Abstract
We recovered two isolates (EP1 and EP2) of Escherichia coli from the same patient that had identical pulsed-field gel electrophoresis patterns but required different MICs of ciprofloxacin (CIP): 16 and 256 mg/liter for EP1 and EP2, respectively. Both isolates had mutations in the quinolone resistance-determining regions of GyrA (Ser83Leu and Asp87Tyr) and ParC (Ser80Ile), but not in those regions of GyrB or ParE. Isolate EP2 was also more resistant to chloramphenicol, tetracyclines, cefuroxime, and organic solvents. A deletion of adenine (A) 1821 was found in marR of isolate EP2, which resulted in an 18-amino-acid C-terminal deletion in the MarR protein. The causative relationship between ΔA1821 and the Mar phenotype was demonstrated both by the replacement of the wild-type marR by marR ΔA1821 in isolate EP1 and by complementation with the wild-type marR in trans in isolate EP2. In isolate EP2 complemented with wild-type marR, susceptibility to chloramphenicol was restored completely, whereas susceptibility to CIP was restored only incompletely. Northern blotting demonstrated increased expression of marA and acrAB but not of soxS in isolate EP2 compared to EP1. In conclusion, the deletion of A1821 in marR in the clinical isolate EP2 caused an increase in the MICs of CIP and unrelated antibiotics. Presumably, the C-terminal part of MarR is necessary for proper repressor function.
Resistance mechanisms of Escherichia coli against fluoroquinolones (FQ) have been well studied, and three mechanisms have been identified. Point mutations in the quinolone resistance-determining regions (QRDRs) of topoisomerases II (gyrAB) and IV (parCE) lead to a stepwise acquisition of resistance (7, 9, 12). Active efflux of FQ by multidrug resistance pumps like AcrAB, and reduced uptake due to OmpF, both regulated by the transcription factor MarA, are also implicated in resistance (5, 18). These mechanisms, often in combination, have been found in strains from both in vitro and clinical investigations. However, few data are available about the development of resistance in patients, that is, the order of acquisition of the respective mechanisms and their contributions to the resistance phenotype. We investigated two clinical isolates of E. coli, with different levels of resistance to ciprofloxacin (CIP) but identical pulsed-field gel electrophoresis (PFGE) patterns, from one patient. By gene exchange and complementation we demonstrated the role of a C-terminal deletion in MarR resulting in increased efflux of FQ in the more resistant strain.
(This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September, 1999.)
MATERIALS AND METHODS
Bacterial strains.
E. coli isolates EP1 and EP2 were isolated from the vagina and the urine of a patient with generalized follicular lymphoma who had received 750 mg of CIP twice daily for selective decontamination of the gut. E. coli ATCC 25922 was obtained from the American Type Culture Collection (ATCC). E. coli EP2 acrA::Tn10-Km was obtained from the clinical isolate EP2 by transposon mutagenesis and screening for susceptibility against FQ. E. coli S17 λpir harboring the plasmid pLOF/Km was kindly donated by V. de Lorenzo (6). E. coli S17 containing plasmid pBP591 with wild-type marR has been described previously (V. Hüllen, P. Heisig, and B. Wiedemann, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-64, p. 57, 1997).
PFGE.
A rapid PFGE procedure was performed as described previously (8). Macrorestriction was performed with XbaI at 37°C for 3 h. Electrophoresis was performed with the contour-clamped homogeneous electric field (CHEF) mapper system (Bio-Rad, Hercules, Calif.). The run time was 14 h, with an initial switch time of 2.16 s and a final switch time of 35 s.
Susceptibility testing.
MICs of CIP were determined according to NCCLS recommendations for microdilution assays (16). MICs of chloramphenicol and tetracycline were determined by Etest (AB BIODISK, Solna, Sweden). For testing of organic solvent tolerance (OST), isolates were inoculated on Mueller-Hinton agar at a concentration of 105 CFU per spot and the plate was overlaid with hexane (H), cyclohexane (CH), and H-CH mixtures at ratios of 3:1, 1:1, and 1:3 to generate different levels of organic solvent toxicity. n-Hexane has a pow of 3.9, and cyclohexane has a pow of 3.4 (3). The plates were checked for growth after 2 days.
DNA amplification and nucleotide sequence determination.
Primers (Table 1) and PCR conditions for amplification of gyrA, gyrB, and parE were used as previously described (7, 12). Primers and annealing temperatures for the amplification of parC, the mar operon, marA and marB, and acrA are listed in Table 1. Complementary strands were sequenced in duplicate on a 310 DNA sequencer (Perkin-Elmer, Foster City, Calif.) using either PCR primer (6 μmol).
TABLE 1.
Primers used in this study
| Target | Primer | Oligonucleotide (5′→3′)a | Position | Tanb (°C) | Remarks |
|---|---|---|---|---|---|
| Subunit A topoisomerase IV | parC-f58 | TGA ATT TAC GGA AAA CGC CTA C | 58–79 | 60 | Sequencing |
| parC-r591 | GCC ACT TCA CGC AGG TTA TG | 591–572 | 60 | Sequencing | |
| marA operon, marR | maropR-f1394 | TTG CCT GGG CAA TAT TAT C | 1394–1411 | 45 | Sequencing |
| maropR-r1950 | GTC CAA AAT GCT ATG AAT G | 1950–1928 | 45 | Sequencing | |
| marop-f1140 | GCCAGGCCAAGAAATAAC | 1140–1157 | 50 | Sequencing | |
| marop-r1556 | ATCCAGCGGAGACAGATAC | 1556–1538 | 50 | Sequencing | |
| marA, marB | marAB-f1840 | GGC AAC ACT TGA GTA TTT GC | 1840–1859 | 50 | Sequencing |
| marAB-r2704 | AGT AGG ACT GGC AAG TGC | 2704–2687 | 50 | Sequencing | |
| marA | marA-f1893 | ATT AAG CTT ATG ACG ATG TCC AGA CG | 1893–1909 | 50 | HindIII site |
| marA-r2281 | ATT TCT AGA ACT AGC TGT TGT AAT G | 2282–2267 | 50 | XbaI site | |
| marR | marR-f1452 | AAT GAA TTC GTA CCA GCG ATC TGT TCA ATG | 1452–1472 | 50 | EcoRI site |
| marR-r2229 | AAT CTG CAG TGG TCA TCC GGT ATT TAT GC | 2229–2210 | 50 | PstI site | |
| soxS | soxS-f331 | ATA GAA TTC ACC AAT AAA ATT ACA GGC GG | 3331–3350 | 50 | EcoRI site |
| soxS-r1319 | ATA CTC GAG ATG TCC CAT CAG AAA ATT ATT CAG | 1319–1297 | 50 | XhoI site | |
| acrA | acrA-f31 | GCG GTC GTT CTG ATG CTC TC | 31–48 | 50 | Northern probe |
| acrA-r330 | ACC TTT CGC ACT GTC GTA TG | 330–310 | 50 | Northern probe |
Restriction sites are italicized.
Tan, annealing temperature.
Plasmids and DNA manipulations.
Strains and plasmids used in this study are listed in Table 2. Introduction of the marR frameshift mutation from isolate EP2 into the wild-type marR of isolate EP1 required subcloning of the EcoRI/PstI-digested PCR fragment (with primers marR-f1452 and marR-r2229) into the corresponding pUC18 site to yield plasmid pmarR-ΔC18. Probes for Northern blot analysis were generated by subcloning of marA and soxS into plasmid pcDNA3 (Invitrogen, Groningen, The Netherlands) and in vitro transcription from the SP6 and T7 promoters, respectively. The complete marA gene (1893 to 2282) was amplified in a DNA thermocycler using primers marA-f1893 and marA-r2281. For the amplification and cloning of soxS, primers soxS-f331 and soxS-r1319 were used. Genomic DNA from E. coli ATCC 25922 served as a template. The PCR fragments were cloned directionally into plasmid pcDNA3 to generate plasmids pc-marA and pc-soxS, respectively. Recombinant DNA techniques, transformation, and restriction enzyme digestions followed standard protocols (19).
TABLE 2.
Bacterial strains and plasmids used in this study
| E. coli strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| ATCC 25922 | E. coli | ATCC |
| EP1 | MIC of CIP, 16 mg/liter | Clinical isolate |
| EP2 | MIC of CIP, 256 mg/liter | Clinical isolate |
| EP2 acrA::Tn10-Km | Derivative of isolate EP2 in which acrA was inactivated by transposon mutagenesis | This study |
| S17 λpir + pLOF/Km | Suicide vector for insertion mutagenesis | 10 |
| S17 + p591 | Wild-type marR in trans | Hüllen et al., 37th ICAAC |
| Plasmids | ||
| pmarR-ΔC18 | Derived from pUC18 containing the PCR product of primers marR-f1452 and marR-r2229 | This study |
| pc-marA | Derived from pcDNA3 containing the PCR product of primers marA-f1893 and marA-r2281 | This study |
| pc-soxS | Derived from pcDNA3 containing the PCR product of primers soxS-f331 and soxS-r1319 | This study |
Gene replacement.
Introduction of the marR mutation into the wild-type marR gene of isolate EP1 was accomplished by homologous recombination between plasmid pmarR-ΔC18 and the bacterial chromosome, essentially as described previously (11). Plasmid pmarR-ΔC18 was introduced into isolate EP1 by electroporation (Genepulser; Bio-Rad). Resulting transformants were grown on Luria-Bertani (LB) agar plates supplemented with 50 mg of ampicillin/liter overnight at 37°C. Recombinant bacteria were then propagated in LB broth containing 32 mg of CIP/liter for selection of E. coli carrying the desired recombination. Segregation of the plasmid after passaging of single colonies for 1 week was shown by growth inhibition of recombinant clones on LB agar plates containing ampicillin and by inability to amplify the specific marR DNA fragment by PCR with plasmid DNA preparations as templates using plasmid-specific primers.
Insertion mutagenesis.
Transposon mutagenesis was performed using a suicide vector system as described previously (6, 10). The exconjugates were selected for reduced CIP MICs by transfer of single colonies to agar plates containing 64 or 2 μg of CIP/ml. Colonies growing selectively on plates with 2 μg of CIP/ml were further analyzed.
RNA extraction and Northern blot analysis.
Overnight cultures were diluted 100-fold in LB broth and grown with shaking to mid-logarithmic phase at 37°C. Paraquat was added for the induction of the sox operon (45 min; final concentration, 1.3 mM; Sigma, Deisenhofen, Germany). For production of the marA probe, plasmid pc-marA was linearized by HindIII digestion and in vitro RNA runoff transcription was performed with the RiboProbe Kit (Promega). Similarly, the soxS probe was obtained by XhoI digestion of plasmid pc-soxS and in vitro transcription. Digoxigenin (DIG)-labeled RNA probes were purified with the RNeasy Purification Kit (QIAGEN). The acrA probe was obtained by PCR. Northern blotting was performed using standard techniques (19).
Complementation assays.
For the complementation of the mutant marR of isolate EP2, wild-type marR under the control of the bla promoter was introduced into isolate EP2 by mobilization with the filter mating technique. The donor strain, E. coli S17 carrying plasmid pBP591, and isolate EP2 were mixed in a 1:1 ratio and incubated at 37°C for 12 h on a minimal agar plate. Cells were resuspended in LB broth and plated on LB agar plates containing 50 μg of kanamycin/ml for selection.
Data analysis.
SPSS 8.0 for Windows was used for calculation of Mann-Whitney U test results.
RESULTS AND DISCUSSION
PFGE typing generated identical patterns of XbaI-digested total genomic DNA for isolates EP1 and EP2 but different patterns for three epidemiologically unrelated strains used as a control. Further evidence for the high genetic relatedness of the two isolates was given by the finding of a 61-bp deletion and a 785-bp insertion at the same site in both strains between bp 1252 and 1313 of the published mar wild-type sequence (4) and by observation of identical patterns in randomly primed PCR using five different primers (data not shown).
The MICs of CIP for EP1 and EP2 were different, 16 and 256 mg/liter, respectively; however, identical changes in critical residues of the QRDRs of gyrA (Ser83Leu, Asp87Tyr) and parC (Ser80Ile) were found. This combination of amino acid alterations in critical residues can explain a CIP MIC of 16 mg/liter (12); however, no other substitutions in the QRDR of gyrB or parE were detected in any strain.
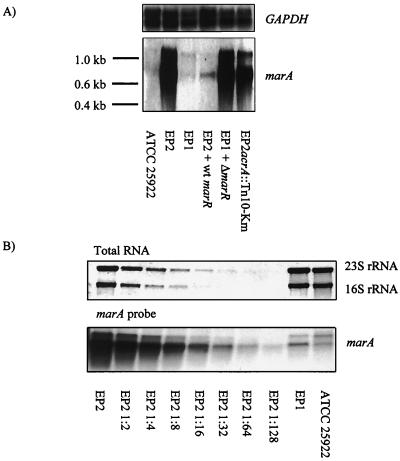
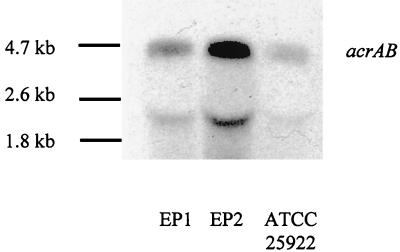
Compared to EP1, EP2 also required higher MICs of chloramphenicol (32 versus 4 mg/liter), tetracycline (32 versus 8 mg/liter), and cefuroxime (>16 versus 4 mg/liter), and EP2 but not EP1 grew on Mueller-Hinton agar overlaid with hexane or a 3:1 hexane-cyclohexane mixture. These properties of EP2 were consistent with a Mar phenotype, and consequently increased expression of the marA transcript was demonstrated by Northern blotting (Fig. 1A). Increased expression was demonstrated independently by quantitative PCR using TaqMan technology after a reverse PCR step (data not shown). Compared to wild-type transcription of marA in strain ATCC 25922, transcription of marA in isolate EP2 was estimated to be 64-fold, as determined by twofold serial dilutions of the target sequence of isolate EP2 (Fig. 1B). Expression of soxS, another transcription factor of the acr locus (15), with and without stimulation with paraquat, was similar in both isolates (Northern blot, data not shown). The increased transcription of marA in isolate EP2 led to increased expression of acrA, as could be expected (1). In the acrA Northern blot, two transcripts of approximately 4.5 and 2.2 kb were observed (Fig. 2). The size of the larger transcript corresponds to the expected length of the acrAB transcript of 4.344 kb, and this transcript is also dominant. Sequencing of the mar operons of isolates EP1 and EP2 indicated a deletion of adenine 1821 of marR in EP2. This deletion resulted in a frameshift and a concomitant loss of 18 amino acids in the C-terminal region of the MarR protein.
FIG. 1.
Northern blot analyses of marA. (A) Total RNA of E. coli was prepared, transferred to Hybond-N+ membranes, and probed with DIG-labeled marR RNA. The prominent transcripts and Boehringer RNA molecular weight standard III are indicated. (B) A twofold serial dilution of total RNA of E. coli isolate EP2 and undiluted RNAs from E. coli isolate EP1 and strain ATCC 25922 were transferred to Hybond-N+ membranes, stained with methylene blue, and probed with DIG-labeled marR RNA.
FIG. 2.
Northern blot analysis of acrAB. Total RNA was prepared, transferred to Hybond-N+ membranes and probed with DIG-labeled acrA PCR product. The prominent transcript and Boehringer RNA molecular weight standard II are indicated.
To investigate the possible role of the truncation of MarR, gene exchange and complementation experiments were designed. When we introduced the defective marR into the chromosomal DNA of isolate EP1 by homologous recombination, the MIC of CIP rose to 64 to 128 mg/liter, suggesting diminished repressor activity of the truncated MarR. Likewise, in isolate EP2, in trans complementation with the wild-type marR resulted in a lower CIP MIC of 64 mg/liter. The putative role of the frameshift in marR affecting the regulation of transcription of the AcrAB efflux pump was corroborated by the knockout mutant EP2 acrA::Tn10-Km, for which the MIC of CIP fell back to 32 mg/liter. The characteristics of EP1, EP2, and their derivatives are summarized in Table 3.
TABLE 3.
Characteristics of E. coli ATCC 25922, EP1, EP2, EP2 acrA::Tn10-Km, EP1 ΔmarR, and EP2 complemented with wild-type marR
| E. coli straina | MIC (mg/liter)b
|
OST
|
mar sequence | Transcription of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | Tetracycline | Chloramphenicol | Cefazolin | Cefuroxime | H | H-CH, 3:1 | H-CH, 1:1 | CH | marA | acrA | ||
| ATCC 25922 | 0.006 | 3 | 3 | ND | ND | − | − | − | − | wt | + | + |
| EP1 | 16 | >256 (8)c | 4 | <2 | 4 | − | − | − | − | wt | ++ | + |
| EP2 | 256 | >256 (32)c | 32 | 4 | >16 | + | + | − | − | ΔA1821 | ++++ | ++ |
| EP2 acrA::Tn10-Km | 32 | ND | 0.75 | <2 | <2 | − | − | − | − | ΔA1821 | ++++ | ND |
| EP1 ΔmarR | 64–128 | ND | 32 | ND | ND | ND | ND | ND | ND | ΔA1821 | ++++ | ND |
| EP2 + wt marR | 64 | ND | 4 | ND | ND | ND | ND | ND | ND | wt | ++ | ND |
wt, wild type.
ND, not determined.
Two subpopulations.
Neither the introduction of the wild-type marR into isolate EP2 nor the introduction of the defective marR into isolate EP1 resulted in a complete reversal of CIP MICs, indicating an additional, yet unknown resistance mechanism(s). The presence of additional resistance mechanisms is also suggested by the author of a recent study of E. coli with GyrA and MarA mutations generated in vitro, in which no clinically relevant resistance to CIP was detectable in acrAB knockout mutants (17). Efflux pump inhibitors, which restored susceptibility to FQ in the presence of target mutations (13), may not be effective in the E. coli clinical isolate EP2 analyzed in this study.
The multiple antibiotic resistance locus (mar) of E. coli controls intrinsic susceptibility to multiple antibiotics, organic solvents, oxidative stress agents, and the disinfectant triclosan (for reviews see references 1, 2, and 14). Presumably, the N-terminal and central regions of MarR, where a helix-turn-helix motif has been identified, are responsible for the specific interactions with the two binding sites in marO (1). The finding of this study indicates that the C terminus of MarR is also necessary for proper repressor function.
In conclusion, using genetic exchange and complementation techniques in an otherwise genetically indistinguishable pair of clinical isolates of E. coli, we have identified a unique C-terminal deletion in MarR resulting in a Mar phenotype affecting the MICs of FQ, tetracycline, chloramphenicol, and cefuroxime, as well as OST. Changes in the target enzyme and active efflux both add to the resistance phenotype. This is yet another example of the versatility of bacterial acquisition of antimicrobial resistance.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Emmi Fuchs and Christine Irtenkauf. Nucleotide sequence determination was performed by Holger Melzl and Josef Köstler.
REFERENCES
- 1.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun M N, Levy S B. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 1999;7:410–413. doi: 10.1016/s0966-842x(99)01589-9. [DOI] [PubMed] [Google Scholar]
- 3.Aono R. Improvement of organic solvent tolerance level of Escherichia coli by overexpression of stress-responsive genes. Extremophiles. 1998;2:239–248. doi: 10.1007/s007920050066. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S P, Hachler H, Levy S B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S P, McMurry L M, Hooper D C, Wolfson J S, Levy S B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989;33:1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 7.Everett M J, Jin Y F, Ricci V, Piddock L J. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob Agents Chemother. 1996;40:2380–2386. doi: 10.1128/aac.40.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautom R K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel J A, Vossen J P, Venema G. A general method for the construction of Escherichia coli mutants by homologous recombination and plasmid segregation. Mol Gen Genet. 1987;207:294–301. doi: 10.1007/BF00331592. [DOI] [PubMed] [Google Scholar]
- 12.Lehn N, Stoewer-Hoffmann J, Kott T, Strassner C, Wagner H, Schneider-Brachert W. Characterization of clinical isolates of Escherichia coli showing high levels of fluoroquinolone resistance. J Clin Microbiol. 1996;34:597–602. doi: 10.1128/jcm.34.3.597-602.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lomovskaya O, Lee A, Hoshino K, Ishida H, Mistry A, Warren M S, Boyer E, Chamberland S, Lee V J. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1340–1346. doi: 10.1128/aac.43.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurry L M, Oethinger M, Levy S B. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS Microbiol Lett. 1998;166:305–309. doi: 10.1111/j.1574-6968.1998.tb13905.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller P F, Sulavik M C. Overlaps and parallels in the regulation of intrinsic multiple-antibiotic resistance in Escherichia coli. Mol Microbiol. 1996;21:441–448. doi: 10.1111/j.1365-2958.1996.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 17.Oethinger M, Kern W V, Jellen-Ritter A S, McMurry L M, Levy S B. Ineffectiveness of topoisomerase mutations in mediating clinically significant fluoroquinolone resistance in Escherichia coli in the absence of the AcrAB efflux pump. Antimicrob Agents Chemother. 2000;44:10–13. doi: 10.1128/aac.44.1.10-13.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oethinger M, Podglajen I, Kern W V, Levy S B. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob Agents Chemother. 1998;42:2089–2094. doi: 10.1128/aac.42.8.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]