Abstract
Background:
In older age, the benefits of antihypertensive treatment (AHT) become less evident, with greater associated risk. Of particular concern is compromising cerebral blood flow (CBF), especially in those with cognitive impairment.
Methods:
We created a synthesis of the published evidence by searching multiple electronic databases from 1970 to May 2021. Included studies had participants with mean age ≥50 years, hypertension or cognitive impairment, and assessed CBF before and after initiating AHT. Two authors independently determined eligibility and extracted data. Study quality was assessed using The Risk of Bias in Nonrandomized Studies of Interventions tool. We summarized study characteristics (qualitative synthesis) and performed random-effects meta-analyses (quantitative synthesis).
Results:
Thirty-two studies (total n=1306) were included, of which 23 were eligible for meta-analysis. In line with the qualitative synthesis, the meta-analysis indicated no effect of AHT initiation on CBF (standardized mean difference, 0.08 [95% CI, −0.07 to 0.22]; P=0.31, I2=42%). This was consistent across subgroups of acute versus chronic AHT, drug class, study design, and CBF measurement. Subgroups by age demonstrated an increase in CBF after AHT in those aged >70 years (standardized mean difference, 4.15 [95% CI, 0.16–8.15]; P=0.04, I2=42%), but not in those aged 50 to 65 and 65 to 70 years (standardized mean difference, 0.18 [95% CI,−2.02 to 2.38]; P=0.87, I2=49%; standardized mean difference, 1.22 [95% CI, −0.45 to 2.88]; P=0.15, I2=68%). Overall, risk of bias was moderate-to-high and quality of evidence (Grading of Recommendations Assessment, Development and Evaluation) was very low, reflecting the observational nature of the data.
Conclusions:
Accepting the observed limitations, current evidence does not suggest a harmful effect of AHT on CBF. Concerns over CBF should not preclude treatment of hypertension.
Keywords: cerebrovascular circulation, cerebral blood flow, dementia, emission-computed, hypertension, tomography, vascular diseases
Novelty and Relevance.
What Is New?
Initiation of antihypertensive treatment in older adults does not result in changes in cerebral blood flow.
However, quality of the current evidence is low and there is lack of high-quality studies.
What Is Relevant?
In older adults, there is an ongoing debate on the risks versus benefits of antihypertensive treatment.
This work indicates that concerns regarding compromised cerebral blood flow might be unfounded.
Clinical/Pathophysiological Implications?
Hypertension management in older adults should consider both the expected benefits and risks of treatment, taking into account that a reduction in CBF following treatment is not expected
The prevalence of hypertension increases with age to about 66% of older adults (aged ≥65 years).1 With increasing age, the treatment goals, specifically the blood pressure (BP) targets, for hypertension become less evident, leading to uncertainty around treatment decisions.1,2 Some of this uncertainty relates to the theoretical risks associated with a too rapid or extreme reduction in BP from antihypertensive treatment (AHT). Studies in older adults have shown links between low BP or use of AHT and adverse health outcomes, including increased mortality3 and various markers of cognitive impairment or dementia.4–6 A frequently suggested underlying mechanism for such associations is a reduction in cerebral blood flow (CBF), caused by the reduction in systemic BP.
In health, a stable level of CBF is ensured by cerebral autoregulation, a regulatory mechanism that counteracts fluctuations in systemic BP by adjusting the resistance of cerebral arteries and arterioles.7 The arteriosclerosis associated with increasing age and prolonged hypertension could lead to dysfunctional cerebral autoregulation mechanisms.8 In that case, a reduction in BP may no longer be fully compensated for, potentially resulting in cerebral hypoperfusion.7–9
Against the theoretical risk of reduced CBF are the proven benefits of BP reduction in terms of stroke, cardiovascular disease, and heart failure,10 even in older adults.11 Hypertension is associated with structural and functional changes in the cerebral circulation, some of which may reversible.12,13 Thus, long-term BP reduction could improve, rather than worsen, CBF and its regulation. Indeed, in older adults, lowering BP could prevent or slow down cognitive decline while reversing the reduction in CBF.12 As cognitive decline is associated with decreased CBF in older adults,14 preventing this reduction in CBF by treating hypertension may preserve cognitive function.
The apparently conflicting evidence around AHT and CBF may represent differences in study methods or biases in the study design. Many studies of CBF in older adults have small sample sizes and thus, modest but important associations may be missed. In this situation a systematic review of the published literature, with critical appraisal of studies and quantitative meta-analysis can help make sense of the available data. Several factors contribute to the complexity of the effects of AHT on CBF. Any study of AHT and CBF should include assessment of the techniques used to quantify CBF, should account for varying CBF baseline levels with age15 and describe potential differential class effects of AHT on CBF.16
Therefore, the aim of this systematic review and meta-analysis was to evaluate published evidence on the effects of AHT on CBF in hypertensive older adults and older adults with mild cognitive impairment (MCI) or dementia.
Methods
The review protocol was preregistered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020193911). The meta-analysis was not preregistered but added later after the systematic search yielded sufficient studies to be included in a quantitative analysis. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline.17 All aspects of title searching data extraction and risk of bias assessment were performed by 2 reviewers working independently (A.E. van Rijssel and B.C. Stins) with access to a third reviewer (R.A.A. de Heus and M.L. Sanders) to make final decisions in case of disagreement. The authors declare that all supporting data are available within the article and its online supplementary files.
Eligibility Criteria
Studies included were interventional studies in which the effect of AHT on CBF was evaluated by measuring CBF preinitiation and postinitiation of AHT, including: randomized controlled trials, nonrandomized controlled trials, and uncontrolled prestudies and poststudies. Populations of interest were older patients, (mean age ≥50 years), with hypertension (as defined by authors in the primary papers), dementia, or MCI as primary disease of interest. The following methods of measuring CBF were included: single photon emission computed tomography, xenon-enhanced computed tomography, dynamic perfusion computed tomography, magnetic resonance imaging dynamic susceptibility contrast, arterial spin labeling, transcranial Doppler.18 Articles with no full text available were excluded.
Sources and Search Strategy
A comprehensive search of PubMed (Medline), Embase (OVID), the Cochrane Library (CENTRAL), and Web of Science (Thomson Reuters) was performed. The articles were limited to humans and English language. The full search strategy is detailed in the data supplement.
Study Selection and Data Extraction
Study selection was performed using the Rayyan QCRI webtool.19 Data on systolic BP (SBP) and CBF before and after AHT were extracted. When SBP was not reported we used mean arterial pressure. Our focus was global CBF (primary outcome), but CBF in cerebral regions was also extracted. If the study did not report global CBF, the average of the combined regions was calculated as a proxy for global CBF, and the SD’s were pooled to give a summary estimate. When CBF values were only presented in a figure, the values were read from the figure by hand. Where necessary, we contacted authors of relevant articles to request additional data. All data are presented as value±SD, unless noted otherwise. SEM were converted to SD. We included CBF measurement using any metric but anticipated that the majority of data would be in the form CBF in mL/100 g/min, cerebral blood velocity in cm/s and Z scores. To ensure consistency of direction of effect, we multiplied data by minus one where necessary. For repeated measurements in an individual, a change in cerebral blood velocity measured with transcranial Doppler (eg, a 10% reduction) reflects a change in CBF of the same magnitude, under the assumption that the vessel diameter remained constant.20
Quality Assessment
Risk of bias for each study was assessed using the Risk of Bias in Nonrandomized Studies of Interventions tool.21,22 For cross-over designs, a supplement was used to address some additional issues.23 Discrepancies were resolved by an additional reviewer (R.A.A. de Heus or M.L. Sanders). In keeping with best practice guidance, any studies from our group were independently assessed by experienced reviewers who were not authors on the articles being assessed. The strength of evidence in this review was assessed using the Grading of Recommendations Assessment, Development and Evaluation criteria.24
Statistical Analysis
Revman Version 5.4 was used to conduct the quantitative meta-analysis. The inverse variance method25 for continuous outcomes with a random effects model was used for all analyses. The primary analysis included all studies examining the chronic effects of AHT, using a standardized mean difference (MD) due to different outcome measures. We conducted the following sensitivity analyses: studies reporting (1) CBF in mL/100 g/min (to allow an analysis using MD, rather than a standardized effect), (2) acute (<24 hours) or chronic treatment (>2 weeks; standardized mean difference), (3) studies investigating chronic effects only: antihypertensive drug class, age, method of measuring CBF and study design (standardized mean difference). Forest plots with pooled estimates were created with 95% CI. The heterogeneity between studies was explored with the I2 statistic25 and publication bias using funnel plots.
Results
Study Selection
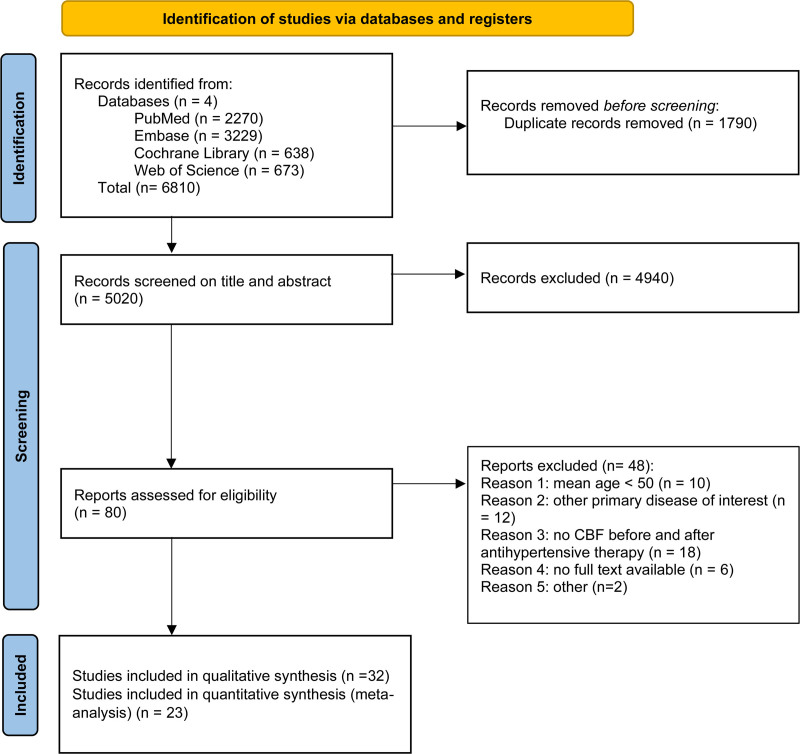
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram. After duplicate removal, 5020 records were screened for eligibility, of which 79 were assessed as full text. Thirty-two studies were included in the qualitative synthesis and 23 in the quantitative synthesis. Reasons for exclusion from this review after full-text review are presented in Table S1.
Figure 1.
Study flow diagram.
N indicates number.
Study Characteristics
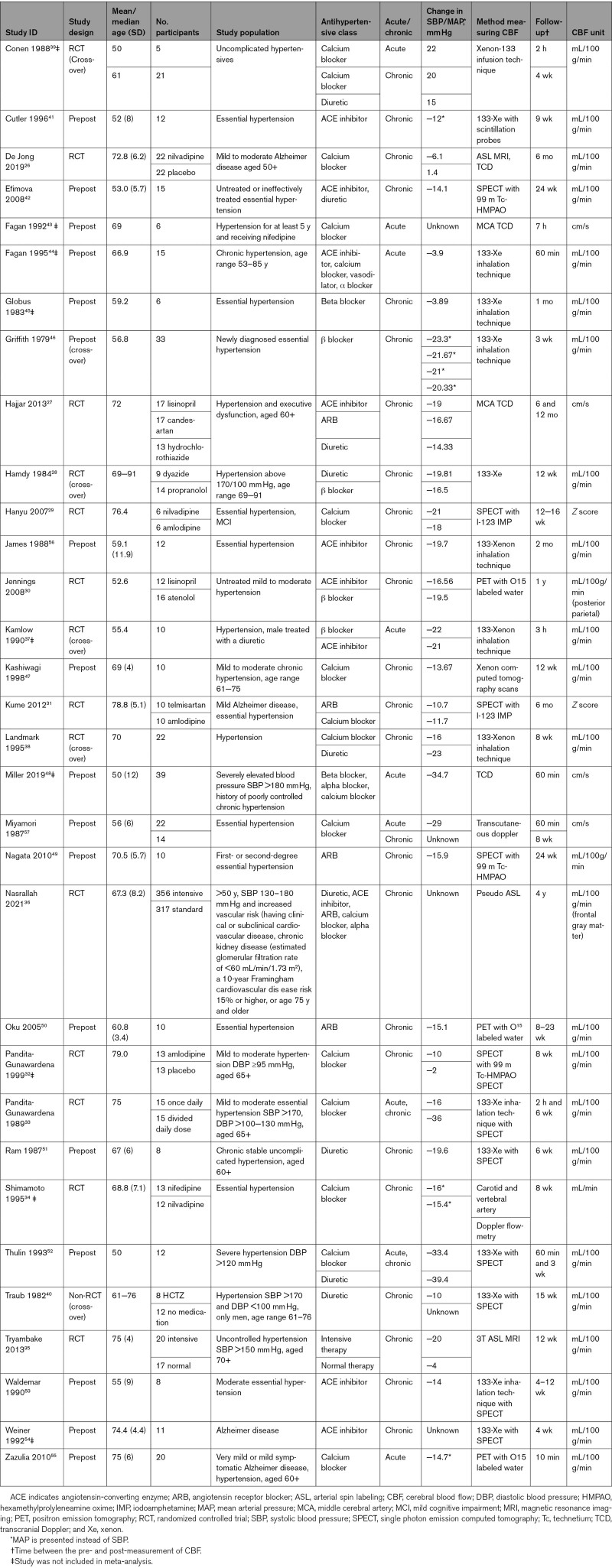
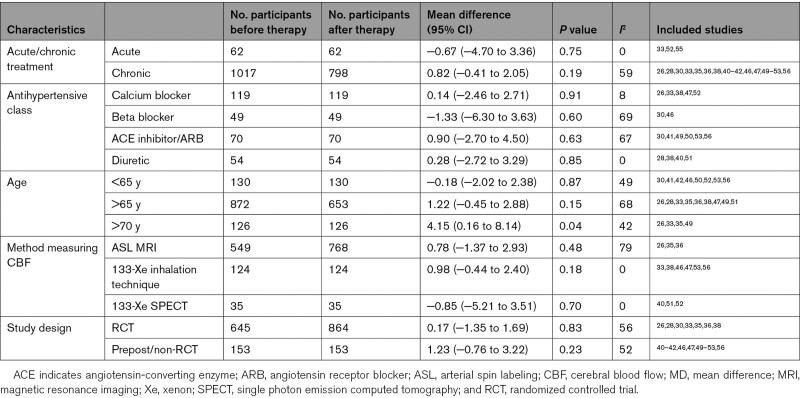
The study characteristics of the 32 included studies are presented in Table 1. Fourteen studies were randomized controlled trial’s,26–39 one was a nonrandomized trial (comparing hydrochlorothiazide with no medication without randomizing the groups)40 and seventeen were prestudies and poststudies,41–57 6 of which used a cross-over design.28,37–40,46 Twenty-three studies investigated chronic effects of AHT,26–32,34–36,40–42,45–47,49–51,53,54 5 investigated acute effects,43,44,48,55 and 4 studies investigated both.33,39,52,57 Sample sizes ranged from 6 to 673 participants with the mean age ranging from 50 to 79 years. Thirty studies included participants with hypertension.27–54,56,57 Six studies included participants with dementia or MCI.26,27,29,31,54,55 Fifteen studies used a calcium channel blocker,26,29,31,32,34,36,38,39,43,44,47,48,52,55,57 but our data also included angiotensin receptor blockers,27,31,36,49,50 diuretics,27,28,36,38–40,42,51,52 angiotensin-converting enzyme inhibitors,27,30,36,37,41,42,44,53,54,56 β blockers,28,30,37,45,46,48 and α blockers.36,44,48 Measurement techniques for CBF were 133-Xenon inhalation technique,28,33,37–39,44–46,53,56 arterial spin labeling,26,35,36 single photon emission computed tomography,29,31,32,40,42,49,51,52,54 transcranial Doppler,26,27,48,57 and PET.30,50,55 The follow-up time ranged from 10 minutes to 1 year.
Table 1.
Study Characteristics
Quality Assessment and Grading of Recommendations Assessment, Development and Evaluation Rating
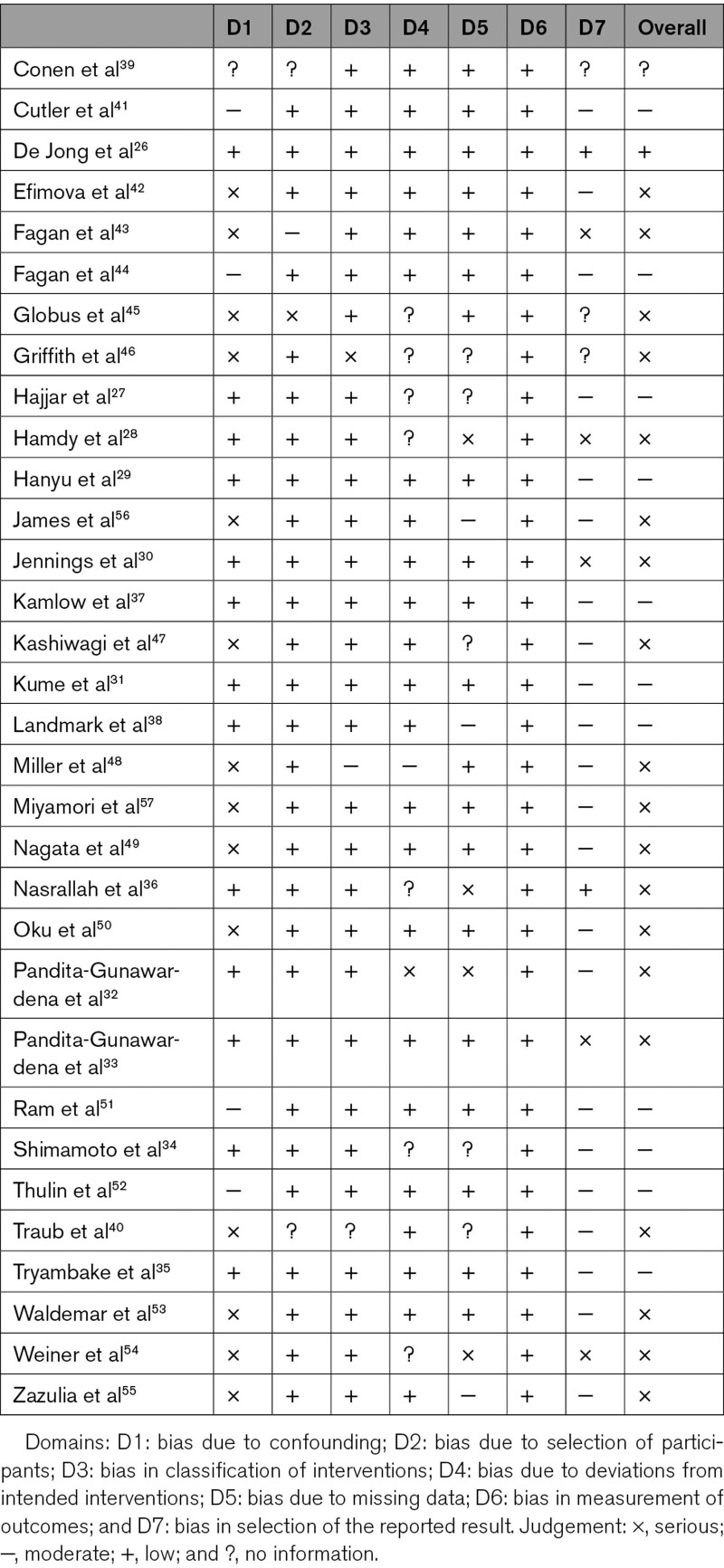
Quality assessment of studies included in the review is presented in Table 2. Risk of bias was judged to be low in only one trial, moderate in 11 trials, serious in 19 trials, and no information in 1 trial. The large number of trials rated at serious risk was mainly due to lack of adequate control for potential confounding factors (age, duration of follow-up). Risk of bias was judged to be moderate in the reporting bias domain for most studies as few prepublished an adequate trial protocol. Grading of Recommendations Assessment, Development and Evaluation rating of the quality of evidence was very low (Table S2). The funnel plot asymmetry suggests publication bias towards studies demonstrating a reduction in CBF (Figures S1 and S3).
Table 2.
Quality Assessment
Qualitative Synthesis
All studies showed a significant decrease in SBP or mean arterial pressure following AHT, expect for Globus et al.45 The fall in SBP ranged between −3.9 and −39.4 mm Hg. Five studies did not report the change in BP.
Global CBF
None of the 9 studies assessing the acute effect of AHT demonstrated a significant effect on global CBF. Eighteen studies investigating the chronic effect of AHT equally showed no significant change in global CBF. Only one study showed a significant decrease in global CBF after treatment with ceronapril for 9 weeks.41 In contrast, 5 studies showed a significant increase in global CBF with chronic AHT.34,35,42,45,49 Of these, 3 studies investigated the effect of AHT over time in one intervention group.42,45,49 One study compared the effects of nifedipine and nilvadipine, demonstrating a significant increase in global CBF in the nilvadipine group.34 Another study compared the effect of intensive BP lowering (target <120 mm Hg SBP) with usual BP lowering (target <140 mm Hg), with a significant increase in global CBF after intensive BP lowering.35
Regional CBF
Thirteen studies investigated regional CBF.26,28,29,31,32,34,36,42,47,49,50,52,54 Only 5 studies showed significant changes (mostly increases) in CBF in specific brain regions.26,29,31,52,54 However, these regions were not consistent across the 5 studies. One study showed a significant increase in CBF in the left hippocampal region after treatment with nilvadipine.26 Another study showed a significant increase in CBF in the left frontal region after treatment with nilvadipine and a significant decrease in the left temporal region after treatment with amlodipine.29 One study investigated the effect of telmisartan and amlodipine,31 with a significant increase in CBF in the right subcallosal gyrus, right superior parietal lobe, right cuneus, and right lingual gyrus with telmisartan. Whereas, with amlodipine, a significant increase was only found in the right cingulate gyrus. Another study showed a significant increase in CBF in the left temporal region after treatment with ceronapril.54 Finally, a significant increase in CBF was found in the left cortex after acute treatment with felodipine.52
CBF in Patients With Cognitive Impairment or Dementia
Six studies included participants with dementia or MCI. These studies showed results consistent with those that had included cognitively intact participants with hypertension. None found significant changes in global CBF. Four studies demonstrated a significant change (mostly increases) in specific cerebral regions: left hippocampus, left frontal, left temporal, right subcallosal gyrus, right superior parietal lobe, right cuneus, right lingual gyrus, right cingulate gyrus.26,29,31,54
Meta-Analysis
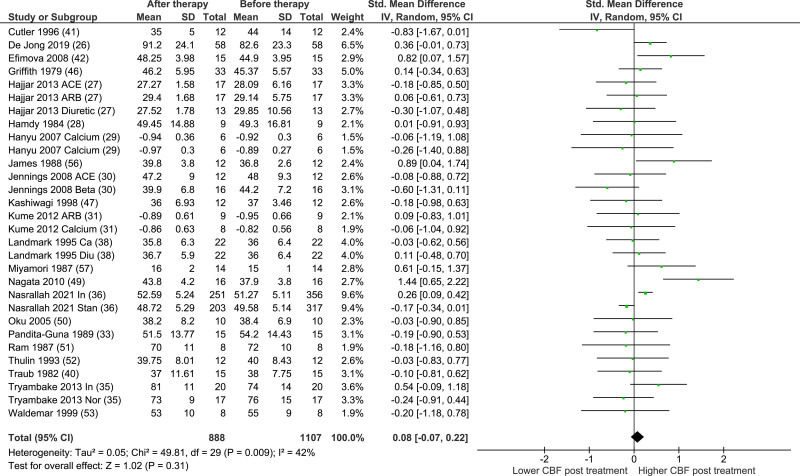
The results of the primary analysis are presented in Figure 2. Twenty-two studies were included in the primary analysis. The number of participants was 1107 before therapy and 888 after therapy. There was no significant effect of AHT on global CBF on pooled analyses across all studies (standardized mean difference, 0.08 [95% CI, −0.07 to 0.22]; P=0.31, I2=42%).
Figure 2.
Standardized mean difference (SMD) in cerebral blood flow before and after chronic antihypertensive treatment.
Total indicates number of participants. Some randomized controlled trials investigated the effect of 2 different therapies, these have been added to the analysis separately. Most studies expressed cerebral blood flow (CBF) in mL/100 g/min, except for Fagan 1992 (cm/s), Hajjar 2013 (cm/s), Hanyu 2007 (Z score), Kume 2012 (Z score), Miller 2019 (cm/s), Miyamori 1987 (cm/s), and Shimamoto 1995 (mL/min). ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; Ca, calcium blocker; Diu, diuretic; IV, inverse variance; In, intensive; Nor, normal; and Stan, standard.
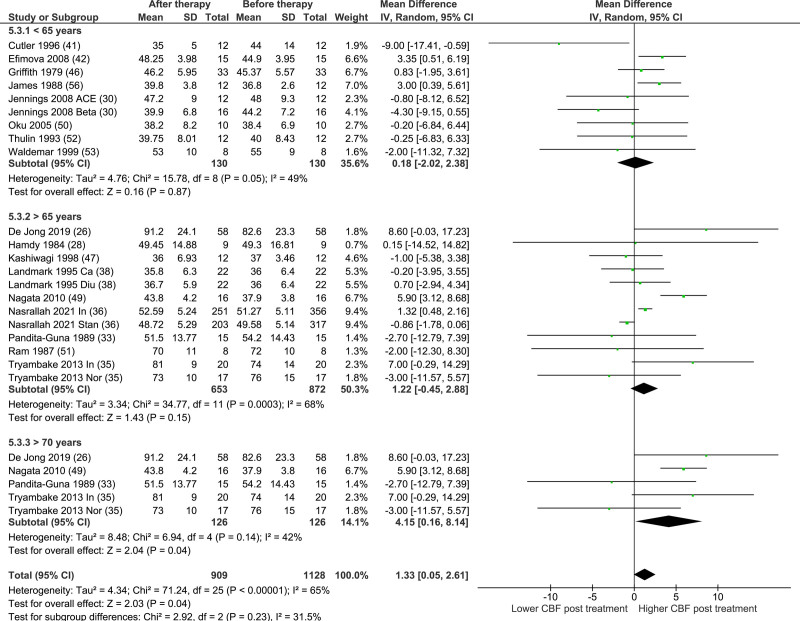
Sensitivity Analysis
The results of the analysis by treatment type (acute/chronic) are presented in Figure S2. There was no significant effect of AHT on global CBF in either the acute or chronic treatment groups (MD, −0.67 [95% CI, −4.70 to 3.36]; P=0.75, I2=0%; MD, 0.82 [95% CI, −0.41 to 2.05]; P=0.19, I2=59%, respectively). Results of the subgroup analyses by study characteristics are presented in Table 3. The forest plot of the subgroup analysis age is presented in Figure 3. There was a significant increase in CBF with AHT in the study populations with a mean age of >70 years (n=126; MD, 4.15 [95% CI, 0.16–8.14]); P=0.04, I2=42%). The majority of subgroup analyses showed high heterogeneity (I2 >50%), except the calcium blocker and mean age >70 years subgroups (I2=30% and 42%, respectively), and the acute, diuretic, 133-Xe inhalation technique, and 133-XE single photon emission computed tomography subgroups (I2=0%). The forest plots of the other subgroup analyses are presented in Figures S4 through S6.
Table 3.
Subgroup analyses MD in Cerebral Blood Flow After Chronic Antihypertensive Treatment
Figure 3.
Subgroup analysis age in mean difference.
Total indicates number of participants. Some randomized controlled trials investigated the effect of 2 different therapies. ACE indicates angiotensin-converting enzyme; Ca, calcium blocker; CBF, cerebral blood flow; Diu, diuretic; In, intensive; IV, inverse variance; Nor, normal; and Stand, standard.
Discussion
Summary of Evidence
This systematic review assessed the effects of AHT on CBF in older adults with hypertension, as well as in people with cognitive impairment. Overall, we identified no significant effect of AHT on global CBF. This finding did not vary by class of antihypertensive drug used, method of measuring CBF, and study design. Five studies showed significant changes (mostly increases) in CBF in specific brain regions, but these regions were not consistent. Studies including participants with dementia or MCI demonstrated similar findings to cognitively normal participants with hypertension. However, the quantitative synthesis was limited by significant heterogeneity and moderate-to-serious risk of bias.
Results in Context
This review focused on older patients (mean age in our included studies ranged from 50 to 79 years) with hypertension, including patients with cognitive impairment and dementia, due to clinical concerns around BP lowering in these groups. Chronic hypertension is highly prevalent in older adults.2 Longstanding hypertension causes remodeling of cerebral blood vessels, leading to higher vascular resistance due to thicker walls and smaller lumens.58 Moreover, there is a higher risk of developing stenotic atherosclerotic plaques with chronic hypertension.59 In theory, increased cerebrovascular resistance and cerebrovascular stenosis could require higher BP to maintain CBF. Indeed, a rightward shift of the autoregulation curve has been demonstrated in older adults with chronic hypertension, where CBF is maintained at high levels of BP.60,61 It is often suggested that this phenomenon implies an impairment in cerebral autoregulation, where CBF would no longer be maintained if BP were reduced, due to irreversible remodeling and stenosis.60,61 Finally, the selfish brain hypothesis suggests that the brain drives hypertension to ensure adequate CBF.62 All these theories suggest that the brain and its vasculature have adapted to the chronic hypertension state and that consequently, lowering BP might disrupt CBF and cause deterioration of brain function.
However, the findings of this review do not support this notion. In contrast, we found that BP reduction in hypertension, even in patients with MCI and dementia, does not reduce CBF. Recent studies in older adults with hypertension and common comorbidities have demonstrated consistent findings with those reported here. In patients with small vessel disease and carotid artery occlusive disease, there was no significant change in CBF with AHT.63,64 Similarly, in patients with type 2 diabetes, a progressive reduction in CBF was only seen with BP lowering in patients with microvascular complications.65 In contrast, in patients with metabolic syndrome, CBF increased following BP reduction.66 Similarly, studies in younger populations (mean age <50 years) demonstrated comparable findings of stable CBF after BP lowering with AHT.13,67,68
Different classes of AHT could differ in their effects on CBF due to their varying mechanisms of action. Angiotensin converting enzyme inhibitors are known to successfully reverse hypertensive vascular hypertrophy and remodeling.69 Angiotensin receptor blockers reverse the cerebrovascular pathological growth and inflammation and can improve vessel compliance.68,70 Lastly, calcium antagonists may have a neuroprotective effect on cerebral ischemia through inhibition of intracellular calcium accumulation which may serve as a trigger for irreversible cellular injury. In particular, nilvadipine can reach higher concentrations in the brain than other calcium blockers, as a result of the high brain blood ratio and longer half-life in the brain compared with blood.34 Despite these different theoretical benefits, this review did not demonstrate different effects between different AHT classes. The effect of specific AHT classes on the risk of dementia has also been studied in a meta-analysis, again finding no evidence for class effects.71
We observed a significant increase in CBF with BP reduction in studies with participants with a mean age >70 years, but not in studies with a lower age cut off. While this should be interpreted with caution, given the small number of studies and the risk for multiplicity, and ecological bias in subgroup analyses, this is an interesting finding. Previous studies have shown that hypertension may reduce CBF, and this finding suggests this could be partially reversible. Mechanistically, antihypertensive drugs might be involved in remodeling of cerebral arteries, although direct evidence is lacking.13,26,72,73
Strengths and Limitations
This review had some limitations. First, a large number of trials were rated as moderate or serious risk of bias, mainly due to confounding and reporting bias. Reporting bias was high because many studies (published before 2000) did not prepublish their protocol. However, this was not standard practice before 2000 and, therefore, the risk of bias may be inflated. Second, not all studies presented exact values of BP and CBF. As a result, the values were derived from a figure or global CBF was calculated from the average of specific regions, potentially reducing the reliability of these estimates.
The strength of this review is that the article selection, data extraction and risk of bias assessment were performed independently by 2 reviewers, ensuring reliability. In addition, a comprehensive search strategy was conducted to detect as many studies as possible, with a quantitative synthesis of the available evidence.
Perspectives
There is clear evidence of a beneficial effect of BP reduction on stroke, cardiovascular disease, and heart failure,10 even in older adults.11 However, concerns that BP reduction may reduce CBF in older adults with longstanding hypertension may deter physicians or patients from adequately treating hypertension. The results from this systematic review and meta-analysis support treatment of hypertension in older adults, given that there was no evidence for a reduction in CBF. However, there were too few studies to draw definitive conclusions on the effects of AHT in older adults with cognitive impairment. Further research is needed to understand if AHT is equally beneficial and safe in this population.
Conclusions
With the best current available evidence this review does not suggest a reduction in CBF following treatment with AHT in older patients, although quality of evidence is low, and heterogeneity was high. Despite these limitations, it seems warranted to conclude no harmful effects on CBF occur in the populations studied. A well-designed follow-up study is needed to confirm this in (very) frail older adults (eg, >75 years, clinical frailty scale >4 or with dementia/MCI), using treatment algorithms used in clinical practice for a reasonable duration (eg, at least 3 months). Preliminary power calculations suggest that such a trial would need about 50 patients.
Article Information
Sources of Funding
L.C. Beishon is a research training fellow funded by the Dunhill Medical Trust (RTF97/0117).
Disclosures
None.
Supplemental Materials
Methods 1
References1–81
Tables S1–S4
Figures S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- AHT
- antihypertensive treatment
- ARB
- angiotensin receptor blocker
- BP
- blood pressure
- CBF
- cerebral blood flow
- MCI
- mild cognitive impairment
- MD
- mean difference
- SBP
- systolic blood pressure
This article was sent to Costantino Iadecola, Guest Editor, for review by expert referees, editorial decision, and final disposition.
A.E. van Rijssel and B.C. Stins are joint first authors.
J.A.H.R. Claassen and Rianne A.A. de Heus are joint senior authors.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.18255.
For Sources of Funding and Disclosures, see page 1076.
Contributor Information
Anniek E. van Rijssel, Email: Anniek.vanRijssel@radboudumc.nl.
Bram C. Stins, Email: bram.stins@radboudumc.nl.
Lucy C. Beishon, Email: lb330@le.ac.uk.
Marit L. Sanders, Email: marit.sanders@radboudumc.nl.
Terence J. Quinn, Email: terry.quinn@glasgow.ac.uk.
Rianne A.A. de Heus, Email: rianne.deheus@radboudumc.nl.
References
- 1.Nguyen QT, Anderson SR, Sanders L, Nguyen LD. Managing hypertension in the elderly: a common chronic disease with increasing age. Am Health Drug Benefits. 2012;5:146–153. [PMC free article] [PubMed] [Google Scholar]
- 2.Butt DA, Harvey PJ. Benefits and risks of antihypertensive medications in the elderly. J Intern Med. 2015;278:599–626. doi: 10.1111/joim.12446 [DOI] [PubMed] [Google Scholar]
- 3.Kagiyama S, Takata Y, Ansai T, Matsumura K, Soh I, Awano S, Sonoki K, Yoshida A, Torisu T, Hamasaki T, et al. Does decreased diastolic blood pressure associate with increased mortality in 80-year-old Japanese? Clin Exp Hypertens. 2009;31:639–647. doi: 10.3109/10641960903407009 [DOI] [PubMed] [Google Scholar]
- 4.Heijer Td, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, Breteler MM. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003;24:307–313. doi: 10.1016/s0197-4580(02)00088-x [DOI] [PubMed] [Google Scholar]
- 5.Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JC, Lernfelt B, van Harskamp F, Hofman A, Breteler MM. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. 2001;12:33–39. doi: 10.1159/000051233 [DOI] [PubMed] [Google Scholar]
- 6.Euser SM, van Bemmel T, Schram MT, Gussekloo J, Hofman A, Westendorp RG, Breteler MM. The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc. 2009;57:1232–1237. doi: 10.1111/j.1532-5415.2009.02264.x [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veglio F, Paglieri C, Rabbia F, Bisbocci D, Bergui M, Cerrato P. Hypertension and cerebrovascular damage. Atherosclerosis. 2009;205:331–341. doi: 10.1016/j.atherosclerosis.2008.10.028 [DOI] [PubMed] [Google Scholar]
- 9.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13 [DOI] [PubMed] [Google Scholar]
- 10.Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, Casiglia E, Kerlikowske K, Coope J. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA Group. Lancet. 1999;353:793–796. doi: 10.1016/s0140-6736(98)08127-6 [DOI] [PubMed] [Google Scholar]
- 11.Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, Bulpitt C, Peters R. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78. doi: 10.1186/s12916-015-0328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. doi: 10.1161/01.HYP.0000153094.09615.11 [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Witkowski S, Fu Q, Claassen JA, Levine BD. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension. 2007;49:1149–1155. doi: 10.1161/HYPERTENSIONAHA.106.084939 [DOI] [PubMed] [Google Scholar]
- 14.Benedictus MR, Leeuwis AE, Binnewijzend MA, Kuijer JP, Scheltens P, Barkhof F, van der Flier WM, Prins ND. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur Radiol. 2017;27:1169–1175. doi: 10.1007/s00330-016-4450-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng SL, Chen X, Li Y, Rodrigue KM, Park DC, Lu H. Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. Neuroimage. 2018;174:257–262. doi: 10.1016/j.neuroimage.2018.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu CH, Yang CC, Kuo TB. Effects of different classes of antihypertensive drugs on cerebral hemodynamics in elderly hypertensive patients. Am J Hypertens. 2005;18(12 Pt 1):1621–1625. doi: 10.1016/j.amjhyper.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559. doi: 10.1152/physrev.00022.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorond FA, Hollenberg NK, Panych LP, Fisher ND. Brain blood flow and velocity: correlations between magnetic resonance imaging and transcranial Doppler sonography. J Ultrasound Med. 2010;29:1017–1022. doi: 10.7863/jum.2010.29.7.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Eldridge S, Li T. (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook. [Google Scholar]
- 24.Schunemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook 2013; guidelinedevelopment.org/handbook. Accessed October 19, 2020.
- 25.Deeks JJ, Higgins J, Altman DG. (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane handbook for systematic reviews of interventions version 6.2 (updated february 2021). Cochrane, 2021. [Google Scholar]
- 26.de Jong DLK, de Heus RAA, Rijpma A, Donders R, Olde Rikkert MGM, Günther M, Lawlor BA, van Osch MJP, Claassen JAHR. Effects of nilvadipine on cerebral blood flow in patients with Alzheimer disease. Hypertension. 2019;74:413–420. doi: 10.1161/HYPERTENSIONAHA.119.12892 [DOI] [PubMed] [Google Scholar]
- 27.Hajjar I, Hart M, Chen YL, Mack W, Novak V, C Chui H, Lipsitz L. Antihypertensive therapy and cerebral hemodynamics in executive mild cognitive impairment: results of a pilot randomized clinical trial. J Am Geriatr Soc. 2013;61:194–201. doi: 10.1111/jgs.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamdy RC, Davies A, Arnold K, Tovey JD, Saimbi SS, Short MD, Exton-Smith AN. The short-term effects of reducing elevated blood pressure in elderly patients with propranolol and dyazide. Age Ageing. 1984;13:83–88. doi: 10.1093/ageing/13.2.83 [DOI] [PubMed] [Google Scholar]
- 29.Hanyu H, Hirao K, Shimizu S, Iwamoto T, Koizumi K, Abe K. Favourable effects of nilvadipine on cognitive function and regional cerebral blood flow on SPECT in hypertensive patients with mild cognitive impairment. Nucl Med Commun. 2007;28:281–287. doi: 10.1097/MNM.0b013e32804c58aa [DOI] [PubMed] [Google Scholar]
- 30.Jennings JR, Muldoon MF, Price J, Christie IC, Meltzer CC. Cerebrovascular support for cognitive processing in hypertensive patients is altered by blood pressure treatment. Hypertension. 2008;52:65–71. doi: 10.1161/HYPERTENSIONAHA.108.110262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kume K, Hanyu H, Sakurai H, Takada Y, Onuma T, Iwamoto T. Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr Gerontol Int. 2012;12:207–214. doi: 10.1111/j.1447-0594.2011.00746.x [DOI] [PubMed] [Google Scholar]
- 32.Pandita-Gunawardena ND, Clarke SE. Amlodipine lowers blood pressure without affecting cerebral blood flow as measured by single photon emission computed tomography in elderly hypertensive subjects. Age Ageing. 1999;28:451–457. doi: 10.1093/ageing/28.5.451 [DOI] [PubMed] [Google Scholar]
- 33.Pandita-Gunawardena ND, Dorrance DE, MacDonald G. Efficacy of nitrendipine in the treatment of elderly hypertensive subjects, and its effect on cerebral blood flow. J Cardiovasc Pharmacol. 1989;14 Suppl 10:S52–S58. [PubMed] [Google Scholar]
- 34.Shimamoto H, Shimamoto Y. Nilvadipine increases cerebral blood flow in elderly hypertensives: comparison with nifedipine. J Hum Hypertens. 1995;9:271–279. [PubMed] [Google Scholar]
- 35.Tryambake D, He J, Firbank MJ, O’Brien JT, Blamire AM, Ford GA. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension. 2013;61:1309–1315. doi: 10.1161/HYPERTENSIONAHA.112.200972 [DOI] [PubMed] [Google Scholar]
- 36.Nasrallah IM, Gaussoin SA, Pomponio R, Dolui S, Erus G, Wright CB, Launer LJ, Detre JA, Wolk DA, Davatzikos C, et al. ; SPRINT Research Group. Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of alzheimer disease: secondary analysis of the SPRINT MIND randomized trial. JAMA Neurol. 2021;78:568–577. doi: 10.1001/jamaneurol.2021.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamlow F, Cruickshank JM, Neil-Dwyer G, Dorrance DE, Hayes Y, Patel S, Wainwright RJ. First-dose effects of enalapril and atenolol upon blood pressure and cerebral blood flow in patients with mild hypertension on diuretic therapy. J Hum Hypertens. 1990;4:281–285. [PubMed] [Google Scholar]
- 38.Landmark K, Forsman M, Lindberg K, Ryman T, Martmann-Moe K, Haaverstad S, Wiel S. Nitrendipine and mefruside in elderly hypertensives: effects on blood pressure, cardiac output, cerebral blood flow and metabolic parameters. J Hum Hypertens. 1995;9:281–285. [PubMed] [Google Scholar]
- 39.Conen D, Rüttimann S, Noll G, Schneider K, Müller J. Short- and long-term cerebrovascular effects of nitrendipine in hypertensive patients. J Cardiovasc Pharmacol. 1988;12 Suppl 4:S64–S68. doi: 10.1097/00005344-198806124-00012 [DOI] [PubMed] [Google Scholar]
- 40.Traub YM, Shapiro AP, Dujovny M, Nelson D. Cerebral blood flow changes with diuretic therapy in elderly subjects with systolic hypertension. Clin Exp Hypertens A. 1982;4:1193–1201. doi: 10.3109/10641968209060783 [DOI] [PubMed] [Google Scholar]
- 41.Cutler NR, Sramek JJ, Luna A, Mena I, Brass EP, Kurtz NM, Brennan JJ. Effect of the ACE inhibitor ceronapril on cerebral blood flow in hypertensive patients. Ann Pharmacother. 1996;30:578–582. doi: 10.1177/106002809603000601 [DOI] [PubMed] [Google Scholar]
- 42.Efimova IY, Efimova NY, Triss SV, Lishmanov YB. Brain perfusion and cognitive function changes in hypertensive patients. Hypertens Res. 2008;31:673–678. doi: 10.1291/hypres.31.673 [DOI] [PubMed] [Google Scholar]
- 43.Fagan SC, Bindlish V, Robert S, Steigerwalt SP, Ramadan NM. Transcranial Doppler to evaluate the effects of antihypertensive medication on cerebral blood flow velocity. J Clin Pharmacol. 1992;32:66–69. doi: 10.1002/j.1552-4604.1992.tb03790.x [DOI] [PubMed] [Google Scholar]
- 44.Fagan SC, Levine SR, Ewing JR, Ramadan NM, Welch KM. Age and carotid artery occlusive disease are important determinants of cerebral blood flow changes after antihypertensive therapy. Pharmacotherapy. 1995;15:573–578. doi: 10.1002/j.1875-9114.1995.tb02865.x [DOI] [PubMed] [Google Scholar]
- 45.Globus M, Keren A, Eldad M, Granot C, Tzivoni D, Lavy S, Stern S. The effect of chronic propranolol therapy on regional cerebral blood flow in hypertensive patients. Stroke. 1983;14:964–967. doi: 10.1161/01.str.14.6.964 [DOI] [PubMed] [Google Scholar]
- 46.Griffith DN, James IM, Newbury PA, Woollard ML. The effect of beta-adrenergic receptor blocking drugs on cerebral blood flow. Br J Clin Pharmacol. 1979;7:491–494. doi: 10.1111/j.1365-2125.1979.tb00991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kashiwagi S, Yoshikawa K, Yamashita K, Kato S, Ito H. Effects of nitrendipine on the cerebral hemodynamics of elderly hypertensive patients. Curr Ther Res. 1998;59:521–527. 10.1016/S0011-393X(98)85090-X [Google Scholar]
- 48.Miller JB, Calo S, Reed B, Thompson R, Nahab B, Wu E, Chaudhry K, Levy P. Cerebrovascular risks with rapid blood pressure lowering in the absence of hypertensive emergency. Am J Emerg Med. 2019;37:1073–1077. doi: 10.1016/j.ajem.2018.08.052 [DOI] [PubMed] [Google Scholar]
- 49.Nagata R, Kawabe K, Ikeda K. Olmesartan, an angiotensin II receptor blocker, restores cerebral hypoperfusion in elderly patients with hypertension. J Stroke Cerebrovasc Dis. 2010;19:236–240. doi: 10.1016/j.jstrokecerebrovasdis.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 50.Oku N, Kitagawa K, Imaizumi M, Takasawa M, Piao R, Kimura Y, Kajimoto K, Matsumoto M, Hori M, Hatazawa J. Hemodynamic influences of losartan on the brain in hypertensive patients. Hypertens Res. 2005;28:43–49. doi: 10.1291/hypres.28.43 [DOI] [PubMed] [Google Scholar]
- 51.Ram CV, Meese R, Kaplan NM, Devous MD, Sr, Bonte FJ, Forland SC, Cutler RE. Antihypertensive therapy in the elderly. Effects on blood pressure and cerebral blood flow. Am J Med. 1987;82(1A):53–57. doi: 10.1016/0002-9343(87)90145-8 [DOI] [PubMed] [Google Scholar]
- 52.Thulin T, Fagher B, Grabowski M, Ryding E, Elmqvist D, Johansson BB. Cerebral blood flow in patients with severe hypertension, and acute and chronic effects of felodipine. J Hypertens. 1993;11:83–88. doi: 10.1097/00004872-199301000-00012 [DOI] [PubMed] [Google Scholar]
- 53.Waldemar G, Ibsen H, Strandgaard S, Andersen AR, Rasmussen S, Paulson OB. The effect of fosinopril sodium on cerebral blood flow in moderate essential hypertension. Am J Hypertens. 1990;3(6 Pt 1):464–470. doi: 10.1093/ajh/3.6.464 [DOI] [PubMed] [Google Scholar]
- 54.Weiner MF, Bonte FJ, Tintner R, Ford N, Svetlik D, Riall T. Ace inhibitor lacks acute effect on cognition or brain blood flow in alzheimer’s disease. Drug Dev Res. 1992;26:467–471. doi: 10.1002/ddr.430260410 [Google Scholar]
- 55.Zazulia AR, Videen TO, Morris JC, Powers WJ. Autoregulation of cerebral blood flow to changes in arterial pressure in mild Alzheimer’s disease. J Cereb Blood Flow Metab. 2010;30:1883–1889. doi: 10.1038/jcbfm.2010.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.James IM, Dickenson EJ, Burgoyne W, Jeremy JY, Barradas MA, Mikhailidis DP, Dandona P. Treatment of hypertension with captopril: preservation of regional blood flow and reduced platelet aggregation. J Hum Hypertens. 1988;2:21–25. [PubMed] [Google Scholar]
- 57.Miyamori I, Yasuhara S, Matsubara T, Takasaki H, Takeda R. Effects of a calcium entry blocker on cerebral circulation in essential hypertension. J Clin Hypertens. 1987;3:528–535. [PubMed] [Google Scholar]
- 58.Rizzoni D, Agabiti-Rosei C, Agabiti-Rosei E. Hemodynamic consequences of changes in microvascular structure. Am J Hypertens. 2017;30:939–946. doi: 10.1093/ajh/hpx032 [DOI] [PubMed] [Google Scholar]
- 59.Hurtubise J, McLellan K, Durr K, Onasanya O, Nwabuko D, Ndisang JF. The Different facets of dyslipidemia and hypertension in atherosclerosis. Curr Atheroscler Rep. 2016;18:82. doi: 10.1007/s11883-016-0632-z [DOI] [PubMed] [Google Scholar]
- 60.Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559. doi: 10.1152/physrev.00022.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strandgaard S, Paulson OB. Cerebral blood flow in untreated and treated hypertension. Neth J Med. 1995;47:180–184. doi: 10.1016/0300-2977(95)00065-u [DOI] [PubMed] [Google Scholar]
- 62.Hart EC. Human hypertension, sympathetic activity and the selfish brain. Exp Physiol. 2016;101:1451–1462. doi: 10.1113/EP085775 [DOI] [PubMed] [Google Scholar]
- 63.Croall ID, Tozer DJ, Moynihan B, Khan U, O’Brien JT, Morris RG, Cambridge VC, Barrick TR, Blamire AM, Ford GA, et al. ; PRESERVE Study Team. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease: the PRESERVE randomized clinical trial. JAMA Neurol. 2018;75:720–727. doi: 10.1001/jamaneurol.2017.5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel RV, Ramadan NM, Levine SR, Welch KM, Fagan SC. Effects of ramipril and enalapril on cerebral blood flow in elderly patients with asymptomatic carotid artery occlusive disease. J Cardiovasc Pharmacol. 1996;28:48–52. doi: 10.1097/00005344-199607000-00008 [DOI] [PubMed] [Google Scholar]
- 65.Kim YS, Davis SC, Truijen J, Stok WJ, Secher NH, van Lieshout JJ. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension. 2011;57:738–745. doi: 10.1161/HYPERTENSIONAHA.110.160523 [DOI] [PubMed] [Google Scholar]
- 66.Efimova NY, Chernov VI, Efimova IY, Lishmanov YB. Influence of antihypertensive therapy on cerebral perfusion in patients with metabolic syndrome: relationship with cognitive function and 24-h arterial blood pressure monitoring. Cardiovasc Ther. 2015;33:209–215. doi: 10.1111/1755-5922.12136 [DOI] [PubMed] [Google Scholar]
- 67.Minematsu K, Yamaguchi T, Tsuchiya M, Ito K, Ikeda M, Omae T. Effect of angiotensin converting enzyme inhibitor (captopril) on cerebral blood flow in hypertensive patients without a history of stroke. Clin Exp Hypertens A. 1987;9:551–557. doi: 10.3109/10641968709164223 [DOI] [PubMed] [Google Scholar]
- 68.Rüttimann S, Noll G, Dreifuss M, Müller-Brand J. Cerebral blood flow is not altered by treatment with nitrendipine in patients with mild to moderate hypertension. J Cardiovasc Pharmacol. 1991;18 Suppl 1:S109–S111. [PubMed] [Google Scholar]
- 69.Iadecola C, Gorelick PB. Hypertension, angiotensin, and stroke: beyond blood pressure. Stroke. 2004;35:348–350. doi: 10.1161/01.STR.0000115162.16321.AA [DOI] [PubMed] [Google Scholar]
- 70.Saavedra JM, Benicky J, Zhou J. Mechanisms of the anti-ischemic effect of Angiotensin II AT(1) receptor antagonists in the brain. Cell Mol Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0 [DOI] [PubMed] [Google Scholar]
- 71.Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, Pase MP, Himali JJ, Gwen Windham B, Griswold M, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70. doi: 10.1016/S1474-4422(19)30393-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuriyama Y, Hashimoto H, Nagatsuka K, Sawada T, Omae T. Effects of dihydropyridines on cerebral blood vessels. J Hypertens Suppl. 1993;11:S9–12. [PubMed] [Google Scholar]
- 73.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension. 1999;33:856–861. doi: 10.1161/01.hyp.33.3.856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.