ABSTRACT
Antiestrogen resistance is a major clinical limitation in treatment of breast cancer. We have recently reported that Aurora A and Mcl-1 (myeloid cell leukemia 1) are potential novel treatment targets in antiestrogen-resistant breast cancer cells and that Aurora A expression is a biomarker for tamoxifen resistance in breast cancer patients.
Abbreviations: Bcl-2, B-cell lymphoma 2; EGF, epidermal growth factor; ERα, estrogen receptor α; Mcl-1, myeloid cell leukemia 1; VEGF, vascular endothelial growth factor.
KEYWORDS: Breast cancer, antiestrogen resistance, tamoxifen, fulvestrant, kinase inhibitors, Mcl-1, Aurora A
Antiestrogens, such as tamoxifen and fulvestrant, are commonly used for treatment of estrogen receptor α (ERα) positive breast cancer; however, their efficacy is significantly limited by development of resistance. Up to one-third of patients receiving adjuvant tamoxifen treatment and almost all patients with advanced disease eventually develop resistance,1 emphasizing the need for new therapeutic strategies. Molecular mechanisms underlying resistance to antiestrogen therapy are complex and possibly multifactorial, and it is still unclear which signaling pathways are the main drivers of the resistant cell growth.2
We have recently reported the finding of two new players in antiestrogen resistance: the cell cycle regulator Aurora kinase A (also known as Aurora A) and the anti-apoptotic protein myeloid cell leukemia 1 (Mcl-1).3 We used a large kinase inhibitor screen to identify compounds with preferential growth inhibition of tamoxifen- and fulvestrant-resistant breast cancer cell lines compared with their parental antiestrogen-sensitive cell line, and by exploring selected inhibitor targets, we could disclose important drivers of antiestrogen-resistant growth. Our data showed that tamoxifen- and fulvestrant-resistant cell lines used common signaling pathways, e.g. Mcl-1 was found upregulated and essential for growth of both tamoxifen- and fulvestrant-resistant cell lines, whereas tamoxifen-resistant cell lines also used specific pathways for growth, such as signaling through Aurora A.3 A schematic presentation of a tamoxifen-resistant breast cancer cell utilizing Aurora A and Mcl-1 for cell proliferation and survival, respectively, is shown (Figure 1).
Figure 1.
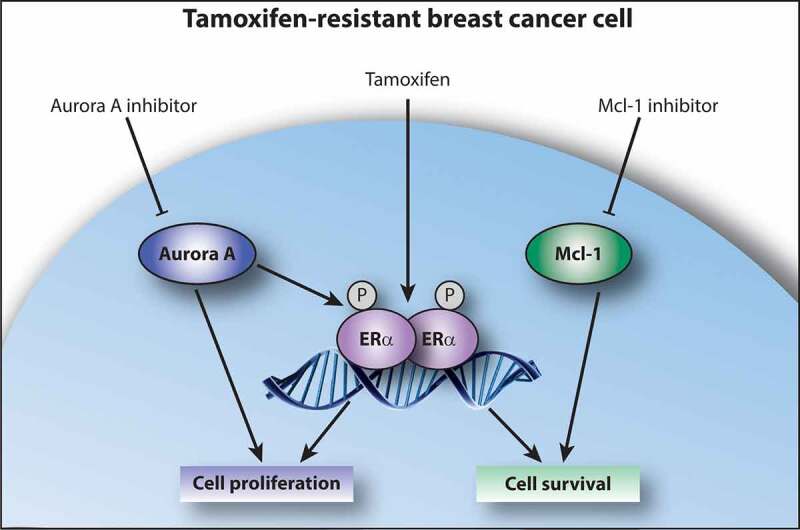
Aurora A and Mcl-1 signaling in tamoxifen-resistant breast cancer cells. Signaling through Aurora A results in cell proliferation, which is, at least partly, mediated by ligand-independent activation of ERα via phosphorylation. Tamoxifen acts as an agonist in tamoxifen-resistant cells, thus stimulating cell growth, and inhibition of Aurora A re-sensitizes resistant cells to tamoxifen treatment. Signaling through Mcl-1 results in cell survival, and inhibition of Mcl-1 causes apoptotic cell death in tamoxifen-resistant cells. ERα, estrogen receptor α; Mcl-1, myeloid cell leukemia 1; P indicates phosphorylation of ERα.
Aurora A is a highly conserved serine/threonine protein kinase, which regulates centrosome maturation, assembly of the bipolar spindle, and chromosome separation at mitosis.4 Overexpression of Aurora A is commonly found in cancer, including breast cancer, where a correlation between high Aurora A expression and poor outcome was shown.5 This correlation was limited to ERα positive breast cancer, suggesting a link between Aurora A and ERα. We analyzed the expression of Aurora A in ERα positive primary breast tumors from 244 patients, who had received adjuvant tamoxifen therapy, and our data revealed a significant association between higher Aurora A expression and shorter time to recurrence and death.3 These data indicate that Aurora A expression is an independent biomarker for selection of patients, who will progress on tamoxifen treatment.
The vast majority of breast cancers are classified as ERα positive. ERα regulates numerous genes involved in cell proliferation and survival, thus promoting breast cancer growth.2,6 Tamoxifen prevents the estrogen-dependent activation of ERα; however, ERα can also be activated in a ligand-independent manner, e.g. by phosphorylation via growth factor signaling pathways, which may result in tamoxifen resistance.6 In addition, tamoxifen can exert agonistic effects on ERα, depending on the co-regulators recruited to ERα bound at estrogen response elements on the DNA, and it has been shown that ERα binding profiles can predict outcome in tamoxifen-treated breast cancer patients.7 We have previously shown that ERα is important for growth of tamoxifen-resistant cells,8 and our finding, that treatment with an Aurora kinase inhibitor or knockdown of Aurora A re-sensitized the tamoxifen-resistant cells to tamoxifen,3 suggests that Aurora A is involved in ERα activation. In support of this, a recent work by Zheng et al. showed that Aurora A can abrogate the sensitivity of breast cancer cells to tamoxifen through phosphorylation of ERα.9 Therefore, we presume that the growth-promoting function of Aurora A in our tamoxifen-resistant cell lines is, at least partly, mediated through ERα (Figure 1). In fulvestrant-resistant cells, binding of fulvestrant results in ERα degradation, thus ligand-independent ERα activation is not an option, which might explain why Aurora A inhibitors did not preferentially target fulvestrant-resistant cell lines in our study.3
Anti-apoptotic proteins are often overexpressed in drug resistance to enable survival of the resistant cancer cells and these proteins may represent attractive new therapeutic targets.2 We showed that both tamoxifen- and fulvestrant-resistant cell lines expressed increased levels of the anti-apoptotic protein Mcl-1 compared with their parental cell lines, and knockdown of Mcl-1 in resistant cells was sufficient for inducing cell death.3 These results indicate that Mcl-1 upregulation might represent a mechanism used by antiestrogen-resistant cells to survive treatment. Mcl-1 is a member of the B-cell lymphoma 2 (Bcl-2) family and exerts its anti-apoptotic function by binding and sequestering of pro-apoptotic Bcl-2 family members, thus preventing cytochrome c release from the mitochondria.10 Its stability is tightly regulated by ubiquitination, phosphorylation, and cleavage, and MCL1 gene expression can be induced by different growth factors, such as epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF).10 Notably, signaling pathways activated from these growth factors have been associated with antiestrogen resistance.2 In contrast to inhibition of Aurora A, Mcl-1 inhibition did not re-sensitize to antiestrogen treatment.3 Thus, Mcl-1 presumably works through ERα-independent mechanisms, suggesting that Mcl-1 and Aurora A may represent different mechanisms underlying antiestrogen resistance.
It is our hope that our recent work will contribute to the scientific field with new insights into the molecular mechanisms of antiestrogen resistance, which prospectively could lead to better treatment options for breast cancer patients. Notably, Aurora kinases have lately drawn much attention as novel treatment targets in cancer and several inhibitors of Aurora A are currently undergoing clinical trials as anti-cancer therapy.4 Thus, clinical studies are highly warranted to evaluate the potential of Aurora A and Mcl-1 as new biomarkers and drug targets in antiestrogen-resistant breast cancer.
Acknowledgments
I thank Anne E. Lykkesfeldt for helpful comments to the manuscript.
Funding Statement
This work was supported by A Race Against Breast Cancer, Astrid Thaysen’s grant, Danish Cancer Research Foundation, Danish Cancer Society, and Harboe Foundation.
Disclosure statement
No potential conflicts of interest was reported by the author.
References
- 1.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musgrove EA, Sutherland RL.. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 3.Thrane S, Pedersen AM, Thomsen MB, Kirkegaard T, Rasmussen BB, Duun-Henriksen AK, Laenkholm AV, Bak M, Lykkesfeldt AE, Yde CW. A kinase inhibitor screen identifies Mcl-1 and Aurora kinase A as novel treatment targets in antiestrogen-resistant breast cancer cells. Oncogene. 2014;34:4199–4210. doi: 10.1038/onc.2014.351. [DOI] [PubMed] [Google Scholar]
- 4.Goldenson B, Crispino JD. The Aurora kinases in cell cycle and leukemia. Oncogene. 2014;34:537–545. doi: 10.1038/onc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali HR, Dawson SJ, Blows FM, Provenzano E, Pharoah PD, Caldas C. Aurora kinase A outperforms Ki67 as a prognostic marker in ER-positive breast cancer. Br J Cancer. 2012;106:1798–1806. doi: 10.1038/bjc.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 7.Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012;481:389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thrane S, Lykkesfeldt AE, Larsen MS, Sorensen BS, Yde CW. Estrogen receptor alpha is the major driving factor for growth in tamoxifen-resistant breast cancer and supported by HER/ERK signaling. Breast Cancer Res Treat. 2013;139:71–80. doi: 10.1007/s10549-013-2485-2. [DOI] [PubMed] [Google Scholar]
- 9.Zheng XQ, Guo JP, Yang H, Kanai M, He LL, Li YY, Koomen JM, Minton S, Gao M, Ren XB, et al. Aurora-A is a determinant of tamoxifen sensitivity through phosphorylation of ERalpha in breast cancer. Oncogene. 2013;33:4985–4996. doi: 10.1038/onc.2013.444. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]


