Abstract
Systemic infections with pathogenic or facultative pathogenic bacteria are associated with activation and aggregation of platelets leading to thrombocytopenia and activation of the clotting system. Bacterial proteins leading to platelet activation and aggregation have been identified, and while platelet receptors are recognized, induced signal transduction cascades are still often unknown. In addition to proteinaceous adhesins, pathogenic bacteria such as Staphylococcus aureus and Streptococcus pneumoniae also produce toxins such as pneumolysin and alpha-hemolysin. They bind to cellular receptors or form pores, which can result in disturbance of physiological functions of platelets. Here, we discuss the bacteria-platelet interplay in the context of adhesin–receptor interactions and platelet-activating bacterial proteins, with a main emphasis on S. aureus and S. pneumoniae. More importantly, we summarize recent findings of how S. aureus toxins and the pore-forming toxin pneumolysin of S. pneumoniae interfere with platelet function. Finally, the relevance of platelet dysfunction due to killing by toxins and potential treatment interventions protecting platelets against cell death are summarized.
Keywords: Streptococcus pneumoniae, platelet killing, platelet activation, pore formation, surface proteins, toxin, pneumolysin, Staphylococcus aureus, MSCRAMMs
1. Introduction
Platelets are anucleated, discoid shaped cells of the blood with a size of 2–4 µm in diameter [1]. Platelets are derived from megakaryocyte (MK) shedding in the bone marrow and the lungs [2,3]. Hence, their translation repertoire is limited to stable MK-derived mRNA [4]. Important platelet functions contribute to coagulation and closure of vascular damage occurring during, e.g., microbial infections. The main players during this processes are the surface expressed group of platelet glycoproteins. They are expressed in high numbers such as, e.g., integrin αIIbβ3, which is highly abundant on the surfaces of platelets and megakaryocytes and is involved in the crosslinking/aggregation of single platelets after activation [5]. Integrin αIIbβ3 is the fibrinogen receptor but also interacts with other extracellular matrix (ECM) proteins containing an RGD-like motif such as von-Willebrand-Factor (vWF), thrombospondin-1 (TSP-1), or fibronectin [6]. Platelet factors playing a role in hemostasis and infection are stored in different cytoplasmic granules. These granules undergo exocytosis upon platelet stimulation/activation and release their content into the circulation or granule proteins re-associate to the platelet surface [7]. Three types of granules can be distinguished: alpha-granules, dense granules, and lysosomal granules. Dense granules contain adenosine diphosphate (ADP), adenosine triphosphate (ATP), serotonin, histamine, and ions such as Ca2+. Alpha granules contain platelet factor 4 (PF-4, CXCL4), P-selectin, coagulation factors such as factor V, mitogenic factors, adhesive glycoproteins such as vWF, TSP-1, and fibrinogen, but also microbicidal proteins. Glycosidases, which are important for clot retraction, are mainly stored in the lysosomal granules [7].
However, in addition to their role in hemostasis, platelets have other important functions. In recent years, platelets have been increasingly recognized as immune and inflammatory cells. Several studies have highlighted the role of platelets in acute and chronic inflammatory processes such as stroke, myocardial infarction, infections, and sepsis [8,9,10,11,12]. Indeed, platelets are the most abundant circulating cell type with important immune functions. They display their function as immune cells either locally at sites of platelet activation or systemically by interacting with, e.g., leukocytes or via release of immune modulatory molecules [13,14]. Platelets store more than 300 proteins in their granules [15]. Besides the abovementioned proteins, granules also contain proteins acting as chemokines and cytokines such as, e.g., RANTES (Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted) or CXCL12 (stromal cell-derived factor 1), and recruit and stimulate other cells of the immune system or induce endothelial inflammation [16]. Granule release also leads to changes in the platelet membrane composition. An increased number of integrin αIIbβ3 molecules and increased surface expression of P-selectin (CD62P) can be found on the surface after platelet activation. Exposure of P-selectin mediates initial interactions between platelets and leukocytes via immobilization of leukocytes at the site of lesion [17] and is used as a binding partner for initiation of complement activation [18]. In addition, platelets are a peripheral source of serotonin, stored in dense granules, which leads to differentiation of monocytes into dendritic cells (DCs) and also T-cell activation [19].
2. Platelets as Immune Cells in Infections
The first systemic response of the body to any kind of infection, tissue injury, or trauma is known as the acute phase response (APR). During APR, proinflammatory cytokines are released and acute phase proteins are produced [20]. By impairing microbial growth and promoting procoagulant activity to trap pathogens in local blood clots, platelets play a crucial role in the APR [21]. In addition, next to inflammatory and immune cells, platelets also produce interleukin 1-β (IL-1β) [21], which is not stored in granules as ready-to-use protein, but instead is translated from megakaryocyte (MK)-derived mRNA and released upon stimulation [22]. In mouse models of malaria, IL-1β has been demonstrated to play a major role in induction of the APR [21]. Another aspect that makes platelets part of the innate immune system is the expression of pattern recognition receptors (PRR) such as toll-like receptors (TLRs). TLR4, for example, is able to recognize bacterial lipopolysaccharides (LPS), which leads to platelet activation and release of IL-1β-rich microparticles, promoting interaction and activation with, e.g., endothelial cells [23]. However, others did not observe activation of washed platelets after incubation with LPS. Only incubation of whole blood with LPS led to increased P-selectin levels [24], indicating an indirect effect of LPS on platelets. During bacterial infections, TLR2 can be stimulated, leading to formation of platelet/neutrophil aggregates, which enhances adhesion of the aggregates to sites of injury or infection [25]. In addition, TLR2 stimulation of MKs is followed by increased maturation of MKs and elevated protein content, suggesting effects on platelet count, platelet function, and inflammation [26].
Besides their role in innate immunity, platelets are also pivotal for the acquired immune response. Platelets stimulate T-cell activation, trafficking, and also differentiation [27]. T cells are divided, with MHC class II recognizing CD4+ cells and MHC class I recognizing cytotoxic CD8+ cells. CD4+ cells release cytokines regulating the activity of B cells and innate immune cells and can be divided into immune effector cells (Th1, Th2, Th17) and T-regulatory cells. Platelet-derived chemokines such as RANTES trigger activation and arrest of T cells at sites of infections. Upon release, RANTES is immobilized on the endothelium and triggers the arrest of monocytes and monocyte-derived cells at the endothelium but not on adherent platelets in a shear resistant manner (Figure 1) [28]. Adhesion of rolling monocytes on activated platelets as well as on the exposed endothelium is P-selectin-dependent, and this interaction further triggers expression and secretion of cytokines such as TNF-α [29,30,31]. Furthermore, CXCL4 (PF-4) can mediate T-cell trafficking to sites of injury or infection by direct and indirect stimulation of CXCR3 expression on T cells [14]. In addition, T cells are also able to activate platelets via CD40L/CD40 interaction, leading to the release of chemokines such as RANTES and an increase in T cell recruitment [32,33]. Platelets further recruit and activate DCs via CD11b-JAM-C interactions, and the increased expression of CD80 and CD86 leads to enhanced T-cell response [34,35].
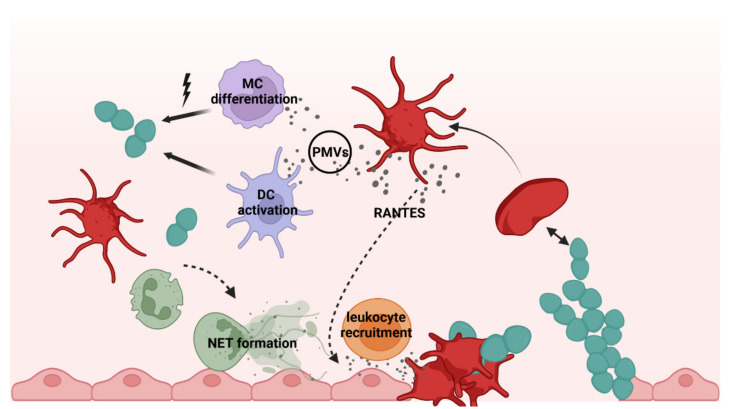
Figure 1.
Scheme illustrating different platelet functions in the immune response. Platelets sense and bind invading bacteria and injured endothelium, resulting in platelet activation. Upon activation, platelets release chemokines and cytokines such as RANTES, triggering leukocyte recruitment and PMVs acting on gene expression of monocytes and monocyte (MC)-derived cells such as dendritic cells (DC). In addition, neutrophils are attracted, and NET formation occurs at the site of infection via platelet-dependent mechanisms. Created with BioRender.com (accessed on 21 March 2022).
Another immune cell function of platelets is displayed by the release of microvesicles. The so-called platelet microvesicles (PMVs) are lipid membrane vesicles with a size of 0.1–1.0 µm that are mediators of cell–cell communication. Elevated numbers of PMVs in the circulation are associated with inflammation and diseases such as arthritis [36], development of an acute coronary artery syndrome, and stroke [37,38]. PMVs carry adhesion molecules (CD62P, RANTES) that facilitate monocyte arrest at sites of deposited PMVs (vessel walls) and additional recruitment of activated platelets [39]. PMVs contain up to 250 different microRNAs (miRNAs, 20–22 nucleotides long). These miRNAs and also other stored molecules can be transferred to other cell types such as immune cells, vascular cells, and also smooth muscle cells, thereby changing their gene expression [40,41]. Changes in gene expression include phenotypic switches of monocytes and monocyte-derived cell lines such as macrophages towards a phagocytic phenotype (Figure 1) [42]. Furthermore, not only PMVs released upon platelet activation but also upon apoptosis of platelets have immunomodulatory effects, as shown by differentiation of monocytes into phagocytes [43]. In bacterial infections, platelets can contribute to clearance of bacteria via release of antimicrobial peptides present in PMVs or stimulation of immune cells via release of immunomodulatory molecules. PMVs can be distinguished in kinocidins, defensins, thymosins, and derivatives of antimicrobial peptides, which act against Staphylococcus aureus (S. aureus) and Candida albicans [44,45].
3. Interactions of Platelets with Bacteria
Bacteria are able to spread from the site of infection, thereby often crossing host barriers and entering the circulatory system, leading to bacteremia and sepsis. Complications of bacteremia associated with abnormal platelet functions are, e.g., infective endocarditis and disseminated intravascular coagulation (DIC) [46]. Interactions between platelets and bacteria are characterized by direct (Figure 2) or indirect binding of bacteria to platelets or via released bacterial factors [24,46,47]. Indirect binding occurs via bridging molecules of the extracellular matrix (ECM), thereby linking bacterial surface proteins with platelet receptors [48]. The adhesive properties of bacteria towards platelets are essential for colonization of, e.g., cardiac valves during infective endocarditis [49,50]. Colonization can lead to local and/or systemic infections, and this might result in platelet activation in the bloodstream. Some bacterial infections cause severe thrombocytopenia without preceding bacteremia. To these belong, e.g., Helicobacter pylori-mediated immune thrombocytopenia driven by autoantibody-destroying platelets [51,52] or the hemolytic uremic syndrome (HUS) caused by Shiga toxin-producing Escherichia coli (E. coli) [52,53], which is due to amplification of the platelet activation process. Thrombocytopenia associated with bacterial infections appears as a secondary effect of systemic platelet activation, coagulation, and DIC, due to boosted platelet consumption [54].
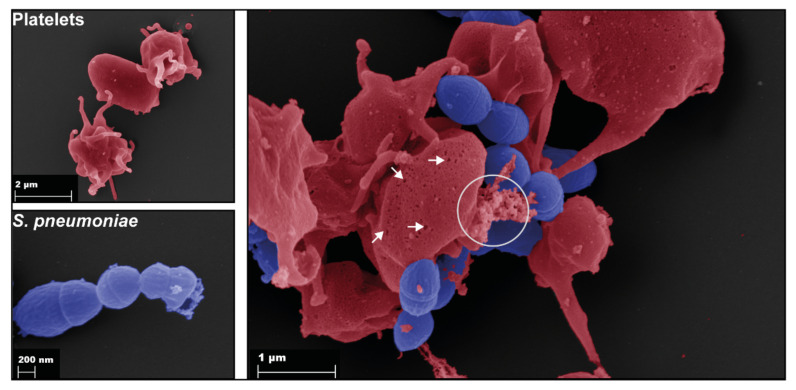
Figure 2.
Binding of S. pneumoniae (blue) to platelets (red). Scanning electron microscopy of single platelets (upper left), single pneumococci (bottom left), and platelets incubated with the pneumococcal TIGR4 strain for 1 h (right). The right image shows binding of pneumococci to platelets. In addition, pneumolysin pores are formed in platelet membranes (arrows), and released granule content is visible (circle).
Besides platelet activation as a result of bacterial binding, another mode of bacteria–platelet interaction has been discovered, namely, internalization of bacteria by platelets. S. aureus was the first bacterium that was described to be internalized by platelets [55,56]. A prerequisite for internalization of S. aureus is a simultaneous platelet stimulation by ADP. Slightly differently, Porphyromonas gingivales was shown to be internalized by platelets without additional stimulation of platelets [57,58]. Furthermore, the platelet FcγRII receptor might also be able to initiate internalization of IgG–bacteria complexes as it has been shown for beads (0.5–1.5 µm in size) coupled with IgG or E. coli pre-opsonized with IgGs [59,60]. Other studies demonstrated a kind of searching and shuttling of invading bacteria by platelets. Adherent platelets can migrate over their substrate, collecting all substrate-bound material including bacteria, resulting in boosted activity of phagocytes [61]. In addition, platelets were shown to deliver the intracellular bacterium Listeria monocytogenes to dendritic cells [62]. However, the fate of the internalized bacteria is still unclear. On the one hand, they could be killed by antimicrobial substances of α-granules. On the other hand, the intracellular fate might help the bacteria to escape from the host immune system.
4. Platelet Receptors in Bacterial Infections and Bacterial Adhesins
Platelets express a large number of receptors involved in interactions with pathogens. This includes integrins, G-protein-coupled receptors, ADP receptors (purinergic receptors, P2Y), leucine-rich repeat glycoproteins (GP), Toll-like receptors (TLRs), IgG superfamily receptors (GPVI, FcγRIIa), and tyrosine kinase receptors [63]. The FcγRIIa receptor is a low-affinity IgG receptor binding to the Fc part of immunogloblins [64]. About 2000 to 3000 FcγRIIa receptors are expressed on the surface of a single platelet [65], which enable binding and internalization of immune complexes containing IgGs (Figure 3) [59]. Binding of, e.g., IgG-covered pathogens leads to platelet activation and aggregation. Binding and activation of other platelet receptors by bacteria often requires simultaneous stimulation of FcγRIIa. For example, platelet aggregation induced by E. coli is dependent on simultaneous stimulation of integrin αIIbβ3 and FcγRIIa [66]. For S. aureus and S. epidermidis, a clustering of integrin αIIbβ3 or TLR with FcγRIIa is necessary for platelet activation [67]. Some pathogens such as S. aureus or H. pylori require plasma proteins such as fibrinogen or vWF to crosslink FcγRIIa with GPIb receptors for platelet activation [68].
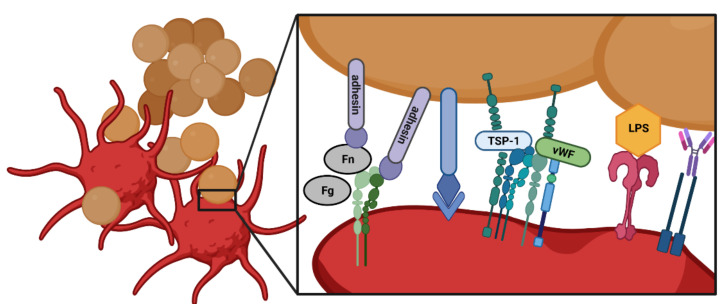
Figure 3.
Binding of bacteria to platelets occurs either directly or indirectly. Bacterial adhesins with specific repeating units can utilize ECM proteins such as fibronectin (Fn), fibrinogen (Fg), TSP-1, or vWF as molecular bridges to bind to, e.g., integrin αIIβ3 or other complexes of glycoproteins. Furthermore, some bacterial factors can directly bind to integrins, TLRs, or other platelet surface proteins. Bacteria already covered by IgGs are recognized by FcγRIIa. Created with BioRender.com (accessed on 21 March 2022).
As mentioned above, integrin αIIbβ3 interacts with ECM proteins containing an RGD-like motif [6]. Some bacteria express surface proteins with domains rich in serine-aspartate repeats. This is highlighted by a family of S. aureus surface components called microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). They exhibit sequence repeats mediating adherence to platelets or other host cell tissues as one of the first steps during infection [69]. Well-characterized members of this family are clumping factor A and B (ClfA and ClfB), fibronectin-binding protein A and B (FnBPA and FnBPB), and serine-aspartate repeat-containing protein E (SdrE). Fibrinogen bridges ClfA and ClfB via their fibrinogen binding domains to integrin αIIbβ3 [70], whereas fibrinogen or fibronectin is used by FnBPA and FnBPB for bridging [71,72]. In addition, integrin αIIbβ3 is also directly targeted by ClfA, but not ClfB, resulting in platelet activation (Figure 3) [73,74]. Further, integrin αIIβ3 can be directly bound by the S. aureus proteins iron-regulated surface determinant B (IsdB) [75]. Next to pneumococci, fibrinogen also bridges proteins of other bacteria such as serine-aspartate dipeptide repeat G (SdrG) protein of S. epidermidis to integrin αIIbβ3 [76]. A further platelet protein target of bacteria is the surface-associated protein-disulfide polymerase (PDI). The S. aureus extracellular adherence protein (Eap), a member of the SERAM (secretable expanded repertoire adhesive molecules) family, binds directly but also indirectly utilizes fibrinogen as a bridging molecule to PDI, resulting in platelet activation [77]. Direct activation and aggregation of platelets is also achieved by other secreted S. aureus proteins such as the chemotaxis inhibitory protein (CHIPS), the formyl peptide receptor-like 1 inhibitory protein (FLIPr), and the major autolysin (AtlA) (Figure 3) [24]. Upon activation of platelets, the extracellular fibrinogen-binding protein EfB of S. aureus binds to surface-exposed P-selectin and inhibits interactions between platelets and leukocytes [78,79].
GPIbα is a type-1 glycosylated membrane receptor that is highly abundant on the surface of platelets and megakaryocytes [80]. GPIbα is found in a complex with GPIbβ, GPIX, and GPV [81] and is the receptor for vWF but also for TSP-1, α-thrombin, and CD62P [80]. Different streptococcal species have been shown to bind to GPIbα via so-called glycosylated adhesins containing serine-rich repeats. This protein family was described in Streptococcus gordonii (glycosylated streptococcal protein B, GspB) [82] and Streptococcus sanguinis (serine-rich protein A, SrpA, and hemagglutinin salivary antigen, HSA) [83] and was shown to bind to sialic acid residues. In addition, vWF is used by bacteria for bridging to GPIbα as was shown for S. aureus surface protein A (SpA) and an uncharacterized H. pylori surface protein [68,84].
TLRs are type 1 transmembrane proteins widely expressed on eukaryotic cells and best characterized on immune cells such as macrophages and dendritic cells. The ectodomain of TLRs contains leucine-rich β-sheets interacting with PAMPs and a Toll-interleukin-1 receptor domain for signal transduction [85]. Platelets express TLR1, TLR2, TLR4, TLR7, and TLR9, with TLR4 being the most abundant [86,87]. Next to the LPS-recognizing TLR4, TLR2 is also important in bacterial infections. TLR2 contains a glycosylated N-terminal ligand-binding domain with leucine-rich repeats [88] and recognizes bacterial lipoproteins [89]. Platelet TLR2 has been shown to be a target of S. pneumoniae and group B streptococci (GBS). Binding induces activation of the phosphoinositide-3 (PI3)-kinase pathway, finally leading to platelet activation and aggregation [90,91]. The interaction between pneumococci and platelet TLR2 induces further activation of integrin αIIbβ3 as well as release of dense granules [90]. Furthermore, TLR9 is also involved in bacteria-induced platelet aggregation. TLR9 recognizes cell-free bacterial DNA, which is increased in the blood of septic patients, leading to activation of coagulation [92].
In addition to the above-mentioned platelet receptors, the zinc-dependent metalloproteinase ADAM10 is a receptor for the S. aureus α-hemolysin (Hla), and binding leads to cleavage of GPVI [93]. Hla is a β-barrel pore-forming toxin, forming pores of 1–3 nm in diameter in the lipid bilayer of eukaryotic cells. These pores allow molecules up to a size of 4 kDa to pass through [94,95]. Hla is expressed by most S. aureus clinical isolates, and expression levels have been reported to correlate with virulence and disease severity [96,97]. Interactions of platelets with Hla have been reported to cause platelet activation and aggregation [98,99]. In addition, we recently demonstrated that Hla-mediated platelet activation is followed by loss of platelet function, leading to impaired thrombus formation and reduced stability of formed thrombi [100].
5. Platelets in S. pneumoniae Infections
S. pneumoniae exhibits the typical characteristics of Gram-positive bacteria. Pneumococci are enclosed by a flexible bilipid membrane, which is surrounded by a thick, multi-layered peptidoglycan sacculus composed of highly cross-linked glycan strands [101]. Besides peptidoglycan, teichoic acids are a general constituent of the Gram-positive cell wall [102]. Pneumococci possess rather complex, structurally unique, peptidoglycan-anchored wall teichoic (WTA) and membrane-anchored lipoteichoic acids (LTA), which are built up of identical repeating sugar units, highly decorated with phosphorylcholine [103,104]. Pneumococci shield themselves from the environment by a thick polysaccharide capsule enveloping the cell wall, whose composition is serotype-specific [105]. The capsule is the main virulence factor of pneumococci and protects the bacteria effectively from opsonization and phagocytosis by the host immune system [106]. Furthermore, four classes of proteins can be found on the surface of pneumococci, which can be classified by their mode of anchoring. The largest group with 37 predicted members is the group of lipoproteins, which are anchored to the bacterial cell membrane via an N-acyl diacylglycerol group [107,108]. Most of the lipoproteins are predicted to be part of ABC-transporters, which are essential for nutrient uptake and therefore directly involved in bacterial fitness [109]. Other lipoproteins have been shown to have essential functions in protein folding, cell wall biosynthesis, stress response, or pathogenicity [110,111,112,113]. The second group of pneumococcal surface proteins is the unique group of choline-binding proteins (CBPs). This group consists of 13–16 proteins (strain dependent), which contain N- or C-terminally a choline-binding domain composed of highly conserved choline-binding modules. CBPs are non-covalently bound to the phosphorylcholine moiety of the repeating units of WTA and LTA [114]. Well-characterized CBPs are the major autolysin LytA, which plays an important role in autolysis and virulence and the pneumococcal surface protein C (PspC), which is essential for pneumococcal colonization and pathogenesis [115,116,117,118,119]. The third class of pneumococcal surface proteins (about 13–19 members) contains a C-terminal cell wall-sorting signal beginning with a LPXTG amino acid motif [120,121]. The transpeptidase sortase A (SrtA) recognizes this motif; cleaves between the threonine and glycine residues; and anchors the protein to lipid II, which is subsequently incorporated into the peptidoglycan of the cell wall [122,123]. Known representatives from this group are, for example, the neuraminidase A (NanA) and the pneumococcal adhesion and virulence factor B (PavB), which were shown to be involved in pneumococcal adhesion and pathogenesis. The fourth group of pneumococcal surface proteins is the so-called moonlighting proteins, also known as non-classical surface proteins. This group includes enzymes that are actually ubiquitous intracellularly but can also be found on the surface of bacteria, where they play an additional role, often associated with virulence [124]. One example of such a protein is the pneumococcal enolase, which intracellularly converts 2-phosphoglycerate to phosphoenolpyruvate during glycolysis, but can also be found on the bacterial surface. Here, the enolase was shown to bind host plasminogen as well as the human complement inhibitor C4b-binding protein, leading to enhanced adherence to epithelial and endothelial cells and complement evasion [125,126].
5.1. Community Acquired Pneumonia (CAP)
S. pneumoniae is one of the leading causes of community-acquired pneumonia (CAP). CAP is a potentially life-threatening disease, with a mortality rate of up to 14% in hospitalized patients [127]. The highest risk for an infection with severe outcomes is in young children, the elderly, immunocompromised patients, and those with comorbidities [128]. During severe CAP, systemic platelet activation [129] and dropping platelet counts have been reported. The development of thrombocytopenia correlates with increased mortality [130,131]. As discussed in Section 5.4, S. pneumoniae directly and indirectly stimulates platelets, leading to activation and release of granule content, which is accompanied with the release of antimicrobial peptides (AMPs). However, although AMPs are released, S. pneumoniae is not affected by platelet releasates, but in turn destroys platelets themselves [132]. Neutrophils are one of the most important players in the progression of inflammation and also sepsis. In response to bacteria, neutrophils form NETs built up of neutrophil DNA, histones, and granular proteins such as defensins, thereby often leading to bacterial trapping and antimicrobial actions [133,134]. Neutrophil-derived defensins inhibit the synthesis of bacterial DNA, RNA, and proteins. In addition, lysozymes degrade the bacterial cell wall, and elastase cleaves bacterial surface virulence factors [135]. NETs and their extracellular histones mediate initiation and proceeding of platelet activation and coagulation, leading to a prothrombotic phenotype [136]. Pneumococci evade trapping in NETs by protective effects mediated by D-alanylation of LTA [137], blocking of NET binding via pneumococcal surface protein A [138], and by degrading the neutrophil DNA scaffold via endonuclease A [139]. Taken together, S. pneumoniae is able to evade the platelet induced immune response, and on the contrary triggers an inflammatory and coagulant phenotype of platelets during severe CAP.
5.2. Sepsis
Sepsis is a common complication of pneumococcal infections, with mortality rates of up to 30%. Sepsis leads to an activation of the coagulation cascade with consumption of circulating coagulation factors, platelets, and the generation of platelet–leukocyte complexes. As a result, patients develop thrombocytopenia and DIC, finally leading to hypoxic organ damage. Up to 50% of patients with severe sepsis develop DIC [140]. Several factors lead to DIC. First, coagulation is activated via different pathways [134,141,142]. Second, there is an increase of endothelial adhesion molecules, leading to platelet adhesion to endothelial cells and exposed subendothelial collagen. During these processes, platelet activation, aggregation, microthrombus formation, and finally vessel occlusion are triggered [99,143,144,145]. In addition, neutrophils are attracted and affected during sepsis. High numbers of circulating immature forms of neutrophils in the peripheral, impaired migration but also prolonged presence of NETS are commonly observed in sepsis [146,147]. NET formation indirectly triggers tissue factor release of endothelial cells, which is then captured in NETs, further triggering coagulation [144,148]. Another factor is the massive amount of released reactive oxygen species (ROS). ROS favor vasoconstriction and have an activating effect on platelets [149]. Next to aggregation and thrombus formation, platelets also play a role in inflammatory processes, which are associated with sepsis. Platelet-derived soluble CD40L is increased in the circulation of septic patients [150,151,152] and plays a central role in activation and recruitment of neutrophils by activation of the β2 integrin Mac-1 of neutrophils and indirectly via macrophage inflammatory protein-2 and subsequent CXCR2 signaling [153,154]. Under septic conditions, activation of CXCL4 is also increased. This results in platelet degranulation, release of proinflammatory factors, and stimulation of the coagulation cascade [155]. Taken together, bacterially induced microthrombus and NET formation can cause uncontrolled coagulation and inflammation, leading to thrombocytopenia and DIC during sepsis.
5.3. Infective Endocarditis
Development of infective endocarditis is a complication of bacteremia caused by S. pneumoniae. In the pre-antibiotic era, S. pneumoniae caused about 15% of IE cases. Upon usage of penicillin or cephalosporine, the prevalence dropped to approximately 3% in the 1990s, and currently, prevalence data are missing [156,157]. IE caused by pneumococci mainly affects the aortic valves, and patients often also suffer from acute pneumonia; a common complication of pneumococcal IE is embolism [158]. Before bacteria can colonize the valves and cause IE, the surface structure of the endothelial valves has to be altered, as observed after previously occurred endocarditis or valve replacement [159]. On these structural changes, fibrin and platelets adhere, forming the so called non-bacterial thrombotic vegetation (NBTV) [160,161]. These NBTV serve as a niche for colonization in transient bacteremia or in the case of oral streptococci, after dental treatment [162,163,164], leading to further platelet aggregation on the surface and fibrin deposition [160]. By further acquisition of fibrin and platelets, bacteria are shielded from the immune system, allowing bacteria to reach very high densities [24,165].
5.4. Pneumococcal Interactions with Platelets
In contrast to S. aureus, little is known about the interaction between S. pneumoniae and platelets. The first studies describing platelet activation due to pneumococci were published in the 1970s. These studies showed that some pneumococcal serotypes induced platelet activation and aggregation in vitro, whereas other serotypes had no effect on platelet activation [166,167]. Later, platelet aggregation was shown to be induced only in interactions with encapsulated strains via an interaction with TLR2, but not with nonencapsulated strains [90]. However, other studies reported contradictory results. De Stoppelaar and colleagues did not observe platelet aggregation in encapsulated strains [168]. In addition, a TLR2-independent platelet degranulation was observed, which could be confirmed in mice with knockouts for several TLRs [168]. However, recent studies, including our own, demonstrated a direct binding of pneumococci to platelets. One study showed aggregate formation between platelets and pneumococci. This aggregate formation was dependent on the presence of soluble fibrin and the presence of TSP-1 derived from activated platelets [169]. The pneumococcal adhesins PavB and PspC are hypothesized to bind to platelet GPII/bIII via bridging of TSP-1 [170,171].
One of the major virulence factors of S. pneumoniae is pneumolysin (Ply), a cholesterol-dependent cytolysin that oligomerizes into the membrane after binding to the target cell, leading to pore formation [172]. Ply has been shown earlier to activate and aggregate platelets depending on Ca 2+-influx through pneumolysin pores [173,174]. However, our own data demonstrate platelet killing by pneumolysin (Figure 4) [47].
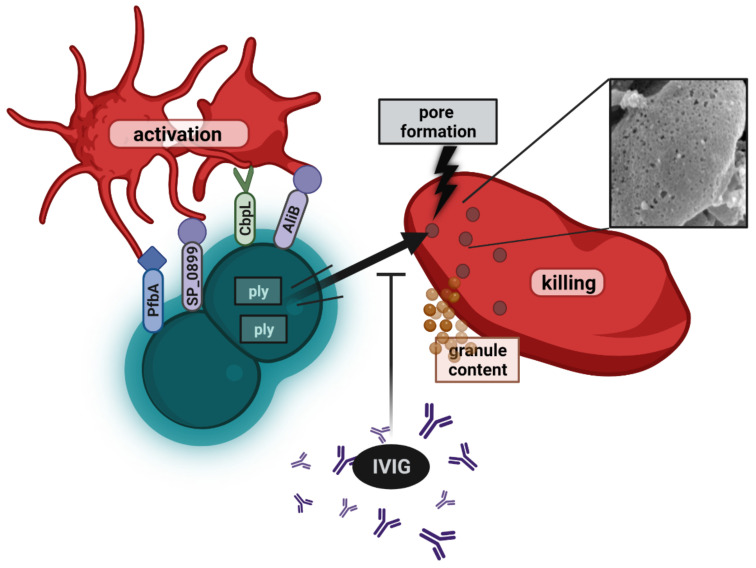
Figure 4.
Scheme of different interactions between platelets and pneumococci. Individual pneumococcal surface proteins can induce direct activation of platelets. On the other hand, the intracellular pneumolysin (Ply) kills platelets by extensive pore formation in platelet membranes, as shown by the SEM image of a Ply-treated platelet on the right side. Pneumolysin is released in the circulation upon autolysis of pneumococci, and its action on platelets can be neutralized by the addition of pharmaceutical IgG preparations. Created with BioRender.com (accessed on 21 March 2022).
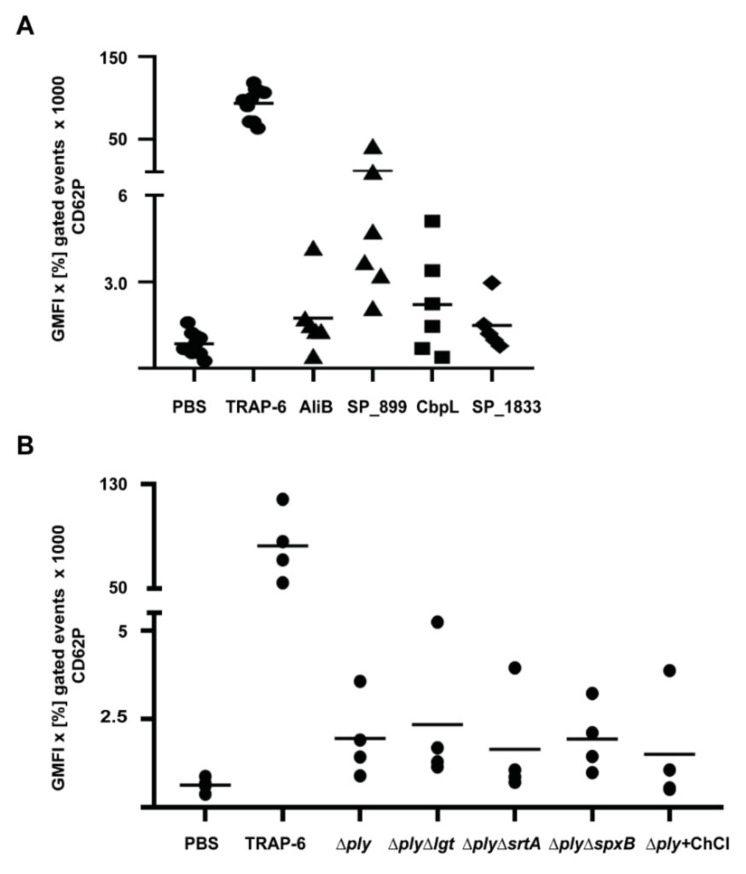
In accordance with other studies, we also observed highly increased P-selectin signals in flow cytometry, suggesting massive platelet activation. However, instead, as shown by confocal imaging, only intracellular stores of P-selectin were stained due to antibodies diffusing through the pneumolysin pores. In addition, the increase in light transmission of the platelet suspension after incubation with pneumolysin is due to cell lysis instead of aggregation. Platelet lysis occurred immediately after addition of pneumolysin, even at very low concentrations. A loss of platelet function was also observed in whole blood experiments [47]. Nevertheless, we also observed increased P-selectin signals in lysates of ply knockout strains, which appear independent of pneumococcal-derived H2O2, since P-selectin levels were similar in a ply-mutant and a ΔspxBΔply double mutant (Figure 5B). Therefore, we hypothesized that other surface-associated proteins of S. pneumoniae trigger platelet activation. A screening of our library of pneumococcal surface proteins (Table 1) in activation assays with washed platelets revealed candidate proteins, which directly activate platelets. Among them were SP_0899, a lipoprotein of thus far unknown function, and CbpL, a choline-binding protein and putative adhesion contributing to colonization [175]. In addition, AliB and SP_1833 also induced at least a slightly increased P-selectin staining. AliB is a lipoprotein and functions as a substrate-binding protein for oligopeptides [176], and SP_1833 (PfbA) is a sortase-anchored protein with plasmin- and fibronectin-binding capacity [177] (Figure 3A). All tested pneumococcal lipoproteins were heterologously expressed without the lipid moiety. The naturally occurring lipidation of lipoproteins has been shown to trigger TLR2-dependent signaling in leukocytes [178,179]. Therefore, lipidated proteins were tested in comparison to their non-lipidated forms [179], but no activation leading to P-selectin surface staining was detected. Nevertheless, it is noteworthy to know that TLR expression levels in resting platelets is low, but they become upregulated, and levels increase on the platelet surface upon activation [87,180]. Since proteins with platelet activation potential were identified in all groups of pneumococcal surface proteins, mutants were applied in activation assays lacking pneumolysin or whole groups of surface proteins [107,177]. This was achieved by the deletion of genes encoding essential enzymes involved in the anchoring of proteins (prolipoprotein diacylglyceryl transferase Lgt for lipoproteins and SrtA for sortase-anchored proteins) to the bacterial surface. To remove CBPs from the surface of pneumococci, a ply mutant was treated with choline chloride. During severe invasive infections, antibiotic treatment autolysis as well as antibiotic-induced lysis occurs in the bloodstream. Therefore, lysates generated from these deletion or choline chloride-treated strains were also tested for their platelet activation potential. Independent of the genetic background, all lysates induced increased P-selectin signals at similar levels (Figure 1B), leading to the assumption that another factor, e.g., components of the cell wall could be responsible for platelet activation. However, isolated and structurally defined protein-free pneumococcal lipoteichoic acids as well as wall teichoic acids [181] had no impact on platelet P-selectin surface expression (Table 1). Taken together, besides the identified surface proteins, another thus far unknown factor might be able to induce direct platelet activation. Nevertheless, the lytic effect of pneumolysin on platelets, even at very low concentrations, probably overshoots any other effect of pneumococcal proteins on platelets in invasive infections (Figure 1 and Figure 4).
Figure 5.
Individual pneumococcal proteins and pneumococcal lysates directly activate human platelets. Washed platelets of a defined set of donors were incubated with different concentrations of pneumococcal proteins (A) (Table 1) for 30 min or pneumococcal lysates (B) with the indicated genetic backgrounds for 60 min at 37 °C. CD62P was used as an activation marker and was detected by flow cytometry, using a PE-Cy5-labelled P-selectin antibody. PBS was used as negative control, and 20 µM TRAP-6 was used as a positive control. The data are presented as geometric mean of fluorescence intensity (GMFI) of positive gated events multiplied with the percentage of positive gated events in the dot plots.
Table 1.
List of pneumococcal proteins and cell wall components, which were tested to activate platelets and led to CD62P expression. * The last column provides the highest tested concentration of each protein in the platelet activation assay. The molarities were chosen on the basis of previous publications determining platelet activating potential of bacterial proteins [24].
| Protein Class | No. | Protein Name SP Number |
Function | Activation of Washed Platelets | Protein Concentration (µM) * |
|---|---|---|---|---|---|
| Lipoproteins | 1 | AdcAII (SP_1002) | substrate-binding protein of ABC transporter for zinc(II) ions | - | 4 |
| 2 | AliB (SP_1527) | substrate-binding protein of ABC transporter for oligopeptides | + | 2 | |
| 3 | AliC | substrate-binding protein of ABC transporter for oligopeptides | - | 4 | |
| 4 | AliD | substrate-binding protein of ABC transporter for oligopeptides | - | 4 | |
| 5 | AmiA (SP_1891) | substrate-binding protein of ABC transporter for oligopeptides | - | 4 | |
| 6 | DacB (SP_0629) | L,D-carboxypeptidase, peptidoglycan turnover | - | 4 | |
| 7 | Lipidated DacB | L,D-carboxypeptidase, peptidoglycan turnover | - | 4 | |
| 8 | Etrx1 (SP_0659) | extracellular thioredoxin protein 1 | - | 4 | |
| 9 | Etrx2 (SP_1000) | extracellular thioredoxin protein 2 | - | 4 | |
| 10 | MetQ (SP_0149) | substrate-binding protein of ABC transporter for methionine | - | 4 | |
| 11 | Lipidated MetQ | substrate-binding protein of ABC transporter for methionine | - | 4 | |
| 12 | PccL (SP_0198) | transport of small hydrophobic molecules such as siderophores | - | 4 | |
| 13 | PiaA (SP_1032) | substrate-binding protein of ABC transporter for iron | - | 4 | |
| 14 | PnrA (SP_0845) | substrate-binding protein of ABC transporter for nucleosides | - | 4 | |
| 15 | PpmA (SP_0981) | proteinase maturation protein, peptidyl-prolyl isomerase | - | 4 | |
| 16 | PsaA (SP_1650) | substrate-binding protein of ABC transporter for manganese | - | 4 | |
| 17 | SlrA (SP_0771) | streptococcal lipoprotein rotamase, peptidyl-prolyl isomerase | - | 4 | |
| 18 | GshT (SP_0148) | substrate-binding protein of ABC transporter for glutathione | - | 4 | |
| 19 | SP_0191 | unknown function | - | 4 | |
| 20 | SP_0899 | unknown function | +++ | 2/4 | |
| 21 | FusA (SP_1796) | substrate-binding protein of ABC transporter for fructo-oligosaccharides |
- | 2 | |
| 22 | RafE (SP_1897) | substrate-binding protein of ABC transporter for multiple sugars |
- | 4 | |
| 23 | PstS (SP_2084) | substrate-binding protein of ABC transporter for phosphate ions |
- | - | |
| 24 | SP_1690 | substrate-binding protein of ABC transporter | - | 4 | |
| 25 | MalX (SP_2108) | substrate-binding protein of ABC transporter for maltose/maltodextrin | - | 4 | |
| 26 | SatA (SP_1683) | substrate-binding protein of ABC transporter for sialic acid | - | 4 | |
| CBPs | 27 | CbpC (SP_0377) | regulatory function for autolysis by inhibiting autolysin LytC | - | 4 |
| 28 | CbpF (SP_0391) | putative adhesin | - | 4 | |
| 29 | CbpL (SP_0667) | putative adhesin | ++ | 4 | |
| 30 | Chimeric (PspA+PspC) | fusion of N-terminal domains of PspA and PspC | - | 4 | |
| 31 | PcpA (SP_2136) | adhesin | - | 2 | |
| 32 | PspA_QP2 (SP_0117) | virulence factor, binds lactoferrinand inhibits complement activation | - | 4 | |
| 33 | PspC_SH2 (SP_2190) | adhesion, IgA inactivation, major factor H–binding protein | - | 4 | |
| Sortase– anchored proteins |
34 | PfbA (SP_1833) | plasmin- and fibronectin-binding protein | + | 2 |
| 35 | PitB (spt_1059) | pilin of pneumococcal pilus-2, adhesin | - | 2 | |
| 36 | PsrP (SP_1772) | adhesion, biofilm formation | - | 4 | |
| 37 | RrgB (SP_0463) | pilus-1 anchorage protein | - | 4 | |
| 38 | RrgC (SP_0464) | pilus-1 backbone protein, pilin | - | 4 | |
| 39 | SP_1992 | adhesin architecture, bind to collagen and lactoferrin in vitro | - | 4 | |
| Cell wall components | 40 | lipoteichoic acids | - | 4 | |
| 41 | wall teichoic acids | - | 40 µg/mL |
6. Relevance of Findings for Disease
Thus far, there are only a few studies focusing on platelet activation during pneumococcal infections. One study showed that the expression of pblB, a phage-derived gene, was associated with increased platelet activation and mortality in hospitalized patients suffering from CAP caused by S. pneumoniae [129]. Another study showed increased platelet activation and platelet hyperreactivity in a porcine model of invasive S. pneumoniae infections [182]. In a follow-up in vitro study, the authors demonstrated that desialylation of platelets by the pneumococcal neuraminidase A (NanA) results in hyperreactivity of platelets to ADP stimulation [183].
One approach to interfere with platelet dysfunction in pneumococcal infections is directly targeting and neutralizing the pore forming pneumolysin with antibodies. Promising candidates are the pharmaceutically available IgG preparation IVIG (Privigen, 98% IgG) or the mixed immunoglobulin preparation trimodulin (21% IgA, 23% IgM, 56% IgG). Both immunoglobulin preparations contain antibodies against pneumolysin and have been shown to efficiently inhibit platelet damage in vitro, as shown by rescued platelet function and viability even in the presence of high pneumolysin concentrations (Figure 4) [47,184]. The presence of IgM and IgA in trimodulin had no beneficial effect for pneumolysin neutralization. In fact, neutralization efficiency was dependent on IgG content in the immunoglobulin preparation [184]. In a phase 2 clinical trial (CIGMA study), patients with severe community-acquired pneumonia were treated with trimodulin in addition to standard care. In the trimodulin group, patients had higher platelet counts and a nominally lower mortality compared to the placebo group [47]. However, patients included in the study were only 160, and larger clinical trials are necessary to confirm this observation.
A major cause for thrombocytopenia is coagulopathy conditions, observed in 80% of septic patients, with DIC being the most severe form [185]. Therefore, treatment of coagulopathies is highly needed to reduce mortality rates and coagulation-associated tissue damage. The focus of ongoing research lies on inhibition of coagulation or the use of anti-platelet drugs. Anti-platelet therapy during sepsis or ARDS is not part of standard care, but several studies conclude this as a promising approach to reduce disease severity [186,187,188,189]. However, the benefit of anti-platelet drugs such as, e.g., acetylsalicylic acid during sepsis is under debate [189,190], and more stringent clinical studies are needed. The same accounts for inhibition of coagulation. Although some clinical studies conclude damped disease severity upon usage of, e.g., antithrombin, its benefit is controversial, and more research on this topic is needed [191,192].
7. Conclusions
In this review, we highlight interactions between platelets and S. pneumoniae or S. aureus in vitro and in disease. Multiple factors of both bacteria result in direct or indirect platelet activation and aggregation. However, the predominant effect seems to be exerted by the pore-forming toxins pneumolysin (pneumococci) and alpha-hemolysin (Hla, S. aureus). Whereas Hla first activates and later on lyses platelets, pneumolysin directly lyses platelets. The pathomechanisms of these toxins and their impact on platelet function are of high clinical importance because thrombocytopenia and DIC are typical complications in severe invasive infections caused by these pathogens. Future clinical studies targeting either bacterial components such as pneumolysin and Hla and their receptors or systemic coagulation as seen in dependence of Hla are highly needed to improve clinical outcomes.
Acknowledgments
The authors thank Jan Wesche, Peggy Stremlow, and Jessica Fuhrmann for technical support. We further thank Manfred Rohde for electron microscopy image courtesy. We are grateful to Thomas Thiele (Transfusion Medicine, University Medicine Greifswald) for suggestions and critical reading.
Author Contributions
Conceptualization, S.H., K.J., T.P.K.; methodology, K.J., L.-S.S., S.W.; software, S.H.; validation, K.J., L.-S.S.:, T.P.K., S.H.; formal analysis, K.J., T.P.K., L.-S.S., S.W.; investigation, K.J., T.P.K., L.-S.S., S.W.; resources, S.H.; data curation, K.J., T.P.K., L.-S.S., S.W.; writing—original draft preparation, K.J.; writing—review and editing, S.H., K.J., T.P.K.; visualization, K.J.; supervision, S.H., T.P.K.; project administration, S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), grant number 374031971–TRR 240 to S.H. and DFG HA 3125/5-2 to S.H.
Institutional Review Board Statement
The use of whole blood and washed platelets from healthy adult individuals was ap-proved by the Ethics Committee of the University Medicine Greifswald (BB 044/18).
Informed Consent Statement
All volunteers gave written informed consent in accordance with the Declaration of Helsinki. All experiments were carried out in accordance with the approved guide-lines.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robier C. Platelet morphology. J. Lab. Med. 2020;44:231–239. doi: 10.1515/labmed-2020-0007. [DOI] [Google Scholar]
- 2.Lefrançais E., Looney M.R. Platelet Biogenesis in the Lung Circulation. Physiology. 2019;34:392–401. doi: 10.1152/physiol.00017.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machlus K.R., Italiano J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macaulay I.C., Carr P., Gusnanto A., Ouwehand W.H., Fitzgerald D., Watkins N.A. Platelet genomics and proteomics in human health and disease. J. Clin. Investig. 2005;115:3370–3377. doi: 10.1172/JCI26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner C.L., Mascelli M.A., Neblock D.S., Weisman H.F., Coller B.S., Jordan R.E. Analysis of GPIIb/IIIa Receptor Number by Quantification of 7E3 Binding to Human Platelets. Blood. 1996;88:907–914. doi: 10.1182/blood.V88.3.907.907. [DOI] [PubMed] [Google Scholar]
- 6.Bledzka K., Smyth S.S., Plow E.F. Integrin αIIbβ3: From discovery to efficacious therapeutic target. Circ. Res. 2013;112:1189–1200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar-Kharghan V., Agah R., Andrews R.K., Aster R.H., Atkinson B., Awtry E.H., Bahou W.F., Barnard M.R., Bavry A.A., Bayer A.S., et al. Contributors. In: Michelson A.D., editor. Platelets. 2nd ed. Academic Press; Cambridge, MA, USA: 2007. [DOI] [Google Scholar]
- 8.Klinger M.H., Jelkmann W. Role of blood platelets in infection and inflammation. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 9.Portier I., Campbell R.A. Role of Platelets in Detection and Regulation of Infection. Arterioscler. Thromb. Vasc. Biol. 2021;41:70–78. doi: 10.1161/ATVBAHA.120.314645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assinger A. Platelets and infection-an emerging role of platelets in viral infection. Front. Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkard P., Vögtle T., Nieswandt B. Platelets in Thrombo-Inflammation: Concepts, Mechanisms, and Therapeutic Strategies for Ischemic Stroke. Hamostaseologie. 2020;40:153–164. doi: 10.1055/a-1151-9519. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Gao X.M., Fang L., Jennings N.L., Su Y., Xu Q., Samson A.L., Kiriazis H., Wang X.F., Shan L., et al. Novel role of platelets in mediating inflammatory responses and ventricular rupture or remodeling following myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2011;31:834–841. doi: 10.1161/ATVBAHA.110.220467. [DOI] [PubMed] [Google Scholar]
- 13.Smyth S.S., McEver R.P., Weyrich A.S., Morrell C.N., Hoffman M.R., Arepally G.M., French P.A., Dauerman H.L., Becker R.C. Platelet functions beyond hemostasis. J. Thromb. Haemost. JTH. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava K., Cockburn I.A., Swaim A., Thompson L.E., Tripathi A., Fletcher C.A., Shirk E.M., Sun H., Kowalska M.A., Fox-Talbot K., et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppinger J.A., Cagney G., Toomey S., Kislinger T., Belton O., McRedmond J.P., Cahill D.J., Emili A., Fitzgerald D.J., Maguire P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 16.Blair P., Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palabrica T., Lobb R., Furie B.C., Aronovitz M., Benjamin C., Hsu Y.M., Sajer S.A., Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 18.Del Conde I., Crúz M.A., Zhang H., López J.A., Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh N., Soga F., Nara T., Tamagawa-Mineoka R., Nin M., Kotani H., Masuda K., Kishimoto S. Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin. Exp. Immunol. 2006;146:354–361. doi: 10.1111/j.1365-2249.2006.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 21.Aggrey A.A., Srivastava K., Ture S., Field D.J., Morrell C.N. Platelet Induction of the Acute-Phase Response Is Protective in Murine Experimental Cerebral Malaria. J. Immunol. 2013;190:4685–4691. doi: 10.4049/jimmunol.1202672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindemann S., Tolley N.D., Dixon D.A., McIntyre T.M., Prescott S.M., Zimmerman G.A., Weyrich A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown G.T., McIntyre T.M. Lipopolysaccharide signaling without a nucleus: Kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. J. Immunol. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binsker U., Palankar R., Wesche J., Kohler T.P., Prucha J., Burchhardt G., Rohde M., Schmidt F., Bröker B.M., Mamat U., et al. Secreted Immunomodulatory Proteins of Staphylococcus aureus Activate Platelets and Induce Platelet Aggregation. Thromb. Haemost. 2018;118:745–757. doi: 10.1055/s-0038-1637735. [DOI] [PubMed] [Google Scholar]
- 25.Blair P., Rex S., Vitseva O., Beaulieu L., Tanriverdi K., Chakrabarti S., Hayashi C., Genco C.A., Iafrati M., Freedman J.E. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ. Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu L.M., Lin E., Morin K.M., Tanriverdi K., Freedman J.E. Regulatory effects of TLR2 on megakaryocytic cell function. Blood. 2011;117:5963–5974. doi: 10.1182/blood-2010-09-304949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green S.A., Smith M., Hasley R.B., Stephany D., Harned A., Nagashima K., Abdullah S., Pittaluga S., Imamichi T., Qin J., et al. Activated platelet-T-cell conjugates in peripheral blood of patients with HIV infection: Coupling coagulation/inflammation and T cells. AIDS. 2015;29:1297–1308. doi: 10.1097/QAD.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hundelshausen P.v., Weber K.S.C., Huo Y., Proudfoot A.E.I., Nelson P.J., Ley K., Weber C. RANTES Deposition by Platelets Triggers Monocyte Arrest on Inflamed and Atherosclerotic Endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.CIR.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 29.Theilmeier G., Lenaerts T., Remacle C., Collen D., Vermylen J., Hoylaerts M.F. Circulating Activated Platelets Assist THP-1 Monocytoid/Endothelial Cell Interaction Under Shear Stress. Blood. 1999;94:2725–2734. doi: 10.1182/blood.V94.8.2725.420k18_2725_2734. [DOI] [PubMed] [Google Scholar]
- 30.Weyrich A.S., McIntyre T.M., McEver R.P., Prescott S.M., Zimmerman G.A. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J. Clin. Investig. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weyrich A.S., Elstad M.R., McEver R.P., McIntyre T.M., Moore K.L., Morrissey J.H., Prescott S.M., Zimmerman G.A. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Investig. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cognasse F., Duchez A.C., Audoux E., Ebermeyer T., Arthaud C.A., Prier A., Eyraud M.A., Mismetti P., Garraud O., Bertoletti L., et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022;13:825892. doi: 10.3389/fimmu.2022.825892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danese S., de la Motte C., Reyes B.M., Sans M., Levine A.D., Fiocchi C. Cutting edge: T cells trigger CD40-dependent platelet activation and granular RANTES release: A novel pathway for immune response amplification. J. Immunol. 2004;172:2011–2015. doi: 10.4049/jimmunol.172.4.2011. [DOI] [PubMed] [Google Scholar]
- 34.Langer H.F., Daub K., Braun G., Schönberger T., May A.E., Schaller M., Stein G.M., Stellos K., Bueltmann A., Siegel-Axel D., et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler. Thromb. Vasc. Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 35.Hagihara M., Higuchi A., Tamura N., Ueda Y., Hirabayashi K., Ikeda Y., Kato S., Sakamoto S., Hotta T., Handa S., et al. Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro. J. Immunol. 2004;172:5297–5303. doi: 10.4049/jimmunol.172.9.5297. [DOI] [PubMed] [Google Scholar]
- 36.Boilard E., Nigrovic P.A., Larabee K., Watts G.F., Coblyn J.S., Weinblatt M.E., Massarotti E.M., Remold-O’Donnell E., Farndale R.W., Ware J., et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernal-Mizrachi L., Jy W., Jimenez J.J., Pastor J., Mauro L.M., Horstman L.L., de Marchena E., Ahn Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 38.Mallat Z., Benamer H., Hugel B., Benessiano J., Steg P.G., Freyssinet J.M., Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.CIR.101.8.841. [DOI] [PubMed] [Google Scholar]
- 39.Suades R., Padró T., Vilahur G., Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb. Haemost. 2012;108:1208–1219. doi: 10.1160/TH12-07-0486. [DOI] [PubMed] [Google Scholar]
- 40.Vajen T., Benedikter B.J., Heinzmann A.C.A., Vasina E.M., Henskens Y., Parsons M., Maguire P.B., Stassen F.R., Heemskerk J.W.M., Schurgers L.J., et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell. Vesicles. 2017;6:1322454. doi: 10.1080/20013078.2017.1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badimon L., Suades R., Fuentes E., Palomo I., Padró T. Role of Platelet-Derived Microvesicles As Crosstalk Mediators in Atherothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis. Front. Pharmacol. 2016;7:293. doi: 10.3389/fphar.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laffont B., Corduan A., Rousseau M., Duchez A.C., Lee C.H., Boilard E., Provost P. Platelet microparticles reprogram macrophage gene expression and function. Thromb. Haemost. 2016;115:311–323. doi: 10.1160/th15-05-0389. [DOI] [PubMed] [Google Scholar]
- 43.Vasina E.M., Cauwenberghs S., Feijge M.A., Heemskerk J.W., Weber C., Koenen R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011;2:e211. doi: 10.1038/cddis.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali R.A., Wuescher L.M., Worth R.G. Platelets: Essential components of the immune system. Curr. Trends Immunol. 2015;16:65–78. [PMC free article] [PubMed] [Google Scholar]
- 45.Kraemer B.F., Campbell R.A., Schwertz H., Cody M.J., Franks Z., Tolley N.D., Kahr W.H., Lindemann S., Seizer P., Yost C.C., et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamzeh-Cognasse H., Damien P., Chabert A., Pozzetto B., Cognasse F., Garraud O. Platelets and infections-complex interactions with bacteria. Front. Immunol. 2015;6:82. doi: 10.3389/fimmu.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jahn K., Handtke S., Palankar R., Weißmüller S., Nouailles G., Kohler T.P., Wesche J., Rohde M., Heinz C., Aschenbrenner A.F., et al. Pneumolysin induces platelet destruction, not platelet activation, which can be prevented by immunoglobulin preparations in vitro. Blood Adv. 2020;4:6315–6326. doi: 10.1182/bloodadvances.2020002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerrigan S.W., Cox D. Platelet-bacterial interactions. Cell. Mol. Life Sci. CMLS. 2010;67:513–523. doi: 10.1007/s00018-009-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford I., Douglas C.W. The role of platelets in infective endocarditis. Platelets. 1997;8:285–294. doi: 10.1080/09537109777159. [DOI] [PubMed] [Google Scholar]
- 50.Jung C.-J., Yeh C.-Y., Shun C.-T., Hsu R.-B., Cheng H.-W., Lin C.-S., Chia J.-S. Platelets Enhance Biofilm Formation and Resistance of Endocarditis-Inducing Streptococci on the Injured Heart Valve. J. Infect. Dis. 2012;205:1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 51.Franchini M., Veneri D. Helicobacter pylori-associated immune thrombocytopenia. Platelets. 2006;17:71–77. doi: 10.1080/09537100500438057. [DOI] [PubMed] [Google Scholar]
- 52.Karpman D., Manea M., Vaziri-Sani F., Ståhl A.L., Kristoffersson A.C. Platelet activation in hemolytic uremic syndrome. Semin. Thromb. Hemost. 2006;32:128–145. doi: 10.1055/s-2006-939769. [DOI] [PubMed] [Google Scholar]
- 53.Joseph A., Cointe A., Mariani Kurkdjian P., Rafat C., Hertig A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins. 2020;12:67. doi: 10.3390/toxins12020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Assinger A., Schrottmaier W.C., Salzmann M., Rayes J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019;10:1687. doi: 10.3389/fimmu.2019.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clawson C.C. Platelet interaction with bacteria. 3. Ultrastructure. Am. J. Pathol. 1973;70:449–471. [PMC free article] [PubMed] [Google Scholar]
- 56.Clawson C.C., Rao G.H., White J.G. Platelet interaction with bacteria. IV. Stimulation of the release reaction. Am. J. Pathol. 1975;81:411–420. [PMC free article] [PubMed] [Google Scholar]
- 57.Li X., Iwai T., Nakamura H., Inoue Y., Chen Y., Umeda M., Suzuki H. An ultrastructural study of Porphyromonas gingivalis-induced platelet aggregation. Thromb. Res. 2008;122:810–819. doi: 10.1016/j.thromres.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Youssefian T., Drouin A., Massé J.M., Guichard J., Cramer E.M. Host defense role of platelets: Engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 59.Worth R.G., Chien C.D., Chien P., Reilly M.P., McKenzie S.E., Schreiber A.D. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp. Hematol. 2006;34:1490–1495. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Antczak A.J., Vieth J.A., Singh N., Worth R.G. Internalization of IgG-coated targets results in activation and secretion of soluble CD40 ligand and RANTES by human platelets. Clin. Vaccine Immunol. 2011;18:210–216. doi: 10.1128/CVI.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaertner F., Ahmad Z., Rosenberger G., Fan S., Nicolai L., Busch B., Yavuz G., Luckner M., Ishikawa-Ankerhold H., Hennel R., et al. Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell. 2017;171:1368–1382. doi: 10.1016/j.cell.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Verschoor A., Neuenhahn M., Navarini A.A., Graef P., Plaumann A., Seidlmeier A., Nieswandt B., Massberg S., Zinkernagel R.M., Hengartner H., et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat. Immunol. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 63.Rivera J., Lozano M.L., Navarro-Núñez L., Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Capel P.J., van de Winkel J.G., van den Herik-Oudijk I.E., Verbeek J.S. Heterogeneity of human IgG Fc receptors. ImmunoMethods. 1994;4:25–34. doi: 10.1006/immu.1994.1004. [DOI] [PubMed] [Google Scholar]
- 65.McCrae K.R., Shattil S.J., Cines D.B. Platelet activation induces increased Fc gamma receptor expression. J. Immunol. 1990;144:3920–3927. [PubMed] [Google Scholar]
- 66.Moriarty R.D., Cox A., McCall M., Smith S.G., Cox D. Escherichia coli induces platelet aggregation in an FcγRIIa-dependent manner. J. Thromb. Haemost. JTH. 2016;14:797–806. doi: 10.1111/jth.13226. [DOI] [PubMed] [Google Scholar]
- 67.Fitzgerald J.R., Loughman A., Keane F., Brennan M., Knobel M., Higgins J., Visai L., Speziale P., Cox D., Foster T.J. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol. Microbiol. 2006;59:212–230. doi: 10.1111/j.1365-2958.2005.04922.x. [DOI] [PubMed] [Google Scholar]
- 68.Byrne M.F., Kerrigan S.W., Corcoran P.A., Atherton J.C., Murray F.E., Fitzgerald D.J., Cox D.M. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology. 2003;124:1846–1854. doi: 10.1016/S0016-5085(03)00397-4. [DOI] [PubMed] [Google Scholar]
- 69.Josefsson E., McCrea K.W., Eidhin D.N., O’Connell D., Cox J., Hook M., Foster T.J. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Pt 12Microbiology. 1998;144:3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- 70.Ní Eidhin D., Perkins S., Francois P., Vaudaux P., Höök M., Foster T.J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 71.Heilmann C., Niemann S., Sinha B., Herrmann M., Kehrel B.E., Peters G. Staphylococcus aureus fibronectin-binding protein (FnBP)-mediated adherence to platelets, and aggregation of platelets induced by FnBPA but not by FnBPB. J. Infect. Dis. 2004;190:321–329. doi: 10.1086/421914. [DOI] [PubMed] [Google Scholar]
- 72.Flock J.I., Fröman G., Jönsson K., Guss B., Signäs C., Nilsson B., Raucci G., Höök M., Wadström T., Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Brien L., Kerrigan S.W., Kaw G., Hogan M., Penades J., Litt D., Fitzgerald D.J., Foster T.J., Cox D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: Roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol. Microbiol. 2002;44:1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 74.Miajlovic H., Loughman A., Brennan M., Cox D., Foster T.J. Both complement-and fibrinogen-dependent mechanisms contribute to platelet aggregation mediated by Staphylococcus aureus clumping factor B. Infect. Immun. 2007;75:3335–3343. doi: 10.1128/IAI.01993-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miajlovic H., Zapotoczna M., Geoghegan J.A., Kerrigan S.W., Speziale P., Foster T.J. Direct interaction of iron-regulated surface determinant IsdB of Staphylococcus aureus with the GPIIb/IIIa receptor on platelets. Microbiology. 2010;156:920–928. doi: 10.1099/mic.0.036673-0. [DOI] [PubMed] [Google Scholar]
- 76.Brennan M.P., Loughman A., Devocelle M., Arasu S., Chubb A.J., Foster T.J., Cox D. Elucidating the role of Staphylococcus epidermidis serine–aspartate repeat protein G in platelet activation. J. Thromb. Haemost. 2009;7:1364–1372. doi: 10.1111/j.1538-7836.2009.03495.x. [DOI] [PubMed] [Google Scholar]
- 77.Bertling A., Niemann S., Hussain M., Holbrook L., Stanley R.G., Brodde M.F., Pohl S., Schifferdecker T., Roth J., Jurk K., et al. Staphylococcal extracellular adherence protein induces platelet activation by stimulation of thiol isomerases. Arterioscler. Thromb. Vasc. Biol. 2012;32:1979–1990. doi: 10.1161/ATVBAHA.112.246249. [DOI] [PubMed] [Google Scholar]
- 78.Wallis S., Wolska N., Englert H., Posner M., Upadhyay A., Renné T., Eggleston I., Bagby S., Pula G. A peptide from the staphylococcal protein Efb binds P-selectin and inhibits the interaction of platelets with leukocytes. J. Thromb. Haemost. 2021;20:729–741. doi: 10.1111/jth.15613. [DOI] [PubMed] [Google Scholar]
- 79.Posner M.G., Upadhyay A., Abubaker A.A., Fortunato T.M., Vara D., Canobbio I., Bagby S., Pula G. Extracellular Fibrinogen-binding Protein (Efb) from Staphylococcus aureus Inhibits the Formation of Platelet-Leukocyte Complexes. J. Biol. Chem. 2016;291:2764–2776. doi: 10.1074/jbc.M115.678359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li R., Emsley J. The organizing principle of the platelet glycoprotein Ib–IX–V complex. J. Thromb. Haemost. 2013;11:605–614. doi: 10.1111/jth.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bennett J.S., Berger B.W., Billings P.C. The structure and function of platelet integrins. J. Thromb. Haemost. 2009;7((Suppl. 1)):200–205. doi: 10.1111/j.1538-7836.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- 82.Bensing B.A., López J.A., Sullam P.M. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect. Immun. 2004;72:6528–6537. doi: 10.1128/IAI.72.11.6528-6537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plummer C., Wu H., Kerrigan S.W., Meade G., Cox D., Ian Douglas C.W. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br. J. Haematol. 2005;129:101–109. doi: 10.1111/j.1365-2141.2005.05421.x. [DOI] [PubMed] [Google Scholar]
- 84.Claes J., Vanassche T., Peetermans M., Liesenborghs L., Vandenbriele C., Vanhoorelbeke K., Missiakas D., Schneewind O., Hoylaerts M.F., Heying R., et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–1676. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Botos I., Segal D.M., Davies D.R. The Structural Biology of Toll-like Receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cognasse F., Nguyen K.A., Damien P., McNicol A., Pozzetto B., Hamzeh-Cognasse H., Garraud O. The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors. Front. Immunol. 2015;6:83. doi: 10.3389/fimmu.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cognasse F., Hamzeh H., Chavarin P., Acquart S., Genin C., Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 88.Gautam J.K., Comeau L.D., Krueger J.K., Smith M.F., Jr. Structural and functional evidence for the role of the TLR2 DD loop in TLR1/TLR2 heterodimerization and signaling. J. Biol. Chem. 2006;281:30132–30142. doi: 10.1074/jbc.M602057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zähringer U., Lindner B., Inamura S., Heine H., Alexander C. TLR2–promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Keane C., Tilley D., Cunningham A., Smolenski A., Kadioglu A., Cox D., Jenkinson H.F., Kerrigan S.W. Invasive Streptococcus pneumoniae trigger platelet activation via Toll-like receptor 2. J. Thromb. Haemost. 2010;8:2757–2765. doi: 10.1111/j.1538-7836.2010.04093.x. [DOI] [PubMed] [Google Scholar]
- 91.Liu X., Liu H., Luo X., Zhang P., Gao Y., Xie S., Xu K., Chang J., Ma L. Strains of Group B streptococci from septic patients induce platelet activation via Toll-like Receptor 2. Clin. Exp. Pharmacol. Physiol. 2017;44:335–343. doi: 10.1111/1440-1681.12707. [DOI] [PubMed] [Google Scholar]
- 92.El Kebir D., Damlaj A., Makhezer N., Filep J.G. Toll-like receptor 9 signaling regulates tissue factor and tissue factor pathway inhibitor expression in human endothelial cells and coagulation in mice. Crit. Care Med. 2015;43:e179–e189. doi: 10.1097/CCM.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Powers M.E., Becker R.E., Sailer A., Turner J.R., Bubeck Wardenburg J. Synergistic Action of Staphylococcus aureus α-Toxin on Platelets and Myeloid Lineage Cells Contributes to Lethal Sepsis. Cell Host Microbe. 2015;17:775–787. doi: 10.1016/j.chom.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berube B.J., Bubeck Wardenburg J. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins. 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jenkins A., Diep B.A., Mai T.T., Vo N.H., Warrener P., Suzich J., Stover C.K., Sellman B.R. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. MBio. 2015;6:e02272-14. doi: 10.1128/mBio.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kebaier C., Chamberland R.R., Allen I.C., Gao X., Broglie P.M., Hall J.D., Jania C., Doerschuk C.M., Tilley S.L., Duncan J.A. Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhakdi S., Muhly M., Mannhardt U., Hugo F., Klapettek K., Mueller-Eckhardt C., Roka L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J. Exp. Med. 1988;168:527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schubert S., Schwertz H., Weyrich A.S., Franks Z.G., Lindemann S., Otto M., Behr H., Loppnow H., Schlitt A., Russ M., et al. Staphylococcus aureus α-Toxin Triggers the Synthesis of B-Cell Lymphoma 3 by Human Platelets. Toxins. 2011;3:120. doi: 10.3390/toxins3020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jahn K., Handtke S., Palankar R., Kohler T.P., Wesche J., Wolff M., Bayer J., Wolz C., Greinacher A., Hammerschmidt S. α-hemolysin of Staphylococcus aureus impairs thrombus formation. bioRxiv. 2021 doi: 10.1111/jth.15703. [DOI] [PubMed] [Google Scholar]
- 101.Vollmer W., Blanot D., de Pedro M.A. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 102.Weidenmaier C., Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 103.Lopez R. Streptococcus pneumoniae and its bacteriophages: One long argument. Int. Microbiol. 2004;7:163–171. [PubMed] [Google Scholar]
- 104.Gisch N., Kohler T., Ulmer A.J., Muthing J., Pribyl T., Fischer K., Lindner B., Hammerschmidt S., Zahringer U. Structural reevaluation of Streptococcus pneumoniae Lipoteichoic acid and new insights into its immunostimulatory potency. J. Biol. Chem. 2013;288:15654–15667. doi: 10.1074/jbc.M112.446963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bentley S.D., Aanensen D.M., Mavroidi A., Saunders D., Rabbinowitsch E., Collins M., Donohoe K., Harris D., Murphy L., Quail M.A., et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006;2:e0020031. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hyams C., Camberlein E., Cohen J.M., Bax K., Brown J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pribyl T., Moche M., Dreisbach A., Bijlsma J.J.E., Saleh M., Abdullah M.R., Hecker M., van Dijl J.M., Becher D., Hammerschmidt S. Influence of Impaired Lipoprotein Biogenesis on Surface and Exoproteome of Streptococcus pneumoniae. J. Proteome Res. 2014;13:650–667. doi: 10.1021/pr400768v. [DOI] [PubMed] [Google Scholar]
- 108.Kohler S., Voß F., Gómez Mejia A., Brown J.S., Hammerschmidt S. Pneumococcal lipoproteins involved in bacterial fitness, virulence, and immune evasion. FEBS Lett. 2016;590:3820–3839. doi: 10.1002/1873-3468.12352. [DOI] [PubMed] [Google Scholar]
- 109.Bergmann S., Hammerschmidt S. Versatility of pneumococcal surface proteins. Microbiology. 2006;152:295–303. doi: 10.1099/mic.0.28610-0. [DOI] [PubMed] [Google Scholar]
- 110.Manzano C., Contreras-Martel C., El Mortaji L., Izore T., Fenel D., Vernet T., Schoehn G., Di Guilmi A.M., Dessen A. Sortase-mediated pilus fiber biogenesis in Streptococcus pneumoniae. Structure. 2008;16:1838–1848. doi: 10.1016/j.str.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 111.Abdullah M.R., Gutierrez-Fernandez J., Pribyl T., Gisch N., Saleh M., Rohde M., Petruschka L., Burchhardt G., Schwudke D., Hermoso J.A., et al. Structure of the pneumococcal l,d-carboxypeptidase DacB and pathophysiological effects of disabled cell wall hydrolases DacA and DacB. Mol. Microbiol. 2014;93:1183–1206. doi: 10.1111/mmi.12729. [DOI] [PubMed] [Google Scholar]
- 112.Saleh M., Bartual S.G., Abdullah M.R., Jensch I., Asmat T.M., Petruschka L., Pribyl T., Gellert M., Lillig C.H., Antelmann H., et al. Molecular architecture of Streptococcus pneumoniae surface thioredoxin-fold lipoproteins crucial for extracellular oxidative stress resistance and maintenance of virulence. EMBO Mol. Med. 2013;5:1852–1870. doi: 10.1002/emmm.201202435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hermans P.W., Adrian P.V., Albert C., Estevao S., Hoogenboezem T., Luijendijk I.H., Kamphausen T., Hammerschmidt S. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J. Biol. Chem. 2006;281:968–976. doi: 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- 114.Maestro B., Sanz J.M. Choline Binding Proteins from Streptococcus pneumoniae: A Dual Role as Enzybiotics and Targets for the Design of New Antimicrobials. Antibiotics. 2016;5:21. doi: 10.3390/antibiotics5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perez-Dorado I., Galan-Bartual S., Hermoso J.A. Pneumococcal surface proteins: When the whole is greater than the sum of its parts. Mol. Oral Microbiol. 2012;27:221–245. doi: 10.1111/j.2041-1014.2012.00655.x. [DOI] [PubMed] [Google Scholar]
- 116.Lopez R., Garcia E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 2004;28:553–580. doi: 10.1016/j.femsre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J.R., Mostov K.E., Lamm M.E., Nanno M., Shimida S., Ohwaki M., Tuomanen E. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell. 2000;102:827–837. doi: 10.1016/S0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 118.Elm C., Braathen R., Bergmann S., Frank R., Vaerman J.P., Kaetzel C.S., Chhatwal G.S., Johansen F.E., Hammerschmidt S. Ectodomains 3 and 4 of human polymeric Immunoglobulin receptor (hpIgR) mediate invasion of Streptococcus pneumoniae into the epithelium. J. Biol. Chem. 2004;279:6296–6304. doi: 10.1074/jbc.M310528200. [DOI] [PubMed] [Google Scholar]
- 119.Hammerschmidt S. Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol. 2006;9:12–20. doi: 10.1016/j.mib.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Hoskins J., Alborn W.E., Jr., Arnold J., Blaszczak L.C., Burgett S., DeHoff B.S., Estrem S.T., Fritz L., Fu D.J., Fuller W., et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 2001;183:5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tettelin H., Nelson K.E., Paulsen I.T., Eisen J.A., Read T.D., Peterson S., Heidelberg J., DeBoy R.T., Haft D.H., Dodson R.J., et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 122.Perry A.M., Ton-That H., Mazmanian S.K., Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 2002;277:16241–16248. doi: 10.1074/jbc.M109194200. [DOI] [PubMed] [Google Scholar]
- 123.Strominger J.L., Izaki K., Matsuhashi M., Tipper D.J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: Penicillin-sensitive enzymatic reactions. Fed. Proc. 1967;26:9–22. [PubMed] [Google Scholar]
- 124.Henderson B., Martin A. Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011;79:3476–3491. doi: 10.1128/IAI.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bergmann S., Schoenen H., Hammerschmidt S. The interaction between bacterial enolase and plasminogen promotes adherence of Streptococcus pneumoniae to epithelial and endothelial cells. Int. J. Med. Microbiol. 2013;303:452–462. doi: 10.1016/j.ijmm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Agarwal V., Hammerschmidt S., Malm S., Bergmann S., Riesbeck K., Blom A.M. Enolase of Streptococcus pneumoniae binds human complement inhibitor C4b-binding protein and contributes to complement evasion. J. Immunol. 2012;189:3575–3584. doi: 10.4049/jimmunol.1102934. [DOI] [PubMed] [Google Scholar]
- 127.Fine M.J., Smith M.A., Carson C.A., Mutha S.S., Sankey S.S., Weissfeld L.A., Kapoor W.N. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275:134–141. doi: 10.1001/jama.1996.03530260048030. [DOI] [PubMed] [Google Scholar]
- 128.Bartlett J.G., Dowell S.F., Mandell L.A., File T.M., Jr., Musher D.M., Fine M.J. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000;31:347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tunjungputri R.N., Mobegi F.M., Cremers A.J., van der Gaast-de Jongh C.E., Ferwerda G., Meis J.F., Roeleveld N., Bentley S.D., Pastura A.S., van Hijum S.A., et al. Phage-Derived Protein Induces Increased Platelet Activation and Is Associated with Mortality in Patients with Invasive Pneumococcal Disease. MBio. 2017;8:e01984-16. doi: 10.1128/mBio.01984-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luquero-Bueno S., Galván-Román J.M., Curbelo J., Lancho-Sánchez A., Roy-Vallejo E., Ortega-Gómez M., Gómez M., Mateo-Jimenez G., Manzaneque-Pradales M., Aspa Marco J. Platelet count as an evolution marker of late mortality and cardiovascular events after an episode of community-acquired pneumonia. Eur. Respir. J. 2019;54:PA4532. doi: 10.1183/13993003.congress-2019.PA4532. [DOI] [Google Scholar]
- 131.Feldman C., Kallenbach J.M., Levy H., Reinach S.G., Hurwitz M.D., Thorburn J.R., Koornhof H.J. Community-acquired pneumonia of diverse aetiology: Prognostic features in patients admitted to an intensive care unit and a “severity of illness” core. Intensive Care Med. 1989;15:302–307. doi: 10.1007/BF00263865. [DOI] [PubMed] [Google Scholar]
- 132.Wolff M., Handtke S., Palankar R., Wesche J., Kohler T.P., Kohler C., Gruel Y., Hammerschmidt S., Greinacher A. Activated platelets kill Staphylococcus aureus, but not Streptococcus pneumoniae-The role of FcγRIIa and platelet factor 4/heparinantibodies. J. Thromb. Haemost. 2020;18:1459–1468. doi: 10.1111/jth.14814. [DOI] [PubMed] [Google Scholar]
- 133.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 134.Vardon-Bounes F., Ruiz S., Gratacap M.P., Garcia C., Payrastre B., Minville V. Platelets Are Critical Key Players in Sepsis. Int. J. Mol. Sci. 2019;20:3494. doi: 10.3390/ijms20143494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 136.Massberg S., Grahl L., von Bruehl M.L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A.B., et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 137.Wartha F., Beiter K., Albiger B., Fernebro J., Zychlinsky A., Normark S., Henriques-Normark B. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 138.Martinez P.J., Farhan A., Mustafa M., Javaid N., Darkoh C., Garrido-Sanabria E., Fisher-Hoch S.P., Briles D.E., Kantarci A., Mirza S. PspA facilitates evasion of pneumococci from bactericidal activity of neutrophil extracellular traps (NETs) Microb. Pathog. 2019;136:103653. doi: 10.1016/j.micpath.2019.103653. [DOI] [PubMed] [Google Scholar]
- 139.Beiter K., Wartha F., Albiger B., Normark S., Zychlinsky A., Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 140.Levi M. The coagulant response in sepsis and inflammation. Hamostaseologie. 2010;30:14–16. doi: 10.1055/s-0037-1617143. [DOI] [PubMed] [Google Scholar]
- 141.Hoshino K., Kitamura T., Nakamura Y., Irie Y., Matsumoto N., Kawano Y., Ishikura H. Usefulness of plasminogen activator inhibitor-1 as a predictive marker of mortality in sepsis. J. Intensive Care. 2017;5:42. doi: 10.1186/s40560-017-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tipoe T.L., Wu W.K.K., Chung L., Gong M., Dong M., Liu T., Roever L., Ho J., Wong M.C.S., Chan M.T.V., et al. Plasminogen Activator Inhibitor 1 for Predicting Sepsis Severity and Mortality Outcomes: A Systematic Review and Meta-Analysis. Front. Immunol. 2018;9:1218. doi: 10.3389/fimmu.2018.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iskander K.N., Osuchowski M.F., Stearns-Kurosawa D.J., Kurosawa S., Stepien D., Valentine C., Remick D.G. Sepsis: Multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol. Rev. 2013;93:1247–1288. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 145.Waller A.K., Sage T., Kumar C., Carr T., Gibbins J.M., Clarke S.R. Staphylococcus aureus lipoteichoic acid inhibits platelet activation and thrombus formation via the Paf receptor. J. Infect. Dis. 2013;208:2046–2057. doi: 10.1093/infdis/jit398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daix T., Guerin E., Tavernier E., Mercier E., Gissot V., Hérault O., Mira J.P., Dumas F., Chapuis N., Guitton C., et al. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest. 2018;154:617–627. doi: 10.1016/j.chest.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 147.Camicia G., Pozner R., de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 148.Folco E.J., Mawson T.L., Vromman A., Bernardes-Souza B., Franck G., Persson O., Nakamura M., Newton G., Luscinskas F.W., Libby P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1α and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018;38:1901–1912. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tyml K. Critical role for oxidative stress, platelets, and coagulation in capillary blood flow impairment in sepsis. Microcirculation. 2011;18:152–162. doi: 10.1111/j.1549-8719.2010.00080.x. [DOI] [PubMed] [Google Scholar]
- 150.Lorente L., Martín M.M., Varo N., Borreguero-León J.M., Solé-Violán J., Blanquer J., Labarta L., Díaz C., Jiménez A., Pastor E., et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit. Care. 2011;15:R97. doi: 10.1186/cc10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang S., Rahman M., Zhang S., Qi Z., Thorlacius H. Simvastatin antagonizes CD40L secretion, CXC chemokine formation, and pulmonary infiltration of neutrophils in abdominal sepsis. J. Leukoc. Biol. 2011;89:735–742. doi: 10.1189/jlb.0510279. [DOI] [PubMed] [Google Scholar]
- 152.Chew M., Rahman M., Ihrman L., Erson A., Zhang S., Thorlacius H. Soluble CD40L (CD154) is increased in patients with shock. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2010;59:979–982. doi: 10.1007/s00011-010-0213-5. [DOI] [PubMed] [Google Scholar]
- 153.Rahman M., Zhang S., Chew M., Ersson A., Jeppsson B., Thorlacius H. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann. Surg. 2009;250:783–790. doi: 10.1097/SLA.0b013e3181bd95b7. [DOI] [PubMed] [Google Scholar]
- 154.Jin R., Yu S., Song Z., Zhu X., Wang C., Yan J., Wu F., Nanda A., Granger D.N., Li G. Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integrin Mac-1 and protein kinase C zeda (PKCζ) in neutrophils: Implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS ONE. 2013;8:e0064631. doi: 10.1371/journal.pone.0064631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yost C.C., Weyrich A.S., Zimmerman G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martínez E., Miró J.M., Almirante B., Aguado J.M., Fernandez-Viladrich P., Fernandez-Guerrero M.L., Villanueva J.L., Dronda F., Moreno-Torrico A., Montejo M., et al. Effect of penicillin resistance of Streptococcus pneumoniae on the presentation, prognosis, and treatment of pneumococcal endocarditis in adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002;35:130–139. doi: 10.1086/341024. [DOI] [PubMed] [Google Scholar]
- 157.Kan B., Ries J., Normark B.H., Chang F.Y., Feldman C., Ko W.C., Rello J., Snydman D.R., Yu V.L., Ortqvist A. Endocarditis and pericarditis complicating pneumococcal bacteraemia, with special reference to the adhesive abilities of pneumococci: Results from a prospective study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2006;12:338–344. doi: 10.1111/j.1469-0691.2006.01363.x. [DOI] [PubMed] [Google Scholar]
- 158.de Egea V., Muñoz P., Valerio M., de Alarcón A., Lepe J.A., Miró J.M., Gálvez-Acebal J., García-Pavía P., Navas E., Goenaga M.A., et al. Characteristics and Outcome of Streptococcus pneumoniae Endocarditis in the XXI Century: A Systematic Review of 111 Cases (2000-2013) Medicine. 2015;94:e1562. doi: 10.1097/MD.0000000000001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liesenborghs L., Meyers S., Vanassche T., Verhamme P. Coagulation: At the heart of infective endocarditis. J. Thromb. Haemost. 2020;18:995–1008. doi: 10.1111/jth.14736. [DOI] [PubMed] [Google Scholar]
- 160.Durack D.T., Beeson P.B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 161.Angrist A.A., Oka M. Pathogenesis of bacterial endocarditis. JAMA. 1963;183:249–252. doi: 10.1001/jama.1963.63700040009010b. [DOI] [PubMed] [Google Scholar]
- 162.Herzberg M.C., Gong K., MacFarlane G.D., Erickson P.R., Soberay A.H., Krebsbach P.H., Manjula G., Schilling K., Bowen W.H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect. Immun. 1990;58:515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Del Giudice C., Vaia E., Liccardo D., Marzano F., Valletta A., Spagnuolo G., Ferrara N., Rengo C., Cannavo A., Rengo G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms. 2021;9:1218. doi: 10.3390/microorganisms9061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ito H.O. Infective endocarditis and dental procedures: Evidence, pathogenesis, and prevention. J. Med. Investig. 2006;53:189–198. doi: 10.2152/jmi.53.189. [DOI] [PubMed] [Google Scholar]
- 165.Xiong Y.-Q., Van Wamel W., Nast C.C., Yeaman M.R., Cheung A.L., Bayer A.S. Activation and Transcriptional Interaction between agr RNAII and RNAIII in Staphylococcus aureus In Vitro and in an Experimental Endocarditis Model. J. Infect. Dis. 2002;186:668–677. doi: 10.1086/342046. [DOI] [PubMed] [Google Scholar]
- 166.Guckian J.C. Effect of pneumococci on blood clotting, platelets, and polymorphonuclear leukocytes. Infect. Immun. 1975;12:910–918. doi: 10.1128/iai.12.4.910-918.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Clawson C.C., White J.G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am. J. Pathol. 1971;65:367–380. [PMC free article] [PubMed] [Google Scholar]
- 168.de Stoppelaar S.F., Claushuis T.A.M., Schaap M.C.L., Hou B., van der Poll T., Nieuwland R., van ‘t Veer C. Toll-Like Receptor Signalling Is Not Involved in Platelet Response to Streptococcus pneumoniae In Vitro or In Vivo. PLoS ONE. 2016;11:e0156977. doi: 10.1371/journal.pone.0156977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Niemann S., Kehrel B.E., Heilmann C., Rennemeier C., Peters G., Hammerschmidt S. Pneumococcal association to platelets is mediated by soluble fibrin and supported by thrombospondin-1. Thromb. Haemost. 2009;102:735–742. doi: 10.1160/th09-01-0049. [DOI] [PubMed] [Google Scholar]
- 170.Binsker U., Kohler T.P., Krauel K., Kohler S., Schwertz H., Hammerschmidt S. Pneumococcal Adhesins PavB and PspC Are Important for the Interplay with Human Thrombospondin-1*. J. Biol. Chem. 2015;290:14542–14555. doi: 10.1074/jbc.M114.623876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Binsker U., Kohler T.P., Krauel K., Kohler S., Habermeyer J., Schwertz H., Hammerschmidt S. Serotype 3 pneumococci sequester platelet-derived human thrombospondin-1 via the adhesin and immune evasion protein Hic. J. Biol. Chem. 2017;292:5770–5783. doi: 10.1074/jbc.M116.760504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marshall J.E., Faraj B.H.A., Gingras A.R., Lonnen R., Sheikh M.A., El-Mezgueldi M., Moody P.C.E., Andrew P.W., Wallis R. The Crystal Structure of Pneumolysin at 2.0 Å Resolution Reveals the Molecular Packing of the Pre-pore Complex. Sci. Rep. 2015;5:13293. doi: 10.1038/srep13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ohkuni H., Nagamune H., Ozaki N., Tabata A., Todome Y., Watanabe Y., Takahashi H., Ohkura K., Kourai H., Ohtsuka H., et al. Characterization of recombinant Streptococcus mitis-derived human platelet aggregation factor. Acta Pathol. Microbiol. Immunol. Scand. 2012;120:56–71. doi: 10.1111/j.1600-0463.2011.02813.x. [DOI] [PubMed] [Google Scholar]
- 174.Nel J.G., Durandt C., Mitchell T.J., Feldman C., Anderson R., Tintinger G.R. Pneumolysin Mediates Platelet Activation In Vitro. Lung. 2016;194:589–593. doi: 10.1007/s00408-016-9900-5. [DOI] [PubMed] [Google Scholar]
- 175.Gutiérrez-Fernández J., Saleh M., Alcorlo M., Gómez-Mejía A., Pantoja-Uceda D., Treviño M.A., Voß F., Abdullah M.R., Galán-Bartual S., Seinen J., et al. Modular Architecture and Unique Teichoic Acid Recognition Features of Choline-Binding Protein L (CbpL) Contributing to Pneumococcal Pathogenesis. Sci. Rep. 2016;6:38094. doi: 10.1038/srep38094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schmidt F., Kakar N., Meyer T.C., Depke M., Masouris I., Burchhardt G., Gómez-Mejia A., Dhople V., Håvarstein L.S., Sun Z., et al. In vivo proteomics identifies the competence regulon and AliB oligopeptide transporter as pathogenic factors in pneumococcal meningitis. PLOS Pathog. 2019;15:e1007987. doi: 10.1371/journal.ppat.1007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yamaguchi M., Terao Y., Mori Y., Hamada S., Kawabata S. PfbA, a novel plasmin- and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis. J. Biol. Chem. 2008;283:36272–36279. doi: 10.1074/jbc.M807087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tomlinson G., Chimalapati S., Pollard T., Lapp T., Cohen J., Camberlein E., Stafford S., Periselneris J., Aldridge C., Vollmer W., et al. TLR-mediated inflammatory responses to Streptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins. J. Immunol. 2014;193:3736–3745. doi: 10.4049/jimmunol.1401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Voß F., van Beek L.F., Schwudke D., Ederveen T.H.A., van Opzeeland F.J., Thalheim D., Werner S., de Jonge M.I., Hammerschmidt S. Lipidation of Pneumococcal Antigens Leads to Improved Immunogenicity and Protection. Vaccines. 2020;8:310. doi: 10.3390/vaccines8020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aslam R., Speck E.R., Kim M., Crow A.R., Bang K.W., Nestel F.P., Ni H., Lazarus A.H., Freedman J., Semple J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 181.Heß N., Waldow F., Kohler T.P., Rohde M., Kreikemeyer B., Gómez-Mejia A., Hain T., Schwudke D., Vollmer W., Hammerschmidt S., et al. Lipoteichoic acid deficiency permits normal growth but impairs virulence of Streptococcus pneumoniae. Nat. Commun. 2017;8:2093. doi: 10.1038/s41467-017-01720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tunjungputri R.N., de Jonge M.I., de Greeff A., van Selm S., Buys H., Harders-Westerveen J.F., Stockhofe-Zurwieden N., Urbanus R.T., de Groot P.G., Smith H.E., et al. Invasive pneumococcal disease leads to activation and hyperreactivity of platelets. Thromb. Res. 2016;144:123–126. doi: 10.1016/j.thromres.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 183.Kullaya V., Jonge M.I.d., Langereis J.D., Jongh C.E.v.d.G.-d., Büll C., Adema G.J., Lefeber D., Cremers A.J., Mmbaga B.T., Groot P.G.d., et al. Desialylation of Platelets by Pneumococcal Neuraminidase A Induces ADP-Dependent Platelet Hyperreactivity. Infect. Immun. 2018;86:e00213-18. doi: 10.1128/IAI.00213-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wiebe F., Handtke S., Wesche J., Schnarre A., Palankar R., Wolff M., Jahn K., Voß F., Weißmüller S., Schüttrumpf J., et al. Polyvalent immunoglobulin preparations inhibit pneumolysin-induced platelet destruction. Thromb. Haemost. 2021:1723–1880. doi: 10.1055/a-1723-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ishikura H., Nishida T., Murai A., Nakamura Y., Irie Y., Tanaka J., Umemura T. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: A prospective single-center observational study. Crit. Care. 2014;18:R19. doi: 10.1186/cc13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tiwari N.R., Chaudhari K.S., Sharma R., Haas K.P., Sharma V.R. Antiplatelet Agents in Sepsis-Putting it all together: A Call to Action. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2020;24:483–484. doi: 10.5005/jp-journals-10071-23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen C.M., Lu H.C., Tung Y.T., Chen W. Antiplatelet Therapy for Acute Respiratory Distress Syndrome. Biomedicines. 2020;8:230. doi: 10.3390/biomedicines8070230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Y., Ouyang Y., Liu B., Ma X., Ding R. Platelet activation and antiplatelet therapy in sepsis: A narrative review. Thromb. Res. 2018;166:28–36. doi: 10.1016/j.thromres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 189.O’Neal H.R., Jr., Koyama T., Koehler E.A., Siew E., Curtis B.R., Fremont R.D., May A.K., Bernard G.R., Ware L.B. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit. Care Med. 2011;39:1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Davì G., Santilli F., Vazzana N. Thromboxane receptors antagonists and/or synthase inhibitors. Handb. Exp. Pharmacol. 2012:261–286. doi: 10.1007/978-3-642-29423-5_11. [DOI] [PubMed] [Google Scholar]
- 191.Tagami T., Matsui H., Horiguchi H., Fushimi K., Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: An observational nationwide study. J. Thromb. Haemost. 2014;12:1470–1479. doi: 10.1111/jth.12643. [DOI] [PubMed] [Google Scholar]
- 192.Levi M. Antithrombin in sepsis revisited. Crit. Care. 2005;9:624–625. doi: 10.1186/cc3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.