Abstract
Laminaria digitata is a novel feedstuff that can be used in pig diets to replace conventional feedstuffs. However, its resilient cell wall can prevent the monogastric digestive system from accessing intracellular nutrients. Carbohydrate-active enzyme (CAZyme) supplementation is a putative solution for this problem, degrading the cell wall during digestion. The objective of this work was to evaluate the effect of 10% L. digitata feed inclusion and CAZyme supplementation on the meat quality and nutritional value of weaned piglets. Forty weaned piglets were randomly allocated to four experimental groups (n = 10): control, LA (10% L. digitata, replacing the control diet), LAR (LA + CAZyme (0.005% Rovabio® Excel AP)) and LAL (LA + CAZyme (0.01% alginate lyase)) and the trial lasted for two weeks. The diets had no effect on any zootechnical parameters measured (p > 0.05) and meat quality traits, except for the pH measured 24 h post-mortem, which was higher in LAL compared to LA (p = 0.016). Piglets fed with seaweed had a significantly lower n-6/n-3 PUFA ratio compared to control, to which the higher accumulation of C20:5n-3 (p = 0.001) and C18:4n-3 (p < 0.0001) contributed. In addition, meat of seaweed-fed piglets was enriched with bromine (Br, p < 0.001) and iodine (I, p < 0.001) and depicted a higher oxidative stability. This study demonstrates that the nutritional value of piglets’ meat could be improved by the dietary incorporation of L. digitata, regardless of CAZyme supplementation, without negatively affecting growth performance in the post-weaning stage.
Keywords: Laminaria digitata, CAZyme, piglets, meat quality
1. Introduction
The human population is estimated to increase above 9 billion people by 2050 [1]. This is expected to increase the demand for animal products, such as pork, thereby increasing the pressure on natural resources such as water and land. It is therefore essential to increase productivity of production systems while maintaining and/or improving environmental sustainability. Feedstuffs commonly used in pig diets, such as soybean meal, have a major environmental impact in both production and transport [2]. In Europe, this impact is worsened by dependency on imports from countries such as the USA and Brazil [3]. Hence, alternative feeds are necessary to reduce Europe’s dependency on these feedstuffs whilst maintaining environmental and economical sustainability. In recent years, researchers have devoted much attention to alternative feedstuffs whose use also reduces food–feed–fuel competition, such as food industry by-products [4], insects [5], microalgae [6,7], and macroalgae [8,9].
Macroalgae (or seaweeds) are a diverse group of multicellular algae with three main categories: Phaeophyceae (brown algae), Rhodophyceae (red algae), and Chlorophyceae (green algae) [10]. Their nutritional composition is highly variable, depending on factors such as species, production/harvesting location, post-harvesting treatment, etc. Green and red algae can have levels of crude protein comparable to those of soybean meal (42–44% on a dry matter–DM-basis), whereas their crude fat content is generally low, below 7% on a DM basis [11]. They also have several bioactive components: n-3 polyunsaturated fatty acids (n-3 PUFA), including eicosapentaenoic acid (EPA), iodine (I), and polysaccharides such as laminarin [12]. Laminaria digitata is a brown seaweed whose laminarin and fucoidan extracts have been widely reported to improve growth and gut health of weaned piglets, as well as meat quality of finishing pigs [13,14,15,16]. To our knowledge, the use of the whole biomass of this seaweed as a feed ingredient (above 3% dietary incorporation) has not been reported. This is likely due to the high abundance of recalcitrant polysaccharides, indigestible by monogastric endogenous enzymes. To take advantage of these nutritional properties, the feed supplementation with carbohydrate-active enzymes (CAZymes) is a viable approach. CAZymes act upon glyosidic bonds of polysaccharides [17]. The commercially available enzyme mixture Rovabio® Excel AP was designed for use in cereal-based diets to degrade non-starch polysaccharides and has been used in microalgae-containing diets [6,7,18]. Alginate lyase, an alginate-degrading enzyme, has been reported to degrade the cell walls of L. digitata in vitro [19], being a putative candidate for in vivo studies.
Weaning is a critical stage in pig production, where piglets endure social, nutritional, and environmental-related stress [20]. Antibiotics were intensively used in the past to deal with post-weaning stress, until their use for such purpose was banned in the European Union due to public health concerns. The prebiotic properties and components of seaweeds could help mitigate this issue. For instance, authors have reported that laminarin extracted from L. digitata improved the microbiome of weaned piglets, without detrimental effects on growth performance [13], while another study reports growth improvement by feeding piglets with a laminarin extract [14]. Other authors have reported that feeding pigs with laminarin and fucoidan extracts reduced saturated fatty acids and lowered lipid oxidation in meat [15]. This is particularly interesting in the Southern European context, given that there is a tradition of spit-roast piglet consumption in countries such as Portugal (Leitão de Negrais).
Following the discussion above, the objective of this work was to evaluate the effect of 10% dietary L. digitata and CAZyme supplementation on the quality and nutritional value of weaned piglets’ meat.
2. Materials and Methods
2.1. Animals and Experimental Diets
The animal trial was conducted at the Animal Production Section of ISA (University of Lisbon, Portugal). It was approved by ISA’s Ethics Commission and accepted by the National Veterinary Authority (ref. 0421/000/000/2020) following current legislation of the European Union (2010/63/EU Directive). Forty male piglets (Large White × Duroc), 35-day old, with 10.49 ± 0.62 kg (mean ± SD) body weight were bought from a commercial farm, where they had been weaned at 28 days of age. They were housed individually in metabolic crates, equipped with individual heating lamps and nipple drinkers. The piglets were then randomly allocated to each of the four experimental groups (n = 10): control (maize, wheat, and soybean meal-based diet), LA (10% L. digitata, replacing the control diet), LAR (LA + CAZyme-0.005% Rovabio® Excel AP from Adisseo (Antony, France)) and LAL (LA + CAZyme-0.01% pre-selected alginate lyase as described by Costa et al. [19]). Experimental diet composition is presented in Table S1. The seaweed was wild caught, bought from Aleor (Lézardrieux, France), and used as supplied (dried powder, <250 µm). The seaweed had 4.85% and 1.31% (on a dry matter basis) of crude protein and crude fat, respectively. Diets were formulated to be isocaloric and isonitrogenous.
2.2. Diet Composition Analysis
2.2.1. Proximal Analysis
Procedures for proximate analysis of diets were previously described [6]. Briefly, dry matter (DM) was assessed by drying diets at 103 °C overnight. Ash was determined by calcinating the dried sample at 500 °C in a muffle furnace overnight. Crude protein was measured using the Kjeldahl method, using 6.25 as conversion factor. Crude fat was determined by hydrolysis followed by automatic Soxhlet extraction with petroleum ether (Gerhardt Analytical Systems, Königswinter, Germany). Crude energy was analysed by adiabatic calorimeter (Parr 1261; Parr Instrument Company, Moline, IL, USA). The AOAC guidelines were followed [21].
2.2.2. Fatty Acid Composition
Fatty acid composition of diets was assessed by one-step extraction and acid transesterification [22]. Afterwards, fatty acid methyl esters (FAME) derivatives were separated, identified, and quantified through gas chromatography (GC), following previously reported conditions [22]. The identification of fatty acids was done using the reference standard (FAME mixture of 37 compounds, Supelco Inc., Bellefonte, PA, USA). The internal standard was nonadecanoic acid (C19:0) methyl ester. Fatty acids were quantified as percentage of total fatty acids.
2.2.3. Pigment Profiling
Diterpene profile and β-carotene content of diets were evaluated based on direct saponification and single extraction with n-hexane and then analysed by HPLC as carried out by Prates et al. [23]. Pigments in diets were determined according to Teimouri et al. [24]. Approximately 0.5 g of diets were incubated with acetone in the dark at room temperature during 12 h under agitation. After extraction, samples were centrifuged and analysed by UV-VIS spectrophotometry (Ultrospec 3100; Amersham Biosciences, Little Chalfont, UK). The amount of pigments was calculated using the Hynstova et al. [25] equations as follows: clorophyll-a (Ca) = 11.24 A662 − 2.04 A645; clorophyll-b (Cb) = 20.13 A645 − 4.19 A662; total chlorophylls (Ca + Cb) = 7.05 A662 + 18.09 A645; total carotenoids (Cx + c) = (1000 A470 − 1.90 Ca − 63.14 Cb)/214; and total chlorophylls and total carotenoids (Ca + Cb) + (Cx + c).
2.2.4. Mineral Profiling
The mineral profile of experimental diets was analysed as described by Ribeiro et al. [26]. Diets were incubated in a ventilated chamber with concentrated nitric acid plus hydrochloric acid, during 16 h, followed by the addition of hydrogen peroxide and heated using a digestion plate (DigiPREP MS, SCP Science, Baie-D’Urfe, QC, Canada). Then, diets were diluted with distilled water, filtered, and analysed by Inductively Coupled Plasma–Optical Emission Spectrometry (ICP-OES, iCAP 7200 duo Thermo Scientific, Waltham, MA, USA). The analysis of I and bromine (Br) was performed by Inductively Coupled Plasma Mass Spectrometer (ICP-MS) (Thermo X series II, Thermo Fisher Scientific, Waltham, MA, USA), according to Delgado et al. [27]. Briefly, tetramethylammonium hydroxide (TMAH) solution (25%, v/v) and ultra-pure water (Milli-Q Element system, Millipore Corporation, Saint-Quentin, France) were added to samples followed by extraction, in triplicate, using a Heating Graphite Block System (DigiPREP MS, SCP Science, Baie-D’Urfe, QC, Canada) at 90 °C during 3 h.
The detailed chemical composition of the experimental diets is presented in Table 1.
Table 1.
Chemical composition of experimental diets.
| Dietary Treatments | ||||
|---|---|---|---|---|
| Control | LA | LAR | LAL | |
| Proximate composition (% dry matter) | ||||
| Dry matter | 89.4 | 89.6 | 89.7 | 89.5 |
| Crude protein | 18.5 | 17.0 | 17.0 | 17.4 |
| Crude fat | 3.9 | 4.0 | 4.6 | 4.1 |
| Ash | 5.9 | 6.4 | 6.5 | 6.3 |
| Crude energy (cal/g DM) | 4390.23 | 4306.12 | 4287.53 | 4339.46 |
| Fatty acid composition (% total FA) | ||||
| Myristic acid (C14:0) | 0.435 | 0.612 | 0.502 | 0.476 |
| Palmitic acid (C16:0) | 10.6 | 11.3 | 11.0 | 10.9 |
| Palmitoleic acid (C16:1c9) | 0.163 | 0.258 | 0.254 | 0.236 |
| Margaric acid (C17:0) | 0.074 | 0.075 | 0.073 | 0.071 |
| cis-9 Margaric acid (C17:1c9) | 0.037 | 0.044 | 0.044 | 0.040 |
| Stearic acid (C18:0) | 3.32 | 3.23 | 3.26 | 3.31 |
| Oleic acid (C18:1c9) | 25.6 | 25.2 | 25.3 | 25.3 |
| Linoleic acid (C18:2n-6) | 55.8 | 54.1 | 54.6 | 54.8 |
| Linolenic acid C18:3n-3 | 1.33 | 1.45 | 1.44 | 1.48 |
| Stearidonic acid (C18:4n-3) | 0.009 | 0.193 | 0.193 | 0.193 |
| Arachidic acid (C20:0) | 0.311 | 0.328 | 0.331 | 0.334 |
| Arachidonic acid (C20:4n-6) | 0.007 | 0.308 | 0.314 | 0.278 |
| Eicosapentaenoic acid (C20:5n-3) | n.d. | 0.382 | 0.396 | 0.351 |
| Diterpene profile (μg/g DM) | ||||
| α-Tocopherol | 59.5 | 51.8 | 45.7 | 45.4 |
| β-Tocopherol | 0.978 | 0.733 | 0.708 | 0.830 |
| γ-Tocopherol | 2.35 | 1.59 | 1.51 | 1.94 |
| δ-Tocopherol | 0.525 | 0.447 | 0.448 | 0.475 |
| γ-Tocotrienol | 1.62 | 1.41 | 1.31 | 1.50 |
| Pigments 1 (µg/g DM) | ||||
| β-Carotene | 0.418 | 1.46 | 1.44 | 1.31 |
| Chlorophyll-a | 0.324 | 36.2 | 36.8 | 36.0 |
| Chlorophyll-b | 3.791 | 1.21 | 0.727 | 0.714 |
| Total chlorophylls | 4.02 | 35.4 | 36.6 | 34.7 |
| Total carotenoids | 0.461 | 12.5 | 12.0 | 10.5 |
| Total chlorophylls + carotenoids | 4.49 | 48.0 | 48.6 | 45.3 |
| Mineral profile (mg/kg DM) | ||||
| Bromine | 15.1 | 83.1 | 80.8 | 87.7 |
| Calcium | 17,445 | 16,022 | 16,675 | 15,931 |
| Copper | 274 | 244 | 269 | 236 |
| Iodine | 9.56 | 652 | 647 | 713 |
| Iron | 304 | 226 | 253 | 246 |
| Magnesium | 1751 | 2615 | 2605 | 2569 |
| Manganese | 149 | 123 | 123 | 113 |
| Phosphorous | 11,131 | 6381 | 6445 | 6167 |
| Potassium | 12,789 | 15,694 | 15,651 | 15,680 |
| Sodium | 4542 | 6647 | 6462 | 6321 |
| Sulphur | 3094 | 4787 | 4726 | 4550 |
| Zinc | 229 | 254 | 269 | 233 |
Control, LA, LAR, and LAL diets represent corn-soybean meal-based diets containing 0% L. digitata (Control), 10% L. digitata (LA), 10% L. digitata + 0.005% of Rovabio® Excel AP (LAR); and 10% L. digitata + 0.01% of alginate lyase recombinant CAZyme (LAL). DM, dry matter; FA, fatty acids; n.d., not detected. 1 Chlorophyll-a, chlorophyll-b, total chlorophylls, and total carotenoids were calculated as described by Hynstova et al. [25].
2.3. Growth Performance and Slaughter of Piglets
After 5 days of adaptation to diets and environmental conditions, the trial lasted for two weeks, and piglets had free access to water. Feed refusals were recorded daily. Piglets were weighed at the beginning and end of each week to calculate growth performance parameters. At the end of trial, all piglets were slaughtered by electrical stunning and exsanguination, following commercial practices. The longissimus lumborum (LL) muscle was removed from each carcass between the third and fifth lumbar vertebrae. Meanwhile, LL muscle from the right carcass was used for meat quality traits and sensory analyses, and LL muscle from the left carcass side was minced, vacuum packed, and stored at −20 °C for biochemical analysis.
2.4. Measurement of Meat Quality Traits and Sensory Analysis
Meat pH was measured, in triplicate, at different positions, on LL muscle at 24 h post-mortem using a pH meter (Hanna Instruments, Woonsocket, RI, USA) equipped with an insertion glass electrode. At 24 h post-mortem, meat colour parameters lightness (L*), redness (a*), and yellowness (b*) were measured three times on the exposed (after blooming for 60 min at 4 °C) cut surface of the LL muscle by using a CR-300 Minolta colorimeter (Tokyo, Japan). Chroma (C*, colour intensity also known as saturation index) was calculated as (a*2 + b*2)1/2. Hue angle (H*) was calculated as tan−1 (b*/a*) × 57.29, expressed in degrees. To determine shear force and cooking loss, meat samples were thawed at 4 °C for 24 h and cooked in a water bath programmed at 80 °C with a meat internal temperature of 78 °C monitored by a thermocouple (Lufft C120; Lufft, München, Germany). Shear force was determined in meat samples, along the direction of the muscle fibres with a 1 cm2 cross-section, using a Warner-Bratzler blade coupled to a texture analyser (TA-XT Plus texture analyser; Stable Micro Systems, Surrey, UK). Cooking loss was calculated as a percentage of weight before and after cooking.
Twelve panellists, specifically trained in five panel sessions with 8 random meat samples per session, were selected in accordance with Cross et al. [28]. The sensory descriptors were tenderness, juiciness, flavour, off-flavour, and overall acceptability. A graduated scale from 1 to 8 was used to quantify these descriptors, where 1 represents the lowest score and 8 the highest score. For off-flavour, the scale used was from 0 (absence) to 8 (maximum).
2.5. Intramuscular Fat Content and Fatty Acid Composition Determination
Lipid fraction from lyophilized LL muscle samples was extracted by combining the traditional Folch method [29] with that applied by Carlson [30], in which dichloromethane-methanol (2:1, v/v) was used as binary solvent mixture. After solvent evaporation, intramuscular fat was measured gravimetrically by weighing the residue. To profile meat fatty acids, lipid extracts were transmethylated in combined alkaline and acid conditions, according to Raes et al. [31]. FAME were identified and quantified by gas chromatography (HP6890A; Hewlett-Packard, Avondale, PA, USA), as reported by Coelho et al. [18]. As mentioned above for diets, nonadecanoic acid (C19:0) methyl ester was the internal standard and fatty acids are expressed as percentage of total fatty acids.
2.6. Total Cholesterol, Diterpene Profile, and Lipid Oxidation Determination
The quantitative analysis of total cholesterol, β-carotene, and vitamin E homologues (tocopherols and tocotrienols) in LL muscle, in duplicate, was performed as described by Prates et al. [23].
The lipid oxidation status of meat was evaluated in terms of thiobarbituric acid reactive substances (TBARS) at days 0 and 8, maintained at 4 °C, according to Grau et al. [32]. TBARS values, in duplicate, were obtained and expressed as mg of malondialdehyde (MDA) per kg of meat.
2.7. Mineral Profile Determination
The determination of mineral profile in LL muscle was done following the same procedure as diet samples (for further information see details above, Section 2.2.4).
2.8. Statistics
All data were analysed with the GLM procedure of the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA), except TBARS that were analysed with MIXED procedure of SAS. The dietary treatment was considered as the single effect, and the piglet was the experimental unit. Upon detection of significant effects (p < 0.05), least-square means were compared using the PDIFF option, adjusted for the Tukey post hoc test. The statistical models used were Yi = µ + τi + εi, (Proc GLM) and Yi = µ + τi + ω(piglet)i + εi (Proc Mixed). Yi is the response of piglet in treatment i, µ is the global average of the effect, τi is the effect of treatment i, ω(piglet) is the effect of time within piglet and εi is the residual error.
A principal component analysis (PCA) was carried out for the fatty acid profile of LL muscle using the PCA and fviz_pca_biplot functions of the FactoMineR [33] and factoextra packages (respectively) of the R software (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria [34]).
3. Results
3.1. Effect of the Experimental Diets on Zootechnical Parameters
Diets had no statistically significant effect on live weight (p > 0.05). Piglets had, on average, 11.6 kg and 16.8 kg of initial and final weight, respectively. The average daily gain, average daily feed intake, and feed conversion ratio were also unaffected, with average 371 g, 645 g, and 1.8, respectively.
3.2. Effect of the Experimental Diets on Meat Quality Traits and Sensory Panel Analysis
Table 2 summarizes the effect of experimental diets on meat quality traits and sensory panel analysis in weaned piglet meat. The pH 24 h post-mortem was higher in the LAL compared with the LA treatment (p = 0.016). However, experimental diets had no significant effect on colour parameters, cooking loss and shear force (p > 0.05). Regarding the sensory panel analysis, there were no significant differences for tenderness, juiciness, flavour, off-flavour, and overall acceptability (p > 0.05).
Table 2.
pH, colour, cooking loss, shear force and sensory panel analysis in longissimus lumborum of piglets fed the experimental diets.
| Dietary Treatments | ||||||
|---|---|---|---|---|---|---|
| Control | LA | LAR | LAL | SEM | p-Value | |
| pH 24 h | 5.57 ab | 5.53 a | 5.63 ab | 5.66 b | 0.029 | 0.016 |
| Colour | ||||||
| L* | 48.0 | 48.9 | 47.9 | 46.6 | 0.670 | 0.129 |
| a* | 7.43 | 7.32 | 6.99 | 7.22 | 0.279 | 0.721 |
| b* | 0.256 | 0.587 | 0.216 | 0.324 | 0.280 | 0.785 |
| C* | 7.40 | 7.38 | 7.30 | 7.48 | 0.287 | 0.981 |
| H* | 2.05 | 4.24 | 1.26 | 1.55 | 2.229 | 0.777 |
| Cooking loss (%) | 34.7 | 35.0 | 34.1 | 33.2 | 0.589 | 0.166 |
| Shear force (kg) | 4.02 | 3.75 | 4.94 | 5.05 | 0.463 | 0.132 |
| Sensory panel scores | ||||||
| Tenderness | 5.52 | 5.92 | 5.72 | 5.86 | 0.120 | 0.084 |
| Juiciness | 5.61 | 5.89 | 5.76 | 5.70 | 0.109 | 0.324 |
| Flavour | 5.60 | 5.53 | 5.63 | 5.57 | 0.093 | 0.908 |
| Off-flavour | 0.192 | 0.367 | 0.242 | 0.227 | 0.068 | 0.285 |
| Overall acceptability | 5.62 | 5.74 | 5.76 | 5.79 | 0.106 | 0.678 |
Control, LA, LAR, and LAL diets represent corn-soybean meal-based diets containing 0% L. digitata (Control), 10% L. digitata (LA), 10% L. digitata + 0.005% of Rovabio® Excel AP (LAR); and 10% L. digitata + 0.01% of alginate lyase recombinant CAZyme (LAL). SEM, standard error of the mean. a,b Different superscript letters within a row are significantly different (p < 0.05).
3.3. Effect of the Experimental Diets on Intramuscular Fat, Total Cholesterol and Vitamin E Content, and Fatty Acid Composition
The effect of experimental diets on intramuscular fat (IMF), total cholesterol, and fatty acid profile of LL muscle is shown in Table 3. The IMF of LAR piglets tended to be higher than its counterparts (p = 0.063), whereas for total cholesterol and α-tocopherol, no statistically significant effect due to experimental diets (p > 0.05) was recorded. Among the diterpenes, only α-tocopherol, the major homologue of vitamin E, was detected in LL muscle. Furthermore, chlorophylls and carotenoids (including β-carotene) were not detected in LL muscle, even though they were present in the diets in low amounts.
Table 3.
Intramuscular fat, total cholesterol and α-tocopherol contents, and fatty acid (FA) composition (% of total FA) in longissimus lumborum muscle of piglets fed the experimental diets.
| Dietary Treatments | ||||||
|---|---|---|---|---|---|---|
| Control | LA | LAR | LAL | SEM | p-Value | |
| Intramuscular fat (g/100 g of muscle) |
1.43 | 1.60 | 1.88 | 1.58 | 0.117 | 0.063 |
| Total cholesterol (mg/100 g muscle) |
35.6 | 36.8 | 30.8 | 33.3 | 0.038 | 0.621 |
| α-Tocopherol (µg/100 g) |
73.0 | 86.3 | 70.2 | 64.9 | 6.1 | 0.103 |
| Fatty acid profile (% of total FA) |
||||||
| Lauric acid (C12:0) | 0.035 | 0.022 | 0.038 | 0.032 | 0.008 | 0.503 |
| Myristic acid (C14:0) | 0.879 | 0.803 | 0.864 | 0.891 | 0.069 | 0.814 |
| Pentadecanoic acid (C15:0) | 0.160 | 0.180 | 0.181 | 0.193 | 0.016 | 0.546 |
| Palmitic acid (C16:0) | 23.3 | 22.5 | 23.1 | 23.7 | 0.491 | 0.419 |
| Margaric acid (C17:0) | 0.607 | 0.774 | 0.721 | 0.786 | 0.060 | 0.151 |
| Stearic acid (C18:0) | 13.7 | 13.8 | 13.5 | 14.0 | 0.443 | 0.831 |
| Arachidic acid (C20:0) | 0.139 | 0.149 | 0.156 | 0.150 | 0.009 | 0.589 |
| Behenic acid (C22:0) | 0.050 | 0.106 | 0.077 | 0.046 | 0.023 | 0.233 |
| Total SFA | 38.9 | 38.4 | 38.6 | 39.8 | 0.669 | 0.458 |
| Myristoleic acid (C14:1c9) | 0.006 | 0.004 | 0.005 | 0.010 | 0.004 | 0.724 |
| c7-Hexadecenoic acid (C16:1c7) | 0.373 | 0.376 | 0.375 | 0.367 | 0.014 | 0.964 |
| Palmitoleic acid (C16:1c9) | 2.48 | 2.04 | 2.44 | 2.42 | 0.236 | 0.528 |
| cis-9 Margaric acid (C17:1c9) | 0.284 | 0.299 | 0.365 | 0.331 | 0.035 | 0.369 |
| Oleic acid (C18:1c9) | 24.2 | 21.5 | 23.4 | 23.2 | 1.39 | 0.463 |
| Vaccenic acid (C18:1c11) | 3.83 | 3.68 | 3.80 | 3.68 | 0.173 | 0.903 |
| Eicosenoic acid (C20:1n-9) | 0.375 | 0.346 | 0.370 | 0.371 | 0.023 | 0.813 |
| Erucic acid (C22:1n-9) | 0.062 | 0.117 | 0.084 | 0.056 | 0.025 | 0.303 |
| Total cis-MUFA | 31.6 | 28.0 | 30.8 | 30.4 | 1.59 | 0.426 |
| Linoleic acid (C18:2n-6) | 20.6 | 22.1 | 20.7 | 20.5 | 0.976 | 0.600 |
| γ-linolenic acid (C18:3n-6) | 0.060 | 0.060 | 0.06 | 0.054 | 0.004 | 0.601 |
| Linolenic acid (C18:3n-3) | 0.334 | 0.403 | 0.399 | 0.339 | 0.042 | 0.505 |
| Stearidonic acid (C18:4n-3) | 0.028 a | 0.115 b | 0.120 b | 0.096 b | 0.012 | <0.0001 |
| Eicosadienoic acid (C20:2n-6) | 0.576 | 0.611 | 0.578 | 0.538 | 0.030 | 0.422 |
| γ-homolinolenic acid (C20:3n-6) | 0.471 | 0.537 | 0.494 | 0.491 | 0.048 | 0.794 |
| Arachidonic acid (C20:4n-6) | 4.41 | 5.12 | 4.51 | 4.55 | 0.602 | 0.834 |
| Eicosatrienoic acid (C20:3n-3) | 0.067 | 0.099 | 0.093 | 0.066 | 0.011 | 0.068 |
| Eicosapentaenoic acid (C20:5n-3) | 0.070 a | 0.163 b | 0.114 ab | 0.118 ab | 0.014 | 0.001 |
| Docosadienoic acid (C22:2n-6) | 0.036 | 0.012 | 0.014 | 0.012 | 0.007 | 0.068 |
| Docosapentaenoic acid (C22:5n-3) | 0.368 | 0.606 | 0.511 | 0.505 | 0.061 | 0.066 |
| Docosahexaenoic acid (C22:6n-3) | 0.253 | 0.366 | 0.315 | 0.293 | 0.033 | 0.127 |
| Total PUFA | 27.2 | 30.2 | 27.9 | 27.6 | 1.67 | 0.583 |
| Total n-3 PUFA | 1.12 a | 1.75 b | 1.55 b | 1.42 ab | 0.111 | 0.003 |
| Total n-6 PUFA | 26.1 | 28.5 | 26.4 | 26.2 | 1.58 | 0.673 |
| Other | 2.25 | 3.38 | 2.65 | 2.21 | 0.329 | 0.059 |
| Ratios of fatty acids | ||||||
| PUFA/SFA | 0.710 | 0.803 | 0.730 | 0.697 | 0.052 | 0.478 |
| n-6/n-3 | 24.2 a | 16.5 b | 17.4 b | 18.9 b | 1.04 | <0.0001 |
Control, LA, LAR, and LAL diets represent corn-soybean meal-based diets containing 0% L. digitata (Control), 10% L. digitata (LA), 10% L. digitata + 0.005% of Rovabio® Excel AP (LAR); and 10% L. digitata + 0.01% of alginate lyase recombinant CAZyme (LAL). SEM, standard error of the mean; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. a,b Different superscript letters within a row are significantly different (p < 0.05).
A PCA analysis for the lipid profile of the LL muscle is depicted in Figure S1. There is not a clear clustering of experimental groups, which is explained by the low number of differences found for individual FA, as indicated by the biplot vectors. The concentration of stearidonic acid (C18:4n-3) was significantly higher in L. digitata fed groups compared to control (p < 0.0001), and eicosapentaenoic acid (EPA, C20:5n-3) was 43% higher in LA compared to control (p = 0.001). Docosadienoic acid (C22:2n-6) tended to be higher in control (p = 0.068), whereas docosapentaenoic acid (C22:5n-3) and eicosatrienoic acid (C20:3n-3) tended to be higher in seaweed diets (p = 0.066 and p = 0.068, respectively). Experimental diets had no effect on any SFA and monounsaturated fatty acids (MUFA) (p > 0.05).
There was no significant effect detected in the PUFA/SFA ratio (p > 0.05). However, there was a significant effect (p < 0.0001) in the n-6/n-3 ratio, where control had increased by 68%, 72%, and 78% compared to LA, LAR, and LAL, respectively.
3.4. Effect of the Experimental Diets on Meat Oxidative Stability
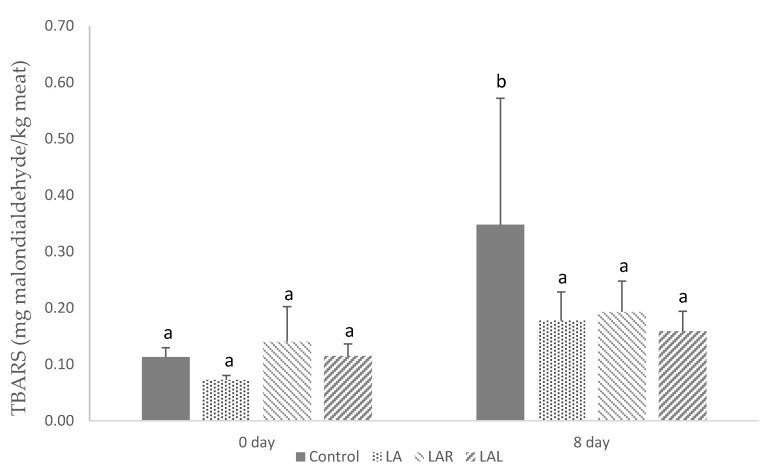
Figure 1 displays the influence of diets on the oxidative stability of piglets’ LL muscle. Data showed no significant effects of lipid oxidation between groups within each time among the experimental diets (p > 0.05). However, there was a significant increase of TBARS concentration in the control group between 0 and 8 days (p < 0.05), which did not occur in the remaining groups.
Figure 1.
TBARS levels (mg malondialdehyde/kg muscle) after 0 and 8 days under refrigeration in longissimus lumborum muscle of piglets fed the experimental diets. Control, LA, LAR, and LAL diets represent corn-soybean meal-based diets containing 0% L. digitata (Control), 10% L. digitata (LA), 10% L. digitata + 0.005% of Rovabio® Excel AP (LAR); and 10% L. digitata + 0.01% of alginate lyase recombinant CAZyme (LAL). a,b Values with different letters are significantly different (p < 0.05).
3.5. Effect of the Experimental Diets on Mineral Profile
The mineral concentration of LL muscle is presented in Table 4. Concerning macrominerals, no significant differences were detected between treatments, albeit having a strong tendency for calcium (Ca, p = 0.05) to be higher in LA compared to the remaining groups. On the other hand, Br (p < 0.001) and I (p < 0.001) were higher in the muscle of piglets fed seaweed diets, which led to a significant increase of total microminerals content when compared with control (p < 0.001). Moreover, the heavy metals arsenic, barium, cadmium, chromium, cobalt, nickel, lead, and vanadium were not detected in the muscle as observed in the experimental diets.
Table 4.
Mineral content in longissimus lumborum muscle of piglets fed the experimental diets.
| Dietary Treatments | ||||||
|---|---|---|---|---|---|---|
| Control | LA | LAR | LAL | SEM | p-Value | |
| Macrominerals (mg/100 g) | ||||||
| Calcium | 22.8 | 25.9 | 22.6 | 21.8 | 1.07 | 0.050 |
| Magnesium | 511 | 508 | 505 | 496 | 7.8 | 0.533 |
| Potassium | 34.4 | 33.5 | 33.9 | 33.5 | 0.54 | 0.595 |
| Phosphorous | 56.6 | 57.1 | 59.8 | 57.8 | 1.31 | 0.367 |
| Sodium | 297 | 293 | 295 | 293 | 8.9 | 0.986 |
| Sulphur | 194 | 191 | 190 | 182 | 3.7 | 0.128 |
| Total | 1116 | 1109 | 1106 | 1083 | 15.9 | 0.503 |
| Microminerals (mg/100 g) | ||||||
| Bromine | 0.108 b | 0.430 a | 0.473 a | 0.491 a | 0.0163 | <0.001 |
| Copper | 0.14 | 0.12 | 0.13 | 0.14 | 0.006 | 0.281 |
| Iodine | 0.002 b | 0.183 a | 0.178 a | 0.194 a | 0.0112 | <0.001 |
| Iron | 1.00 | 1.06 | 0.98 | 0.96 | 0.052 | 0.593 |
| Manganese | 0.045 | 0.039 | 0.045 | 0.043 | 0.0023 | 0.218 |
| Zinc | 1.24 | 1.42 | 1.41 | 1.28 | 0.066 | 0.131 |
| Total | 2.52 b | 3.26 a | 3.22 a | 3.11 a | 0.079 | <0.001 |
| Total macro- and microminerals | 1118 | 1112 | 1109 | 1086 | 15.9 | 0.513 |
Control, LA, LAR, and LAL diets represent corn-soybean meal-based diets containing 0% L. digitata (Control), 10% L. digitata (LA), 10% L. digitata + 0.005% of Rovabio® Excel AP (LAR); and 10% L. digitata + 0.01% of alginate lyase recombinant CAZyme (LAL). SEM, standard error of the mean. a,b Different superscript letters within a row are significantly different (p < 0.05).
4. Discussion
This study is, to our knowledge, the first to report inclusion levels of L. digitata reaching 10%, as well as the effects of CAZyme supplementation on growth performance and meat quality traits of weaned piglets. We found that diets with the inclusion of the seaweed used in this study had no effect on piglet growth or feed intake. This is coherent with previous studies using lower inclusion levels of seaweeds. Brugger et al. [8] have reported that diets containing up to 5% whole Laminaria japonica have no detrimental effect on piglet growth but did improve feed conversion ratio compared to the control diet. Other authors have reported similar results obtained by supplementing piglet diets with fucoidan (250 ppm) extracted from Ascophyllum nodosum. Indeed, the authors found no effect on piglet growth, but an improvement of feed conversion ratio in supplemented piglets compared to the control group [13]. The reason why we did not find such an effect in the present study could be related to the different seaweed species. Nevertheless, our study demonstrates that there is no detrimental effect in growth performance by feeding piglets with up to 10% whole biomass L. digitata, regardless of enzymatic supplementation.
Regarding meat quality traits, there were no differences in traits including meat colour, cooking loss, or any score from the sensory panel evaluation. However, there was a significant increase in 24 h post-mortem pH of LAL meat compared to LA. Meat pH is a determinant factor in the development of pork quality attributes [35], such as tenderness, juiciness, and flavour [36]. The pH of meat is lowered post-mortem, during the transition muscle to meat where, under anaerobic conditions, glycogen metabolization and ATP hydrolysis accumulate lactate and H+, respectively [37]. The normal range of ultimate pH is between 5.5 and 5.7, where desirable meat traits are developed. The values reported in this study are within this range, pointing towards absence of undesirable meat development such as pale-soft and exudative meat (PSE, pH < 5.4). Authors have reported no effects of feeding pigs with Macrocystis pyrifera (up to 4%), a brown seaweed, on meat pH of finishing pigs [38]. Another study has also reported no effect of feeding pigs with a L. digitata polysaccharide extract on pork patties pH [39]. Therefore, the reason why we found a higher pH in LAL compared to LA could be the higher dietary inclusion of L. digitata and enzyme supplementation. This influenced the muscle glycogen content and, ultimately, the rate of pH decline, without detrimental effects on meat sensory properties.
The fatty acid profile has been significantly changed in the meat of seaweed-fed piglets compared to control. Regarding individual fatty acids, control piglets had significantly lower levels of C18:4n-3 compared to the remaining groups, whereas LA accumulated significantly more C20:5n-3 when compared to controls. C18:4n-3 was 21 times more concentrated in L. digitata diets whereas C20:5n-3 was not detected in the control diet, which explains these differences as being due to their dietary availability. In fact, seaweeds have been reported as having low amounts of fat compared to other feedstuffs, but the fatty acid profile is generally rich in n-3 PUFA [11,12], which have a beneficial effect on health. Enrichment of pig meat with n-3 PUFA through dietary manipulation has been achieved with other nutrient sources, including microalgae [6] or linseed [40]. Regarding seaweeds, there are few studies that report the fatty acid profile of meat from animals fed with these novel feedstuffs. Moroney et al. [15] have reported that dietary laminarin extracted from L. digitata reduced SFA in the longissimus dorsi (LD) muscle of pigs, without an effect in n-3 PUFA. This suggests that the enrichment reported in the present study is independent of the seaweed’s bioactive polysaccharides. Indeed, the higher dietary availability of n-3 PUFA in L. digitata diets ultimately contributed to a significantly lower n-6/n-3 ratio, which favours the nutritional value of the meat. Researchers have advised that this ratio should be kept to a maximum of 4 in human diets [41], in order to reduce the incidence of cardiovascular diseases (CVD). Importantly, the long-chain n-3 PUFA, EPA, contributed to these results. This FA has been reported as a contributor for reduced incidence of cancer, obesity, and diabetes, in addition to CVD [42]. Finally, this was achieved without compromising the oxidative stability of meat, which has been reported in n-3 PUFA enriched pork [6], due to the propensity of these long chain PUFA to be oxidised.
We found that control piglets had significantly higher TBARS in meat after 8 days of refrigeration, compared to the remaining groups. This has happened despite an increase of n-3 PUFA accumulation in seaweed-fed piglets. It could be explained by an increased accumulation of pigments with antioxidant activity, but it was not the case because these were not detected in the meat (data not shown). Therefore, this reduced oxidation in L. digitata treatments could occur due to the prebiotic activity of antioxidative polysaccharides such as laminarin and fucoidan. Authors have found that feeding pigs with L. digitata extracts containing them reduces meat oxidation [15]. To our knowledge, the precise action of this mechanism remains to be elucidated [12].
Finally, seaweed diets promoted an accumulation of Br and I in the LL of piglets leading to an increase of total sum of microminerals in relation to control. These results are explained by the high amount of both minerals in L. digitata, which is within the range of values already reported [11], and thus in the respective experimental diets. Accordingly, previous studies described a significant accumulation of I in the muscle of pigs fed brown seaweeds. For instance, feeding piglets with 2% Ascophylum nodosum led to an increase of 36% of I concentration in the LD muscle in comparison with the control [43]. In addition, supplementing pig diets with up to 0.186% L. digitata was shown to enrich gluteus maximus muscle in I by 45% when compared with the control [44]. The organic I from L. digitata is readily metabolised and deposited in piglet muscle [45,46]. To our knowledge, there is no available data concerning the accumulation of Br in the meat of seaweed-fed pigs. The Br:I ratio reached 2.66 in LAR. This ratio should be kept low to prevent goitrogenic effects derived from excess bromine [47]. However, it is known that Br is also an essential nutrient due to its requirements for collagen IV formation [48]. Thus, we demonstrated that feeding piglets with L. digitata provides an important source of microminerals, which are of paramount importance to maintain physiological functions at the critical post-weaning stage [49].
5. Conclusions
The dietary incorporation of L. digitata in piglet diets had no detrimental effect on either growth performance or meat quality. CAZyme supplementation was not necessary to improve several meat nutritional variables, including fatty acid composition, mineral profile, and oxidative stability. This contributes to the production of healthier meat without the need for feed supplementation, promoting the intake of n-3 PUFA, particularly EPA, without recurring to unsustainable sources such as fish oil. Ultimately, this study supports the feasibility of reducing the incorporation of conventional feedstuffs in piglet diets using seaweeds. However, further research is necessary, namely, to evaluate the digestibility of L. digitata at these incorporation levels and its impact in piglet metabolism.
Acknowledgments
The authors acknowledge Teresa Costa from Indukern, Lda. (Sintra, Portugal), for offering the Rovabio® Excel AP. The graphical abstract was created using BioRender.com (https://biorender.com/, accessed on 2 February 2022).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods11071024/s1, Table S1: Dietary composition of control (maize, wheat and soybean meal-based diet), LA (10% Laminaria digitata, replacing the control diet), LAR (LA + CAZyme − 0.005% Rovabio® Excel AP from Adisseo (Antony, France)) and LAL (LA + CAZyme − 0.01% pre-selected alginate lyase). Figure S1: PCA and biplot obtained for the fatty acid profile of longissimus lumborum muscle piglets fed with control (ctrl–maize, wheat and soybean meal-based diet), LA (10% Laminaria digitata), LAR (10% L. digitata + 0.005% of Rovabio® Excel AP) and LAL (10% L. digitata + 0.01% of alginate lyase recombinant CAZyme).
Author Contributions
Conceptualization, J.P.B.F., A.M.A. and J.A.M.P.; methodology, D.M.R., C.M.A., J.M.P., D.F.P.C., M.C., C.F.M., J.P.C.L., M.M., S.G., I.D., P.C., D.C. and I.C.; writing—draft preparation, D.M.R., C.M.A., J.M.P. and M.C.; writing—review and editing, J.P.B.F., A.M.A. and J.A.M.P.; project administration, J.A.M.P.; funding acquisition, J.A.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia, Portugal, through PTDC/CAL-ZOO/30238/2017 grant, associated with a post-doc contract to M.C., PhD fellowships to D.M.R. (SFRH/BD/143992/2019) and D.C. (SFRH/BD/126198/2016), and CIISA (UIDB/00276/2020) and LEAF (UIDB/04129/2020) grants.
Institutional Review Board Statement
ARRIVE Guidelines for in vivo experiments have been followed.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO Global agriculture towards 2050; Proceedings of the High-Level Expert Forum on How to Feed the World in 2050; Rome, Italy. 12–13 October 2009. [Google Scholar]
- 2.Villavicencio-Gutiérrez M.R., Rogers-Montoya N.A., Martínez-Campos R., Gómez-Tenorio G., Martínez-Castañeda F.E. The environmental performance of different pork production scenarios: A life cycle assessment study. Trop. Anim. Health Prod. 2022;54:44. doi: 10.1007/s11250-022-03045-6. [DOI] [PubMed] [Google Scholar]
- 3.De Visser C.L.M., Schreuder R., Stoddard F. The EU’s dependency on soya bean import for the animal feed industry and potential for EU produced alternatives. OCL. 2014;21:D407. doi: 10.1051/ocl/2014021. [DOI] [Google Scholar]
- 4.Correia C.S., Alfaia C.M., Madeira M.S., Lopes P.A., Matos T.J.S., Cunha L.F., Prates J.A.M., Freire J.P.B. Dietary inclusion of tomato pomace improves meat oxidative stability of young pigs. J. Anim. Physiol. Anim. Nutr. 2017;101:1215–1226. doi: 10.1111/jpn.12642. [DOI] [PubMed] [Google Scholar]
- 5.Altmann B.A., Neumann C., Rothstein S., Liebert F., Mörlein D. Do dietary soy alternatives lead to pork quality improvements or drawbacks? A look into micro-alga and insect protein in swine diets. Meat Sci. 2019;153:26–34. doi: 10.1016/j.meatsci.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Martins C.F., Pestana J.M., Alfaia C.M., Costa M., Ribeiro D.M., Coelho D., Lopes P.A., Almeida A.M., Freire P.B., Prates J.A.M. Effects of Chlorella vulgaris as a Feed Ingredient on the Quality and Nutritional Value of Weaned Piglets’ Meat. Foods. 2021;10:1155. doi: 10.3390/foods10061155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins C.F., Pestana Assunção J., Ribeiro Santos D.M., Madeira M.S.M.S., Alfaia C.M.R.P.M., Lopes P.A.A.B., Coelho D.F.M., Cardoso Lemos J.P., Almeida A.M., Mestre Prates J.A., et al. Effect of dietary inclusion of Spirulina on production performance, nutrient digestibility and meat quality traits in post-weaning piglets. J. Anim. Physiol. Anim. Nutr. 2021;105:247–259. doi: 10.1111/jpn.13470. [DOI] [PubMed] [Google Scholar]
- 8.Brugger D., Bolduan C., Becker C., Buffler M., Zhao J., Windisch W.M. Effects of whole plant brown algae (Laminaria japonica) on zootechnical performance, apparent total tract digestibility, faecal characteristics and blood plasma urea in weaned piglets. Arch. Anim. Nutr. 2020;74:19–38. doi: 10.1080/1745039X.2019.1672479. [DOI] [PubMed] [Google Scholar]
- 9.Samarasinghe M.B., van der Heide M.E., Weisbjerg M.R., Sehested J., Sloth J.J., Bruhn A., Vestergaard M., Nørgaard J.V., Hernández-Castellano L.E. A descriptive chemical analysis of seaweeds, Ulva sp., Saccharina latissima and Ascophyllum nodosum harvested from Danish and Icelandic waters. Anim. Feed Sci. Technol. 2021;278:115005. doi: 10.1016/j.anifeedsci.2021.115005. [DOI] [Google Scholar]
- 10.Morais T., Inácio A., Coutinho T., Ministro M., Cotas J., Pereira L., Bahcevandziev K. Seaweed potential in the animal feed: A review. J. Mar. Sci. Eng. 2020;8:559. doi: 10.3390/jmse8080559. [DOI] [Google Scholar]
- 11.Costa M., Cardoso C., Afonso C., Bandarra N.M., Prates J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021;105:1075–1102. doi: 10.1111/jpn.13509. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro D.M., Martins C.F., Costa M., Coelho D., Pestana J., Alfaia C., Lordelo M., de Almeida A.M., Freire J.P.B., Prates J.A.M. Quality Traits and Nutritional Value of Pork and Poultry Meat from Animals Fed with Seaweeds. Foods. 2021;10:2961. doi: 10.3390/foods10122961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigors S., O’Doherty J., Rattigan R., Sweeney T. Effect of Supplementing Seaweed Extracts to Pigs until d35 Post-Weaning on Performance and Aspects of Intestinal Health. Mar. Drugs. 2021;19:183. doi: 10.3390/md19040183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigors S., O’Doherty J.V., Rattigan R., McDonnell M.J., Rajauria G., Sweeney T. Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig. Mar. Drugs. 2020;18:157. doi: 10.3390/md18030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moroney N.C., O’Grady M.N., Robertson R.C., Stanton C., O’Doherty J.V., Kerry J.P. Influence of level and duration of feeding polysaccharide (laminarin and fucoidan) extracts from brown seaweed (Laminaria digitata) on quality indices of fresh pork. Meat Sci. 2015;99:132–141. doi: 10.1016/j.meatsci.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Moroney N.C., O’Grady M.N., Lordan S., Stanton C., Kerry J.P. Seaweed polysaccharides (laminarin and fucoidan) as functional ingredients in pork meat: An evaluation of anti-oxidative potential, thermal stability and bioaccessibility. Mar. Drugs. 2015;13:2447–2464. doi: 10.3390/md13042447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso V., Ribeiro T., Fernandes V., Guerreiro C., Centeno M., Pires V., Ponte P., Goyal A., Najmudin S., Alves V.D., et al. Exogenous Enzymes Improve the nutritive value of cereal-based diets for monogastric animals through different mechanisms. In: Duarte A.F., editor. Advances in Animal Health, Medicine and Production. Springer Nature Switzerland AG; Cham, Switzerland: 2020. [Google Scholar]
- 18.Coelho D., Pestana J., Almeida J.M., Alfaia C.M., Fontes C.M.G.A., Moreira O., Prates J.A.M. A high dietary incorporation level of Chlorella vulgaris improves the nutritional value of pork fat without impairing the performance of finishing pigs. Animals. 2020;10:2384. doi: 10.3390/ani10122384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa M., Pio L., Bule P., Cardoso V., Alfaia C.M., Coelho D., Brás J., Fontes C.M.G.A., Prates J.A.M. An individual alginate lyase is effective in the disruption of Laminaria digitata recalcitrant cell wall. Sci. Rep. 2021;11:9706. doi: 10.1038/s41598-021-89278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo J.M., Opapeju F.O., Pluske J.R., Kim J.C., Hampson D.J., Nyachoti C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 21.Association of Official Analytical Chemists International . Official Methods of Analysis. 17th ed. AOAC International; Arlington, VA, USA: 2000. [Google Scholar]
- 22.Sukhija P.S., Palmquist D.L. Rapid method for determination of total fatty acid content and composition of feedstufs and feces. J. Agric. Food Chem. 1988;36:1202–1206. doi: 10.1021/jf00084a019. [DOI] [Google Scholar]
- 23.Prates J., Quaresma M.A.G., Bessa R.J.B., Fontes C.M.A., Alfaia C.M.M. Simultaneous HPLC quantification of total cholesterol, tocopherols and carotene in Barrosã-PDO veal. Food Chem. 2006;94:469–477. doi: 10.1016/j.foodchem.2005.01.021. [DOI] [Google Scholar]
- 24.Teimouri M., Amirkolaie A.K., Yeganeh S. The effects of Spirulina platensis meal as a feed supplement on growth perfor-mance and pigmentation of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2013;396:14–19. doi: 10.1016/j.aquaculture.2013.02.009. [DOI] [Google Scholar]
- 25.Hynstova V., Sterbova D., Klejdus B., Hedbavny J., Huska D., Adamab V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018;148:108–118. doi: 10.1016/j.jpba.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro D.M., Scanlon T., Kilminster T., Martins C.F., Greeff J., Oldham C., Freire J.P.B., Mourato M.P., Almeida A.M. Mineral profiling of muscle and hepatic tissues of Australian Merino, Damara and Dorper lambs: Effect of weight loss. J. Anim. Physiol. Anim. Nutr. 2020;104:823–830. doi: 10.1111/jpn.13339. [DOI] [PubMed] [Google Scholar]
- 27.Delgado I., Ventura M., Gueifão S., Coelho I., Nascimento A.C., Silva J.A.L., Castanheira I. 12th IFDC 2017 special issue—Iodine, selenium and iron contents in Portuguese key foods as consumed. J. Food Comp. Anal. 2019;79:39–46. doi: 10.1016/j.jfca.2019.03.004. [DOI] [Google Scholar]
- 28.Cross H.R., Moen R., Stanfield M.S. Training and testing of judges for sensory analysis of meat quality. Food Technol. 1979;32:48–54. [Google Scholar]
- 29.Folch J., Lees M., Stanley G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 30.Carlson L.A. Extraction of lipids from human whole serum and lipoproteins and from rat liver tissue with methylene chloridemethanol: A comparison with extraction with chloroform-methanol. Clin. Chim. Acta. 1985;149:89–93. doi: 10.1016/0009-8981(85)90277-3. [DOI] [PubMed] [Google Scholar]
- 31.Raes K., De Smet S., Demeyer D. Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids. Anim. Sci. 2001;73:253–260. doi: 10.1017/S1357729800058227. [DOI] [Google Scholar]
- 32.Grau A., Guardiola F., Boatella J., Barroeta A.C., Codony R. Measurement of 2-thiobarbituric acid values in dark chicken meat through derivative spectrophotometry: Influence of various parameters. J. Agric. Food Chem. 2000;48:1155–1159. doi: 10.1021/jf990518q. [DOI] [PubMed] [Google Scholar]
- 33.Lê S., Josse J., Husson F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008;25:253–258. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 34.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2019. [(accessed on 24 February 2020)]. Available online: https://www.R-project.org/ [Google Scholar]
- 35.Alfaia C.M., Lopes P.A., Madeira M.S., Pestana J.M., Coelho D., Toldrá F., Prates J.A.M. Advances in Food and Nutrition Research. Volume 89. Elsevier; Amsterdam, The Netherlands: 2019. Current feeding strategies to improve pork intramuscular fat content and its nutritional quality; pp. 53–94. [DOI] [PubMed] [Google Scholar]
- 36.Ngapo T.M., Gariépy C. Factors affecting the eating quality of pork. Crit. Rev. Food Sci. Nutr. 2008;48:599–633. doi: 10.1080/10408390701558126. [DOI] [PubMed] [Google Scholar]
- 37.Matarneh S.K., England E.M., Scheffler T.L., Gerrard D.E. Lawrie’s Meat Science. 8th ed. Woodhead Publishing; Sawston, UK: 2017. The Conversion of Muscle to Meat; pp. 159–185. [Google Scholar]
- 38.Jerez-Timaure N., Sánchez-Hidalgo M., Pulido R., Mendoza J. Effect of Dietary Brown Seaweed (Macrocystis pyrifera) Additive on Meat Quality and Nutrient Composition of Fattening Pigs. Foods. 2021;10:1720. doi: 10.3390/foods10081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moroney N.C., O’Grady M.N., O’Doherty J.V., Kerry J.P. Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Sci. 2013;94:304–311. doi: 10.1016/j.meatsci.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 40.De Tonnac A., Guillevic M., Mourot J., Mag E.L. Fatty acid composition of several muscles and adipose tissues of pigs fed n-3 PUFA rich diets. Meat Sci. 2018;140:1–8. doi: 10.1016/j.meatsci.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Simopoulos A.P. Omega-6/Omega-3 Essential Fatty Acid Ratio and Chronic Diseases. Food Rev. Int. 2004;20:77–90. doi: 10.1081/FRI-120028831. [DOI] [Google Scholar]
- 42.Prates J.A.M., Bessa R.J.B. Trans and n-3 fatty acids. In: Nollet L.M.L., Toldrá F., editors. Handbook of Muscle Foods Analysis. CRC Press; Boca Raton, FL, USA: 2008. [Google Scholar]
- 43.Dierick N., Ovyn A., De Smet S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 2009;89:584–594. doi: 10.1002/jsfa.3480. [DOI] [Google Scholar]
- 44.He M.L., Hollwich W., Rambeck W.A. Supplementation of algae to the diet of pigs: A new possibility to improve the iodine content in the meat. J. Anim. Physiol. Anim. Nutr. 2002;86:97–104. doi: 10.1046/j.1439-0396.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- 45.Makkar H.P.S., Tran G., Heuzé V., Giger-Reverdin S., Lessire M., Lebas F., Ankers P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016;212:1–17. doi: 10.1016/j.anifeedsci.2015.09.018. [DOI] [Google Scholar]
- 46.Banoch T., Fajt Z., Drabek J., Svoboda M. Iodine and its importance in human and pigs. Veterinarstvi. 2010;60:690–694. [Google Scholar]
- 47.Sobolev N., Aksenov A., Sorokina T., Chashchin V., Ellingsen D.G., Nieboer E., Varakina Y., Plakhina E., Onuchina A., Thomassen M.S., et al. Iodine and bromine in fish consumed by indigenous peoples of the Russian Arctic. Sci. Rep. 2020;10:5451. doi: 10.1038/s41598-020-62242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCall A.S., Cummings C.F., Bhave G., Vanacore R., Page-McCaw A., Hudson B.G. Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture. Cell. 2014;157:1380–1392. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro D.M., Mourato M.P., Almeida A.M. Assessing mineral status in edible tissues of domestic and game animals: A review with a special emphasis in tropical regions. Trop. Anim. Health Prod. 2019;51:1019–1032. doi: 10.1007/s11250-019-01848-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.