units during 72 h was observed.
TABLE 2.
Pharmacodynamics of HMR 3647 in the in vitro kinetic modela
| Strain | No. of inclusions/insertb for indicated type of exposure
|
|
|---|---|---|
| 10 times MIC initially | Controls | |
| CWL 029 | 35 ± 33 | ∼18,000 |
| G 954 | 23 ± 27 | ∼18,000 |
MIC was reached at 33 h.
Means of three experiments ± standard deviations. Detected by passage after 72 h.
The results from the kinetic studies with HMR 3004 are presented in Table 3. The initial antibiotic concentration was 0.043 ± 0.0043 mg/liter and fell below MIC after 17 h. A significant inhibition was observed already after 72 h, and there was no regrowth in the cultures up to 120 h.
TABLE 3.
Pharmacodynamics of HMR 3004 in the in vitro kinetic modela
| Strain | No. of inclusions/insertb at:
|
||
|---|---|---|---|
| 72 h | 120 h | Controls | |
| CWL 029 | 6 ± 0.7 | 0 | 3,000 ± 1,000 |
| G 954 | 6 ± 6 | 0 | 3,400 ± 1,000 |
MIC was reached at 17 h.
Means of three experiments ± standard deviations. Detected by direct staining.
DISCUSSION
Both ketolides showed low MICs against the two C. pneumoniae strains, which corresponds well with earlier findings by Roblin and Hammerschlag (16). They found a MIC at which 50% of the isolates were inhibited of 0.0625 μg/ml and an MBC ranging from 0.031 to 2 μg/ml for HMR 3647 against 19 isolates of C. pneumoniae. The MIC is well achieved with HMR 3647 for which a dose of 800 mg gives a maximum concentration in serum of 2 mg/liter and a free concentration in serum of 0.6 mg/liter (Hoechst-Marion-Roussel, unpublished data). The killing experiments with a static concentration of 10 times the MIC showed that 72 to 96 h of exposure was needed to obtain a maximal bactericidal effect. This is probably not caused by slow uptake of the drug into the cells since it has been shown for human polymorphonuclear neutrophils and nonphagocytizing cells that the ketolides are avidly accumulated with high ratios of cellular to extracellular concentrations (19, 20; I. Garcia, A. Pascual, S. Ballesta, and E. J. Perea, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-112, 1998). Instead, the time lag to maximal killing may be related to the life cycle of chlamydia. Since ketolides bind to the 50S subunit of the ribosome, the chlamydia elementary bodies have to reorganize to the metabolically active form, reticulate bodies, before the drug can take effect. A study by Nyström-Rosander et al. showed the importance of sufficient antibiotic availability during the complete growth cycle (13). A total reduction could not be expected since elementary bodies are not susceptible to antibiotics and stay in a latent phase even in cell cultures (8).
However, when HMR 3647 at a sub-MIC concentration of 0.5 times the MIC was added after 12 h of exposure of 10 times the MIC, the reduction of inclusions was significant but not as pronounced as after a static exposure of 10 times the MIC for 72 h. The subinhibitory concentration was obviously sufficient to maintain a critical concentration of drug at the site of the ribosomes. This effect corresponds well with studies on macrolides and azithromycin, where long postantibiotic sub-MIC effects have been described and could be explained by the fact that the ketolides form a complex with 23S rRNA which is 10-fold stronger than that formed by erythromycin (10, 14).
In the kinetic model, a half-life of 5 h was chosen for HMR 3004 in order to speed up the elimination and see if regrowth of chlamydia would occur. The experiments exhibited a vast reduction of inclusions after 72 h. Regrowth within 5 days could not be detected in the exposed cultures. For HMR 3647, the half-life was adjusted to 10 h, which corresponds with the elimination half-life of the drug found in humans. These experiments were run for 72 h. A reduction of 2 log units was obtained with one initial dose of 10 times the MIC, as determined by transfer to new cells, which indicates prolonged bactericidal effect even when the concentration fell below the MIC.
Our results have shown that the ketolides HMR 3004 and HMR 3647 have bactericidal activity and show a significant sub-MIC effect on the intracellular pathogen C. pneumoniae.
ACKNOWLEDGMENT
This work was supported by grants from Hoechst-Marion-Roussel, Romainville, France.
REFERENCES
- 1.Barry A L, Fuchs P C, Brown S D. In vitro activities of the ketolide HMR 3647 against recent gram-positive clinical isolates and Haemophilus influenzae. Antimicrob Agents Chemother. 1998;42:2138–2140. doi: 10.1128/aac.42.8.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of HMR 3647, a new ketolide, on respiratory pathogens, enterococci and Bacteroides fragilis demonstrated by studies of time-kill kinetics and postantibiotic effect. J Antimicrob Chemother. 1998;41:149–153. doi: 10.1093/jac/41.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Bryskier A, Agouridas C, Chantot J F. Ketolides: new semi-synthetic 14-membered ring macrolide. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides and streptogramins. New York, N.Y: Marcel Dekker Inc.; 1997. pp. 39–50. [Google Scholar]
- 4.Campbell L A, O'Brien E R, Cappuccio A L, Kuo C, Wang S, Stewart D, Patton D L, Cummings P K, Grayston J T. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 5.Carbon C. Pharmacodynamics of macrolides, azalides and streptogramins: effect on extracellular pathogens. Clin Infect Dis. 1998;27:28–32. doi: 10.1086/514619. [DOI] [PubMed] [Google Scholar]
- 6.Falck G, Gnarpe J, Gnarpe H. Persistent Chlamydia pneumoniae infection in a Swedish family. Scand J Infect Dis. 1996;28:271–273. doi: 10.3109/00365549609027171. [DOI] [PubMed] [Google Scholar]
- 7.Falck G, Engstrand I, Gad A, Gnarpe J, Gnarpe H, Laurila A. Demonstration of Chlamydia pneumoniae in patients with chronic pharyngitis. Scand J Infect Dis. 1997;29:585–589. doi: 10.3109/00365549709035899. [DOI] [PubMed] [Google Scholar]
- 8.Gnarpe J, Eriksson K, Gnarpe H. In vitro activities of azithromycin and doxycyclin against 15 isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 1996;40:1843–1845. doi: 10.1128/aac.40.8.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn D L, Dodge R W, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis and adult-onset asthma. JAMA. 1991;226:225–230. [PubMed] [Google Scholar]
- 10.Hansen L H, Mauvais P, Douthwaite S. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol Microbiol. 1999;31:623–631. doi: 10.1046/j.1365-2958.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 11.Hultén K, Rigo R, Gustafsson I, Engstrand L. New pharmacokinetic in vitro model for studies of antibiotic activity against intracellular microorganisms. Antimicrob Agents Chemother. 1996;40:2727–2731. doi: 10.1128/aac.40.12.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyström-Rosander C, Thelin S, Hjelm E, Lindquist O, Påhlsson C, Friman G. High incidence of Chlamydia pneumoniae in sclerotic heart valves of patients undergoing aortic valve replacement. Scand J Infect Dis. 1997;29:361–365. doi: 10.3109/00365549709011831. [DOI] [PubMed] [Google Scholar]
- 13.Nyström-Rosander C, Hultén K, Gustafsson I, Cars O, Engstrand L, Hjelm E. Susceptibility of Chlamydia pneumoniae to azithromycin and doxycyclin: methodological aspects on the determination of minimal inhibitory and minimal bactericidal concentrations. Scand J Infect Dis. 1997;29:513–516. doi: 10.3109/00365549709011865. [DOI] [PubMed] [Google Scholar]
- 14.Odenholt-Tornqvist I, Löwdin E, Cars O. Postantibiotic effects and postantibiotic sub-MIC effects of roxithromycin, clarithromycin and azithromycin on respiratory tract pathogens. Antimicrob Agents Chemother. 1995;39:221–226. doi: 10.1128/aac.39.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinert R R, Bryskier A, Lütticken R. In vitro activities of the new ketolide antibiotics HMR 3004 and HMR 3647 against Streptococcus pneumoniae in Germany. Antimicrob Agents Chemother. 1998;42:1509–1511. doi: 10.1128/aac.42.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roblin P M, Hammerschlag R. In vitro activity of a new ketolide antibiotic, HMR 3647, against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1998;42:1515–1516. doi: 10.1128/aac.42.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saikku P, Leinonen M, Mattila K, Ekman M R, Nieminen M S, Mäkelä P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 18.Schulin T, Wennersten C B, Ferraro M J, Moellering R C, Jr, Eliopoulos G M. Susceptibilities of Legionella spp. to newer antimicrobials in vitro. Antimicrob Agents Chemother. 1998;42:1520–1523. doi: 10.1128/aac.42.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazifeh D, Preira A, Bryskier A, Labro T. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1998;42:1944–1951. doi: 10.1128/aac.42.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]