Abstract
Ligase chain reaction (LCR) is a recently developed technique that employs a thermostable ligase and allows for the discrimination of DNA sequences differing in only a single base pair. The method has been adapted and applied to differentiation of blaSHV genes. We have developed an LCR typing method to characterize point mutations in genes for SHV-derived extended-spectrum β-lactamases with four different sets of biotinylated LCR primers. To evaluate the applicability of the current technique, we tested seven Escherichia coli strains producing SHV-1, SHV-2, SHV-2a, SHV-3, SHV-4, SHV-5, and SHV-12. With the LCR typing, seven SHV genes can be distinguished according to their incorporating point mutations. In an attempt to characterize SHV β-lactamases by LCR typing in clinical isolates, 46 strains carrying blaSHV genes (32 Klebsiella pneumoniae, 10 Enterobacter cloacae, and 4 E. coli) were subjected to antibiotic susceptibility testing, isoelectric focusing, and LCR typing. LCR typing allowed the characterization of β-lactamases, and genotypes obtained by LCR typing were in accordance with phenotypes such as antibiotic resistance profile and pI value of β-lactamase. Therefore, we concluded that LCR typing may permit defining the SHV families with simplicity and reliability and can be applied to the detailed characterization and molecular epidemiology of SHV-type β-lactamases.
The SHV-type β-lactamases represent one of the most clinically significant families of plasmid-encoded β-lactamases. Point mutations in the nucleotide sequences of the structural genes for the SHV-type β-lactamases can broaden their substrate spectrum towards all β-lactams except cephamycins and carbapenems (12, 28). Detection of such mutations usually requires sequencing of the genes, which is time-consuming and technically demanding. Other approaches used to study the β-lactamases of SHV-group have limitations: isoelectric focusing (IEF) is inadequate since the same pI can correspond to different β-lactamases and characterization of enzymatic substrate profiles does not allow one to differentiate between closely related enzymes.
Here, we report a new strategy for differentiation of blaSHV genes based on a nonradioactive ligase chain reaction (LCR) method. LCR employs a thermostable ligase and allows for the discrimination of DNA sequences differing in only a single base pair (4). In the LCR, a target DNA sequence is denatured at 94°C and the four primers anneal to their complementary strands at 60°C. Then, thermostable ligase only ligates primers that are perfectly complementary to their target sequence and hybridize directly adjacent to each other. Because the oligonucleotide products from one round may serve as substrates during the next round, the signal is amplified exponentially, analogous to PCR amplification. A single-base mismatch at the oligonucleotide junction will not be amplified and is, therefore, distinguished. Thus, LCR allows for the detection and discrimination of parental and mutated nucleotide sequences of SHV enzymes. We have developed an LCR typing method by using four different sets of biotinylated LCR primers to characterize point mutations in genes for SHV-derived extended-spectrum β-lactamases (ESBLs). With LCR typing, we distinguished seven SHV genes encoding SHV-1, SHV-2, SHV-2a, SHV-3, SHV-4, SHV-5, or SHV-12 β-lactamase. (This work was presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 1999.)
MATERIALS AND METHODS
Bacterial strains.
Seven strains, each producing one of seven recognized SHV β-lactamases, were used: Escherichia coli C600(R1010), encoding SHV-1; E. coli C600(pMG229), encoding SHV-2; E. coli J53-2(pUD18), encoding SHV-3; E. coli J53-2(pUD21), encoding SHV-4; E. coli HB101(pAFF2), encoding SHV-5; E. coli J53(pKS39), encoding SHV-2a; and E. coli J53(pKS12), encoding SHV-12 (13, 14). Forty-six clinical isolates harboring blaSHV genes were also included in this study: 4 strains of E. coli, 32 strains of Klebsiella pneumoniae, and 10 strains of Enterobacter cloacae. These isolates were selected by SHV-specific PCR from 82 clinical isolates with reduced susceptibility or resistance to oxyiminocephalosporins that were obtained from blood specimens of pediatric patients at Seoul National University Hospital during 1995 and 1999.
SHV-specific PCR.
An 870-bp fragment of the SHV gene was amplified with the primers S1 (5′-TGGTTATGCGTTATATTCGCC-3′) and S2 (5′-GGTTAGCGTTGCCAGTGCT-3′), corresponding to nucleotides 120 to 140 and 990 to 972, respectively, of the SHV-1 bla gene (18). PCR amplification was performed in 100-μl reaction mixtures containing 1 μl of crude cellular lysate, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.0 mM MgCl2, 0.1 μM oligonucleotide primers, 200 μM deoxynucleoside triphosphate mix, and 2.5 U of Taq DNA polymerase (Promega). PCR assay was performed in a Gene Cycler thermal cycler (Bio-Rad, Hercules, Calif.) with the following cycling parameters: denaturation at 94°C for 5 min; 35 cycles of 94°C for 30 s, 58°C for 1 min, and 72°C for 1 min; and a final extension period of 72°C for 10 min. Two microliters of each PCR product was diluted with 38 μl of distilled water, and then 2 μl of diluted PCR product was used as a DNA template for LCR. Before addition to the LCR mixture, the DNA template was boiled for 8 min.
Oligonucleotide primers.
For discriminatory detection of genes coding for SHV variants, we synthesized four different biotinylated primer sets that were designed to detect the following amino acid substitutions: Gln for Leu at position 35, Leu for Arg at 205, Ser for Gly at 238, and Lys for Glu at 240 (Table 1). Each primer set included four oligonucleotides (i.e., two pairs of oligonucleotides) to amplify the target sequence. One pair of oligonucleotides was complementary to one strand of the target DNA sequence, and the second pair was complementary to the first pair. Two oligonucleotides of each pair were hybridized to denatured target DNA so that the 3′ end of one primer is next to the 5′ end of the other primer. Consensus primers contained a phosphate at the 5′ end that is required for the ligation reaction. Mutant-specific primers of each oligonucleotide set allowed discrimination of parental and mutated nucleotide sequences of SHV enzymes. For colorimetric detection, one of the mutant-specific primers contained a biotin at the 5′ end for capture on a streptavidin-coated microwell, and the other mutant-specific primer contained an additional 21-base sequence (5′-TGGCACTGGCCGTCGTTTTAC-3′) at its 5′ end, complementary to the universal primer sequence. This sequence hybridizes to the detection oligonucleotide provided by the AmpLiTek LCR detection kit (Bio-Rad).
TABLE 1.
Nucleotide sequences of the oligonucleotides used as LCR primer sets
| Genes | Designation | Nucleotide sequence (5′→3′) of primersa
|
|
|---|---|---|---|
| Consensus | Mutant specific | ||
| blaS-2a, -12 | Gln-35 | P-AAGCGAAAGCCAGCTGTCGG (279) | B-GCCGCTTGAGCAAATTAAACA (258) |
| P-GTTTAATTTGCTCAAGCGGCTG (256) | U-GACAGCTGGCTTTCGCTTT (278) | ||
| blaS-3, -4 | Leu-205 | P-GCAGCTGCTGCAGTGGATGG (789) | B-CGCCCGTTCGCAACT (774) |
| P-GTTGCGAACGGGCGCTC (771) | U-CCACTGCAGCAGCTGCA (788) | ||
| blaS-2, -2a, -3, -4, -5, -12 | Ser-238 | P-GCGAGCGGGGTGCGC (887) | B-GCCGATAAGACCGGAGCTA (868) |
| P-AGCTCCGGTCTTATCGGCG (867) | U-GCACCCCGCTCGCT (886) | ||
| blaS-4, -5, -12 | Lys-240 | P-AGCGGGGTGCGCGCG (890) | B-CGATAAGACCGGAGCTAGCA (870) |
| P-GCTAGCTCCGGTCTTATCGGC (868) | U-CGCGCACCCCGCTT (889) | ||
LCR requires four oligonucleotides to amplify the target sequence. Nucleotides shown in boldface indicate the point mutations that lead to amino acid substitutions. These amino acids are the basis for the designation of the primer set, and their numbering is according to the consensus numbering of Ambler et al. (1). Consensus primers contain a phosphate (P) at the 5′ end that is required for the ligation reaction. One of the mutant-specific primers contains a biotin (B) at the 5′ end for capture on a streptavidin-coated microwell. The other mutant-specific primer contains an additional 21-base sequence (U; 5′-TGGCACTGGCCGTCGTTTTAC-3′) at its 5′ end complementary to the universal primer sequence. This sequence hybridizes to the detection oligonucleotide for colorimetric detection. The numbers in parentheses correspond to the first 5′ base of nucleotides according to the coding sequence of SHV-2 (10).
LCR and colorimetric detection.
LCRs were performed with an AmpLiTek LCR kit (Bio-Rad) according to directions provided by the manufacturer. The reactions took place in 25-μl reaction mixtures with 2 μl of target DNA containing 16 fmol of oligonucleotide mix per μl, 50 ng of salmon sperm DNA per μl, 1 U of Taq ligase, and 10× ligase buffer. After samples were covered with 30 μl of mineral oil, they were run in a Gene Cycler thermal cycler (Bio-Rad) with the following cycling parameters: 1 cycle of 94°C for 4 min and 60°C for 4 min and 10 cycles of 91°C for 30 s and 60°C for 5 min. Amplified products from the reaction were identified by colorimetric detection in a microtiter plate with the AmpLiTek LCR detection kit (Bio-Rad) according to the directions provided by the manufacturer. After thermal cycling, 5 μl of each amplified product was diluted with 45 μl of 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate) and was placed in the streptavidin-coated wells. After incubation for 1 h at 37°C, each well was washed five times with 300 μl of 1× well wash solution. After washing, the detection oligonucleotide supplied with the kit was added to each well. This alkaline-phosphatase-conjugated oligonucleotide contained the universal primer sequence that would hybridize to its complementary sequence on the LCR-amplified products bound in the well. After incubation for 1 h at 37°C, each well was washed again five times with 300 μl of 1× well wash solution. The substrate (NADPH) for alkaline phosphatase was added to all wells, and a second reagent, which uses the product of the NADPH-alkaline-phosphatase reaction to generate a redox cycle that produces a red formazan dye, was added. The color change reaction was assayed through endpoint determination at 490 nm in a precision microplate reader (Molecular Device). If the optical density value of the color change was above 0.1 at 10 min after addition of amplifier, we interpreted it as a positive reaction.
Antibiotics and susceptibility testing.
Antimicrobial agents tested were cefotaxime, ceftazidime, aztreonam, cefotetan, piperacillin, and piperacillin-tazobactam. MICs were determined by the agar dilution method according to the guidelines of the NCCLS (20). In the piperacillin-tazobactam combination, the concentration of tazobactam was 4 mg/liter.
Analytical IEF.
Crude preparations of β-lactamases from clinical isolates were obtained by two sonications for 30 s each time in 0.1 M phosphate buffer (pH 7.0). IEF was performed by the method of Matthew et al. (17) with a Mini-IEF cell system (Bio-Rad). Enzyme activities were detected by overlaying the gel with 0.5 mM nitrocefin in 0.1 M phosphate buffer, pH 7.0. β-Lactamases were identified by comparison to reference enzymes run in tracks adjacent to the test samples. Inhibition assay was performed by overlaying the gels with 0.5 mM nitrocefin with and without 0.3 mM cloxacillin or 0.3 mM clavulanic acid in 0.1 M phosphate buffer, pH 7.0 (15).
RESULTS AND DISCUSSION
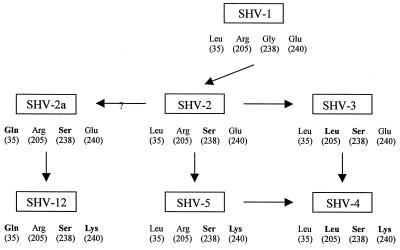
The evolutionary relationship of seven members of the SHV family is summarized in Fig. 1. Many SHV-derived extended-spectrum enzymes have the same change of amino acid at position 238 and thus must all be derived from SHV-2. This first mutation from SHV-1, changing glycine to serine, was associated with a large increase in the MIC of cefotaxime but only a moderate increase in the MIC of ceftazidime. SHV-4 was derived from SHV-2 by substitution of two amino acids through either SHV-3 or SHV-5. A change in the amino acid at position 240 from glutamic acid (SHV-2) to lysine (SHV-5) considerably increased the MIC of ceftazidime, but had a lesser effect on the MIC of cefotaxime (8). On the other hand, although SHV-2a and SHV-12 were indistinguishable from SHV-2 and SHV-5, respectively, by isoelectric point and substrate profiles, SHV-2a and SHV-12 share the same substitution of glutamine for leucine at position 35 as SHV-2 and SHV-5, respectively. SHV-12 conferred a relatively low level of resistance to cefotaxime but higher resistance to ceftazidime and aztreonam (14). The substrate profile and the sites of amino acid variation suggest that SHV-12 may be derived from SHV-2a by replacement of glutamic acid with lysine at position 240. Continued challenge of an SHV-2a-producing strain with 7-oxyiminocephalosporins, particularly ceftazidime, is likely to select for the E240K substitution leading to SHV-12 (14).
FIG. 1.
A diagram of the evolutionary relationship of six SHV-derived ESBLs. Location of selected amino acids is according to the consensus numbering of Ambler et al. (1). Amino acids in boldface type represent changes from SHV-1 at these positions.
For discriminatory detection of genes coding for SHV variants, we performed an LCR typing with the four different primer sets that are designed to detect the following amino acid substitutions: Gln for Leu at position 35, Leu for Arg at 205, Ser for Gly at 238, and Lys for Glu at 240.
Discrimination of SHV enzymes by LCR typing.
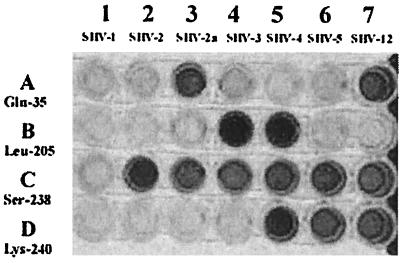
The seven reference strains, each producing one of the seven recognized SHV β-lactamases, were subjected to LCR typing. Each point mutation of blaSHV genes targeted by each primer set was precisely detected (Fig. 2). LCRs with the primer set of Ser-238 gave a positive signal with all blaSHV-ESBL. Similar results were obtained with the primer sets of Gln-35, Leu-205, and Lys-240 on blaSHV-2a and blaSHV-12; blaSHV-3 and blaSHV-4; and blaSHV-4, blaSHV-5, and blaSHV-12 target DNAs, respectively. Target-independent ligation or false-negative reactions were not observed on any occasion. Therefore, we could distinguish seven SHV bla genes rapidly and precisely with LCR typing according to their incorporating point mutations (Table 2).
FIG. 2.
Identification and discrimination of blaSHV genes by LCR typing. The seven reference strains (lanes 1 to 7), each producing one of seven recognized SHV β-lactamases, were subjected to LCR typing with four different biotinylated primer sets (A to D).
TABLE 2.
LCR typing of standard SHV-derived β-lactamases
| β-Lactamase (pI) | LCR typing of primer setsa
|
|||
|---|---|---|---|---|
| Gln-35 | Leu-205 | Ser-238 | Lys-240 | |
| SHV-1 (7.6) | − | − | − | − |
| SHV-2 (7.6) | − | − | + | − |
| SHV-2a (7.6) | + | − | + | − |
| SHV-3 (7.0) | − | + | + | − |
| SHV-4 (7.8) | − | + | + | + |
| SHV-5 (8.2) | − | − | + | + |
| SHV-12 (8.2) | + | − | + | + |
+, positive LCR; −, negative LCR.
The phenotypic characterization of β-lactamases that relies on the determination of their substrate profile, biochemical data, and IEF properties is poorly reproducible from one laboratory to another and can be technically demanding. Recently, the genotypic identification of β-lactamases such as oligotyping (16, 29), PCR–single-strand conformational polymorphism (SSCP) (14), PCR-restriction fragment length polymorphism (2, 6, 22), immunoassay (7), and direct sequencing of PCR product has been developed, and in the future these approaches may be more reliable. Oligotyping using biotinylated probes has been used to characterize TEM variant enzymes (29), and PCR-SSCP has been used to distinguish SHV-1 to -5 (19). We had also tried differentiation of SHV variants by PCR-SSCP according to the method described by M'Zali et al. (19) with some modifications. By that method, SHV-1 to SHV-5 were distinguished consistently, but SHV-2a and SHV-12 were not distinguished from SHV-2 and SHV-5, respectively (data not shown). In contrast, LCR typing enabled us to discriminate all tested SHV enzymes, including SHV-2a and SHV-12. Moreover, LCR typing of this study offers many advantages. First, LCR with the primer set of Ser-238 allows detection of serine-for-glycine substitution at amino acid position 238 incorporated in many blaSHV-ESBL genes, so this can be used to screen clinical isolates producing a majority of SHV ESBLs. Second, LCR with the primer set of Gln-35 allows differentiation of SHV-2a and SHV-12 from SHV-2 and SHV-5, respectively, without sequencing. Third, combining LCR and simple colorimetric detection using the AmpLiTek LCR kit provides an acquisition of the results in 1 day. And finally, the use of stripwell and microplate reader affords the opportunity to screen a large number of strains. Therefore, we believe that the LCR typing may also become a very useful genotypic method to detect and distinguish β-lactamases.
Identification and discrimination of SHV ESBLs in clinical isolates with LCR typing.
To test the applicability of the LCR technique for identification of unknown SHV β-lactamases, clinical isolates were also included in this study. Of 82 strains with reduced susceptibility to extended-spectrum cephalosporins which had been collected, 46 strains (32 K. pneumoniae, 10 E. cloacae, and 4 E. coli) were positively reacted with the PCR of blaSHV genes. By LCR with the primer set of Ser-238, 16 of 32 K. pneumoniae strains were considered to carry chromosomal blaSHV-1 gene giving no color change, and they were excluded from further studies. The remaining 30 strains were subjected to antibiotic susceptibility testing, IEF, and LCR typing. The results obtained are shown in Table 3.
TABLE 3.
Characteristics of clinical isolates carrying blaSHV-ESBL genes
| Strain | MIC (μg/ml) ofa:
|
pI(s) of β-lactamases | Presumptive blaSHV-ESBL gene | |||||
|---|---|---|---|---|---|---|---|---|
| PIP | CTX | CAZ | ATM | CFT | P/T | |||
| E. coli | ||||||||
| E3 | 128 | 2 | 1 | 0.5 | 0.25 | 2 | 7.6, 5.4 | blaSHV-2a |
| E13 | 128 | 2 | 1 | 0.5 | 0.12 | 2 | 7.6 | blaSHV-2a |
| E14 | 128 | 1 | 1 | 0.5 | 0.12 | 2 | 7.6, 5.4 | blaSHV-2a |
| E15 | >256 | 64 | 32 | 4 | 256 | 64 | 7.6, 5.4, 5.7, 8.0 | blaSHV-2a |
| K. pneumoniae | ||||||||
| K4 | >256 | 16 | 64 | 16 | 1 | >128 | 7.6 | blaSHV-2a |
| K5 | >256 | 8 | 4 | 1 | 0.12 | 4 | 7.6 | blaSHV-2a |
| K12 | >256 | 16 | 32 | 4 | 0.5 | 2 | 7.6, 5.9 | blaSHV-2a |
| K14 | 256 | 2 | 2 | 1 | 0.12 | 4 | 7.6, 5.4 | blaSHV-2a |
| K15 | 256 | 2 | 4 | 1 | 0.12 | 0.5 | 7.6 | blaSHV-2a |
| K19 | 128 | 2 | 2 | 1 | 0.12 | 1 | 7.6 | blaSHV-2a |
| K20 | 256 | 4 | 2 | 0.5 | 0.12 | 1 | 7.6 | blaSHV-2a |
| K22 | 256 | 16 | 4 | 4 | 0.25 | 2 | 7.6, 5.4 | blaSHV-2a |
| K25 | 128 | 2 | 2 | 0.5 | <0.06 | 1 | 7.6 | blaSHV-2a |
| K26 | >256 | 16 | 8 | 4 | 1 | 1 | 7.6, 5.4 | blaSHV-2a |
| K29 | 256 | 2 | 2 | 2 | <0.06 | 1 | 7.6 | blaSHV-2a |
| K30 | >256 | 16 | 16 | 8 | 0.25 | 2 | 7.6 | blaSHV-2a |
| K32 | 128 | 2 | 0.5 | 1 | <0.06 | 0.5 | 7.6 | blaSHV-2a |
| K37 | 256 | 2 | 2 | 1 | 0.06 | 0.5 | 7.6 | blaSHV-2a |
| K16 | 128 | 8 | 128 | 32 | 0.25 | 1 | 8.2, 5.4 | blaSHV-12 |
| K27 | 256 | 1 | 8 | 4 | 8 | 2 | 8.2, 7.6, 5.4 | blaSHV-12 |
| E. cloacae | ||||||||
| En11 | 256 | 4 | 32 | 64 | 0.25 | 1 | 8.2, 5.4, 8.1 | blaSHV-12 |
| En14 | 256 | 16 | 128 | 256 | 8 | 2 | 8.2, 5.4, 8.1 | blaSHV-12 |
| En15 | 256 | 8 | 128 | 256 | 0.5 | 4 | 8.2, 5.4 | blaSHV-12 |
| En17 | 128 | 64 | 256 | 16 | 2 | 16 | 8.2, 5.4 | blaSHV-12 |
| En26 | >256 | 64 | >256 | >256 | 4 | 128 | 8.2, 5.4 | blaSHV-12 |
| En29 | >256 | 16 | 128 | 256 | 32 | 2 | 8.2, 8.1 | blaSHV-12 |
| En30 | 128 | 2 | 32 | 16 | 64 | 8 | 8.2, 8.5 | blaSHV-12 |
| En7 | 128 | 8 | 2 | 0.5 | 64 | 2 | 7.6, 8.5 | blaSHV-2a |
| En18 | >256 | 256 | 128 | 8 | >256 | 32 | 7.6, 8.5 | blaSHV-2a |
| En19 | 128 | >256 | 128 | 128 | >256 | 64 | 7.6, 8.5 | blaSHV-2a |
Abbreviations: PIP, piperacillin; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; CFT, cefotetan; P/T, piperacillin-tazobactam combination.
Upon IEF, 21 strains revealed the SHV-type β-lactamase of pI 7.6, and nine isolates revealed the SHV-type β-lactamase of pI 8.2. Additional TEM-type enzymes with pI of 5.4, 5.7, or 5.9 appeared in 14 strains. One isolate of E. coli E15 also produced a class C β-lactamase with a pI of 8.0 which was sensitive to inhibition by cloxacillin but not clavulanic acid, and most strains of E. cloacae also produced a class C chromosomal β-lactamase with a pI of 8.1 or 8.5.
For 21 strains showing the β-lactamase of pI 7.6, LCR with the primer set of Gln-35 enabled us to distinguish blaSHV-2a from blaSHV-2 (10), blaSHV-7 (5), and blaSHV-8 (27). Similarly, for nine strains showing the β-lactamase of pI 8.2, LCR with the primer set of Gln-35 enabled us to distinguish blaSHV-12 from blaSHV-5, blaSHV-9, and blaSHV-10 (25, 26). LCR with the primer set of Lys-240 allowed us to discriminate blaSHV-2a and blaSHV-12. The results revealed that 21 strains carried the presumptive blaSHV-2a gene and nine strains carried the presumptive blaSHV-12 gene. Unfortunately, the present method does not detect SHV ESBLs lacking the four covered substitutions, e.g., SHV-6 (3), SHV-7 (5), and SHV-8 (27). But this can be overcome by implementing two additional primer sets for the amino acid substitutions R43S and D179. It is also noted that there are two different codons that give rise to the Lys-240 substitution: AAG, which our primer set of Lys-240 is based on, and AAA, found in one of the SHV-5 genes deposited in GenBank and in SHV-7. If one of the genes that have AAA would be present, it would be a mismatch for our primer set of Lys-240. (Information for the various ESBL sequences for SHV β-lactamases was obtained from the website http://www.lahey.org/studies/webt.htm#SHV.)
Antimicrobial resistance patterns of these isolates were in accordance with LCR data. MICs of ceftazidime and aztreonam for the strains harboring the blaSHV-2a gene were frequently low, while the strains harboring the blaSHV-12 gene usually showed high resistance to those antibiotics (14). For the strain of E. coli E15, the presence of an enzyme with a pI of 8.0, resembling CMY-1, raised the MICs of cefotaxime, ceftazidime, aztreonam, and cefotetan up to 64, 32, 4, and 256 μg/ml, respectively. Two strains of E. cloacae, En18 and En19, were highly resistant to all antibiotics tested, including cefotetan, indicating that they produced the Bush group 1 β-lactamase constitutively at high levels due to the derepressed mutation of a chromosomal gene, ampD. Therefore, they were considered to be derepressed mutants and SHV-2a β-lactamase producers.
The results obtained indicated that LCR typing was also applied successfully to the identification of SHV β-lactamases from clinical isolates. It is important to note that IEF and biochemical data can be used in conjunction with the LCR typing data to confirm observations. In addition, as LCR typing detects a limited number of amino acid changes but other changes in the sequence may also exist, care is required in the interpretation of the data.
So far, SHV ESBLs have been found predominantly in Klebsiella spp. and E. coli. ESBLs of the TEM and, particularly, the SHV type are very rarely found in other Enterobacteriaceae genera, such as Enterobacter, Serratia, Citrobacter, etc., in which chromosomal AmpC cepahlosporinases predominate (11, 15). Nevertheless, Serratia marcescens isolated in Greece has been reported to produce an SHV-like enzyme (identified by IEF) (9), and S. marcescens as well as E. cloacae have been found to carry SHV-4 (23). In addition, Pitout et al. (24) presented evidence for the production of SHV-3 and SHV-4 by E. cloacae and Enterobacter aerogenes in the United States. A single isolate of E. cloacae from Switzerland was found to carry SHV-2 by DNA sequencing (21). In this context, it is important to note that this is the first report describing a number of strains of E. cloacae producing SHV-2a or SHV-12.
Although the prevalence of SHV-2a and SHV-12 in Enterobacteriaceae in Western Europe or in the United States is not known, they are widespread among K. pneumoniae strains in Korea (14). This study also revealed the prevalence of SHV-2a and SHV-12 even among E. cloacae strains. Although the reason for the widespread distribution of SHV-2a and SHV-12 is not clear, the spread of resistant organisms or mobile elements like transposons or insertion sequences may have played a role in the spread of common ESBLs. Moreover, intrahospital spread of common organisms or similar selective pressure among the institutions could be another possible explanation.
In summary, we have developed a new genotypic method to characterize point mutations in genes for SHV-type β-lactamases based on a nonradioactive LCR technique. This technique permits detailed characterization and molecular epidemiology of SHV-type β-lactamases more easily and rapidly than sequencing. The SHV family of ESBLs is well defined and has proved to be a good model for the development of LCR technology as applied to the characterization of antibiotic resistance genes in bacteria. This technique could also be extended to characterize the mutations that have given rise to the much larger family of TEM-derived ESBLs and any other resistance genes that differ by only point mutations.
ACKNOWLEDGMENTS
We are very grateful to G. A. Jacoby, who provided the five strains carrying plasmids encoding SHV-1, SHV-2, SHV-3, SHV-4, and SHV-5 β-lactamases.
This work was supported by grant KOSEF 971-0712-102-2 from the Korean Science and Engineering Foundation.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frere J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlet G, Brami G, Flippo A, Gaillot O, Lagrange P H, Philippon A. Molecular characterisation by PCR-restriction fragment length polymorphism of TEM β-lactamases. FEMS Microbiol Lett. 1995;134:203–208. doi: 10.1111/j.1574-6968.1995.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 3.Arlet G, Rouveau M, Philippon A. Substitution of alanine for aspartate at position 179 in the SHV-6 extended-spectrum β-lactamase. FEMS Microbiol Lett. 1997;152:163–167. doi: 10.1016/s0378-1097(97)00196-1. [DOI] [PubMed] [Google Scholar]
- 4.Barany F. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc Natl Acad Sci USA. 1991;88:189–193. doi: 10.1073/pnas.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford P A, Urban C, Jaiswal A, Mariano N, Rasmussen B A, Projan S J, Rahal J J, Bush K. SHV-7, a novel cefotaxime-hydrolyzing β-lactamase, identified in Escherichia coli isolated from hospitalized nursing home patients. Antimicrob Agents Chemother. 1995;39:899–905. doi: 10.1128/aac.39.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canica M M, Lu C Y, Krishnamoorthy R, Paul G C. Molecular diversity and evolution of blaTEM genes encoding β-lactamases resistant to clavulanic acid in clinical E. coli. J Mol Evol. 1997;44:57–65. doi: 10.1007/pl00006121. [DOI] [PubMed] [Google Scholar]
- 7.Curran R, Talbot D C, Towner K. A rapid immunoassay method for the direct detection of PCR products: application to detection of TEM β-lactamase genes. J Med Microbiol. 1996;45:76–78. doi: 10.1099/00222615-45-1-76. [DOI] [PubMed] [Google Scholar]
- 8.Du Bois S K, Marriott M S, Amyes S G B. TEM- and SHV-derived extended-spectrum β-lactamases: relationship between selection, structure and function. J Antimicrob Chemother. 1995;35:7–22. doi: 10.1093/jac/35.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Gianneli D, Tzelepi E, Tzouvelekis L S, Mentis A F, Nikolopoulou C. Dissemination of cephalosporin-resistant Serratia marcescens strains producing a plasmidic SHV type β-lactamase in Greek hospitals. Eur J Clin Microbiol Infect Dis. 1994;13:764–767. doi: 10.1007/BF02276063. [DOI] [PubMed] [Google Scholar]
- 10.Huletsky A, Couture F, Levesque R C. Nucleotide sequence and phylogeny of SHV-2 β-lactamase. Antimicrob Agents Chemother. 1990;34:1725–1732. doi: 10.1128/aac.34.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacoby G A. Genetics of extended-spectrum β-lactamases. Eur J Clin Microbiol Infect Dis. 1994;13:2–11. doi: 10.1007/BF02390679. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby G A, Sutton L. Properties of plasmids responsible for extended-spectrum β-lactamase production. Antimicrob Agents Chemother. 1991;35:164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Kwon Y, Pai H, Kim J W, Cho D T. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998;36:1446–1449. doi: 10.1128/jcm.36.5.1446-1449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabilet C, Courvalin P. Development of oligotyping for characterization and molecular epidemiology of TEM β-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990;34:2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthew M, Harris M, Marshall M J, Rose G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 18.Mercier J, Levesque R C. Cloning of SHV-2, OHIO-1, and OXA-6 β-lactamases and cloning and sequencing of SHV-1 β-lactamase. Antimicrob Agents Chemother. 1990;34:1577–1583. doi: 10.1128/aac.34.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.M'Zali F, Gascoyne-Binzi D M, Heritage J, Hawkey P M. Detection of mutations conferring extended-spectrum activity on SHV β-lactamases using polymerase chain reaction single strand conformational polymorphism (PCR-SSCP) J Antimicrob Chemother. 1996;37:797–802. doi: 10.1093/jac/37.4.797. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 7th ed. 1997. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 21.Nuesch-Inderbinen M T, Kayser F H, Hachler H. Survey and molecular genetics of SHV β-lactamase in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob Agents Chemother. 1997;41:943–949. doi: 10.1128/aac.41.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuesch-Inderbinen M T, Hachler H, Kayser F H. Detection of genes coding for extended-spectrum SHV β-lactamases in clinical isolates by a molecular genetic method and comparison with the E test. Eur J Clin Microbiol Infect Dis. 1996;15:398–402. doi: 10.1007/BF01690097. [DOI] [PubMed] [Google Scholar]
- 23.Philippon A, Labia R, Jacoby G A. Extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1989;33:1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitout J D D, Moland E S, Sanders C C, Thomson K S, Fitzsimmons S R. β-Lactamases and detection of β-lactam resistance in Enterobacter spp. Antimicrob Agents Chemother. 1997;41:33–39. doi: 10.1128/aac.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prinarakis E E, Tzelepi E, Gazouli M, Mentis A F, Tzouvelekis L S. Characterization of a novel SHV β-lactamase variant that resembles the SHV-5 enzyme. FEMS Microbiol Lett. 1996;139:229–234. doi: 10.1111/j.1574-6968.1996.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 26.Prinarakis E E, Miriagou V, Tzelepi E, Gazouli M, Tzouvelekis L S. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob Agents Chemother. 1997;41:838–840. doi: 10.1128/aac.41.4.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasheed J K, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros A A, Tenover F C. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41:647–653. doi: 10.1128/aac.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirot D. Extended-spectrum plasmid-mediated β-lactamases. J Antimicrob Chemother. 1995;36:19–34. doi: 10.1093/jac/36.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 29.Tham T N, Mabilet C, Courvalin P, Guesdon J L. Biotinylated oligonucleotide probes for the detection and the characterization of TEM-type extended broad spectrum β-lactamases in Enterobacteriaceae. FEMS Microbiol Lett. 1990;69:109–116. doi: 10.1016/0378-1097(90)90423-n. [DOI] [PubMed] [Google Scholar]