Abstract
Respiratory depression is the proximal cause of death in opioid overdose, yet the mechanisms underlying this potentially fatal outcome are not well understood. The goal of this review is to provide a comprehensive understanding of the pharmacological mechanisms of opioid-induced respiratory depression, which could lead to improved therapeutic options to counter opioid overdose, as well as other detrimental effects of opioids on breathing. The development of tolerance in the respiratory system is also discussed, as are differences in the degree of respiratory depression caused by various opioid agonists. Finally, potential future therapeutics aimed at reversing or avoiding opioid-induced respiratory depression through non- opioid receptor targets are in development and could provide certain advantages over naloxone. By providing an overview of mechanisms and effects of opioids in the respiratory network, this review will benefit future research on countering opioid-induced respiratory depression.
Keywords: addiction, respiratory pharmacology, electrophysiology, opioids, mu opioid receptor, brainstem, control of breathing
1. Introduction
Opioid analgesics are among the most frequently prescribed drugs worldwide, and a mainstay of modern medicine. This is despite the existence of serious side effects, including abuse liability and respiratory depression (Khanna et al., 2020). The combination of the high abuse liability and lethality of respiratory depression has led to an exponentially increasing death toll from opioid overdose in the past 15 years and a serious public health crisis (Wilson et al., 2020). Of recent concern is a surge in opioid overdose during the COVID-19 pandemic (Haley and Saitz, 2020). The current opioid epidemic is largely driven by overdoses of fentanyl and fentanyl derivatives, though heroin and prescription opioids also contribute (Wilson et al., 2020). Certainly, the addictive nature of opioids is a primary driver of the opioid epidemic and improved treatment of opioid use disorder is essential to curbing misuse. However, preventing fatalities from opioid-induced respiratory depression is a more immediately addressable approach and an important focus for pharmacological research. In addition, respiratory depression does not only occur with misuse. Respiratory depression also occurs in hospitalized patients (Khanna et al., 2020), in individuals taking opioids for chronic pain (Algera et al., 2020), in the case of accidental exposure (Madadi et al., 2013) or as chemical warfare (Riches et al., 2012). Certain patients are at higher risk, including those who are morbidly obese, have sleep apnea, chronic heart failure or certain neuromuscular diseases, or are on the age extremes (i.e. geriatric or premature infants) (Dahan et al., 2010; Khanna et al., 2020). Given the immediate need to curb overdoses and the broad range of affected individuals, countering respiratory depression is a fundamental aspect of combating the opioid crisis.
Fatality from opioid overdose occurs following hypoventilation, sustained apnea (the cessation of breathing) and subsequent cardiorespiratory arrest (Dahan et al., 2010). While respiratory arrest is the most fatal consequence, opioids cause disturbances detrimental to patient outcome prior to complete apnea, many of which occur with doses in the therapeutic range, and with treatments used for addiction (i.e. methadone) (Teichtahl et al., 2005). By highlighting previous work on the pharmacology of opioid-induced respiratory depression and mechanisms of opioids in the pontomedullary respiratory network, this review will provide a framework for future studies aimed at pharmacological approaches to counter, or prevent, opioid-induced respiratory depression.
2. Opioid effects on respiration
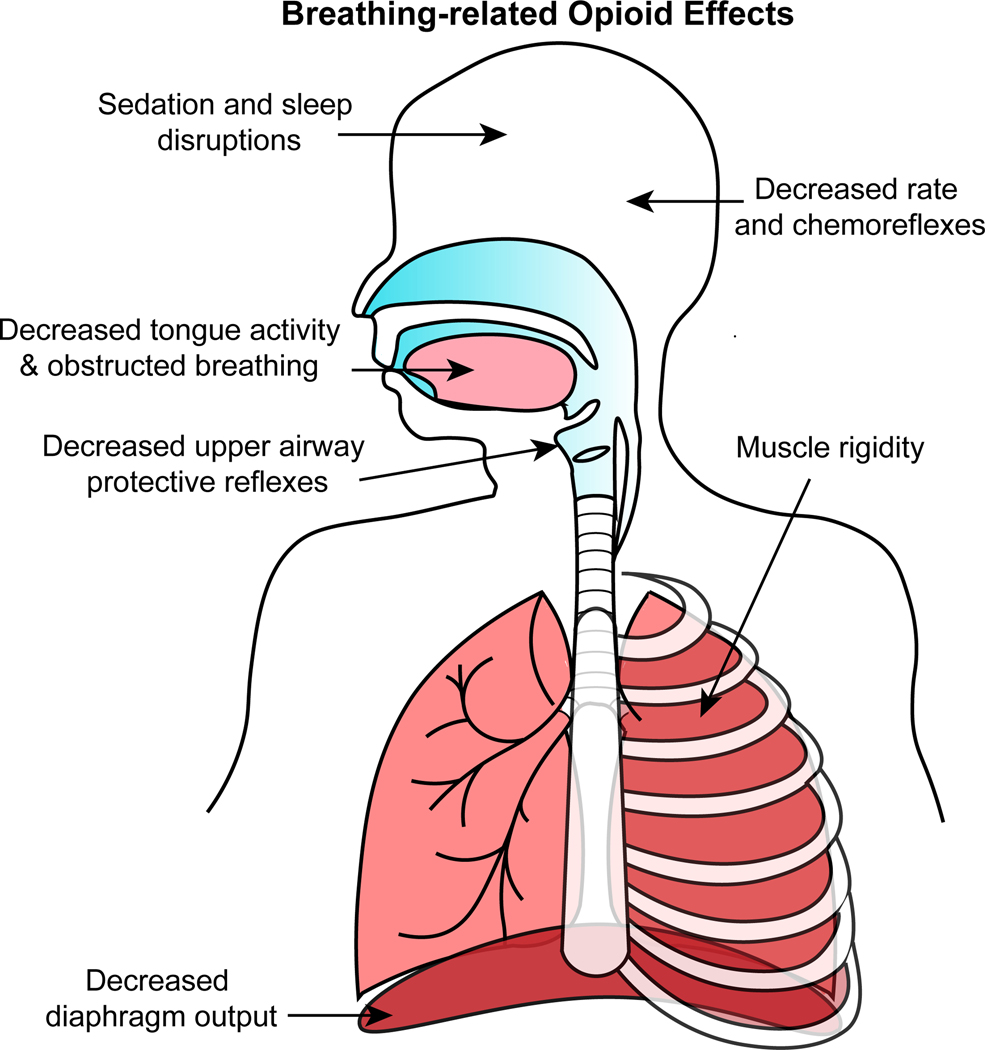
Opioid-induced respiratory depression is characterized by slow, shallow and irregular breathing, which in severe cases leads to respiratory arrest (i.e. sustained apnea). Opioids also impair chemoreflexes and upper airway patency, and induce muscle rigidity, sedation and sleep-disordered breathing - all of which compromise patient outcomes and will be reviewed in more detail below (Figure 1).
Figure 1.
Summary diagram of breathing-related effects of opioids observed in humans. Opioids cause sedation, sleep disruption, sleep-disordered breathing, obstructed breathing and muscle rigidity. Opioids decrease respiratory rate, chemoreflexes, and diaphragm activity. Description of these effects and potential underlying mechanisms are reviewed in the text (section 2. Opioid effects on respiration).
Hypoventilation and variability
The most studied effects of opioids on breathing are changes in ventilation, including rate, amplitude and variability, which occurs across many species, including humans, rats, mice, dogs, cats and rabbits (Lalley, 2003; Ferguson and Drummond, 2006; Prkic et al., 2012; Miller et al., 2017; Varga et al., 2020b). Respiratory rate is the most severely and consistently depressed component of ventilation (Ferguson and Drummond, 2006). Opioids also cause a dose-dependent change in tidal volume. At low doses, tidal volume remains unchanged, or even slightly elevated due to compensation from chemoreflexes (Lalley, 2003; Ferguson and Drummond, 2006; Hill et al., 2020). At higher doses, tidal volume decreases, which is more pronounced with certain agonists, like fentanyl (Hill et al., 2020). The reduction in tidal volume occurs with decreased activity of phrenic motor neurons, which innervate the diaphragm (Ren et al., 2009; Prkic et al., 2012; Saunders and Levitt, 2020), and is confounded in some cases by rigidity of chest wall muscles ((Torralva and Janowsky, 2019), and discussed further below).
Pharmacokinetics influences the progression of hypoventilation to apnea. Hypoventilation impairs pulmonary gas exchange resulting in oxygen desaturation and CO2 retention. Gradual administration of opioids permits central chemoreceptors to compensate for slowly rising arterial carbon dioxide levels (PaCO2) and is therefore less likely to induce apnea (Pattinson, 2008). With rapid or bolus administration, apnea can occur before PaCO2 rises to stimulate compensatory increases in ventilation. This pharmacokinetic effect contributes to the high lethality of fentanyl, which has a rapid rate of onset (Pattinson, 2008; Kiyatkin, 2019; Hill et al., 2020). In addition to hypoventilation, opioids increase breathing variability. This variability often appears at low doses and interestingly may be a better predictor for severity of opioid-induced respiratory depression than respiratory frequency (Bouillon et al., 2003).
Chemosensory reflexes
Hypercapnic (HCVR) and hypoxic (HVR) ventilatory responses are measurements of compensatory increases in ventilation induced by hypercapnia and hypoxia, respectively. Opioids inhibit the hypercapnic and hypoxic ventilatory reflexes in rodents and humans (Weil et al., 1975; Dahan et al., 2001) through opioid receptor-mediated inhibition of central and peripheral chemoreceptors (Poole et al., 2007; Zhang et al., 2007; Zhuang et al., 2017), as discussed further below. Briefly, the inhibition of chemosensory neurons impairs feedback to respiratory control centers and results in inadequate responses to increasing PaCO2 and decreasing PaO2 levels during hypoventilation. Thus, in addition to direct inhibition of ventilation, inhibition of compensatory chemoreflexes further compounds opioid-induced respiratory depression.
Sedation/sleep state
Opioids induce a sedative state with electrocortical activity that is distinct from sleep (Montandon et al., 2016a; Montandon and Horner, 2019). During periods of decreased cortical arousal, such as sedation or sleep, breathing is more dependent on respiratory rhythm generation mechanisms in the brainstem (McKay et al., 2005). Unfortunately, many brainstem neurons important for generating respiration are sensitive to opioids (see Brainstem mechanisms of opioid-induced respiratory depression section below), including brainstem neurons that mediate arousal (such as locus coeruleus and lateral parabrachial neurons). Therefore, opioids compromise not only cortical and sensory drive, but also the brainstem neurons that control breathing when arousal is low. Not surprisingly, degree of sedation, as determined by electrocortical activity, is associated with severity of opioid-induced rate depression in both rats and humans (Montandon et al., 2016a; Montandon and Horner, 2019).
Sleep-disordered breathing
Sleep-disordered breathing frequently manifests in patients that are chronically using opioids (Walker et al., 2007). The incidence of sleep-disordered breathing, including central sleep apnea (CSA) and obstructive sleep apnea (OSA), with chronic opioid use is high (up to 75 % of patients taking opioids) and correlates with opioid dose (Walker et al., 2007). However, opioids contribute to worsening of sleep-disordered breathing even at low doses (Wu et al., 2020), and in patients taking the partial opioid receptor agonist buprenorphine (Farney et al., 2013). In addition, opioids disrupt slow-wave and REM sleep (Wu et al., 2020), which can contribute to worsening of sleep-disordered breathing because ventilation in light sleep stages is inherently unstable. Indeed, opioid-induced apneic episodes occur primarily during non-REM sleep (Walker et al., 2007).
Upper Airways
The pharynx and larynx in the upper airway are important for airway defense and coordination of breathing and non-breathing behaviors (i.e. swallowing and speech). Understandably, opioid inhibition of upper airway patency can have a range of consequences, including obstructive sleep apnea, aspiration and difficulty swallowing (Christ et al., 2006; Walker et al., 2007; Savilampi et al., 2014). Opioids inhibit the activity of hypoglossal motoneurons (which innervate protruder muscles in the tongue), which contributes to worsening of obstructive sleep apnea (Hajiha et al., 2009). Intranasal leptin increases excitatory drive to hypoglossal motoneurons and reduces morphine-induced obstructed breaths (Freire et al., 2020). Opioids also inhibit motor output to the larynx, especially during the post-inspiratory phase of breathing, which is important for controlled expiration and coordination of breathing and swallowing (Levitt et al., 2015; Saunders and Levitt, 2020). Additionally, opioids inhibit upper airway protective reflexes (Tagaito et al., 1998), including swallowing and cough, which increases the risk of aspiration-induced pneumonia particularly in the elderly population (Dublin et al., 2011) and illicit drug users following overdose (Christ et al., 2006). Importantly, aspiration and difficulty swallowing occur not only in overdose, when sedation could contribute, but also with doses of opioids that only mildly suppress respiratory rate (Savilampi et al., 2014).
Muscle rigidity
Rigidity of respiratory skeletal muscles, including the diaphragm, chest wall and upper airway muscles, occurs following administration of opioids (Torralva and Janowsky, 2019). Though rigidity can occur with morphine or heroin, incidence is more common after high or rapid dosing of fentanyl (Torralva and Janowsky, 2019). This muscle rigidity has been called “wooden chest syndrome” and is observed both in illicit fentanyl use and anesthesiology (Kinshella et al., 2018). Increased chest wall tone constrains the ability to expand lung volume, leading to reduced tidal volume and impaired ventilation. Muscle rigidity does not, however, fully account for decreased tidal volume, since in paralyzed animals phrenic nerve output decreases following opioid exposure, suggesting decreases in tidal volume are also caused by loss of drive to phrenic motor neurons (Ren et al., 2009; Prkic et al., 2012; Saunders and Levitt, 2020). In addition, rigidity of intercostal and masseter skeletal muscles of the thorax and jaw can make mechanical ventilation difficult and interfere with effective resuscitation procedures. Fentanyl-induced muscle rigidity can be reversed by opioid receptor antagonists and alpha-2 adrenergic receptor agonists, suggesting it is likely due to opioid receptor-mediated increases in noradrenergic activity (Weinger et al., 1995; Torralva and Janowsky, 2019).
3. Brainstem mechanisms of opioid-induced respiratory depression
Pontomedullary respiratory network overview
Breathing is controlled by a highly interconnected network of brainstem neurons that stretch from the rostral pons to the caudal medulla with output to spinal and cranial motor nerves (Figure 2). A brief overview of the major structures in the control of breathing network is presented below, as several excellent detailed reviews on respiratory rhythm generation already exist.
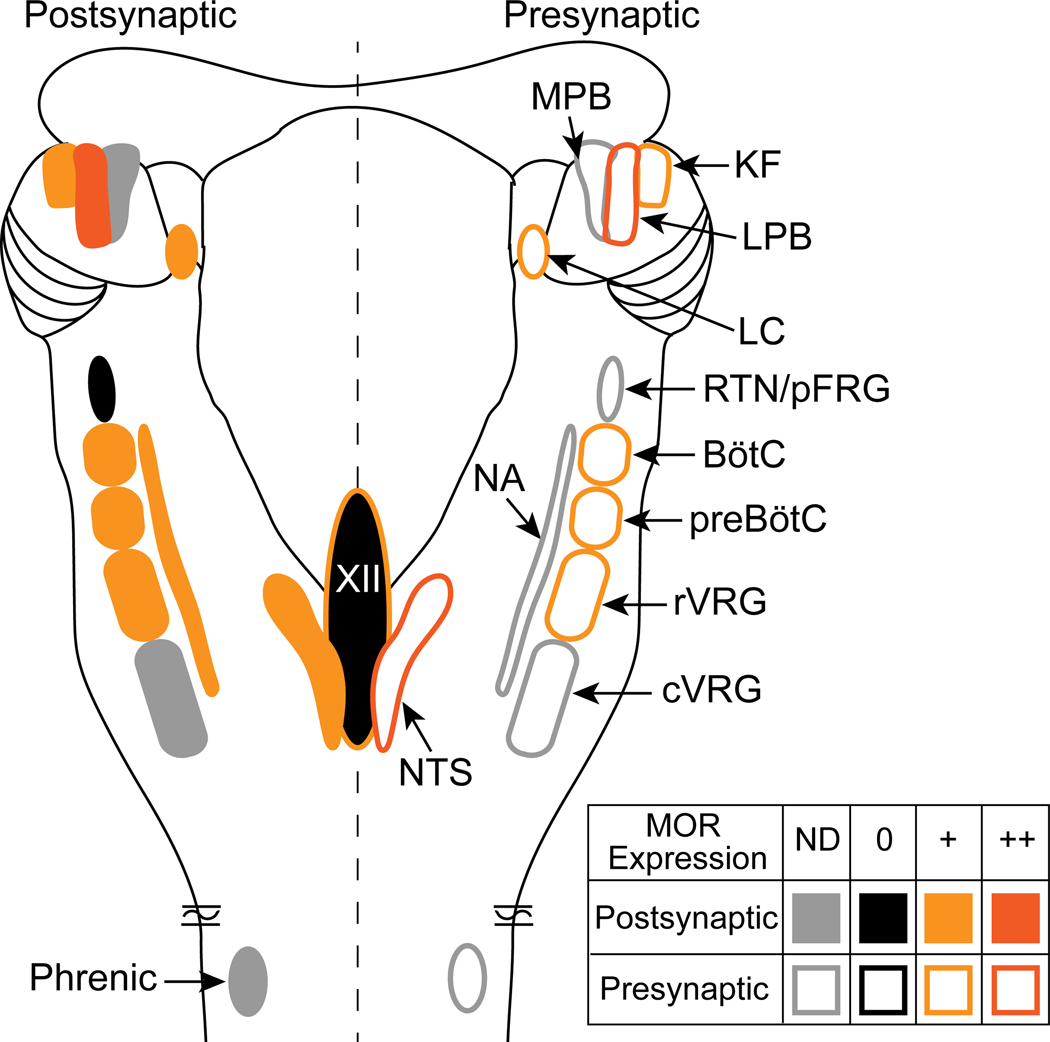
Figure 2.
Mu opioid receptor distribution in the pontomedullary respiratory network. Dorsal view of a rodent brainstem with bilateral pontomedullary respiratory structures is depicted. Mu opioid receptor expression is indicated as postsynaptic (somatodendritic, left side of brainstem, filled symbols) or presynaptic (axon terminals, right side of brainstem, outlined symbols). In each area, mu opioid receptors are expressed (+, orange), highly expressed (++, dark orange), not expressed (-, black) or not determined (ND, gray). Details are in the text (section 3. Brainstem mechanisms of opioid-induced respiratory depression). Abbreviations and references: MOR, mu opioid receptor; KF, Kölliker-Fuse (Levitt et al., 2015); LPB, lateral parabrachial area (Chamberlin et al., 1999); LC, locus coeruleus (Bradaia et al., 2005; Levitt and Williams, 2012); RTN/pFRG, retrotrapezoid nucleus/parafacial respiratory group (Mulkey et al., 2004); BötC, Bötzinger complex (Lonergan et al., 2003); preBötC, preBötzinger complex (Lonergan et al., 2003; Gray et al., 1999; Sun et al., 2019; Bachmutsky et al., 2020b); rVRG, rostral ventral respiratory group (Lonergan et al., 2003); cVRG, caudal ventral respiratory group; NTS, nucleus of the solitary tract (Aicher et al., 2000; Poole et al., 2007); XII, hypoglossal motor nucleus (Lorier et al., 2010); NA, nucleus ambiguous (Erbs et al., 2015); Phrenic, phrenic motor neurons.
Premotor neurons, antecedent to the spinal motor neuron pools, are in the rostral and caudal ventral respiratory groups (rVRG and cVRG, respectively) of the ventrolateral medulla. Continuing rostral from the premotor neurons are the preBötzinger complex (preBötC), the Bötzinger complex (BötC), the retrotrapezoid/parafacial nucleus (RTN/pFRG) and the post-inspiratory complex (PiCo), which is embedded in the larger intermediate reticular nucleus (Smith et al., 1991; Mulkey et al., 2004; Huckstepp et al., 2015; Anderson et al., 2016; Toor et al., 2019). Central to the respiratory network, the preBötC is necessary for generation of inspiratory bursts (Smith et al., 1991). The RTN/pFRG is involved in active expiration and central chemoreception (Mulkey et al., 2004; Huckstepp et al., 2015). The BötC contains mostly inhibitory neurons that fire during expiration and is a major source of inhibition within the network (Schreihofer et al., 1999; Ezure et al., 2003). These medullary control areas receive dense excitatory input from pontine structures including the Kölliker-Fuse (KF) and lateral parabrachial nucleus (LPB) (Song et al., 2012; Yokota et al., 2015; Geerling et al., 2017), which influence respiratory rate (Chamberlin and Saper, 1994; Navarrete-Opazo et al., 2020; Saunders and Levitt, 2020) and are required for maintenance of normal “eupnic” breathing pattern and coordination of the upper airways (Fung and St-John, 1995; Dutschmann and Herbert, 2006). In addition, the caudomedial nucleus tractus solitarius (NTS) of the dorsal medulla receives and integrates respiratory-related sensory information from peripheral afferents, including pulmonary stretch receptors and bronchopulmonary C-fibers, and chemosensory information from carotid and aortic bodies (Kubin et al., 2006). Together, these areas comprise a pontomedullary network that produces and controls respiratory rhythm (Baertsch et al., 2019; Dhingra et al., 2020).
Studies using global mu opioid receptor (MOR) knockout animals have shown that opioid depression of ventilation is due to activation of MORs (Dahan et al., 2001; Hill et al., 2020; Saunders and Levitt, 2020). MORs are expressed in all the above areas of the respiratory network, except the RTN/pFRG (Figure 2) (Chamberlin et al., 1999; Lonergan et al., 2003; Mulkey et al., 2004; Barnes et al., 2007; Zhuang et al., 2017). The exact mechanisms of opioid-induced respiratory depression are unknown, but (similar to pain and addiction circuitry) likely involve cumulative effects of opioids on receptors distributed throughout the network. In addition, cortical and peripheral MORs likely contribute to respiratory depression via decreases in wakefulness and sensory drive, respectively (Montandon and Horner, 2019; Perekopskiy et al., 2020). Since different nuclei in the respiratory network have different (though often overlapping) physiological functions, understanding the activity of MORs in each brainstem nucleus may help unravel the various effects of opioids on breathing and is reviewed below.
Pontomedullary mechanisms of opioid-induced hypoventilation and variability
Within the brainstem, there are two main areas that have emerged as key contributors to opioid-induced depression of respiratory rate – the preBötC in the medulla and the KF/parabrachial complex in the pons. The importance of the preBötC in opioid-induced respiratory depression was based initially on observations that fictive inspiratory bursts in rhythmic brainstem slices from neonatal rodents are slowed with local application of opioid agonist to the preBötC (Gray et al., 1999). Further studies in rhythmic preBötC slices have shown that the mu opioid agonist DAMGO reduces the frequency of putatively rhythmogenic inspiratory burstlets in a dose-dependent manner, suggesting that opioids modulate an emergent rhythmogenic mechanism in the preBötC (Sun et al., 2019). However, these effects in vitro do not always translate to more complex in vivo respiratory circuits.
The importance of the preBötC in opioid-induced respiratory rate depression is supported in vivo by observations that opioid agonist administration into the preBötC of adult rats depresses respiratory rate (Montandon et al., 2011, 2016b). However, others have found opposite results, reporting increases in respiratory rate following opioid agonist administration to the preBötC of adult animals, including rats, dogs, goats and rabbits (Lonergan et al., 2003; Mustapic et al., 2010; Langer et al., 2017; Cinelli et al., 2020). One possible explanation for the increases in rate observed in vivo is opioid inhibition of GABA or glycine transmission resulting in disinhibition (Langer et al., 2017). Indeed, DAMGO administration into the ventral respiratory column of goats decreases GABA levels (Langer et al., 2017), and MORs are expressed on GABAergic and glycinergic preBötC neurons in mice (Bachmutsky et al., 2020).
Studies using local application of opioid antagonists in vivo have also yielded conflicting results regarding the necessity of direct activation of MORs in the preBötC as a mechanism of opioid-induced suppression of respiratory rate (Mustapic et al., 2010; Montandon et al., 2011; Stucke et al., 2015). Microdialysis infusion of the opioid antagonist naloxone into the preBötC area prevented fentanyl-induced respiratory depression in rats (Montandon et al., 2011). Sequential microinjections of naloxone into the preBötC area partially reversed remifentanil-induced respiratory depression in rabbits (Stucke et al., 2015), and had no impact on remifentanil-induced respiratory depression in dogs (Mustapic et al., 2010). The opposing results in these experiments have been postulated to occur through differences in species, and location, dose and method of drug delivery.
Administration of opioid agonist into the neighboring BötC can cause a myriad of effects including an increase, decrease or no change in respiratory rate, and an increase in variability (Lonergan et al., 2003; Cinelli et al., 2020). In addition, application of opioid agonist into several areas of the medulla besides the preBötC, including rVRG (Lonergan et al., 2003; Cinelli et al., 2020) and rostroventromedial medulla (Phillips et al., 2012) causes reduction of respiratory rate. Given the number of neighboring respiratory nuclei expressing MORs (Figure 2), it is perhaps not surprising that application of opioid agonists outside the preBötC have effects on respiratory rate. These studies highlight that exclusively targeting any of these medullary areas without spillover is extremely difficult.
In contrast to the conflicting results in the preBötC, application of opioid agonists to the dorsolateral pons, including the KF, lateral PB and medial PB, consistently reduces respiratory rate in vivo in all species tested, including rats, cats, dogs and rabbits (Hurle et al., 1985; Prkic et al., 2012; Levitt et al., 2015; Miller et al., 2017). In addition, local application of opioid antagonists into the KF/parabrachial complex reverses systemic opioid-induced suppression of respiratory rate, but not motor nerve amplitude or patterning in dogs, rabbits and rats (Prkic et al., 2012; Miller et al., 2017; Saunders and Levitt, 2020).
Recently, studies from two independent groups have shown that deletion of MORs from either preBötC neurons or KF neurons attenuates respiratory rate depression caused by therapeutically relevant doses of morphine (10 or 20 mg/kg) in mice (Bachmutsky et al., 2020; Varga et al., 2020b). Further, deletion of MORs from both KF/parabrachial and preBötC neurons eliminates respiratory rate depression by morphine (20 mg/kg) (Bachmutsky et al., 2020). MOR density is higher on presynaptic terminals than somas in medullary respiratory areas including BötC, rVRG (Lonergan et al., 2003) and hypoglossal motor nucleus (Lorier et al., 2010). KF projections are also dense to BötC and rVRG (Song et al., 2012; Geerling et al., 2017). It could be that many of the MORs in BötC and rVRG are presynaptic receptors on KF terminals, so that deletion of MORs from KF neurons effectively deletes MORs from these regions as well. At higher doses of opioids (30 and 100 mg/kg), deletion of MORs from KF neurons attenuates morphine-induced rate suppression, but, surprisingly, deletion of MORs from preBötC neurons did not have the same effect (Varga et al., 2020b).
MOR deletion from preBötC neurons enhanced breathing instability following high doses of morphine (30 mg/kg and 100 mg/kg), as evidenced by high prevalence of apneas and ataxic breathing patterns (Varga et al., 2020b). One hypothesis is that deletion of MORs leads to preBötC “hyperactivity” and an increased refractory period leading to apneas (Cregg et al., 2017; Baertsch et al., 2018). Hyperactivity in the preBötC also leads to variability of phrenic nerve bursting in vivo (Bongianni et al., 2010) and in silico (Bacak et al., 2016), akin to variability after opioid injection into preBötC (Cinelli et al., 2020). In addition, the preBötC contains opioid- sensitive and non-sensitive inspiratory neurons (Barnes et al., 2007), so extended apnea in opioid overdose may not only occur through direct inhibition of preBötC inspiratory neurons (Gray et al., 1999; Montandon et al., 2011), but also though an increase in inhibitory input to opioid- insensitive neurons. Tonic activity of expiratory neurons, potentially of the dorsolateral pons (Abdala et al., 2009; Saunders and Levitt, 2020), could silence the opioid-insensitive preBötC population. One also cannot exclude variability stemming from the dorsolateral pons (Dhingra, 2017), as variability significantly increased after opioid injection into the KF of in situ preparations lacking vagal afferents (Levitt, 2015).
Cellular mechanisms of opioids in preBötC and KF
Direct cellular actions of opioids in the preBötC and KF have been identified. Both the preBötC and KF are heterogeneous, and sub-populations of neurons in each area are directly hyperpolarized by somatodendritic opioid receptor-mediated activation of G protein-coupled inwardly rectifying potassium (GIRK) channels (Gray et al., 1999; Montandon et al., 2011, 2016b; Levitt et al., 2015). In the KF, neurons that are hyperpolarized by MORs have different intrinsic properties from neurons that are not opioid sensitive (Levitt et al., 2015), indicating they are a distinct population. However, the neurochemical identity, firing pattern and projection target of these neurons are unknown.
Considerably more is known about the neuronal populations expressing MORs in the preBötC. Inspiratory, NK1R+ preBötC neurons are directly hyperpolarized by DAMGO, supporting somatodendritic expression of MORs (Gray et al., 1999; Montandon et al., 2011). The coexpression of MOR and NK1R transcript is supported by in situ hybridization studies (Sun et al., 2019). Targeted deletion of NK1R+ preBötC neurons leads to an ataxic breathing pattern in vivo (McKay et al., 2005), similar to ataxic breathing observed with high-dose opioids. However, in preBötC MOR knockouts ataxic breathing was not prevented (Varga et al., 2020b), suggesting other neuronal populations can also induce ataxic breathing.
In the preBötC, MOR transcript is found in glutamatergic, glycinergic and GABAergic neurons (Sun et al., 2019; Bachmutsky et al., 2020). Within the population of glutamatergic neurons, overlapping subsets of MOR+ neurons express the developmental transcription factors Dbx1 and/or Foxp2 (Sun et al., 2019; Bachmutsky et al., 2020). Despite the widespread distribution, deletion of MORs from just the subset of glutamatergic neurons that express Dbx1 eliminates opioid inhibition of inspiratory bursts in rhythmic neonatal preBötC slices (Sun et al., 2019; Bachmutsky et al., 2020). Deletion of MORs from the smaller subset of glutamatergic neurons that express Foxp2 attenuates DAMGO effects in rhythmic slices (Bachmutsky et al., 2020). The effect of deletion of MORs from Dbx1 neurons on opioid-induced respiratory depression in adults in vivo remains to be determined.
Dense reciprocal projections exist between KF and BötC/preBötC (Ezure, 2004; Song et al., 2012; Yokota et al., 2015; Geerling et al., 2017; Yang and Feldman, 2018; Yang et al., 2020). Projections from the KF to the BötC and preBötC are glutamatergic (Yokota et al., 2015; Geerling et al., 2017). Projections from the preBötC to the KF are both excitatory and inhibitory (Yang and Feldman, 2018). Projections from the BötC to the KF are inhibitory (Schreihofer et al., 1999; Ezure, 2004). The regulation of preBötC neurons by presynaptic opioid receptors has been examined (Ballanyi et al., 2010; Wei and Ramirez, 2019). However, in these studies the source of synaptic projections was unknown. Further studies to elucidate the location and function of presynaptic opioid receptors in the respiratory circuitry are needed.
Opioid mechanisms in other respiratory-related brainstem areas
Other locations in the brainstem also have opioid receptors, where they mediate effects of opioids other than rate suppression. The caudomedial NTS has dense expression of MORs (Aicher et al., 2000; Zhuang et al., 2017) that are involved in opioid suppression of respiratory-related sensory reflexes (Zhuang et al., 2017). Local application of the opioid agonist DAMGO into the caudomedial NTS attenuates the hypercapic and hypoxic ventilatory responses and the bronchopulmonary C-fiber reflex (Zhuang et al., 2017). A large portion of MORs in the NTS are presynaptic on peripheral vagal afferents (Aicher et al., 2000), and inhibit glutamate release onto second order neurons (Poole et al., 2007). In addition, some second order neurons in the caudomedial NTS have somatodendritic MORs and are hyperpolarized by opioid agonist (Aicher et al., 2000; Poole et al., 2007). However, MORs are rarely present on both afferent terminals and their dendritic target (Aicher et al., 2000).
Other brainstem areas potentially involved in depression of the hypercapnic ventilatory response include the caudal medullary raphe and the locus coeruleus. Opioid application to the caudal medullary raphe depresses the hypercapnic ventilatory response (Zhang et al., 2007). Locus coeruleus neurons are intrinsically chemoresponsive (Nichols et al., 2008), contribute to the hypercapnic ventilatory response (Magalhães et al., 2018) and are hyperpolarized by MORs (Levitt and Williams, 2012).
MORs are also expressed in motoneuron pools. The nucleus ambiguous contains laryngeal motoneurons that express somatodendritic MORs (Erbs et al., 2015). In the hypoglossal motor nucleus, presynaptic MORs suppress glutamate release onto hypoglossal motoneurons (Lorier et al., 2010; Freire et al., 2020), which could be involved in the impairments in the upper airways (ie. obstructed breaths) caused by opioids (Hajiha et al., 2009).
The carotid bodies, which play the primary role in the hypoxic ventilatory response, may contain opioid receptors (Kirby and McQueen, 1986), and therefore could contribute to opioid-induced decreases in respiratory chemoreception, and the beneficial effects of peripherally-restricted opioid antagonist (Perekopskiy et al., 2020). However, removal of carotid body input via transection of the carotid sinus nerve worsened opioid-induced suppression of HVR and HCVR, suggesting that carotid bodies are protective rather than participate in opioid-induced respiratory depression (Baby et al., 2018).
4. Agonist dependent respiratory depression
Though all mu opioid agonists have the potential to cause respiratory depression, several studies have sought to compare the degree of respiratory depression produced by different opioid agonists. One study compared the two most common opioids in overdose, fentanyl and heroin, with morphine, the active metabolite of heroin (Hill et al., 2020). All three agonists suppressed minute ventilation, but fentanyl was about 70 times more potent than morphine. The rate of onset of respiratory suppression was most rapid for fentanyl, followed by heroin, and then morphine, which correlates with their lipophilicity. Fentanyl also produced a greater reduction in tidal volume than heroin or morphine. A separate study compared decreases in brain hypoxia following fentanyl, heroin, morphine and oxycodone (Kiyatkin, 2019). Though all four produced a similar maximum level of brain hypoxia, oxycodone, heroin and fentanyl were about 4, 40 and 400 times more potent than morphine, respectively. Hypoxia induced by fentanyl and heroin occurred more rapidly than morphine or oxycodone.
Partial opioid receptor agonists, which have lower intrinsic efficacy and activate receptors to a lesser extent even at maximal doses, have been proposed as safer analgesics. In rodents, the degree of respiratory depression correlates with intrinsic efficacy (lower efficacy agonists produce less respiratory depression) (Hill et al., 2018b; Gillis et al., 2020a). Partial opioid receptor agonists that can be used clinically, like buprenorphine and newly approved oliceridine, have shown reduced respiratory depression leading to a greater safety profile in humans (Dahan et al., 2006, 2020). Thus, opioid agonists produce graded degrees of respiratory depression that is dose-dependent and correlates largely with agonist efficacy (Hill et al., 2018b; Kiyatkin, 2019; Gillis et al., 2020a; Hill et al., 2020). A major exception is fentanyl, which due to rapid penetration to the brain (Hill et al., 2020) can result apnea before protective CO2 accumulation (as described above and in (Pattinson, 2008)), and can cause muscle rigidity (Torralva and Janowsky, 2019).
5. Tolerance
Long-term use of opioids leads to the development of tolerance, defined as a diminished effect of a drug when given at the same dose. This diminished effect can be overcome by dose escalation, but this will worsen side effects that develop less tolerance. For opioids, significant tolerance develops for analgesia and euphoria, but not constipation and miosis. Tolerance in the respiratory system does occur, but to a lesser degree than analgesic tolerance (Paronis and Woods, 1997; Athanasos et al., 2006; Hill et al., 2016). As a consequence, people taking opioids long-term (i.e. for chronic pain) will be at risk of respiratory side effects upon dose escalation (Algera et al., 2020). The respiratory tolerance that does develop is reversed by ethanol and pregabalin via a mechanism involving PKC, which may partly explain the dangerous combination of these drugs with opioids (Hill et al., 2016, 2018a). Thus, in the respiratory system tolerance would be protective. Enhancing the development of tolerance by PKC overexpression in the preBötC reduced respiratory depression in mice (Lin et al., 2012), but is not a translatable approach. Identification of translatable mechanisms to enhance tolerance selectively in the respiratory system would be beneficial.
Opioid receptors are not all affected similarly following chronic opioid treatment. For instance, receptors can become more active (i.e. constitutively active or sensitized) or less active (i.e. desensitized) (Williams et al., 2013). Acute desensitization of somatodendritic MORs precedes the development of long-term tolerance of the receptors (Levitt and Williams, 2012). Desensitization of MORs occurs during application of saturating concentrations of agonist in a matter of minutes, recovers gradually once opioid agonist is removed, and is due to multi-site phosphorylation of the receptor (Kliewer et al., 2019). By comparison to desensitization of MORs on locus coeruleus neurons, MORs on KF neurons desensitize significantly less, and do not display long-term tolerance following chronic morphine treatment (Levitt and Williams, 2018). Mechanisms are currently unknown but may involve a missing beta-arrestin or PKC dependent process.
6. Current therapy
Naloxone (Narcan®) is the currently available pharmacological agent to reverse opioid-induced respiratory depression. Naloxone, administered via parenteral or intranasal routes, rapidly reverses respiratory depression and saves lives (Somerville et al., 2017). However, naloxone has several limitations that could be improved upon, as reviewed below.
As an opioid receptor antagonist, naloxone will reverse all opioid effects simultaneously leading to uncontrolled pain or severe withdrawal in dependent individuals.
Individuals at high risk of overdose are those taking high-dose opioids for chronic pain or management of opioid use disorder, especially during initiation of methadone therapy (Teichtahl et al., 2005; Chou et al., 2014). A preventative agent to preserve breathing integrity and/or prevent respiratory depression would be very beneficial in these high-risk individuals. Naloxone cannot be used because of interference with opioid-induced analgesia and/or opioid substitution therapy (methadone and buprenorphine) for treatment of opioid use disorder. Intranasal leptin prevents opioid-mediated obstructed breaths in mice and could be a non-opioid receptor-based option to correct obstructive sleep apnea from opioid use (Freire et al., 2020).
The half-life of naloxone is shorter than many opioid agonists necessitating repetitive or continuous administration of naloxone (Dahan et al., 2010; Somerville et al., 2017), and continued monitoring, preferably by a medical professional, to ensure re-emergence of respiratory depression can be appropriately corrected. Advances to develop MOR antagonists with a longer half-life exist, including intranasal nalmefene and methocinnamox (see (France et al., 2020) for review).
Naloxone, when given at the standard dose, often does not effectively antagonize high potency agonists, such as fentanyl and buprenorphine (Mégarbane et al., 2010; Hill et al., 2020). Since naloxone is a competitive antagonist this limitation can be overcome by using higher doses of naloxone (Somerville et al., 2017). Adequate reversal of respiratory depression can be achieved by using multiple doses of naloxone for fentanyl reversal (Somerville et al., 2017), or continuous intravenous administration of high-dose naloxone for buprenorphine reversal (Dahan et al., 2010). However, naloxone is not completely devoid of side effects, which can emerge at higher doses (Levine et al., 2020). An alternate approach would be to use a higher affinity antagonist. The high affinity antagonist diprenorphine is more effective than naloxone at reversing fentanyl-induced respiratory depression in mice (Hill et al., 2020).
Naloxone is unable to counter respiratory depression caused by non-opioid drugs. This is a significant problem given the incidence of polypharmacy (Mégarbane et al., 2010).
Due to the above reasons, a desirable goal is to develop non-opioid receptor-based agents to stimulate breathing selectively without affecting pain relief or precipitating withdrawal (Figure 3). An alternate to opioid antagonists in development are vaccines (for review see (Pravetoni and Comer, 2019)) and monoclonal antibodies (Baehr et al., 2020). In both strategies, anti-opioid antibodies bind to circulating opioid molecules and reduce brain levels of opioid. However, some of the limitations of opioid antagonist still exist, including interruption of opioid-mediated analgesia. Antibodies are specific for a single drug (for instance fentanyl, heroin or oxycodone), which would leave the individual vulnerable to respiratory depression from other opioids. This drug specificity could, however, be used advantageously to afford protection from an opioid with high lethality (such as fentanyl) while allowing use of a chemically unrelated opioid for either pain relief or management of opioid use disorder.
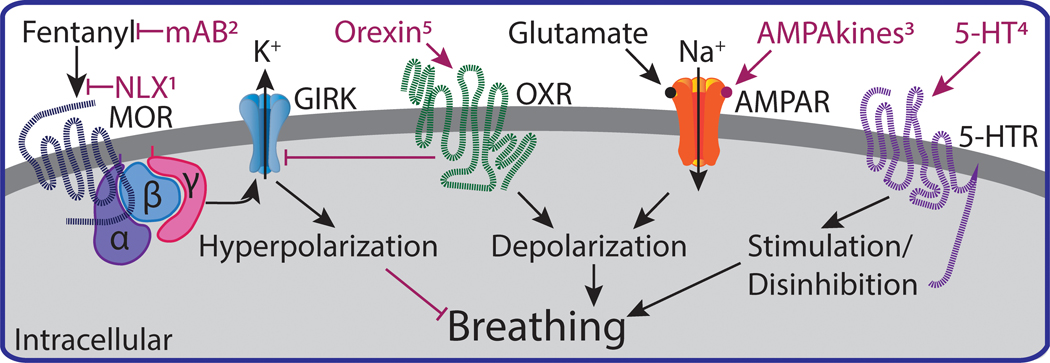
Figure 3.
Schematic of molecular mechanisms of opioid-induced respiratory depression and potential strategies to counter respiratory depression. Fentanyl activates G protein-coupled MORs and GIRK to hyperpolarize neurons and depress breathing. Strategies to counter fentanyl-induced depression of breathing include 1) naloxone (NLX), 2) monoclonal antibodies (mAB), 3) positive allosteric modulators of AMPA receptors (AMPAkines), 4) 5-HT1a and 5-HT4 receptor agonists, 5) orexin receptor agonists. These strategies are further reviewed in the text (sections 6 and 7).
7. Future Therapeutics
Currently, there are two strategies of future therapeutics to combat opioid-induced respiratory depression, while maintaining positive opioid effects. The first is to develop alternative analgesics that have minimal respiratory depression, for example G-protein biased opioid agonists (for review see (Grim et al., 2019; Gillis et al., 2020b)) or mu opioid receptor positive allosteric modulators (Kandasamy et al., 2021). The first agonist to come out of this line of work, olicieridine, was recently FDA-approved and has been shown to have a better safety profile than morphine in humans (Dahan et al., 2020). Whether this is due to biased signaling or intrinsic efficacy remains an active debate (Gillis et al., 2020b).
The other strategy is to develop a non-opioid respiratory stimulant that can be used concomitantly with an opioid and counter opioid-induced respiratory depression (Figure 3). Specifically, we will discuss the respiratory stimulants: A) ampakines, B) serotonin agonists, and C) orexin agonists. We do not intend for this to be an exhaustive review of all respiratory stimulants, since several recent reviews address this issue specifically.
7A. Ampakines
The AMPA receptor is an ionotropic transmembrane receptor that is activated by glutamate and mediates the majority of fast synaptic excitatory neurotransmission. AMPA receptors are expressed throughout the CNS--including brainstem respiratory nuclei--and are important in the neural control of breathing (Greer et al., 1991; Navarrete-Opazo et al., 2020; Varga et al., 2020a). Therefore, a potential strategy to counter opioid-induced respiratory depression is to target AMPA receptors--expressed in respiratory nuclei--to stimulate respiration. However, the broad expression pattern of AMPA receptors throughout the CNS make this task difficult, and it is therefore necessary to target AMPA receptors in a selective manner. Fortunately, AMPA receptors have multiple druggable targets that could increase region selectivity (Chang et al., 2012).
Ampakines are positive allosteric modulators that can reduce desensitization and/or deactivation of AMPA receptors, thereby potentiating AMPA-receptor-mediated synaptic currents (Chang et al., 2012). Through this mechanism, ampakines can act as respiratory stimulants and counter opioid-induced respiratory depression (Oertel et al., 2010). Interestingly, ampakines stimulate respiration more in mice with respiratory dysfunction compared to wild-type mice (ElMallah et al., 2015), improving therapeutic usefulness.
Multiple ampakines have been tested as respiratory stimulants during opioid-induced respiratory depression. The first was CX546, which, in the presence of fentanyl, stimulated breathing amplitude and frequency in vitro, in situ, and in vivo without altering opioid-induced analgesia (Ren et al., 2006). Ampakine CX717 ameliorates fentanyl-induced respiratory depression in a dose-dependent manner in rats (Ren et al., 2009). More recently, the ampakine CX1942 was compared to the respiratory stimulant doxapram (Haw et al., 2016). The study found that although doxapram corrected opioid-induced respiratory depression in goats, it led to arousal and hyperventilation. Conversely, CX1942 gradually attenuated opioid-induced respiratory depression with less arousal (Haw et al., 2016). Furthermore, ampakines (CX717 and CX614) attenuate opioid-induced depression of hypoglossal motor neuron activity, which could improve obstructed breathing that occurs with opioid use (Lorier et al., 2010).
In human studies, CX717 co-administered with alfentanil in healthy males caused significantly more blood oxygenation and a greater ventilatory response to a hypercapnic challenge compared to placebo, without interfering with alfentanil-induced analgesia (Oertel et al., 2010). The ampakine CX1739 was advanced to Phase II clinical trials to determine its ability to antagonize opioid-induced respiratory depression, but as of now, no results have been submitted (ClinicalTrials.gov; Identifier: NCT02735629).
7B. Serotonin
Serotonin (5-hydroxytryptamine; 5-HT) is a neurotransmitter with diverse neural action, including regulating neural excitability within control of breathing networks (Hodges and Richerson, 2008). All serotonin receptor subtypes are G protein-coupled receptors--except the 5- HT3 receptor, which is a ligand-gated ion channel. These receptors--specifically 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT4, and 5-HT7 receptor subtypes--are expressed in the brainstem and implicated in the neural control of breathing (Hodges and Richerson, 2008; Varga et al., 2020a). Because of their expression pattern and/or molecular mechanism, 5-HT1A, 5-HT7 and 5-HT4 receptors have been studied as potential targets to counter opioid-induced respiratory depression and will be reviewed below.
5-HT4 receptors and MORs are both expressed in the preBötC, but couple to different heterotrimeric G proteins (Gs and Gi/o, respectively), and influence respiratory rhythmogenesis in opposite ways (Manzke et al., 2003). BIMU8, a selective agonist of 5-HT4 receptors, stimulates respiratory output to overcome fentanyl-induced respiratory depression both in situ and in vivo, without interfering with opioid analgesia (Manzke et al., 2003). Zacopride, a 5-HT4 receptor agonist and 5-HT3 antagonist, countered the negative effects of etorphine, a highly potent opioid agonist, on respiratory rate, oxygen saturation and CO2 retention (Meyer et al., 2006), supporting the hypothesis that activation of 5-HT4 receptors can ameliorate respiratory depression caused by highly potent opioids. Unfortunately, mosapride, the only clinically available 5-HT4 receptor agonist, failed to alleviate opioid-induced respiratory depression in both rats and humans (Lötsch et al., 2005). This failure is attributed to the poor pharmacokinetics of mosapride (insufficient brain penetration and low drug potency) (Lötsch et al., 2005).
The concept that 5-HT1a receptor agonists could stimulate respiration and attenuate opioid-induced respiratory depression was built on studies demonstrating that 5-HT1a receptor agonists, buspirone and 8-OH-DPAT, could stimulate respiratory output (Garner et al., 1989) and counter apneustic breathing (i.e. prolonged inspiratory effort) (Lalley et al., 1994). In anesthetized rats, buspirone and 8-OH-DPAT counter morphine-induced apnea (Sahibzada et al., 2000). In unanesthetized rats, buspirone and 8-OH-DPAT counter decreases in ventilation but have no effect on morphine antinociception (Kimura et al., 2013). Meyer et al. (2006) demonstrated that 8-OH-DPAT maintains respiratory rate in the presence of the highly potent opioid agonist etorphine, but despite this ventilation did not improve (i.e. PaCO2 remained elevated). However, 8-OH-DPAT did abolish etorphine-induced increases in alveolar-arterial oxygen partial pressure gradients (A-a gradients) (Meyer et al., 2006). This indicates oxygen diffusion in the pulmonary circulation was preserved, which may have facilitated attenuation of etorphine-induced decrease in PaO2 and oxygen saturation. Antithetical to these positive studies in rodents, buspirone had no effect on morphine-induced respiratory depression in humans (Oertel et al., 2007). As buspirone is a weak partial agonist of 5-HT1a receptors, perhaps an agonist with higher intrinsic activity would prove more efficacious in humans.
Agonists other than buspirone and 8-OH-DPAT have also been tested. Repinotan, a selective 5-HT1a agonist, blocks opioid-induced respiratory depression in anesthetized rats (Guenther et al., 2010), while also prolonging opioid analgesia (Guenther et al., 2012). The 5-HT1a receptor agonist befiradol reduced fentanyl-induced respiratory depression, but unfortunately, induced baseline hyperventilation and hyperalgesia, and decreased opioid analgesia (Ren et al., 2015). More research needs to be conducted to validate this work in humans.
Unfortunately, the mechanism and the primary site of action by which 5-HT1a receptors attenuate opioid-induced respiratory depression remains elusive. Possible mechanisms initially seem counterintuitive, since both 5-HT1a and MORs couple to inhibitory G proteins and decrease neuronal excitability. 8-OH-DPAT can also activate 5-HT7 receptors, which couple to stimulatory G proteins (Hedlund et al., 2004). Perhaps activation of 5-HT7 receptors is sufficient to counter the inhibitory effects of 5-HT1a receptor activation. However, another explanation might be that potentiation of glycinergic inhibition causes changes to the respiratory-control network that restore respiratory output (Manzke et al., 2009, 2010). Inhibitory neurotransmission is critical to a functioning respiratory network (Abdala et al., 2015; Cregg et al., 2017; Baertsch et al., 2018). Manzke et al. (2009) found that inhibition of glycine receptors blocked the respiratory-stimulating effects of 8-OH-DPAT. Activated 5-HT1a receptors interact with inhibitory glycine receptor alpha 3 subtypes to potentiate glycine-mediated chloride currents (Manzke et al., 2010). These potentiated glycinergic currents are likely on inhibitory glycinergic neurons themselves. Thus, inhibition of glycinergic inhibitory neurons could produce disinhibition that results in increased neuronal excitability. This glycine-mediated increase in neural excitability could reverse opioid-induced respiratory depression. In fact, a 5-HT1a agonist could not block opioid-induced apneas in a glycine receptor alpha 3 subtype knockout mice (Manzke et al., 2010), emphasizing the role of glycine in serotonergic mechanisms of respiratory stimulation. A better understanding of the mechanisms is needed to identify other ways serotonin receptors counter opioid-induced respiratory depression.
Additionally, the primary site mediating the effects of 5-HT1a agonists remains ambiguous. Until recently, it was hypothesized that systemically administered 5-HT1a receptor agonists act primarily on the preBötC to overcome opioid-induced respiratory depression. However, microinjections of serotonin and 8-OH-DPAT into the ventral respiratory column--including the preBötC--failed to stimulate breathing, suggesting that this effect is mediated by nuclei outside of the ventral respiratory column (Radocaj et al., 2015). One potential site is the KF. 5-HT1a receptors are expressed in the KF where they regulate breathing variability (Dhingra et al., 2016). Clearly, more work is needed to understand how 5-HT receptors could be targeted to counter opioid-induced respiratory depression. Emphasis should be placed on studies in humans and determining the mechanisms and the primary site of action. However, the overall conjecture: 5-HT1a and/or 5-HT4 receptor agonists have potential to attenuate opioid-induced respiratory depression.
7C. Orexin
The neuropeptide orexin plays an important role in many neurophysiological processes, including arousal and respiration (Hagan et al., 1999; Young et al., 2005). Orexin, expressed in A and B splice variants, activates two G-protein coupled receptors (OX1R and OX2R) (Sakurai et al., 1998). Stimulation of the lateral hypothalamus--the site of orexin synthesis--increases respiratory frequency, an effect absent in orexin knockout mice (Kayaba et al., 2003). Orexinergic neurons project to respiratory-related nuclei in the pons and medulla, including the locus coeruleus, KF, retrotrapezoid nucleus, raphe, preBötC, and phrenic motor pool (Hagan et al., 1999; Young et al., 2005; Lazarenko et al., 2011; Yokota et al., 2016)--thereby providing many avenues to regulate breathing. Interestingly, opioid receptor activation suppresses the activity of orexinergic neurons (Li and Pol, 2008), suggesting that opioidergic and orexinergic pathways overlap, and that during opioid use orexin release may be reduced. Furthermore, orexin receptors activate Gq proteins (causing depolarization) and attenuate GIRK channel activity (Hoang et al., 2003). Therefore, orexin pharmacotherapies could perhaps be developed to supplement orexin depletion, counter the opioid-induced hyperpolarization of MOR expressing neurons, and stimulate breathing.
Soon after Sakurai et al. (1998) discovered orexins, studies began to characterize the peptides and their neurophysiological function. For example, the locus coeruleus--responsible for arousal--receives dense orexinergic innervation, and orexin applied to this region increases the basal firing rate of noradrenergic neurons (Hagan et al., 1999). Later, it was discovered that orexin administration to the preBötC or the phrenic motor pool increases diaphragm activity, but does not affect respiratory frequency (Young et al., 2005). A subsequent study found that application of orexin receptor agonists into the KF stimulates respiratory frequency in an in situ preparation (Dutschmann et al., 2007), demonstrating that orexinergic neurons could be targeted to stimulate breathing. In support of this, orexin can excite neurons in the retrotrapezoid nucleus (Lazarenko et al., 2011)--an important regulator of central respiratory chemoreflexes that can stimulate breathing. Administration of an orexin receptor antagonist to the retrotrapezoid nucleus reduces breathing--likely blocking the excitatory effects of endogenous orexin (Dias et al., 2009). In addition, orexin depolarizes inspiratory and pre-inspiratory neurons in the medulla, thereby increasing respiratory activity (Sugita et al., 2014). Together, these studies suggest that orexin receptors are a promising target to stimulate breathing.
Surprisingly, only a few studies have investigated the therapeutic potential of orexin pharmacotherapies to counter opioid-induced respiratory depression. In one study, orexin counters remifentanil-induced respiratory depression ex vivo (Umezawa et al., 2015). In a subsequent study, activation of orexin-2 receptors by a novel nonpeptide orexin agonist (YNT-185) attenuates morphine-induced sedation in vivo (Toyama et al., 2018). Clearly more work is needed, but based on the evidence that orexin stimulates breathing and that OX2R agonists are currently being developed for narcolepsy, it appears that orexin pharmacotherapies could be a strategy to counter opioid-induced respiratory depression.
8. Conclusion
Opioids cause a myriad of detrimental effects on the respiratory system. The most critical to the opioid epidemic are reductions in ventilation, particularly rate, to the point of prolonged apnea and tissue hypoxia, but others, including sleep disordered breathing and impairment of protective upper airway reflexes warrant considerable attention. We have reviewed opioid mechanisms throughout the brainstem respiratory network and predict that improving the understanding of these mechanisms, as well as suprapontine influences, in relation to physiological function will provide a foundation for furthering therapeutics to counter opioid-induced respiratory depression beyond antagonism of opioid receptors. Some examples were presented in this review, and hopefully future studies will identify additional mechanistic-based targets that are able to transcend from preclinical development to clinical use.
Acknowledgements:
We would like to thank Dr. David Baekey for comments on the manuscript. This work was supported by National Institutes of Health grants R00DA038069 and R01DA047978. JTB was supported by NIH grant F31DA053798.
Abbreviations
- 5‐HT
5‐hydroxytryptamine, serotonin
- AMPA
α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid
- BötC
Bötzinger complex
- CNS
central nervous system
- CSA
central sleep apnea
- cVRG
caudal ventral respiratory group
- Dbx1
developing brain homeobox 1
- FoxP2
Forkhead box protein 2
- GABA
γ‐aminobutyric acid
- GIRK
G protein-coupled inwardly-rectifying potassium channel
- GPCR
G‐protein‐coupled receptor
- HCVR
hypercapnic ventilatory response
- HVR
hypoxic ventilatory response
- KF
Kölliker-Fuse nucleus
- LC
locus coeruleus
- LPB
lateral parabrachial nucleus
- MOR
mu opioid receptor
- NK1R
neurokinin 1 receptor
- NTS
nucleus tractus solitarius
- OSA
obstructive sleep apnea
- PaCO2
partial pressure of carbon dioxide in arterial blood
- PaO2
partial pressure of oxygen in arterial blood
- PB
parabrachial
- pFRG
parafacial respiratory group
- PiCo
post-inspiratory complex
- PKC
protein kinase C
- preBötC
preBötzinger complex
- RTN
retrotrapezoid nucleus
- rVRG
rostral ventral respiratory group
Footnotes
Data Availability Statement:
Data sharing is not applicable to this article because no new data were created or analysed in this study.
Authorship statement:
ESL conceptualized the manuscript. JTB, SES and ESL drafted, revised and have given final approval of the manuscript.
Conflict of Interest Statement: Nothing to declare.
References:
- Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, and Paton JFR (2015). Deficiency of GABAergic synaptic inhibition in the Kolliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. The Journal of Physiology. [DOI] [PMC free article] [PubMed]
- Abdala APL, Rybak IA, Smith JC, and Paton JFR (2009). Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. The Journal of Physiology 587: 3539–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Goldberg A, Sharma S, and Pickel VM (2000). μ‐opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol 422: 181–190. [DOI] [PubMed] [Google Scholar]
- Algera MH, Olofsen E, Moss L, Dobbins RL, Niesters M, Velzen M van, et al. (2020). Tolerance to Opioid‐Induced Respiratory Depression in Chronic High‐Dose Opioid Users: A Model‐Based Comparison With Opioid‐Naïve Individuals. Clin Pharmacol Ther. [DOI] [PMC free article] [PubMed]
- Anderson TM, Garcia AJ, Baertsch NA, Pollak J, Bloom JC, Wei AD, et al. (2016). A novel excitatory network for the control of breathing. Nature 536: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasos P, Smith CS, White JM, Somogyi AA, Bochner F, and Ling W (2006). Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain 120: 267–275. [DOI] [PubMed] [Google Scholar]
- Baby SM, Gruber RB, Young AP, MacFarlane PM, Teppema LJ, and Lewis SJ (2018). Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur J Pharmacol 834: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacak BJ, Kim T, Smith JC, Rubin JE, and Rybak IA (2016). Mixed-mode oscillations and population bursting in the pre-Bötzinger complex. Elife 5: e13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmutsky I, Wei XP, Kish E, and Yackle K (2020). Opioids depress breathing through two small brainstem sites. Elife 9: e52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr CA, Kelcher AH, Khaimraj A, Reed DE, Pandit SG, AuCoin D, et al. (2020). Monoclonal antibodies counteract opioid-induced behavioral and toxic effects in mice and rats. J Pharmacol Exp Ther 375: JPET-AR-2020–000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertsch NA, Baertsch HC, and Ramirez JM (2018). The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat Commun 9: 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertsch NA, Severs LJ, Anderson TM, and Ramirez J-M (2019). A spatially dynamic network underlies the generation of inspiratory behaviors. Proc National Acad Sci 116: 201900523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Panaitescu B, and Ruangkittisakul A (2010). Indirect opioid actions on inspiratory pre-Botzinger complex neurons in newborn rat brainstem slices. Advances in Experimental Medicine and Biology 669: 75–79. [DOI] [PubMed] [Google Scholar]
- Barnes BJ, Tuong C-M, and Mellen NM (2007). Functional Imaging Reveals Respiratory Network Activity During Hypoxic and Opioid Challenge in the Neonate Rat Tilted Sagittal Slab Preparation. J Neurophysiol 97: 2283–2292. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Cinelli E, and Pantaleo T (2010). Respiratory responses induced by blockades of GABA and glycine receptors within the Bötzinger complex and the pre-Bötzinger complex of the rabbit. Brain Res 1344: 134–147. [DOI] [PubMed] [Google Scholar]
- Bouillon T, Bruhn J, Roepcke H, and Hoeft A (2003). Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesth 20: 127–133. [DOI] [PubMed] [Google Scholar]
- Bradaia A, Berton F, Ferrari S, and Luscher C (2005). beta-Arrestin2, interacting with phosphodiesterase 4, regulates synaptic release probability and presynaptic inhibition by opioids. Proceedings of the National Academy of Sciences of the United States of America 102: 3034–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Mansour A, Watson SJ, and Saper CB (1999). Localization of mu-opioid receptors on amygdaloid projection neurons in the parabrachial nucleus of the rat. Brain Research 1–7. [DOI] [PubMed]
- Chamberlin NL, and Saper CB (1994). Topographic Organization of Respiratory Responses to Glutamate Microstimulation of the Parabrachial Nucleus in the Rat. Journal of Neuroscience 14: 6500–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PK‐Y , Verbich D, and McKinney RA (2012). AMPA receptors as drug targets in neurological disease – advantages, caveats, and future outlook. Eur J Neurosci 35: 1908–1916. [DOI] [PubMed] [Google Scholar]
- Chou R, Cruciani RA, Fiellin DA, Compton P, Farrar JT, Haigney MC, et al. (2014). Methadone Safety: A Clinical Practice Guideline From the American Pain Society and College on Problems of Drug Dependence, in Collaboration With the Heart Rhythm Society. J Pain 15: 321–337. [DOI] [PubMed] [Google Scholar]
- Christ A, Arranto CA, Schindler C, Klima T, Hunziker PR, Siegemund M, et al. (2006). Incidence, risk factors, and outcome of aspiration pneumonitis in ICU overdose patients. Intensive Care Medicine 32: 1423–1427. [DOI] [PubMed] [Google Scholar]
- Cinelli E, Bongianni F, Pantaleo T, and Mutolo D (2020). Activation of μ-opioid receptors differentially affects the preBötzinger Complex and neighbouring regions of the respiratory network in the adult rabbit. Resp Physiol Neurobi 280: 103482. [DOI] [PubMed] [Google Scholar]
- Cregg JM, Chu KA, Dick TE, Landmesser LT, and Silver J (2017). Phasic inhibition as a mechanism for generation of rapid respiratory rhythms. Proc National Acad Sci 114: 12815–12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Aarts L, and Smith TW (2010). Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology 112: 226–238. [DOI] [PubMed] [Google Scholar]
- Dahan A, Dam CJ van Niesters, M., Velzen M van Fossler MJ, Demitrack MA, et al. (2020). Benefit and Risk Evaluation of Biased μ-Receptor Agonist Oliceridine versus Morphine. Anesthesiology 133: 559–568. [DOI] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, et al. (2001). Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology 94: 824–832. [DOI] [PubMed] [Google Scholar]
- Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, et al. (2006). Buprenorphine induces ceiling in respiratory depression but not in analgesia. Bja Br J Anaesth 96: 627–632. [DOI] [PubMed] [Google Scholar]
- Dhingra RR, Dick TE, Furuya WI, Galán RF, and Dutschmann M (2020). Volumetric mapping of the functional neuroanatomy of the respiratory network in the perfused brainstem preparation of rats. J Physiology 598: 2061–2079. [DOI] [PubMed] [Google Scholar]
- Dhingra RR, Dutschmann M, and Dick TE (2016). Blockade of dorsolateral pontine 5HT1A receptors destabilizes the respiratory rhythm in C57BL6/J wild-type mice. Resp Physiol Neurobi 226: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, and Nattie EE (2009). Antagonism of orexin receptor‐1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiology 587: 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Korff M, et al. (2011). Use of Opioids or Benzodiazepines and Risk of Pneumonia in Older Adults: A Population‐Based Case–Control Study. J Am Geriatr Soc 59: 1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, and Herbert H (2006). The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. European Journal of Neuroscience 24: 1071–1084. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Kron M, Morschel M, and Gestreau C (2007). Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respiratory Physiology Neurobiology 159: 232–235. [DOI] [PubMed] [Google Scholar]
- ElMallah MK, Pagliardini S, Turner SM, Cerreta AJ, Falk DJ, Byrne BJ, et al. (2015). Stimulation of Respiratory Motor Output and Ventilation in a Murine Model of Pompe Disease by Ampakines. Am J Resp Cell Mol 53: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J-L, et al. (2015). A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Structure and Function 220: 677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K (2004). Respiration-related afferents to parabrachial pontine regions. Respiratory Physiology Neurobiology 143: 167–175. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, and Kondo M (2003). Glycine Is Used as a Transmitter by Decrementing Expiratory Neurons of the Ventrolateral Medulla in the Rat. J Neurosci 23: 8941–8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farney RJ, McDonald AM, Boyle KM, Snow GL, Nuttall RT, Coudreaut MF, et al. (2013). Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. The European Respiratory Journal 42: 394–403. [DOI] [PubMed] [Google Scholar]
- Ferguson LM, and Drummond GB (2006). Acute effects of fentanyl on breathing pattern in anaesthetized subjects. Bja Br J Anaesth 96: 384–390. [DOI] [PubMed] [Google Scholar]
- France CP, Ahern GP, Averick S, Disney A, Enright HA, Esmaeli‐Azad B, et al. (2020). Countermeasures for Preventing and Treating Opioid Overdose. Clin Pharmacol Ther. [DOI] [PMC free article] [PubMed]
- Freire C, Pho H, Kim LJ, Wang X, Dyavanapalli J, Streeter SR, et al. (2020). Intranasal Leptin Prevents Opioid-induced Sleep-disordered Breathing in Obese Mice. Am J Resp Cell Mol 63: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung ML, and St-John WM (1995). The functional expression of a pontine pneumotaxic centre in neonatal rats. The Journal of Physiology 489 (Pt 2): 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner SJ, Eldridge FL, Wagner PG, and Dowell RT (1989). Buspirone, an anxiolytic drug that stimulates respiration. Am Rev Respir Dis 139: 946–950. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Yokota S, Rukhadze I, Roe D, and Chamberlin NL (2017). Kölliker–Fuse GABAergic and glutamatergic neurons project to distinct targets. J Comp Neurol 525: 1844–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, et al. (2020a). Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal 13: eaaz3140. [DOI] [PubMed] [Google Scholar]
- Gillis A, Kliewer A, Kelly E, Henderson G, Christie MJ, Schulz S, et al. (2020b). Critical Assessment of G Protein-Biased Agonism at the μ-Opioid Receptor. Trends Pharmacol Sci 41: 947–959. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, and Feldman JL (1999). Modulation of Respiratory Frequency by Peptidergic Input to Rhythmogenic Neurons in the PreBotzinger Complex. Science 286: 1566–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, and Feldman JL (1991). Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiology 437: 727–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Acevedo-Canabal A, and Bohn LM (2019). Toward Directing Opioid Receptor Signaling to Refine Opioid Therapeutics. Biol Psychiat 87: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther U, Theuerkauf NU, Huse D, Boettcher MF, Wensing G, Putensen C, et al. (2012). Selective 5-HT1A-R-agonist Repinotan Prevents Remifentanil-induced Ventilatory Depression and Prolongs Antinociception. Anesthesiology 116: 56–64. [DOI] [PubMed] [Google Scholar]
- Guenther U, Wrigge H, Theuerkauf N, Boettcher MF, Wensing G, Zinserling J, et al. (2010). Repinotan, a selective 5-HT1A-R-agonist, antagonizes morphine-induced ventilatory depression in anesthetized rats. Anesth Analg 111: 901–907. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. (1999). Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc National Acad Sci 96: 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajiha M, DuBord M-A, Liu H, and Horner RL (2009). Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. The Journal of Physiology 587: 2677–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DF, and Saitz R (2020). The Opioid Epidemic During the COVID-19 Pandemic. Jama 324: 1615–1617. [DOI] [PubMed] [Google Scholar]
- Haw AJ, Meyer LC, Greer JJ, and Fuller A (2016). Ampakine CX1942 attenuates opioid‐induced respiratory depression and corrects the hypoxaemic effects of etorphine in immobilized goats (Capra hircus). Vet Anaesth Analg 43: 528–538. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, and Bonaventure P (2004). 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. European Journal of Pharmacology 487: 125–132. [DOI] [PubMed] [Google Scholar]
- Hill R, Dewey WL, Kelly E, and Henderson G (2018a). Oxycodone‐induced tolerance to respiratory depression: reversal by ethanol, pregabalin and protein kinase C inhibition. Brit J Pharmacol 175: 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, et al. (2018b). The novel μ‐opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Brit J Pharmacol 175: 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, et al. (2016). Ethanol Reversal of Tolerance to the Respiratory Depressant Effects of Morphine. Neuropsychopharmacology 41: 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Santhakumar R, Dewey W, Kelly E, and Henderson G (2020). Fentanyl depression of respiration: Comparison with heroin and morphine. Brit J Pharmacol 177: 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang QV, Bajic D, Yanagisawa M, Nakajima S, and Nakajima Y (2003). Effects of Orexin (Hypocretin) on GIRK Channels. J Neurophysiol 90: 693–702. [DOI] [PubMed] [Google Scholar]
- Hodges MR, and Richerson GB (2008). Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Resp Physiol Neurobi 164: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RTR, Cardoza KP, Henderson LE, and Feldman JL (2015). Role of Parafacial Nuclei in Control of Breathing in Adult Rats. J Neurosci 35: 1052–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle MA, Mediavilla A, and Florez J (1985). Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology 24: 597–606. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Hillhouse TM, Livingston KE, Kochan KE, Meurice C, Eans SO, et al. (2021). Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects. Proc National Acad Sci 118: e2000017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. (2003). Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiology-Regulatory Integr Comp Physiology 285: R581–R593. [DOI] [PubMed] [Google Scholar]
- Khanna AK, Bergese SD, Jungquist CR, Morimatsu H, Uezono S, Lee S, et al. (2020). Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesthesia Analgesia 131: 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Ohi Y, and Haji A (2013). Effects of cholinesterase inhibitors and serotonin-1A receptor agonists on morphine-induced ventilatory depression and antinociception in rats. European Journal of Pharmacology 703: 33–41. [DOI] [PubMed] [Google Scholar]
- Kinshella M-LW, Gauthier T, and Lysyshyn M (2018). Rigidity, dyskinesia and other atypical overdose presentations observed at a supervised injection site, Vancouver, Canada. Harm Reduct J 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby GC, and McQueen DS (1986). Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Brit J Pharmacol 88: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA (2019). Respiratory depression and brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin, and fentanyl. Neuropharmacology 151: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, et al. (2019). Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, and McCrimmon DR (2006). Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM (2003). μ-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiology-Regulatory Integr Comp Physiology 285: R1287–R1304. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, and Richter DW (1994). 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. The Journal of Physiology 476: 117–130. [PMC free article] [PubMed] [Google Scholar]
- Langer TM, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, et al. (2017). Effects on breathing of agonists to μ-opioid or GABAA receptors dialyzed into the ventral respiratory column of awake and sleeping goats. Resp Physiol Neurobi 239: 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Stornetta RL, Bayliss DA, and Guyenet PG (2011). Orexin A activates retrotrapezoid neurons in mice. Resp Physiol Neurobi 175: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R, Veliz S, and Singer D (2020). Wooden chest syndrome: Beware of opioid antagonists, not just agonists. Am J Emerg Medicine 38: 411.e5–411.e6. [DOI] [PubMed] [Google Scholar]
- Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, and Williams JT (2015). $\mu$ opioid receptor activation hyperpolarizes respiratory-controlling Kolliker-Fuse neurons and suppresses post-inspiratory drive. The Journal of Physiology 593: 4453–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, and Williams JT (2012). Morphine desensitization and cellular tolerance are distinguished in rat locus ceruleus neurons. Molecular Pharmacology 82: 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, and Williams JT (2018). Desensitization and tolerance of mu opioid receptors on pontine Kolliker-Fuse neurons. Mol Pharmacol 93: mol.117.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, and Pol AN van den (2008). μ-Opioid Receptor-Mediated Depression of the Hypothalamic Hypocretin/Orexin Arousal System. J Neurosci 28: 2814–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-Y, Law P-Y, and Loh HH (2012). Activation of protein kinase C (PKC)$\alpha$ or PKC$\varepsilon$ as an approach to increase morphine tolerance in respiratory depression and lethal overdose. Journal of Pharmacology and Experimental Therapeutics 341: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, and Pilowsky PM (2003). Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respiratory Physiology Neurobiology 138: 165–178. [DOI] [PubMed] [Google Scholar]
- Lorier AR, Funk GD, and Greer JJ (2010). Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PloS One 5: e8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Skarke C, Schneider A, Hummel T, and Geisslinger G (2005). The 5‐hydroxytryptamine 4 receptor agonist mosapride does not antagonize morphine‐induced respiratory depression. Clin Pharmacol Ther 78: 278–287. [DOI] [PubMed] [Google Scholar]
- Madadi P, Hildebrandt D, Lauwers AE, and Koren G (2013). Characteristics of Opioid- Users Whose Death Was Related to Opioid-Toxicity: A Population-Based Study in Ontario, Canada. Plos One 8: e60600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães KS, Spiller PF, Silva MP da Kuntze LB, Paton JFR, Machado BH, et al. (2018). Locus Coeruleus as a vigilance centre for active inspiration and expiration in rats. Sci Rep-Uk 8: 15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Dutschmann M, Schlaf G, Morschel M, Koch UR, Ponimaskin E, et al. (2009). Serotonin targets inhibitory synapses to induce modulation of network functions. Philosophical Transactions of the Royal Society B: Biological Sciences 364: 2589–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, et al. (2003). 5-HT4(a) Receptors Avert Opioid-Induced Breathing Depression Without Loss of Analgesia. Science 301: 226–229. [DOI] [PubMed] [Google Scholar]
- Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hulsmann S, et al. (2010). Serotonin receptor 1A--modulated phosphorylation of glycine receptor $\alpha$3 controls breathing in mice. Journal of Clinical Investigation 120: 4118–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, and Feldman JL (2005). Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci 8: 1142–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégarbane B, Buisine A, Jacobs F, Résière D, Chevillard L, Vicaut E, et al. (2010). Prospective comparative assessment of buprenorphine overdose with heroin and methadone: Clinical characteristics and response to antidotal treatment. J Subst Abuse Treat 38: 403–407. [DOI] [PubMed] [Google Scholar]
- Meyer LCR, Fuller A, and Mitchell D (2006). Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats. Am J Physiology-Regulatory Integr Comp Physiology 290: R405–R413. [DOI] [PubMed] [Google Scholar]
- Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, and Stucke AG (2017). A Subregion of the Parabrachial Nucleus Partially Mediates Respiratory Rate Depression from Intravenous Remifentanil in Young and Adult Rabbits. Anesthesiology 127: 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Cushing SL, Campbell F, Propst EJ, Horner RL, and Narang I (2016a). Distinct Cortical Signatures Associated with Sedation and Respiratory Rate Depression by Morphine in a Pediatric Population. Anesthesiology 125: 889–903. [DOI] [PubMed] [Google Scholar]
- Montandon G, and Horner RL (2019). Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep-Uk 9: 14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, and Horner RL (2011). PreBotzinger Complex Neurokinin-1 Receptor-Expressing Neurons Mediate Opioid-Induced Respiratory Depression. Journal of Neuroscience 31: 1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, et al. (2016b). G-protein–gated Inwardly Rectifying Potassium Channels Modulate Respiratory Depression by Opioids. Anesthesiology 124: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, et al. (2004). Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369. [DOI] [PubMed] [Google Scholar]
- Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, et al. (2010). Clinically Relevant Infusion Rates of -Opioid Agonist Remifentanil Cause Bradypnea in Decerebrate Dogs but not Via Direct Effects in the pre-Botzinger Complex Region. Journal of Neurophysiology 103: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo AA, Cook-Snyder DR, Miller JR, Callison JJ, McCarthy N, Palkovic B, et al. (2020). Endogenous glutamatergic inputs to the Parabrachial Nucleus/Kölliker-Fuse Complex determine respiratory rate. Resp Physiol Neurobi 277: 103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Hartzler LK, Conrad SC, Dean JB, and Putnam RW (2008). Integration in Respiratory Control, From Genes to Systems. Adv Exp Med Biol 605: 348–352. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, et al. (2010). Selective Antagonism of Opioid‐Induced Ventilatory Depression by an Ampakine Molecule in Humans Without Loss of Opioid Analgesia. Clin Pharmacol Ther 87: 204–211. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Schneider A, Rohrbacher M, Schmidt H, Tegeder I, Geisslinger G, et al. (2007). The partial 5-hydroxytryptamine1A receptor agonist buspirone does not antagonize morphine-induced respiratory depression in humans. Clinical Pharmacology and Therapeutics 81: 59–68. [DOI] [PubMed] [Google Scholar]
- Paronis CA, and Woods JH (1997). Ventilation in morphine-maintained rhesus monkeys. II: Tolerance to the antinociceptive but not the ventilatory effects of morphine. The Journal of Pharmacology and Experimental Therapeutics 282: 355–362. [PubMed] [Google Scholar]
- Pattinson KTS (2008). Opioids and the control of respiration. British Journal of Anaesthesia 100: 747–758. [DOI] [PubMed] [Google Scholar]
- Perekopskiy D, Afzal A, Jackson SN, Muller L, Woods AS, and Kiyatkin EA (2020). The Role of Peripheral Opioid Receptors in Triggering Heroin-induced Brain Hypoxia. Sci Rep- Uk 10: 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RS, Cleary DR, Nalwalk JW, Arttamangkul S, Hough LB, and Heinricher MM. (2012). Pain-facilitating medullary neurons contribute to opioid-induced respiratory depression. Journal of Neurophysiology 108: 2393–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole SL, Deuchars J, Lewis DI, and Deuchars SA (2007). Subdivision-Specific Responses of Neurons in the Nucleus of the Tractus Solitarius to Activation of Mu-Opioid Receptors in the Rat. J Neurophysiol 98: 3060–3071. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, and Comer SD (2019). Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158: 107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EAE, Hopp FA, et al. (2012). Pontine-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. Journal of Neurophysiology 108: 2430–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radocaj T, Mustapic S, Prkic I, Stucke AG, Hopp FA, Stuth EAE, et al. (2015). Activation of 5-HT1A receptors in the preBotzinger region has little impact on the respiratory pattern. Respiratory Physiology Neurobiology 212–214: 9–19. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, and Greer JJ (2009). Ampakine CX717 Protects against Fentanyl-induced Respiratory Depression and Lethal Apnea in Rats. Anesthesiology 110: 1364–1370. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, and Greer JJ (2015). 5-HT1A Receptor Agonist Befiradol Reduces Fentanyl-induced Respiratory Depression, Analgesia, and Sedation in Rats. Anesthesiology 122: 424–434. [DOI] [PubMed] [Google Scholar]
- Riches JR, Read RW, Black RM, Cooper NJ, and Timperley CM (2012). Analysis of Clothing and Urine from Moscow Theatre Siege Casualties Reveals Carfentanil and Remifentanil Use. J Anal Toxicol 36: 647–656. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, and Gillis RA (2000). Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. The Journal of Pharmacology and Experimental Therapeutics 292: 704–713. [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. (1998). Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein- Coupled Receptors that Regulate Feeding Behavior. Cell 92: 573–585. [DOI] [PubMed] [Google Scholar]
- Saunders SE, and Levitt ES (2020). Kölliker-Fuse/Parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Resp Physiol Neurobi 275: 103388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savilampi J, Ahlstrand R, Magnuson A, Geijer H, and Wattwil M (2014). Aspiration induced by remifentanil: a double-blind, randomized, crossover study in healthy volunteers. Anesthesiology 121: 52–58. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, and Guyenet PG (1999). Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol 407: 583–597. [DOI] [PubMed] [Google Scholar]
- Smith J, Ellenberger H, Ballanyi K, Richter D, and Feldman J (1991). Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, et al. (2017). Characteristics of Fentanyl Overdose — Massachusetts, 2014–2016. Mmwr Morbidity Mortal Wkly Rep 66: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Wang H, Xu H, and Poon C-S (2012). Kolliker--Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Structure and Function 217: 835–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke AG, Miller JR, Prkic I, Zuperku EJ, Hopp FA, and Stuth EAE (2015). Opioid-induced Respiratory Depression Is Only Partially Mediated by the preBötzinger Complex in Young and Adult Rabbits In Vivo. Anesthesiology 122: 1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita T, Sakuraba S, Kaku Y, Yoshida K, Arisaka H, and Kuwana S (2014). Orexin induces excitation of respiratory neuronal network in isolated brainstem spinal cord of neonatal rat. Resp Physiol Neurobi 200: 105–109. [DOI] [PubMed] [Google Scholar]
- Sun X, Pérez CT, D NH, Shao XM, Greenwood M., Heath S, et al. (2019). Opioids modulate an emergent rhythmogenic process to depress breathing. Elife 8: e50613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaito Y, Isono S, and Nishino T (1998). Upper Airway Reflexes during a Combination of Propofol and Fentanyl Anesthesia. Anesthesiology 88: 1459–1466. [DOI] [PubMed] [Google Scholar]
- Teichtahl H, Wang D, Cunnington D, Quinnell T, Tran H, Kronborg I, et al. (2005). Ventilatory Responses to Hypoxia and Hypercapnia in Stable Methadone Maintenance Treatment Patients. Chest 128: 1339–1347. [DOI] [PubMed] [Google Scholar]
- Toor RUAS, Sun Q-J, Kumar N, Le S, Hildreth C, Phillips J, et al. (2019). Neurons in the intermediate reticular nucleus coordinate post-inspiratory activity, swallowing, and respiratory-sympathetic coupling in the rat. J Neurosci 39: 0502–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva R, and Janowsky A (2019). Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J Pharmacol Exp Ther 371: 453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama S, Shimoyama N, Tagaito Y, Nagase H, Saitoh T, Yanagisawa M, et al. (2018). Nonpeptide Orexin-2 Receptor Agonist Attenuates Morphine-induced Sedative Effects in Rats. Anesthesiology 128: 992–1003. [DOI] [PubMed] [Google Scholar]
- Umezawa N, Arisaka H, Sakuraba S, Sugita T, Matsumoto A, Kaku Y, et al. (2015). Orexin-B antagonized respiratory depression induced by sevoflurane, propofol, and remifentanil in isolated brainstem-spinal cords of neonatal rats. Resp Physiol Neurobi 205: 61–65. [DOI] [PubMed] [Google Scholar]
- Varga AG, Maletz SN, Bateman JT, Reid BT, and Levitt ES (2020a). Neurochemistry of the Kölliker‐Fuse nucleus from a respiratory perspective. J Neurochem. [DOI] [PMC free article] [PubMed]
- Varga AG, Reid BT, Kieffer BL, and Levitt ES (2020b). Differential impact of two critical respiratory centres in opioid‐induced respiratory depression in awake mice. J Physiology 598: 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, et al. (2007). Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine 3: 455–461. [PMC free article] [PubMed] [Google Scholar]
- Wei AD, and Ramirez J-M (2019). Presynaptic Mechanisms and KCNQ Potassium Channels Modulate Opioid Depression of Respiratory Drive. Front Physiol 10: 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV, McCullough RE, Kline JS, and Sodal IE (1975). Diminished Ventilatory Response to Hypoxia and Hypercapnia after Morphine in Normal Man. New Engl J Medicine 292: 1103–1106. [DOI] [PubMed] [Google Scholar]
- Weinger MB, Chen D-Y, Lin T, Lau C, Koob GF, and Smith NT (1995). A role for CNS α−2 adrenergic receptors in opiate-induced muscle rigidity in the rat. Brain Res 669: 10–18. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, Zastrow M von Schulz, et al. (2013). Regulation of $\mu$-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacological Reviews 65: 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H, and Davis NL (2020). Drug and Opioid-Involved Overdose Deaths — United States, 2017–2018. Mmwr Morbidity Mortal Wkly Rep 69: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JG‐A , Wang D, Rowsell L, Wong KK, Yee BJ, Nguyen CD, et al. (2020). The effect of acute exposure to morphine on breathing variability and cardiopulmonary coupling in men with obstructive sleep apnea: A randomized controlled trial. J Sleep Res 29: e12930. [DOI] [PubMed] [Google Scholar]
- Yang CF, and Feldman JL (2018). Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J Comp Neurol 526: 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Kim EJ, Callaway EM, and Feldman JL (2020). Monosynaptic Projections to Excitatory and Inhibitory preBötzinger Complex Neurons. Front Neuroanat 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Kaur S, VanderHorst VG, Saper CB, and Chamberlin NL (2015). Respiratory- related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. The Journal of Comparative Neurology 523: 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Oka T, Asano H, and Yasui Y (2016). Orexinergic fibers are in contact with Kolliker-Fuse nucleus neurons projecting to the respiration-related nuclei in the medulla oblongata and spinal cord of the rat. Brain Research. [DOI] [PubMed]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, et al. (2005). Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 98: 1387–1395. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, and Liang X (2007). Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107: 288–297. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Gao X, Gao F, and Xu F (2017). Mu-opioid receptors in the caudomedial NTS are critical for respiratory responses to stimulation of bronchopulmonary C-fibers and carotid body in conscious rats. Resp Physiol Neurobi 235: 71–78. [DOI] [PubMed] [Google Scholar]