Abstract
To examine the efficacy and safety of short courses of azithromycin and ofloxacin for treating multidrug-resistant (MDR, i.e., resistant to chloramphenicol, ampicillin, and cotrimoxazole) and nalidixic acid-resistant enteric fever, azithromycin (1 g once daily for 5 days at 20 mg/kg/day) and ofloxacin (200 mg orally twice a day for 5 days at 8 mg/kg/day) were compared in an open randomized study in adults admitted to a hospital with uncomplicated enteric fever. A total of 88 blood culture-confirmed patients were enrolled in the study (86 with Salmonella enterica serovar Typhi and 2 with S. enterica serovar Paratyphi A). Of these, 44 received azithromycin and 44 ofloxacin. A total of 68 of 87 (78%) isolates were MDR serovar Typhi, and 46 of 87 (53%) were nalidixic acid resistant. The MIC90 (range) of azithromycin was 8 (4 to 16) μg/ml for the isolates. The MIC90 (range) of ofloxacin for the nalidixic acid-sensitive isolates was 0.03 (0.015 to 0.06) μg/ml and for the nalidixic acid-resistant isolates it was 0.5 (0.25 to 1.0) μg/ml. There was no significant difference in the overall clinical cure rate with ofloxacin and azithromycin (38 of 44 [86.4%] versus 42 of 44 [95.5%]; P = 0.27) or in the patients infected with nalidixic acid-resistant typhoid (17 of 21 [81.0%] versus 24 of 25 [96.0%]; P = 0.16). However, patients with nalidixic acid-resistant typhoid treated with ofloxacin had a longer fever clearance time compared with those treated with azithromycin (174 [60 to 264] versus 135 [72 to 186] h; P = 0.004) and had positive fecal cultures after the end of treatment (7 of 17 [41%] versus 0 of 19 [0%]; P = 0.002). Both antibiotics were well tolerated. A 5-day course of azithromycin was effective for the treatment of enteric fever due to MDR and nalidixic-acid-resistant serovar Typhi, whereas the ofloxacin regimen chosen was less satisfactory for these strains.
In recent years, multidrug-resistant (MDR) strains of Salmonella enterica serovar Typhi (resistant to chloramphenicol, ampicillin, and cotrimoxazole) have emerged in many countries, including Vietnam (20). Third-generation cephalosporins and fluoroquinolones are alternatives for treatment, and the fluoroquinolones have proved particularly effective (29). However, isolates of Salmonella enterica serovars Typhi and Paratyphi A with reduced susceptibility to fluoroquinolones (as indicated in the laboratory by resistance to nalidixic acid) have now appeared in the Indian subcontinent, Vietnam, and Tajikistan (1, 2, 8, 12, 19, 26), and treatment failures with fluoroquinolones have also been reported (5, 11, 23, 25, 26). In 1998, 32 of 151 (21%) of isolates of serovar Typhi in the United Kingdom had reduced susceptibility to ciprofloxacin (23). The majority of patients infected with these isolates had recently returned from the Indian subcontinent. Furthermore, the recent report of an isolate of serovar Typhi from Bangladesh with high-level resistance to ceftriaxone (21) means that untreatable typhoid may become a reality. There is a need for alternative antimicrobial agents to treat such MDR infections.
Azithromycin has moderate in vitro activity against serovar Typhi (10) but achieves high intracellular concentrations and has been shown to be effective in a murine model with S. enterica serovar Typhimurium (3). Treatment courses of 7 days or more in cases of typhoid have been promising (4, 7, 24; T. Butler, C. Palomino, R. B. Johnson, and S. J. Hopkins, Prog. Abstr. 32nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1579, 1992). In a pilot study at our center azithromycin given at 1 g per day for 5 days was successful in five adults with blood culture-positive typhoid fever; the patients showed no relapses or adverse effects. We therefore conducted a randomized comparison of the efficacy of a 5-day course of azithromycin and ofloxacin for the treatment of uncomplicated enteric fever in adults.
(The interim results of this study were presented at the Third Asia-Pacific Symposium on Typhoid Fever and other Salmonellosis, Denspasar, Bali-Indonesia, on December 8 to 10, 1997 [abstr. T-7].)
MATERIALS AND METHODS
The study was performed on the adult typhoid ward at the Centre for Tropical Diseases, Ho Chi Minh City, Vietnam. The hospital is a 500-bed referral center for Ho Chi Minh City and the surrounding provinces. The study had received approval from the Scientific and Ethical Committee of the Centre for Tropical Diseases, and all patients gave informed verbal consent.
Patients.
Adults (≥15 years old) with the clinical features of enteric fever and who were blood culture positive with serovar Typhi or serovar Paratyphi A were enrolled in the study. Patients were excluded if they had evidence of severe or complicated disease (severe gastrointestinal bleeding, intestinal perforation, visible jaundice, myocarditis, pneumonia, renal failure, shock, or coma), a history of significant underlying disease, or a history of hypersensitivity to either of the trial drugs or if they were pregnant. Patients who gave a history of treatment with a quinolone or third-generation cephalosporin or macrolides within 1 week of hospital admission were also excluded.
Treatment.
Patients were allocated to one of two treatment groups in an open randomized comparison. The treatment allocations were kept in serially numbered sealed envelopes that were only opened when the patient had been enrolled into the study. Patients received either azithromycin (Zithromax; Pfizer International) given in doses of 1 g orally once a day for 5 days or ofloxacin (Oflocet; Hoechst Marion Roussel, Paris, France) given in doses of 200 mg orally twice a day for 5 days.
Laboratory procedures.
A full blood count, serum aspartate transaminase (AST), alanine transaminase (ALT), creatinine, and urinalysis were performed before therapy. The AST and ALT analyses were repeated 1 day after the end of therapy. Chest X-ray and other radiological investigations, including abdominal ultrasound, were performed as clinically indicated. Blood cultures were obtained before therapy and 24 h after the end of therapy (day 6). A 5- to 8-ml specimen of blood was inoculated into Bactec 6B aerobic bottles (Becton Dickinson) and incubated in the Bactec 9050 continuous monitoring incubator system for 7 days. Bottles giving a positive signal were subcultured onto sheep blood agar (Oxoid, Basingstoke, United Kingdom). Up to three fecal specimens and a urine specimen were cultured before and 2 to 5 days after the end of treatment. Salmonella isolates were identified by standard biochemical tests and agglutination with Salmonella-specific antisera (Murex diagnostics, Dartford, United Kingdom). Antimicrobial sensitivities were determined by the modified Bauer-Kirby disc diffusion method with zone size interpretation based on NCCLS guidelines (13, 15). Antibiotic disks tested were chloramphenicol (30 μg), ampicillin (10 μg), trimethoprim-sulfamethoxazole (1.25 and 23.75 μg), ceftriaxone (30 μg), ofloxacin (5 μg), azithromycin (15 μg), and nalidixic acid (30 μg). Isolates were stored in Protect beads (Prolabs, Oxford, United Kingdom) at −20°C for later MIC testing by agar plate dilution (14). Antibiotic powders were purchased from Sigma except azithromycin, a gift from Pfizer International, and ofloxacin, a gift from Hoechst Marion Roussel. The azithromycin MIC was also checked by using E-Test (AB Biodisk, Solna, Sweden). An isolate was defined as MDR if it was resistant to chloramphenicol at ≥32 μg/ml, ampicillin at ≥32 μg/ml, and trimethoprim-sulfamethoxazole at ≥8/≥152 μg/ml and nalidixic acid resistant if it was resistant to nalidixic acid at ≥32 μg/ml. The current NCCLS breakpoints for both azithromycin and ofloxacin are ≤2 μg/ml (susceptible) and ≥8 μg/ml (resistant) (15).
Evaluation of treatment response.
Patients were examined daily until discharge from hospital, with particular reference to clinical symptoms, fever clearance time, any side effects of the drug and any complication of the disease. The response to treatment was assessed by clinical parameters (resolution of clinical symptoms and signs), fever defervescence (time from the start of treatment until the body temperature fell below 37.5°C and remained at ≤37.5°C for 48 h), development of complications, and evidence of relapse of infection. A clinical treatment failure was defined as the persistence of fever and symptoms for more than 5 days after the end of treatment or the development of severe complications (severe gastrointestinal bleeding, intestinal perforation, visible jaundice, myocarditis, pneumonia, renal failure, shock, or coma) during treatment, requiring a change in therapy. Patients who failed were re-treated with ofloxacin at 10 to 15 mg/kg/day for 7 to 10 days or ceftriaxone at 2 g/day for 7 to 10 days. Patients were followed up at 4 to 6 weeks posttreatment. At this time any clinical evidence of relapse was sought and one stool culture was analyzed. A blood culture was done if the symptoms and signs suggested relapse. A relapse was defined as a recurrence of symptoms and signs suggestive of enteric fever after the patient had been discharged as well from the hospital. Microbiological treatment failure was defined as isolation of serovar Typhi or serovar Paratyphi A from blood or a sterile site after the completion of treatment.
Sample size and statistical analysis.
Assuming a failure rate in the ofloxacin arm of 5%, a sample size of 43 patients in each group would give an 80% power to detect a 25% difference in failure rate at a 5% significance level. Proportions were compared with the chi-square test with Yates' correction or the Fisher exact test. Normally distributed data were compared using the Student t test, and non-normally distributed data were compared using the Mann-Whitney U test. Fisher exact test and relative risk with a 95% confidence interval (CI) was used for the outcome variables. The fever clearance time and duration of admission after the start of treatment were compared using survival analysis and the log rank test. Statistical analysis was performed using EpiInfo version 6 (Centers for Disease Control, Atlanta, Ga.) and SPSS for Windows version 7.5 (SPSS, Inc., Chicago, Ill.).
RESULTS
Ninety-seven adults with suspected enteric fever were entered into the study. Nine adults were subsequently excluded. In six patients the blood culture was negative. Two patients were found after entry to the study to have taken a fluoroquinolone before admission to hospital, and one patient was entered in the study but later the same day was removed when found to have renal impairment with a serum creatinine of 2.7 mg/dl.
The 88 remaining adults included 86 with a blood culture positive for serovar Typhi and 2 with a blood culture positive for serovar Paratyphi A. One serovar Typhi isolate was not available for sensitivity testing. Of the remaining isolates, 68 of 87 (78%) were MDR and 46 of 87 (53%) were nalidixic acid resistant. Both serovar Paratyphi A isolates were susceptible to all of the antimicrobials tested. A total of 44 patients were randomized to receive azithromycin and 44 were randomized to receive ofloxacin. The epidemiological, clinical, and laboratory data of the two groups of patients are presented in Table 1. There were no significant differences between the admission characteristics of the two groups. The mean MIC90 (range) of azithromycin was 8 (4 to 16) μg/ml and of ofloxacin was 0.5 (0.015 to 1.0) μg/ml for the isolates. The ofloxacin MIC90 (range) for the nalidixic acid-sensitive isolates was 0.03 (0.015 to 0.06) μg/ml and for the nalidixic acid-resistant isolates was 0.5 (0.25 to 1.0) μg/ml. There was no difference in the azithromycin MICs between the nalidixic acid-sensitive and -resistant isolates. By NCCLS breakpoint guidelines, all isolates were susceptible to ofloxacin but 25 of 87 (29%) isolates were intermediate (MIC = 4 μg/ml) and 62 of 87 (71%) isolates were resistant (MIC = 8, 12, or 16 μg/ml) to azithromycin.
TABLE 1.
Epidemiological, clinical, and laboratory features in the 88 patients with culture-confirmed enteric fever
| Patient characteristics | Treatment
|
|
|---|---|---|
| Ofloxacin (n = 44) | Azithromycin (n = 44) | |
| No. of males/no. of females | 26/18 | 20/24 |
| Mean age (95% CI, range) (yr) | 24.7 (22.6–26.8, 15–40) | 26.6 (23.1–30.1, 15–68) |
| Mean wt (95% CI, range) (kg) | 48.7 (46.3–51.0, 29–62) | 47.3 (45.3–49.3, 34–60) |
| Mean duration of fever before admission (95% CI, range) (days) | 13.6 (11.4–15.8, 5–32) | 11.9 (10.4–13.3, 5–25) |
| Abdominal pain (n [%]) | 10 (23) | 8 (18) |
| Vomiting (n [%]) | 3 (7) | 8 (18) |
| Diarrhea (n [%]) | 34 (77) | 32 (73) |
| Hepatomegaly (n [%]) | 13 (30) | 9 (21) |
| Splenomegaly (n [%]) | 1 (2) | 3 (7) |
| Mean hematocrit (95% CI, range) (%) | 39 (37–41, 31–45) | 39 (37–41, 25–47) |
| Mean white cell count (95% CI, range) (109/liter) | 7.5 (6.1–8.9, 3.0–16.3) | 6.5 (5.5–7.5, 2.8–12.0) |
| Mean platelet count (95% CI, range) (109/liter) | 176 (156–196, 57–374) | 177 (162–192, 84–300) |
| Organism isolated from blood sample | 43/1 | 43/1 |
| MDR strains (n [%]) | 35 (80) | 33 (77) |
| Nalidixic acid-resistant strains (n [%]) | 21 (48) | 25 (57) |
| Positive pretreatment fecal cultures (n [%]) | 12 (27) | 12 (27) |
There were eight treatment failures: six in the ofloxacin-treated patients and two in the azithromycin group (relative risk, 3.0; 95% CI, 0.6 to 14.1; P = 0.27) (Table 2). With azithromycin, one patient failed with persistent fever and symptoms after the end of treatment, and the repeat blood culture was positive. The azithromycin MIC for the serovar Typhi isolate in this patient was 12 μg/ml. The second patient deteriorated with gastrointestinal bleeding on the fourth day of treatment. The six patients who failed with ofloxacin included three patients with persisting fever, symptoms, and positive stool cultures after the end of treatment although the blood cultures were negative, one patient with severe gastrointestinal bleeding on the second day of treatment, and two patients who relapsed (one MDR nalidixic acid-resistant serovar Typhi infection and one fully sensitive serovar Paratyphi A infection). Of the patients in whom posttreatment fecal cultures were obtained, 8 of 35 (23%) treated with ofloxacin were still excreting serovar Typhi for 2 to 3 days after the end of treatment compared to 0 of 34 patients treated with azithromycin (P = 0.005). In the patients with a positive fecal culture, the last fecal culture obtained prior to hospital discharge was negative.
TABLE 2.
Outcome of treatment in all patients and in patients infected with a nalidixic acid-resistant isolate
| Parameter(s) | Treatment
|
P | |
|---|---|---|---|
| Ofloxacin | Azithromycin | ||
| All patients (n) | 44 | 44 | |
| Clinical failures (n [%]) | 6 (13.6) | 2 (4.5) | 0.27 |
| Persistent fever and symptoms | 3 (6.8) | 1 (2.3) | |
| Gastrointestinal hemorrhage | 1 (2.3) | 1 (2.3) | |
| Relapse | 2 (4.5) | 0 | |
| Microbiological failures (n [%]) | 2 (4.5) | 1 (2.3) | 1.00 |
| Mean fever clearance time (h) (95% CI, range) | 134 (111–156, 12–264) | 130 (118–142, 60–204) | 0.19 |
| Mean duration (days) of hospitalization after starting treatment (95% CI, range) | 10.5 (9.5–11.5, 5–20) | 9.6 (8.9–10.3, 7–19) | 0.05 |
| Patients infected with nalidixic acid-resistant isolate (n) | 21 | 25 | |
| Clinical failures (n [%]) | 4 (19.0) | 1 (4.0) | 0.16 |
| Persistent fever and symptoms | 3 (14.3) | 0 | |
| Gastrointestinal hemorrhage | 0 | 1 (4.0) | |
| Relapse | 1 (4.8) | 0 | |
| Microbiological failures (n [%]) | 1 (4.8) | 0 | 0.46 |
| Mean fever clearance time (h) (95% CI, range) | 174 (143–205, 60–264) | 135 (119–151, 72–186) | 0.004 |
| Mean duration (days) of hospitalization after starting treatment (95% CI, range) | 11.9 (10.4–13.5, 7–20) | 9.3 (8.5–10.0, 7–14) | 0.001 |
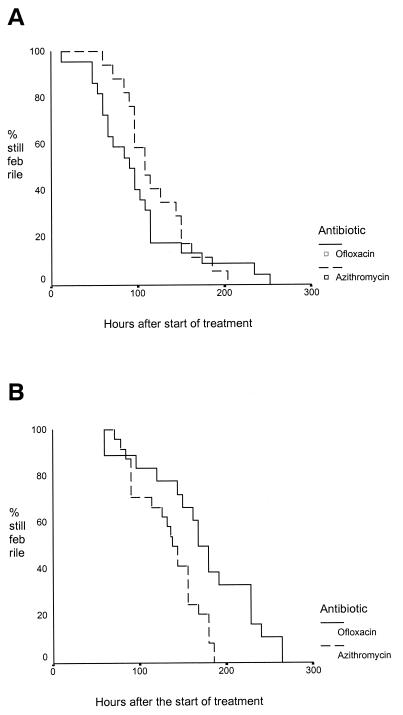
Table 2 also shows the treatment response in the subgroup of patients infected with a nalidixic acid-resistant isolate, and Fig. 1 shows the Kaplan-Meier survival curve for the fever clearance in the patients infected with nalidixic acid-susceptible and -resistant isolates. In the subgroup of patients with nalidixic acid-resistant typhoid, those treated with ofloxacin had a significantly longer fever clearance time (P = 0.004) and duration of hospital admission following the start of treatment (P = 0.001), and there was a higher proportion of patients with transient stool carriage after the end of treatment (7 of 17 [41%] versus 0 of 19; P = 0.002) compared with those treated with azithromycin.
FIG. 1.
(A) Fever clearance times for patients infected with a nalidixic acid-sensitive isolate of serovar Typhi or serovar Paratyphi A. (B) Fever clearance times for patients infected with a nalidixic acid-resistant isolate of serovar Typhi.
A total of 38 of 91 (42%) patients returned for follow-up at 4 to 6 weeks: 21 (48%) treated with azithromycin and 17 (39%) treated with ofloxacin. Two of seventeen (12%) of the ofloxacin-treated patients relapsed, but none of the twenty-one patients treated with azithromycin relapsed (P > 0.05). All the other patients followed up were clinically well with a negative stool culture.
Side effects are summarized in Table 3. Self-limiting gastrointestinal side effects were seen in five of the azithromycin-treated patients. The mean levels of AST and ALT increased in both groups during treatment. These increases had no clinical impact and resolved with time. There were no other significant side effects attributable to either antibiotic.
TABLE 3.
Adverse effects of treatment
| Effecta | Treatment
|
|
|---|---|---|
| Ofloxacin (n = 44) | Azithromycin (n = 44) | |
| Nausea | 1 (2.3) | 5 (11.6) |
| Vomiting | 3 (6.8) | 5 (11.6) |
| Abdominal pain | 4 (9.1) | 4 (9.3) |
| Skin rash | 0 | 1 (2.3) |
| Mean AST (IU/liter, 95% CI range) | ||
| Before treatment (NR, 20–40 IU/liter) | 97 (71–124, 25–294) | 123 (89–157, 35–466) |
| After treatment (NR, 20–40 IU/liter) | 106 (67–145, 17–551) | 127 (80–175, 19–600) |
| Mean ALT (IU/liter, 95% CI range) | ||
| Before treatment (NR, 20–45 IU/liter) | 94 (63–124, 18–251) | 105 (76–135, 23–361) |
| After treatment (NR, 20–45 IU/liter) | 119 (88–150, 17–327) | 138 (101–176, 20–367) |
NR, normal range.
DISCUSSION
This study has shown that a 5-day course of azithromycin is an effective treatment for uncomplicated enteric fever in adults, including those infected with MDR and nalidixic acid-resistant serovar Typhi strains. The average fever clearance of 5.4 days with azithromycin was longer than when using ofloxacin in nalidixic acid-sensitive isolates (4.3 days) but shorter than with nalidixic acid-resistant isolates (7.25 days). This fever clearance time also compares favorably with the third-generation cephalosporins (5.2 to 8.3 days) (29). Azithromycin was effective in eradicating fecal carriage, and there were no relapses. One patient treated with azithromycin was a clinical failure, however, with a positive blood culture after the end of treatment despite infection with an isolate for which the MIC was similar to those for the other isolates in the study. The low number of patients who returned for follow-up is a limitation of this trial. Further studies will be required to confirm that relapse and long-term carriage is not a problem with azithromycin.
The in vitro activity of azithromycin against serovar Typhi in this study (MIC90, 8 μg/ml; range, 3 to 16 μg/ml) was similar to those reported in other studies (10). The MIC is above the reported peak serum level of 0.4 μg/ml following a 500-mg oral dose of azithromycin (6) and 3.1 μg/ml following a 1-g dose by intravenous infusion (9) and is above the NCCLS breakpoint for susceptibility (15). Azithromycin, however, achieves high intracellular concentrations (16) and activity (18). The discordance between in vitro susceptibility and in vivo effectiveness is probably explained by the predominantly intracellular location of serovar Typhi (27).
Previous studies of azithromycin in typhoid fever have used longer courses of treatment. In nonrandomized studies in Chile (n = 10 patients) and Egypt (n = 14 patients) azithromycin at 500 mg given once daily for between 7 and 14 days or in a 1-g dose on the first day followed by 500 mg for 6 additional days was found to be effective in adults with typhoid fever (24; Butler et al., 32nd ICAAC). Fever clearance occurred within 4.3 to 5.4 days. However, in these two studies 3 of 24 (13%) patients were still blood culture positive at day 4. In a study in Bahrain three of four adults failed when azithromycin was given as a 1-g dose on day 1 and then 500 mg was given each day for the next 6 days (28). The three failures had clinically deteriorated by day 4 or 5 of therapy, and one patient infected with serovar Paratyphi A was blood culture positive on day 4. In a randomized comparative study in Egypt of azithromycin (1 g on day 1, 500 mg per day for the next 6 days) and ciprofloxacin (500 mg twice daily for 7 days) in 64 blood culture-positive adults, of whom 33% were MDR, all patients were cured. The mean fever clearance times in days (defined as a maximum daily temperature of ≤38°C) were 3.8 ± 1.1 (range, 2 to 7) for azithromycin and 3.3 ± 1.0 (range, 1 to 5) for ciprofloxacin (7). One patient treated with azithromycin had a positive blood culture on day 4 of therapy. There were no relapses and no fecal carriage posttreatment. In a similar comparative study in India, azithromycin at 500 mg per day for 7 days was 88% clinically successful and 100% microbiologically successful by day 8 in 42 adults with blood culture-positive enteric fever compared with 86 and 94% success rates in 35 adults treated with chloramphenicol at 2 to 3 g per day for 14 days (4).
A 5-day course of azithromycin was chosen to increase compliance and to be of comparable duration to the 5-day course of ofloxacin which had been shown previously to be effective at this center (22). Because of the long half-life of azithromycin, the patient would actually have antibiotic in the tissues for 3 to 7 days after the end of treatment. A dose of 1 g per day was used because of concern about the reports of the blood cultures remaining positive after 4 days of treatment with 500 mg a day. Doses of azithromycin higher than the recommended 5 to 10 mg/kg have been well tolerated (9), and this dose was well tolerated in our patients. Problems with nausea and vomiting occurred in 5 of 44 (11.6%) patients in the first day or two of treatment but were not severe enough to necessitate stopping the treatment. Azithromycin has been associated with elevated liver transaminases. In patients with enteric fever it is common for the transaminase values to be elevated two to three times the normal level on admission. In both the ofloxacin- and azithromycin-treated patients there was a slight increase in the transaminase levels during treatment, but these were not significantly different between the two treatments.
Ofloxacin in a 5-day course was only 86% effective. In the patients with nalidixic-acid-sensitive typhoid the efficacy was 91% and the mean fever clearance time was 4.3 days, a slightly poorer response than our previous experience with this regimen (22). In the patients with nalidixic acid-resistant typhoid the success rate was 81% and the mean fever clearance time 7.25 days. Furthermore, transient stool carriage posttreatment was present in 41% of the patients tested, and this has the potential to allow further transmission of serovar Typhi. The major route of elimination of ofloxacin is in the urine as unchanged drug, whereas with azithromycin and also with ciprofloxacin there is a high degree of intestinal elimination. Convalescent fecal carriage was less of a problem with azithromycin compared to ofloxacin and may potentially be also less of a problem with ciprofloxacin.
Cost and compliance, as well as safety and efficacy, need to be considered when choosing regimens for treating enteric fever in countries with limited resources where the disease is endemic. We had previously shown that short courses of fluoroquinolones are very effective for treating MDR nalidixic-acid-sensitive serovar Typhi (29). Unfortunately, during the course of this study the proportion of serovar Typhi strains that were nalidixic acid resistant increased from 10 to 76% (17). The optimum fluoroquinolone regimen for these resistant infections will require an increase in the dose and possibly also the duration of treatment. This will increase costs and reassert worries about fluoroquinolone usage in children. Azithromycin is relatively expensive (this regimen costs $10 to $50 [U.S. dollars] in Vietnam depending on the manufacturer) but is less expensive than the third-generation cephalosporins (the usual regimen with parenteral cephalosporins is $75 to $200 [U.S. dollars]) and seems to be at least as effective. Fluoroquinolones still remain the cheapest option ($4 to $40 [U.S. dollars] for a 7- to 10-day course).
The antimicrobial susceptibility of serovar Typhi is in a period of rapid change in many areas of the world. Fluoroquinolones are no longer predictably effective for treatment in areas of reduced fluoroquinolone susceptibility. The present study has shown that azithromycin is an effective alternative treatment for uncomplicated enteric fever in adults in a region where MDR and nalidixic acid-resistant serovar Typhi strains are endemic.
ACKNOWLEDGMENTS
We thank the Directors of the Centre for Tropical Diseases and the staff of the adult typhoid ward and the microbiology laboratory for their support of this study. We also thank Debbie House for her valuable assistance. The ofloxacin tablets used in the study were kindly provided by Andre Bryskier of Hoechst Marion Roussel.
This work was supported by The Wellcome Trust of Great Britain.
REFERENCES
- 1.Brown N M, Millar M R, Frost J A, Rowe B. Ciprofloxacin resistance in Salmonella paratyphi A. J Antimicrob Chemother. 1994;33:1258–1259. doi: 10.1093/jac/33.6.1258. [DOI] [PubMed] [Google Scholar]
- 2.Brown J C, Shanahan P M A, Jesudason M V, Thomson C J, Aymes S G B. Mutations responsible for reduced susceptibility to 4-quinolones in clinical isolates of multi-resistant Salmonella typhi in India. J Antimicrob Chemother. 1996;37:891–900. doi: 10.1093/jac/37.5.891. [DOI] [PubMed] [Google Scholar]
- 3.Butler T, Girard A E. Comparative efficacies of azithromycin and ciprofloxacin against experimental Salmonella typhimurium infection in mice. J Antimicrob Chemother. 1993;31:313–319. doi: 10.1093/jac/31.2.313. [DOI] [PubMed] [Google Scholar]
- 4.Butler T, Sridhar C, Daga M, Kani K, Pandit R, Khakhria R, Potkar C, Johnson R. Treatment of typhoid fever with azithromycin versus chloramphenicol in a randomized multicentre trial in India. J Antimicrob Chemother. 1999;44:243–250. doi: 10.1093/jac/44.2.243. [DOI] [PubMed] [Google Scholar]
- 5.Chinh N T, Solomon T, Thong M X, Ly N T, Hoa N T T, Wain J, Diep T S, Smith M D, Day N P J, Phi L T, Parry C M, White N J. Short course of ofloxacin for the treatment of enteric fever. Trans R Soc Trop Med Hyg. 1997;91:347–349. doi: 10.1016/s0035-9203(97)90102-4. [DOI] [PubMed] [Google Scholar]
- 6.Foulds G, Shepard R M, Johnson R B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl. A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 7.Girgis N I, Butler T, Frenck R W, Sultan Y, Brown F M, Tribble D, Khakhria R. Azithromycin versus ciprofloxacin for treatment of uncomplicated typhoid fever in a randomized trial in Egypt that included patients with multidrug resistance. Antimicrob Agents Chemother. 1999;43:1441–1444. doi: 10.1128/aac.43.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jesudason M V, Malathy B, John T J. Trend of increasing levels of minimum inhibitory concentration of ciprofloxacin to Salmonella typhi. Indian J Med Res. 1996;103:247–249. [PubMed] [Google Scholar]
- 9.Luke D R, Foulds G, Cohen S F, Levy B. Safety, toleration and pharmacokinetics of intravenous azithromycin. Antimicrob Agents Chemother. 1996;40:2577–2581. doi: 10.1128/aac.40.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metchock B. In vitro activity of azithromycin compared with other macrolides and oral antibiotics against Salmonella typhi. J Antimicrob Chemother. 1990;25(Suppl. A):29–31. doi: 10.1093/jac/25.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell D H. Ciprofloxacin-resistant Salmonella typhi: an emerging problem. Med J Aust. 1997;167:172. doi: 10.5694/j.1326-5377.1997.tb138827.x. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch D A, Banatvala N A, Bone A, Shoismatulloev B I, Ward L R, Threlfall E J. Epidemic ciprofloxacin-resistant Salmonella typhi in Tajikistan. Lancet. 1998;351:339. doi: 10.1016/s0140-6736(05)78338-0. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 6th ed. 1997. Approved standard M2-A6. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 7th ed. 1997. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial sensitivity testing: eighth informational supplement. 18. M100-S8. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 16.Panteix G, Guillaumond B, Harf R, Desbos A, Sapin V, Leclerq M, Perrin-Fayolle M. In vitro concentration of azithromycin in human phagocytic cells. J Antimicrob Chemother. 1993;31(Suppl. E):1–4. doi: 10.1093/jac/31.suppl_e.1. [DOI] [PubMed] [Google Scholar]
- 17.Parry C, Wain J, Chinh N T, Vinh H, Farrar J J. Quinolone-resistant Salmonella typhi in Vietnam. Lancet. 1998;351:1289. doi: 10.1016/s0140-6736(05)79356-9. [DOI] [PubMed] [Google Scholar]
- 18.Rakita R M, Jaques-Palaz K, Murray B E. Intracellular activity of azithromycin against bacterial enteric pathogens. Antimicrob Agents Chemother. 1994;38:1915–1921. doi: 10.1128/aac.38.9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe B, Threlfall E J, Ward L R. Ciprofloxacin-resistant Salmonella typhi in the UK. Lancet. 1995;346:1302. doi: 10.1016/s0140-6736(95)91906-6. [DOI] [PubMed] [Google Scholar]
- 20.Rowe B, Ward L R, Threlfall E J. Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clin Infect Dis. 1997;24(Suppl. 1):S106–S109. doi: 10.1093/clinids/24.supplement_1.s106. [DOI] [PubMed] [Google Scholar]
- 21.Saha S K, Talukder S Y, Islam M, Saha S. A highly ceftriaxone-resistant Salmonella typhi in Bangladesh. Pediatr Infect Dis J. 1999;18:387. doi: 10.1097/00006454-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Smith M D, Duong N M D, Hoa N T T, Wain J, Ha H D, Diep T S, Day N P J, Hien T T, White N J. Comparison of ofloxacin and ceftriaxone for short-course treatment of enteric fever. Antimicrob Agents Chemother. 1994;38:1716–1720. doi: 10.1128/aac.38.8.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Threlfall E J, Ward L R, Skinner J A, Smith H R, Lacey S. Ciprofloxacin-resistant Salmonella typhi and treatment failure. Lancet. 1999;353:1590–1591. doi: 10.1016/s0140-6736(99)01001-6. [DOI] [PubMed] [Google Scholar]
- 24.Tribble D, Girgis N, Habib N, Butler T. Efficacy of azithromycin for typhoid fever. Clin Infect Dis. 1995;21:1045–1046. doi: 10.1093/clinids/21.4.1045. [DOI] [PubMed] [Google Scholar]
- 25.Umasanker S, Wall R A, Berger J. A case of ciprofloxacin-resistant typhoid fever. CDR Rev. 1992;2:R139–R140. [PubMed] [Google Scholar]
- 26.Wain J, Hoa N T T, Chinh N T, Vinh H, Everett M J, Diep T S, Day N P J, Solomon T, White N J, Parry C M. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin Infect Dis. 1997;25:1404–1410. doi: 10.1086/516128. [DOI] [PubMed] [Google Scholar]
- 27.Wain J, Diep T S, Ho V A, Walsh A M, Hoa N T T, Parry C M, White N J. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility and antibiotic resistance. J Clin Microbiol. 1998;36:1683–1687. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace M R, Yousif A A, Habib N F, Tribble D R. Azithromycin and typhoid. Lancet. 1994;343:1497–1498. doi: 10.1016/s0140-6736(94)92604-2. [DOI] [PubMed] [Google Scholar]
- 29.White N J, Parry C M. The treatment of typhoid fever. Curr Opin Infect Dis. 1996;9:298–302. [Google Scholar]