Summary
The aim of the article is to examine side effects of increased dietary intake of amino acids, which are commonly used as a dietary supplement. In addition to toxicity, mutagenicity and carcinogenicity, attention is focused on renal and gastrointestinal tract functions, ammonia production, and consequences of a competition with other amino acids for a carrier at the cell membranes and enzymes responsible for their degradation. In alphabetic order are examined arginine, β-alanine, branched-chain amino acids, carnosine, citrulline, creatine, glutamine, histidine, β-hydroxy-β-methylbutyrate, leucine, and tryptophan. In the article is shown that enhanced intake of most amino acid supplements may not be risk-free and can cause a number of detrimental side effects. Further research is necessary to elucidate effects of high doses and long-term consumption of amino acid supplements on immune system, brain function, muscle protein balance, synthesis of toxic metabolites, and tumor growth and examine their suitability under certain circumstances. These include elderly, childhood, pregnancy, nursing a baby, and medical condition, such as diabetes and liver disease. Studies are also needed to examine adaptive response to a long-term intake of any substance and consequences of discontinuation of supplementation.
Keywords: Arginine, Branched-chain amino acids, Carnosine, Citrulline, Creatine, Glutamine, Histidine, Tryptophan
Introduction
There are many people who consume chronically high amounts of amino acids as a dietary supplement. These are mainly athletes and bodybuilders who use amino acids and their derivatives to increase muscle mass and strength and delay the onset of fatigue. Humans also consume amino acids to support immune system, improve memory, ameliorate depression, prevent headaches, and to help insomniacs. Several amino acids are recommended or investigated in therapy of muscle wasting disorders, sepsis, multiple trauma, liver cirrhosis, renal insufficiency, eczema, and ageing-related disorders. However, well controlled studies on adverse effects of increased intake of specific amino acids on humans are rare. A growing problem, which this article will not address, is the frequent content of anabolic steroids in supplements that are non-standard, counterfeit and deliberately manufactured to imitate a legitimate product [1].
The aim of the present article is to examine side effects of increased dietary intake of amino acids, which are commonly used as a dietary supplement. First, I will try to outline general risks of excessive amino acid intake. Then I will examine in an alphabetic order what side effects can be induced by increased intake of a specific amino acid.
Side effects of increased amino acid intake
Several studies have examined possible toxic, mutagenic, cancerogenic and teratogenic effects of high doses of specific amino acids and tried to assess the safe limits of their increased intake [2–5]. Many studies have evaluated effects on the digestive system, for example, whether or not nausea, abdominal pain, vomiting, and diarrhea are present [3,6,7]. As the liver and the kidneys are the main organs involved in metabolism and excretion of excess substances from the body, a large part of the studies focusses on hepatic and renal functions [8–10].
However, there are a number of other side effects, which are evaluated only sporadically, although they can be harmful, especially in children, pregnancy, elderly, and illness. For example, experimental studies have clearly demonstrated that some amino acids, such as glutamine (GLN) and arginine (ARG), are essential for growth of tumor cells [4,5,11,12]. The dangerous may be increased production of ammonia in individuals with impaired hepatic or renal function, especially after consumption of high amount of amino acids with several nitrogen atoms, such as GLN, histidine (HIS), and ARG. Unfortunately, the studies examining effects of chronic amino acid intake on tumor growth and ammonia levels in humans are almost non-existent.
Furthermore, increased intake of one or more amino acids can cause imbalance in amino acid concentrations, increase concentrations of its metabolites, and affect the transport of a group of amino acids into cells due to competition for a carrier at the cell membrane. The phenomenon of carrier competition can affect absorption of other amino acids in the gut and subsequently their appearance in the blood, transport across the blood-brain barrier, and supply for protein synthesis.
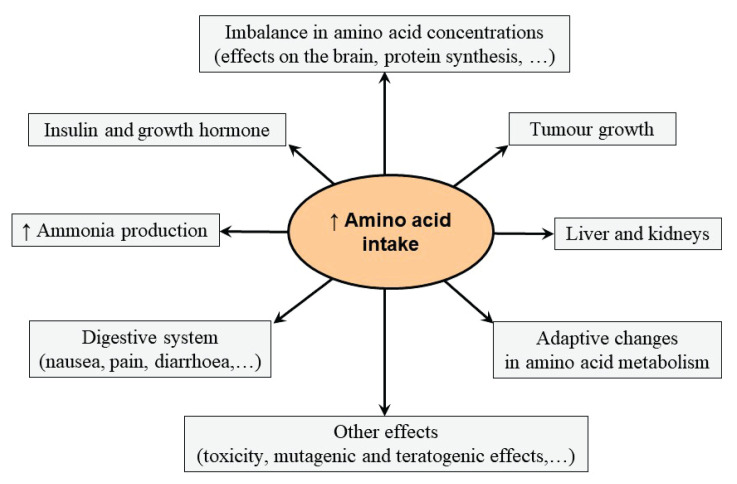
In summary, it may be assumed that chronic intake of high amounts of individual amino acid or its derivatives alters various biochemical pathways and cellular functions (Fig. 1). The consequences might be detrimental effects on the course of the illness and unexpected response to various physiological and pathological conditions.
Fig. 1.
Possible side effects of increased amino acid intake.
Side effects of increased intake of individual amino acids
I will briefly describe the reasons for use of a specific amino acid as a dietary supplement and then possible adverse effects of its increased intake. The amino acids are listed alphabetically.
L-Arginine (ARG)
ARG is a substrate for various isoforms of nitric oxide synthase (NOS) to produce nitric oxide (NO), plays a role in removing ammonia from the body through the urea cycle, serves as a substrate for synthesis of creatine and proline, and has a stimulatory role in secretion of insulin and growth hormone [6,13]. In addition, ARG decreases plasma triglycerides and exerts positive influence on immune system and collagen synthesis [5,12,14,15]. The most of endogenous ARG is synthesized from citrulline in the kidneys. The synthesis may be not sufficient in neonates, in catabolic states, and after small bowel resection [16]. Therefore, ARG is classified as a conditionally essential amino acid. Low plasma ARG concentrations are found in patients with sepsis [17].
Supplementation of ARG has been proposed to increase endothelial NO bioavailability and subsequently improve health status in elderly, treat patients with cardiovascular diseases, and improve sexual functions [18–20]. In addition, ARG is recommended to increase muscle performance in athletes and body builders, treat adiposity and metabolic syndrome, and for wound healing [21,22].
A common dosage is 6 g per day; higher doses, up to 30 g per day have also been reported [13]. It should be emphasized that the majority of orally taken ARG is degraded in enterocytes and the liver to yield ornithine, citrulline, and urea.
Side effects
There are reports of gastrointestinal distress, such as nausea and diarrhea [6,13]. A significant increase in weight and protein content in the kidneys was observed after a two-month intake of ARG-enriched diet in the rat [14]. Overstimulation of NOS by ARG load may induce hypotension due to NO-mediated vasodilatation, which was observed in subjects receiving a large dose of ARG intravenously [23]. Therefore, recommendations to increase ARG intake should aware its negative interaction with blood pressure medications. Unclear are the effects on tumor growth [5,6,12].
A serious problem with the use of ARG in therapy of cardiovascular disorders is that its beneficial effects disappear if it is given chronically. Benefits of ARG (3–9 g/d) in patients with peripheral artery insufficiency observed after 3 months [18] were not observed after 6 months [24]. Similarly, the short-term benefits of ARG in myocardial infarction observed after one month [19] were not observed in myocardial infarction trial with 153 patients randomly assigned to ARG (9 g/d) or placebo for 6 months. In addition, 6 patients in the ARG group died during the study [25]. The authors concluded that ARG should not be recommended following acute myocardial infarction.
It is supposed that the loss of therapeutic effects of ARG upon long-term supplementation in humans (“L-arginine tolerance”) is due to oxidative stress. It was shown that endothelial cells exposed to physiologically relevant concentrations of ARG manifest NOS down-regulation and superoxide and glucose cumulation [26]. In addition, it was shown that long-term ARG supplementation increases formation of ROS and decreases NO production in old mouse aortas and accelerates functional decline of kidney in aging. Hence, it was suggested that ARG supplementation should be avoided in elderly population [27].
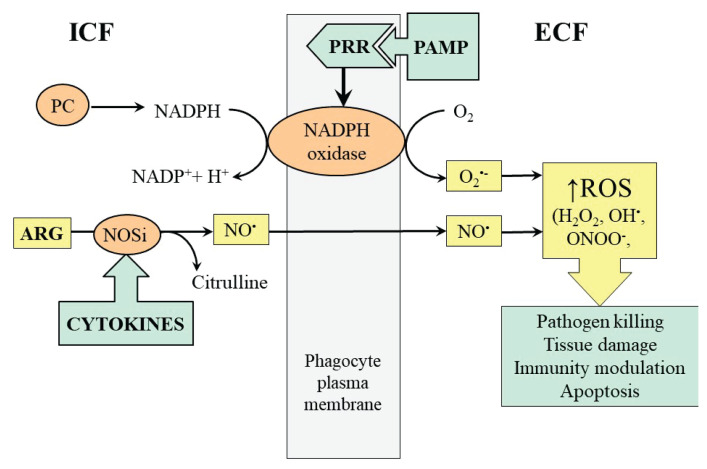
Oxidative stress and ARG consumption for NO synthesis, resulting to decreased concentration of ARG in plasma, is activated during inflammatory response, especially during sepsis. The NO released by phagocytes participates in vasodilation at the site of inflammation and under conditions of simultaneously increased ROS production gives rise to cytotoxic peroxynitrite, which plays a role in potential damage to its own structures (Fig. 2). Only a few studies have evaluated effects of ARG supplementation on individuals with sepsis [17].
Fig. 2.
The role of ARG in NO and ROS formation by phagocytes during sepsis. PRR, pathogen recognition receptor; PAMP; pathogen-associated molecular pat-tern; PC, pentose cycle.
There have been few studies investigating the specific effects of ARG supplementation on the distribution of amino acids in plasma and tissues. In our study, in rats consuming ARG-enriched diet for 2 months increased plasma concentrations of urea, creatinine, ARG, and ornithine and decreased concentrations of most of other amino acids. ARG and ornithine increased also in muscles and kidneys. In most of examined tissues, including liver, soleus and extensor digitorum longus muscles, and kidneys, decreased methionine, phenylalanine, threonine, asparagine, glycine, serine, and taurine. An increase of lysine was observed in muscles [14].
Resume
Careful studies are necessary to determine under which conditions ARG supplementation is appropriate and when it is undesirable. Attention should be focused to markers of oxidative stress and blood pressure stability.
β-Alanine (BA)
BA is the rate-limiting amino acid in the synthesis of carnosine (β-alanyl-L-histidine) a dipeptide with high buffering and antioxidant capacity present in skeletal muscle. BA is more effective at increasing carnosine content than HIS and, currently, BA supplementation is becoming a popular ergogenic strategy [28–30]. The recommended dose is 1–3 g/day. In some studies, more than 6 g/day was administered [31].
Side effects
There is no information on toxic or carcinogenic effects. The only reported side effects are short-term paresthesia and a slight increase in alanine aminotransferase levels [32]. BA supplementation at 6.4 g/day for 24 weeks did not significantly affect clinical markers of renal, hepatic and muscle function, nor did it result in chronic sensory side-effects [31]. However, a substantial decrease in HIS content (~30 %) in muscles and plasma after BA supplementation has been reported [29].
Resume
Obviously, further studies are needed to determine whether BA supplementation requires a concomitant increase in histidine (HIS) intake.
Branched-chain amino acids (BCAAs)
The BCAAs (valine, leucine, and isoleucine) are essential amino acids, which serve as substrates and regulators of protein metabolism, particularly in muscles. Positive effects of BCAA on protein synthesis, especially of leucine, are realized through the mTOR signaling pathway, phosphorylation of translation initiation factors and ribosomal proteins, and stimulatory effect on insulin secretion [33,34]. The inhibitory effects on proteolysis are mediated mainly by branched-chain keto acids (BCKA; α-ketoisocaproate, α-keto-β-methylvalerate, and α-keto-isovalerate) and β-hydroxy-β-methylbutyrate (HMB).
BCAA catabolism is increased in all muscle wasting conditions, including sepsis, trauma, cancer, chronic renal failure, and liver cirrhosis. However, BCAA levels decrease markedly only in liver cirrhosis, whereas increased levels are found in diabetes. Decrease in BCAA levels in liver cirrhosis is due to their use as a donor of amino group for synthesis of glutamate, which is a direct substrate for ammonia detoxification to GLN in muscles [35]. Increase in BCAA levels in diabetes is due to their impaired catabolism related to decreased glycolysis and surplus of NADH from increased fatty acid oxidation [36].
BCAA supplementation is thought to promote anabolic pathways in athletes, mitigate cachexia in muscle wasting disorders, prevent or treat signs of hepatic encephalopathy, attenuate fatigue during exercise, promote wound healing, and stimulate insulin production [37]. A common dose of BCAA supplementation is 10–20 g/day, in some studies 60 g/day, resulting in 4-fold [38] and more than 10-fold [39] increase in plasma BCAA, respectively. On the basis of maintenance studies [40,41] and the ratios of BCAAs in proteins [42], most studies involving pharmacologic BCAA administration have used mixtures containing 50 to 100 % leucine more than isoleucine and valine.
Side effects
There is no evidence of carcinogenesis and mutagenicity, or neurological damage as observed in maple syrup urine disease, the hereditary disorder of BCAA catabolism.
It seems that effects of exogenous BCAA are temporary limited to the early period after their administration and opposite effects are activated later. In our study, muscle protein synthesis in postprandial state was higher in rats fed by BCAA-enriched diet for two months when compared with controls. However, we failed to demonstrate positive effects of BCAA-enriched diet on muscle protein balance. Furthermore, muscle protein synthesis decreased after overnight starvation in animals fed before starvation by BCAA-enriched diet more than in controls [43].
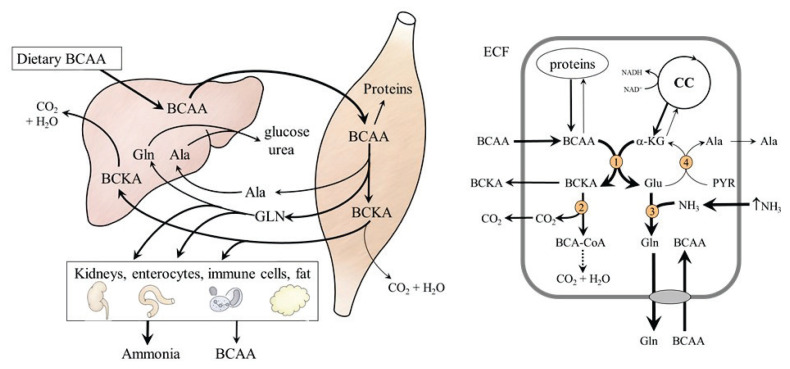
It is well established that the rate of BCAA degradation in skeletal muscle is highly responsive to changes in dietary intake. The Km of BCAA aminotransferases for BCAAs is two- to four-fold higher than tissue BCAA concentrations [44] and, therefore, the rate of transamination leading to the production of BCKA, glutamate, alanine, and GLN responds rapidly to changes in BCAA level (Fig. 3, left side). Therefore, the effects of BCAA load may be detrimental under conditions of impaired or increased ammonia production, such as in subjects with liver disease and during heavy physical performance (Fig. 3, right side). In these conditions, ammonia detoxification to GLN in muscles is activated and α-ketoglutarate (α-KG) synthesis from glutamate is impaired. The result is drain of α-KG from the tricarboxylic acid cycle (cataplerosis) and an increased release of GLN into the blood stream to be catabolized to ammonia in visceral tissues. Hence, the positive effects of BCAAs on muscle protein balance compete with the negative effects of cataplerosis on muscles and increased ammonia levels on the brain [45–50].
Fig. 3.
Effects of BCAA load on amino acid metabolism. On the left: BCAA administration leads to the release of GLN, alanine and BCKA from muscles. Glutamine is catabolized in visceral tissues to form ammonia. Part of the BCKA released from the muscles is used for BCAA synthesis. On the right: Effects of hyperammonaemia. Ammonia detoxification to GLN increases flux of BCAA through BCAA aminotransferase and the drain of α-KG from citric cycle. 1, BCAA aminotransferase; 2, BCKA dehydrogenase; 3, GLN synthetase; 4, alanine aminotransferase.
Because BCAA are transported into the brain via a common transporter for large neutral amino acids, the excess of BCAA may lower brain uptake of other neutral amino acids, such as phenylalanine, tyrosine, HIS, and tryptophan (TRP), which are precursors of dopamine, norepinephrine, histamine, and serotonin. This phenomenon, which is a rationale for use of the BCAA to prevent hepatic encephalopathy and onset of fatigue during exercise, might have detrimental effect on mental functions of patients with neurological and psychiatric disorders. Due to impaired synthesis of serotonin from TRP, increased aggression can be expected in schizophrenic patients and increased quarrelsomeness in people with a tendency to irritability or aggression [51].
Some researchers suggest that increased BCAA concentrations and their metabolites are responsible for insulin resistance and might have detrimental role in pathogenesis of complications associated with diabetes [52,53].
Resume
Criteria should be established for the use of BCAAs in conditions where the increased ammonia production due to enhanced BCAA catabolism might be detrimental. BCAA supplementation in diabetes appears to be inappropriate.
Carnosine
Carnosine (β-alanyl-L-histidine) is an effective buffer, antioxidant, heavy metal chelator, and anti-glycating agent in muscles [54]. Carnosine is predicted to be a more efficient proton-buffering and antioxidant compound than HIS. Hence, several intervention studies have been performed using carnosine to examine its effects on muscle performance in neurodegenerative and age-related disorders, metabolic syndrome, and inflammatory bowel disease. In most studies, daily carnosine supplementation doses ranged from 0.1 to 2 g [30,55].
Side effects
Carnosine administered orally is rapidly degraded by carnosinase to BA and HIS [56]. Therefore, the effects of carnosine supplements are induced mainly by these amino acids, which are discussed separately.
Resume
It should be determined whether or not carnosine supplementation has advantages over BA and HIS administration.
L-Citrulline (CIT)
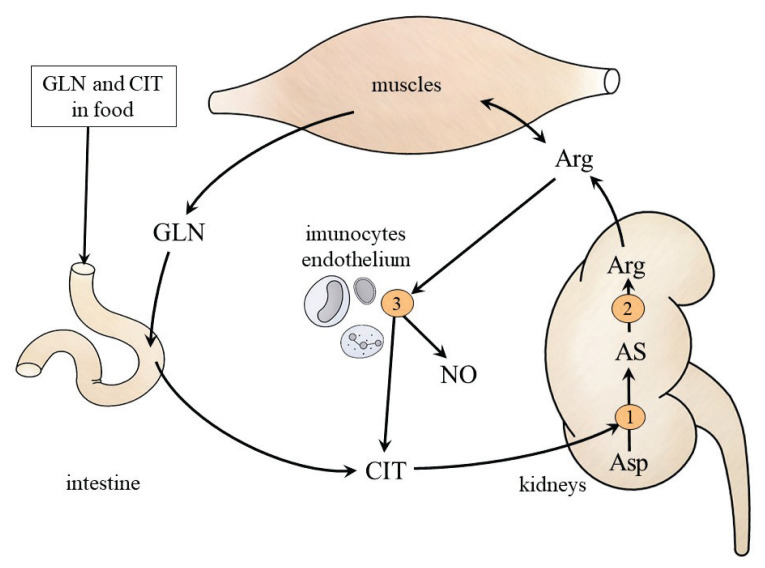
The most CIT provided orally or synthesized from GLN by enterocytes are taken up by the kidneys, and utilized for ARG synthesis (Fig. 4). The main possible indications for increased CIT intake are short bowel syndrome and the indications for ARG. A decrease in the blood CIT concentration in patients with small bowel resection is considered a signal for its parenteral administration [57]. The benefits of short-term therapy are reported on cardiovascular disorders, muscle wasting, intestinal resection, obesity, and insulin resistance [58,59].
Fig. 4.
CIT and ARG metabolism. 1, argininosuccinate synthetase; 2, argininosuccinate lyase; 3, nitric oxide synthase. AS, argininosuccinate.
Side effects and resume
Because the main mediators of the effects of CIT supplementation are ARG and NO, and long-term studies of the effect of ARG supplementation indicate cardiovascular and renal risks [24,25,27], studies examining the safety of CIT supplementation are necessary.
Creatine
Creatine (methylguanidoacetic acid) is the most used dietary supplement for athletes in order to increase the content of creatine phosphate, a ready source of ATP in muscles. There are different available forms, most common is creatine monohydrate. Beneficial effects of exogenous creatine are demonstrated in anaerobic disciplines (e.g. sprint). The recommended dose is 3–5 g/day [60].
Side effects
The most common adverse effect is transient water retention in the early stages of supplementation [61]. Some studies report muscle cramps, dehydration, gastrointestinal distress (vomiting, diarrhea) and liver dysfunction [3,62]. Position statements of International Society of Sports Nutrition published in 2007 and 2017 are that creatine monohydrate is safe and effective ergogenic supplement [60,63]. An internationally renowned team of experts has performed an evidence-based scientific evaluation of literature and confirmed the statements [64].
However, it should be noted that there is insufficient information on the effect of creatine supplementation on the kidneys and the liver in the elderly, individuals with renal disease, and when taken at higher than recommended doses for several months [61,65]. It has been advised that high-dose (>5 g/day) creatine supplementation should not be used by individuals with pre-existing renal disease [3]. Furthermore, it has been shown that enhanced creatine consumption results in its increased conversion to sarcosine and then to cytotoxic and carcinogenic agents methylamine and formaldehyde [66]. Although excretion of methylamine and formaldehyde not reach the limit values for healthy individuals, long term studies are required to evaluate risks of enhanced production of these agents.
Because meat and/or creatine intake increases blood creatinine levels, tests that are not based on plasma levels and urinary creatinine excretion should be used to assess renal function in individuals consuming creatine.
Resume
The conditions under which increased creatine intake is inappropriate should be defined.
Glutamine (GLN)
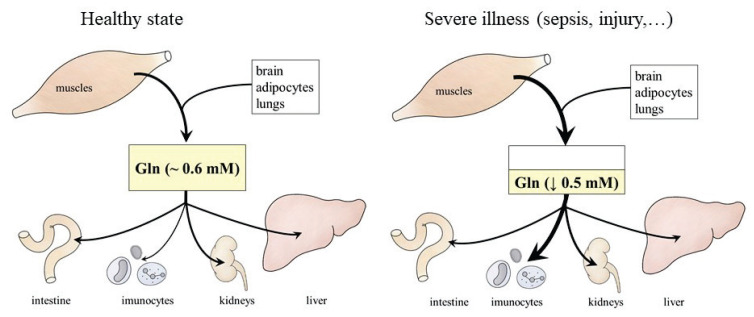
GLN is proteinogenic amino acid known as an important energy fuel for rapidly growing cells (especially immune cells, enterocytes, and tumor cells), as precursor for synthesis of glucose, ammonia, and nucleic acids, and it plays a signalling role in many processes, including expression of genes. Most GLN are synthesized in muscles, in smaller amounts it is released into the blood from the brain, lungs and adipose tissue (Fig. 5).
Fig. 5.
GLN synthesis and utilization in healthy conditions and severe illness. In a healthy individual, there is a balance between synthesis and utilization of GLN. In severe disease, GLN utilization often exceeds its synthesis.
GLN concentration in plasma and tissues decreases postoperatively, after multiple trauma, in exhaustive exercise, major burns, and during sepsis and contributes to negative protein balance, immunosuppression and enhanced morbidity and mortality on underlying illness [67–71]. Therefore, GLN is considered as a “conditionally essential” amino acid with proposed favorable effects on immune system, gut, and protein balance [72–75]. GLN supplementation doses range from 2 g/day to 40 g/day (high-dose GLN supple-mentation). Due to the instability of GLN and its limited solubility, its dipeptides (glycyl-GLN or alanyl-GLN) are sometimes used instead of GLN.
Side effects
GLN supplementation is without signs of toxicity or mutagenic activity [67,76–78]. However, enhanced GLN intake, especially by parenteral route, substantially increases glomerular filtration rate, renal plasma flow, and ammonia production and protein content in the kidneys [8–10,79]. Higher concentrations of serum urea nitrogen and creatinine and decrease in glomerular filtration rate were found in older volunteers who received 0.5 g GLN/kg/day than in middle-aged individuals [80]. It has been shown that chronic intake of GLN-enriched diet has negative influence on protein balance in muscles and alters amino acid concentrations in plasma and tissues in healthy rats [79,81].
Tumor tissue cells utilize GLN much faster than normal cells and die rapidly in a medium lacking GLN [4,11] and many tumors exhibit special transporters responsible for fast transport of GLN across their membranes [82]. Studies dealing with hepatoma cells demonstrated that GLN transporters referred to as ASCT2 [83] are not expressed in normal liver cells. It was found that GLN was acting as a cellular signal to maintain ASCT2 expression and that enhanced glutaminase activity mediates signalling events coupled with c-myc oncogenesis [84–86]. Hence, there is an obvious risk that increased GLN availability will promote tumor growth. On the other side, several studies have demonstrated that GLN supplementation improves the protein balance in tumor-bearing animals [87,88], the function of natural killer cells [89,90] and has no effect on tumor weight or protein and DNA synthesis [87,91,92].
Because most of the GLN administered is utilized to form ammonia, increased GLN consumption may exert adverse effects in subjects with hyperammonaemia, such as subjects with liver disease or urea cycle disorders [81]. There is strong evidence that GLN accumulation in astrocytes contributes to the cerebral edema in acute hepatic failure and to the Alzheimer type II astrocytes in chronic hyperammonemia [93]. Impairment in the electroencephalogram and neurological deficits during oral GLN challenge in cirrhotic patients indicate that GLN intake in patients with liver disease should be restricted [94,95].
Resume
It is inappropriate to administer high doses of GLN in conditions not associated with GLN deficiency, especially in elderly and individuals with hepatic and renal impairment. Research is needed to elucidate whether it is safe to consume GLN supplements by patients with cancer.
Histidine (HIS)
The imidazole ring in HIS structure determines its role as an important buffer, antioxidant, and chelating agent. HIS content is high in hemoproteins (hemoglobin, myoglobin, cytochromes, hem peroxidases, etc.) and epidermal barrier protein termed filaggrin [30]. Atopic dermatitis and anemia are the first consequences of prolonged consumption of HIS-deficient diet [96].
HIS and HIS-containing dipeptides, notably carnosine, are investigated in order to increase muscle performance and delay fatigue in athletes, reduce the effect of reactive oxygen and nitrogen species in aging-related disorders, neurodegenerative diseases, metabolic syndrome, rheumatoid arthritis, and for therapy of eczema [30]. HIS supplementation doses range from 1 to 4 g/day. High amounts of HIS are in solutions employed for organ preservation before transplantation and myocardial protection in cardiac surgery [97].
Side effects
There are no data on the toxic or mutagenic effects of increased HIS intake. Although HIS is a precursor of histamine, there are no data on allergies or peptic ulcer disease. Of practical significance might be anorexia, decreased folic acid levels, and increased urinary zinc losses [7,98,99]. Several articles have reported that HIS administration may increase ammonia (three nitrogen atoms are present in one HIS molecule) and affect the level of several amino acids. The most common are increased levels of alanine, glutamine, and glutamate and decreased levels of glycine and BCAA [100]. In addition, hypercholesterolemia and liver enlargement have been reported after long-term intake of HIS [101–104].
Resume
A long-term intake of high amounts of HIS is not suitable for people with impaired liver function.
β-Hydroxy-β-methylbutyrate (HMB)
HMB is a metabolite of leucine with a positive effect on protein balance, repair, strength and function of skeletal muscle [105]. HMB is used as a dietary supplement mainly in athletes, studies performed with older people have demonstrated that HMB attenuates development of sarcopenia [106,107]. In a recent study was shown that HMB restores muscle protein homeostasis in liver disease [108]. HMB is commercially available in the form of calcium salts, less often as the free form. The recommended dose is 3 g/day.
Side effects
Most studies report that HMB is well tolerated and has no toxic effects [2,109,110]. However, probably due to protein anabolic effect of HMB on muscles, decreased GLN level has been reported in rats at 24 h after HMB treatment [111]. Decreased ATP concentra-tions and increased AMP/ATP ratios were found in the liver and muscles of HMB-treated rats with diabetes [112].
Resume
It should be examined whether the positive effects of HMB on protein balance in muscles are not associated with adverse effects on amino acid concentrations and ATP metabolism.
Leucine (LEU)
Of all the amino acids, LEU is the most powerful anabolic agent [34,113–115]. Proponents of use of LEU alone as a dietary supplement claim that competition between LEU and other neutral amino acids for transport into muscle cells hinders the unique effects of LEU on protein synthesis and, therefore, using LEU alone is more effective than a mixture of all three BCAA (leucine, valine, and isoleucine).
The effects of a dietary supplement of LEU alone are studied mainly in athletes and old people. Several articles indicate that LEU may overcome the blunted muscle protein synthesis response to food ingestion in the elderly, treat sarcopenia, and improve glycemic control in type 2 diabetes [114–117]. A commonly used dose is 5 g/day.
Side effects
Most studies reporting positive effects of LEU on muscles are short-term studies, which report positive effects on protein synthesis and not on mass of muscle protein [115,118–120]. Positive influence of LEU supplementation on protein balance was not observed by most of long-term studies [121–123], and there are also reports of negative effects [43,124].
Several studies evaluating the consequences of enhanced LEU intake revealed a decrease in concentrations of valine and isoleucine in plasma [43,124,125], and even in muscles [43]. Decreased levels of valine and isoleucine are due to induction of BCKA dehydrogenase (rate limiting enzyme in BCAA catabolism) by ketoisocaproate, a product of LEU transamination, in liver, muscles, and adipose tissue [44,125–128]. A role in depletion of valine and isoleucine pools may play also their competition with LEU for transport via the L-carrier system. This already occurs in the gut. Higher intracellular concentrations of valine and isoleucine in enterocytes of jejunum of animals fed by LEU-enriched diet indicate impaired transport through basolateral membrane of enterocytes [43]. The availability of valine and isoleucine may therefore become rate limiting for protein synthesis when LEU alone is consumed.
Resume
There are significant limitations if LEU alone is used as a dietary supplement, although its stimulatory effect on protein synthesis in muscles has been confirmed. Due to deficiency of valine and isoleucine, administration of LEU alone could have only a transitory stimulatory effect on protein synthesis.
Tryptophan (TRP)
TRP is an essential proteinogenic amino acid used to treat depression and insomnia. Its effect is induced by TRP-induced increase in synthesis of serotonin (5-hydroxytryptamine), a neurotransmitter known to regulate neuronal circuits that control sleep and mood. Next to its role as a neurotransmitter, serotonin is the precursor of melatonin, modulates gut and immune functions, and plays a role in hemostasis.
TRP supplementation has been employed as a potential treatment for depression and sleep disturbances since the early 1960s. In addition, there is considerable evidence for beneficial effects of TRP on mood and social behavior. TRP can reduce aggression in schizophrenic patients while increasing agreeableness in people with a tendency to irritability or aggression [51].
The well-known affair of adverse effect of enhanced consumption of TRP as a supplement is the eosinophilia–myalgia syndrome reported in the 1989 [129]. Several thousand cases have occurred in users of TRP for insomnia and mood elevation, several dozen of which have resulted in deaths. The cause was traced to impurities in synthetic TRP supplements from one Japanese manufacturer. This issue will not be considered as a possible side effect of TRP supplementation.
Single doses of TRP from 1 g to 15 g were used both acutely and chronically for periods up to 2 years [130]. A commonly used dose is 3 g/day.
Side effects
Although TRP has been studied for 6 decades, few side effects, which include tremor, nausea, and dizziness, have been reported [130]. A more common effect of high doses of TRP, which can be expected due to the stimulating effect of TRP on serotonin synthesis, is fatigue or drowsiness.
A potentially life-threatening condition is “serotonin syndrome” (also referred to as serotonin toxicity), which includes neuromuscular abnormalities, autonomic hyperactivity, and mental state changes [131]. Serotonin syndrome is usually precipitated by the simultaneous administration of two or more drugs, which enhance serotonin availability, such as serotonin reuptake or MAO inhibitors.
In humans, intestinal cells and gut microbiota play an important role in TRP metabolism. The kynurenine-anthranilate route produces kynurenine and its derivatives (kynurenic acids, 3-hydroxykynurenine, 3-hydroxyanthranilate, and anthranilic acid). In enterochromaffin cells, hydroxylation of TRP yields 5-hydroxytryptophan, which is decarboxylated to serotonin. The degradation of side chain of TRP results in indole and a number of related substances, such as indole propionate, indole acetate, methyl indole (skatole) and indole lactate. Most of metabolites of TRP are classified as uremic toxins and act as ligands for aryl hydrocarbon receptors (AHR), which play a crucial role in immune response, epithelial renewal, barrier integrity, and metastasis of cancer cells [132]. In a recent study was shown that TRP supplementation in mice and human volunteers increases kynurenine metabolites in circulation [133].
Resume
Careful studies examining effects of TRP supplementation on AHR activation and concentrations of TRP metabolites should be performed. Because TRP is a precursor to serotonin, caution should be exercised when supplementing it with drugs that affect serotonin metabolism.
Conclusions
Amino acids and their derivatives are commonly used dietary supplement and there are a number of studies on their beneficial effects both in healthy and sick individuals. However, data on their possible side effects, including those that may be harmful to consumers, are rare. Only sporadically are mentioned consequences of adaptive responses on long-term amino acid intake, such as altered degradation and excretion of a supplement, expression of amino acid transporters, and synthesis of toxic metabolites. Almost absent are reports on side effects of supplements based on combination of individual amino acids or combination with other substances or medications. Adverse influence on metabolism may have also the withdrawal of a supplement. Starvation for 24 h of animals previously fed by GLN-enriched diet resulted in a significant decrease in GLN concentration in body fluids and more pronounced decrease in protein synthesis in the liver, jejunum, colon, and spleen when compared with animals fed before starvation by a basal diet [79].
In conclusion, the reports summarized in this article demonstrate that enhanced intake of most amino acid supplements can cause adverse side effects. Further research is necessary to elucidate effects of high doses and long-term consumption of amino acids on immune system, brain function, muscle protein balance, synthesis of toxic metabolites, and tumor growth and examine their suitability under certain circumstances. These include elderly, childhood, pregnancy, and nursing a baby and medical condition, such as diabetes and liver disease. Studies are also needed to examine adaptive response to a long-term intake of any substance and consequences of discontinuation of supplementation.
Acknowledgements
Supported by the program PROGRES Q40/02 of Charles University.
Abbreviations
- AHR
aryl hydrocarbon receptors
- ARG
arginine
- BA
β-alanine
- BCAA
branched-chain amino acids
- BCKA
branched-chain keto acids
- CAR
carnosine
- CIT
citrulline
- GLN
glutamine
- HIS
histidine
- HMB
β-hydroxy-β-methylbutyrate
- LEU
leucine
- NO
nitric oxide
- NOS
nitric oxide synthase
- ROS
reactive oxygen species
- TRP
tryptophan
- α-KG
α-ketoglu-tarate
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Fabresse N, Gheddar L, Kintz P, Knapp A, Larabi IA, Alvarez JC. Analysis of pharmaceutical products and dietary supplements seized from the black market among bodybuilders. Forensic Sci Int. 2021;322:110771. doi: 10.1016/j.forsciint.2021.110771. [DOI] [PubMed] [Google Scholar]
- 2.Baxter JH, Carlos JL, Thurmond J, Rehani RN, Bultman J, Frost D. Dietary toxicity of calcium beta-hydroxy-beta-methyl butyrate (CaHMB) Food Chem Toxicol. 2005;43:1731–1741. doi: 10.1016/j.fct.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Kim CK, Carpentier A, Poortmans JR. Studies on the safety of creatine supplementation. Amino Acids. 2011;40:1409–1418. doi: 10.1007/s00726-011-0878-2. [DOI] [PubMed] [Google Scholar]
- 4.Bode BP, Kaminski DL, Souba WW, Li AP. Glutamine transport in isolated human hepatocytes and transformed liver cells. Hepatology. 1995;21:511–520. [PubMed] [Google Scholar]
- 5.Park KG. The Sir David Cuthbertson Medal Lecture 1992. The immunological and metabolic effects of L-arginine in human cancer. Proc Nutr Soc. 1993;52:387–401. doi: 10.1079/pns19930080. [DOI] [PubMed] [Google Scholar]
- 6.Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 1986;10:227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- 7.Geliebter AA, Hashim SA, Van Itallie TB. Oral L-histidine fails to reduce taste and smell acuity but induces anorexia and urinary zinc excretion. Am J Clin Nutr. 1981;34:119–120. doi: 10.1093/ajcn/34.1.119. [DOI] [PubMed] [Google Scholar]
- 8.Tietze IN, S⊘rensen SS, Eiskjaer H, Thomsen K, Pedersen EB. Tubular handling of amino acids after intravenous infusion of amino acids in healthy humans. Nephrol Dial Transplant. 1992;7:493–500. [PubMed] [Google Scholar]
- 9.Gougoux A, Vinay P, Halperin ML. Regulation of renal ammoniagenesis in the dog with chronic metabolic acidosis: effect of a glutamine load. Am J Physiol. 1985;249:F745–F752. doi: 10.1152/ajprenal.1985.249.5.F745. [DOI] [PubMed] [Google Scholar]
- 10.Canessa-Fischer M, Shalhoub R, Glabman S, de Haas J, Pitts RF. Effects of infusions of ammonia, amides, and amino acids on excretion of ammonia. Am J Physiol. 1963;204:192–196. doi: 10.1152/ajplegacy.1963.204.2.192. [DOI] [PubMed] [Google Scholar]
- 11.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albaugh VL, Pinzon-Guzman C, Barbul A. Arginine-dual roles as an onconutrient and immunonutrient. J Surg Oncol. 2017;115:273–280. doi: 10.1002/jso.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans RW, Fernstrom JD, Thompson J, Morris SM, Jr, Kuller LH. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J Nutr Biochem. 2004;15:534–539. doi: 10.1016/j.jnutbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Holecek M, Sispera L. Effects of arginine supplementation on amino acid profiles in blood and tissues in fed and overnight-fasted rats. Nutrients. 2016;8:206. doi: 10.3390/nu8040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze F, Glos S, Petruschka D, Altenburg C, Maas R, Benndorf R, Schwedhelm E, Beil U, Böger RH. L-Arginine enhances the triglyceride-lowering effect of simvastatin in patients with elevated plasma triglycerides. Nutr Res. 2009;29:291–297. doi: 10.1016/j.nutres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Flynn NE, Knabe DA, Mallick BK, Wu G. Postnatal changes of plasma amino acids in suckling pigs. J Anim Sci. 2000;7:2369–2375. doi: 10.2527/2000.7892369x. [DOI] [PubMed] [Google Scholar]
- 17.Luiking YC, Poeze M, Deutz NE. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin Sci (Lond) 2015;128:57–67. doi: 10.1042/CS20140343. [DOI] [PubMed] [Google Scholar]
- 18.Oka RK, Szuba A, Giacomini JC, Cooke JP. A pilot study of L-arginine supplementation on functional capacity in peripheral arterial disease. Vasc Med. 2005;10:265–274. doi: 10.1191/1358863x05vm637oa. [DOI] [PubMed] [Google Scholar]
- 19.Bednarz B, Jaxa-Chamiec T, Maciejewski P, Szpajer M, Janik K, Gniot J, Kawka-Urbanek T, Drozdowska D, Gessek J, Laskowski H. Efficacy and safety of oral l-arginine in acute myocardial infarction. Results of the multicenter, randomized, double-blind, placebo-controlled ARAMI pilot trial. Kardiol Pol. 2005;62:421–427. [PubMed] [Google Scholar]
- 20.Balasubramanian A, Thirumavalavan N, Srivatsav A, Yu J, Hotaling JM, Lipshultz LI, Pastuszak AW. An analysis of popular online erectile dysfunction supplements. J Sex Med. 2019;16:843–852. doi: 10.1016/j.jsxm.2019.03.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, Shen WQ, Chen HL. Efficacy of arginine-enriched enteral formulas for the healing of pressure ulcers: A systematic review. J Wound Care. 2017;26:319–323. doi: 10.12968/jowc.2017.26.6.319. [DOI] [PubMed] [Google Scholar]
- 22.Tan B, Li X, Yin Y, Wu Z, Liu C, Tekwe CD, Wu G. Regulatory roles for L-arginine in reducing white adipose tissue. Front Biosci (Landmark Ed) 2012;17:2237–2246. doi: 10.2741/4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta S, Stewart DJ, Levy RD. The hypotensive effect of L-arginine is associated with increased expired nitric oxide in humans. Chest. 1996;109:1550–555. doi: 10.1378/chest.109.6.1550. [DOI] [PubMed] [Google Scholar]
- 24.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 25.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Mohan S, Wu CC, Shin S, Fung HL. Continuous exposure to L-arginine induces oxidative stress and physiological tolerance in cultured human endothelial cells. Amino Acids. 2012;43:1179–1188. doi: 10.1007/s00726-011-1173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Ladeiras D, Yu Y, Ming XF, Yang Z. Detrimental effects of chronic L-arginine rich food on aging kidney. Front Pharmacol. 2021;11:582155. doi: 10.3389/fphar.2020.582155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Favero S, Roschel H, Solis MY, Hayashi AP, Artioli GG, Otaduy MC, Benatti FB, Harris RC, Wise JA, Leite CC, Pereira RM, de Sá-Pinto AL, Lancha-Junior AH, Gualano Beta-alanine (Carnosyn™) supplementation in elderly subjects (60–80 years): effects on muscle carnosine content and physical capacity. Amino Acids. 2012;43:49–56. doi: 10.1007/s00726-011-1190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blancquaert L, Everaert I, Missinne M, Baguet A, Stegen S, Volkaert A, Petrovic M, Vervaet C, Achten E, De Maeyer M, De Henauw S, Derave W. Effects of histidine and β-alanine supplementation on human muscle carnosine storage. Med Sci Sports Exerc. 2017;49:602–609. doi: 10.1249/MSS.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 30.Holeček M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients. 2020;12:848. doi: 10.3390/nu12030848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders B, Franchi M, De Oliveira LF, Da Eira Silva V, Da Silva RP, De Salles Painelli V, Costa LAR, Sale C, Harris RC, Roschel H, Artioli GG, Gualano B. 24-Week β-alanine ingestion does not affect muscle taurine or clinical blood parameters in healthy males. Eur J Nutr. 2020;59:57–65. doi: 10.1007/s00394-018-1881-0. [DOI] [PubMed] [Google Scholar]
- 32.Dolan E, Swinton PA, Painelli VS, Stephens Hemingway B, Mazzolani B, Infante Smaira F, Saunders B, Artioli GG, Gualano B. A systematic risk assessment meta-analysis on the use of oral β-alanine supplementation. Adv Nutr. 2019;10:452–463. doi: 10.1093/advances/nmy115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135(6 Suppl):1547S–1552S. doi: 10.1093/jn/135.6.1547S. [DOI] [PubMed] [Google Scholar]
- 35.Holeček M. The role of skeletal muscle in the pathogenesis of altered concentrations of branched-chain amino acids (valine, leucine, and isoleucine) in liver cirrhosis, diabetes, and other diseases. Physiol Res. 2021;70:293–305. doi: 10.33549/physiolres.934648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holeček M. Why are branched-chain amino acids increased in starvation and diabetes? Nutrients. 2020;12:3087. doi: 10.3390/nu12103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond) 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K. Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Horm Metab Res. 1998;30:188–194. doi: 10.1055/s-2007-978864. [DOI] [PubMed] [Google Scholar]
- 39.Gijsman HJ, Scarnà A, Harmer CJ, McTavish SB, Odontiadis J, Cowen PJ, Goodwin GM. A dose-finding study on the effects of branch chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology (Berl) 2002;160:192–197. doi: 10.1007/s00213-001-0970-5. [DOI] [PubMed] [Google Scholar]
- 40.Rose WC, Wixom RL, Lockhart HB, Lambert GF. The amino acid requirements of man. XV. The valine requirement; summary and final observations. J Biol Chem. 1955;217:987–995. [PubMed] [Google Scholar]
- 41.Heger J, Van Phung T, Krízová L, Sustala M, Simecek K. Efficiency of amino acid utilization in the growing pig at suboptimal levels of intake: branched-chain amino acids, histidine and phenylalanine + tyrosine. J Anim Physiol Anim Nutr (Berl) 2003;87:52–65. doi: 10.1046/j.1439-0396.2003.00406.x. [DOI] [PubMed] [Google Scholar]
- 42.Bikker P, Verstegen MW, Bosch MW. Amino acid composition of growing pigs is affected by protein and energy intake. J Nutr. 1994;124:1961–1969. doi: 10.1093/jn/124.10.1961. [DOI] [PubMed] [Google Scholar]
- 43.Holecek M, Siman P, Vodenicarovova M, Kandar R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr Metab (Lond) 2016;13:12. doi: 10.1186/s12986-016-0072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 45.Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, Sandlers Y, Van Wagoner DR, Flask CA, Hoppel C, Kasumov T, Dasarathy S. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis oxidative stress. J Physiol. 2016;594:7341–7360. doi: 10.1113/JP272796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagenmakers AJ, Coakley JH, Edwards RH. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle’s disease. Int J Sports Med. 1990;11(Suppl 2):S101–S113. doi: 10.1055/s-2007-1024861. [DOI] [PubMed] [Google Scholar]
- 47.Holecek M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis. 2014;29:9–17. doi: 10.1007/s11011-013-9428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holeček M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80–5. doi: 10.1016/j.nut.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 49.MacLean DA, Graham TE. Branched-chain amino acid supplementation augments plasma ammonia responses during exercise in humans. J Appl Physiol (1985) 1993;74:2711–2717. doi: 10.1152/jappl.1993.74.6.2711. [DOI] [PubMed] [Google Scholar]
- 50.MacLean DA, Graham TE, Saltin B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J Physiol. 1996;493:909–922. doi: 10.1113/jphysiol.1996.sp021433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibson EL. Tryptophan supplementation and serotonin function: genetic variations in behavioural effects. Proc Nutr Soc. 2018;77:174–188. doi: 10.1017/S0029665117004451. [DOI] [PubMed] [Google Scholar]
- 52.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10:350–52. doi: 10.1111/1753-0407.12645. [DOI] [PubMed] [Google Scholar]
- 53.Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8:405. doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc) 2000;65:757–765. [PubMed] [Google Scholar]
- 55.Liu Y, Cotillard A, Vatier C, Bastard JP, Fellahi S, Stévant M, Allatif O, Langlois C, Bieuvelet S, Brochot A, Guilbot A, Clément K, Rizkalla SW. A dietary supplement containing cinnamon, chromium and carnosine decreases fasting plasma glucose and increases lean mass in overweight or obese pre-diabetic subjects: A randomized, placebo-controlled trial. PLoS One. 2015;10:e0138646. doi: 10.1371/journal.pone.0138646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park YJ, Volpe SL, Decker EA. Quantitation of carnosine in humans plasma after dietary consumption of beef. J Agric Food Chem. 2005;53:4736–4739. doi: 10.1021/jf047934h. [DOI] [PubMed] [Google Scholar]
- 57.Crenn P, Coudray-Lucas C, Cynober L, Messing B. Post-absorptive plasma citrulline concentration: a marker of intestinal failure in humans. Transplant Proc. 1998;30:2528. doi: 10.1016/s0041-1345(98)00711-8. [DOI] [PubMed] [Google Scholar]
- 58.Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. L-Citrulline supplementation: Impact on cardiometabolic health. Nutrients. 2018;10:921. doi: 10.3390/nu10070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osowska S, Moinard C, Neveux N, Loï C, Cynober L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut. 2004;53:1781–1786. doi: 10.1136/gut.2004.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J. International Society of Sports Nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr. 2007;4:6. doi: 10.1186/1550-2783-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall M, Trojian TH. Creatine supplementation. Curr Sports Med Rep. 2013;12:240–44. doi: 10.1249/JSR.0b013e31829cdff2. [DOI] [PubMed] [Google Scholar]
- 62.Juhn MS, O’Kane JW, Vinci DM. Oral creatine supplementation in male collegiate athletes: a survey of dosing habits and side effects. J Am Diet Assoc. 1999;99:593–595. doi: 10.1016/s0002-8223(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 63.Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, Candow DG, Kleiner SM, Almada AL, Lopez HL. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017;14:18. doi: 10.1186/s12970-017-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antonio J, Candow DG, Forbes SC, Gualano B, Jagim AR, Kreider RB, Rawson ES, Smith-Ryan AE, VanDusseldorp TA, Willoughby DS, Ziegenfuss TN. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? J Int Soc Sports Nutr. 2021;18:13. doi: 10.1186/s12970-021-00412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davani-Davari D, Karimzadeh I, Ezzatzadegan-Jahromi S, Sagheb MM. Potential adverse effects of creatine supplement on the kidney in athletes and bodybuilders. Iran J Kidney Dis. 2018;12:253–260. [PubMed] [Google Scholar]
- 66.Poortmans JR, Kumps A, Duez P, Fofonka A, Carpentier A, Francaux M. Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Med Sci Sports Exerc. 2005;37:1717–1720. doi: 10.1249/01.mss.0000176398.64189.e6. [DOI] [PubMed] [Google Scholar]
- 67.Ziegler TR, Szeszycki EE, Estívariz CF, Puckett AB, Leader LM. Glutamine: from basic science to clinical applications. Nutrition. 1996;12(11–12 Suppl):S68–S70. doi: 10.1016/s0899-9007(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 68.Décombaz J, Reinhardt P, Anantharaman K, von Glutz G, Poortmans JR. Biochemical changes in a 100 km run: free amino acids, urea, and creatinine. Eur J Appl Physiol Occup Physiol. 1979;41:61–72. doi: 10.1007/BF00424469. [DOI] [PubMed] [Google Scholar]
- 69.Brodan V, Kuhn E, Pechar J, Tomková D. Changes of free amino acids in plasma of healthy subjects induced by physical exercise. Eur J Appl Physiol Occup Physiol. 1976;35:69–77. doi: 10.1007/BF00444658. [DOI] [PubMed] [Google Scholar]
- 70.Roth E, Funovics J, Mühlbacher F, Schemper M, Mauritz W, Sporn P, Fritsch A. Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr. 1982;1:25–41. doi: 10.1016/0261-5614(82)90004-8. [DOI] [PubMed] [Google Scholar]
- 71.Holecek M, Sispera L. Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats. Amino Acids. 2014;46:1377–1384. doi: 10.1007/s00726-014-1701-7. [DOI] [PubMed] [Google Scholar]
- 72.Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lawin P, Fürst P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989;1:231–233. doi: 10.1016/s0140-6736(89)91254-3. [DOI] [PubMed] [Google Scholar]
- 73.Wernerman J, Hammarqvist F, Vinnars E. Alpha-ketoglutarate and postoperative muscle catabolism. Lancet. 1990;335:701–703. doi: 10.1016/0140-6736(90)90811-i. [DOI] [PubMed] [Google Scholar]
- 74.Newsholme E, Hardy G. Supplementation of diets with nutritional pharmaceuticals. Nutrition. 1997;13:837–839. doi: 10.1016/s0899-9007(97)00253-0. [DOI] [PubMed] [Google Scholar]
- 75.Hardy G, Hardy IJ. Can glutamine enable the critically ill to cope better with infection? JPEN J Parenter Enteral Nutr. 2008;32:489–491. doi: 10.1177/0148607108319796. [DOI] [PubMed] [Google Scholar]
- 76.Ziegler TR, Benfell K, Smith RJ, Young LS, Brown E, Ferrari-Baliviera E, Lowe DK, Wilmore DW. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990;14(4 Suppl):137S–146S. doi: 10.1177/0148607190014004201. [DOI] [PubMed] [Google Scholar]
- 77.Tsubuku S, Hatayama K, Mawatari K, Smriga M, Kimura T. Thirteen-week oral toxicity study of L-glutamine in rats. Int J Toxicol. 2004;23:107–112. doi: 10.1080/10915810490435677. [DOI] [PubMed] [Google Scholar]
- 78.Wong AW, Magnuson BA, Nakagawa K, Bursey RG. Oral subchronic and genotoxicity studies conducted with the amino acid, L-glutamine. Food Chem Toxicol. 2011;49:2096–2102. doi: 10.1016/j.fct.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 79.Holeček M. Adverse effects of chronic intake of glutamine-supplemented diet on amino acid concentrations and protein metabolism in rat: effect of short-term starvation. Clin Nutr ESPEN. 2011;6:E190–E196. doi: 10.1016/j.eclnm.2011.05.002. [DOI] [Google Scholar]
- 80.Galera SC, Fechine FV, Teixeira MJ, Coelho ZC, de Vasconcelos RC, de Vasconcelos PR. The safety of oral use of L-glutamine in middle-aged and elderly individuals. Nutrition. 2010;26:375–381. doi: 10.1016/j.nut.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 81.Holecek M. Side effects of long-term glutamine supplementation. JPEN J Parenter Enteral Nutr. 2013;37:607–616. doi: 10.1177/0148607112460682. [DOI] [PubMed] [Google Scholar]
- 82.Matsuno T, Satoh T. Glutamine metabolism in the avian host bearing transplantable hepatomatous growth induced by MC-29 virus. Int J Biochem. 1986;18:187–189. doi: 10.1016/0020-711x(86)90155-2. [DOI] [PubMed] [Google Scholar]
- 83.McGivan JD, Bungard CI. The transport of glutamine into mammalian cells. Front Biosci. 2007;12:874–882. doi: 10.2741/2109. [DOI] [PubMed] [Google Scholar]
- 84.Bungard CI, McGivan JD. Glutamine availability up-regulates expression of the amino acid transporter protein ASCT2 in HepG2 cells and stimulates the ASCT2 promoter. Biochem J. 2004;382:27–32. doi: 10.1042/BJ20040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao P, Tchernyshyov I, Chang TC, Lee Y-S, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A. 2009;106:14878–14883. doi: 10.1073/pnas.0901221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Bricon T. Effect of glutamine supplementation on protein metabolism and glutathione in tumor-bearing rats. Clin Nutr. 1996;15:211. doi: 10.1016/s0261-5614(96)80244-5. [DOI] [PubMed] [Google Scholar]
- 88.Kaibara A, Yoshida S, Yamasaki K, Ishibashi N, Kakegawa T. Effect of glutamine and chemotherapy on protein metabolism in tumor-bearing rats. J Surg Res. 1994;57:143–149. doi: 10.1006/jsre.1994.1122. [DOI] [PubMed] [Google Scholar]
- 89.Fahr MJ, Kornbluth J, Blossom S, Schaeffer R, Klimberg VS, Harry M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. JPEN J Parenter Enteral Nutr. 1994;18:471–476. doi: 10.1177/0148607194018006471. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida S, Kaibara A, Yamasaki K, Ishibashi N, Noake T, Kakegawa T. Effect of glutamine supplementation on protein metabolism and glutathione in tumor-bearing rats. JPEN J Parenter Enteral Nutr. 1995;19:492–497. doi: 10.1177/0148607195019006492. [DOI] [PubMed] [Google Scholar]
- 91.Klimberg VS, Souba WW, Salloum RM, Plumley DA, Cohen FS, Dolson DJ, Bland KI, Copeland EM. Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J Surg Res. 1990;48:319–323. doi: 10.1016/0022-4804(90)90066-b. [DOI] [PubMed] [Google Scholar]
- 92.Austgen TR, Dudrick PS, Sitren H, Bland KI, Copeland E, Souba WW. The effects of glutamine-enriched total parenteral nutrition on tumor growth and host tissues. Ann Surg. 1992;215:107–113. doi: 10.1097/00000658-199202000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Albrecht J, Zielińska M, Norenberg MD. Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem Pharmacol. 2010;80:1303–1308. doi: 10.1016/j.bcp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oppong KN, Al-Mardini H, Thick M, Record CO. Oral glutamine challenge in cirrhotics pre- and post-liver transplantation: a psychometric and analyzed EEG study. Hepatology. 1997;26:870–76. doi: 10.1002/hep.510260411. [DOI] [PubMed] [Google Scholar]
- 95.Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(Suppl 1):S45–S53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 96.Kopple JD, Swendseid ME. Evidence that histidine is an essential amino acid in normal and chronically uremic man. J Clin Invest. 1975;55:881–891. doi: 10.1172/JCI108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edelman JJ, Seco M, Dunne B, Matzelle SJ, Murphy M, Joshi P, Yan TD, Wilson MK, Bannon PG, Vallely MP, Passage J. Custodiol for myocardial protection and preservation: a systematic review. Ann Cardiothorac Surg. 2013;2:717–728. doi: 10.3978/j.issn.2225-319X.2013.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheiner JB, Morris P, Anderson GH. Food intake suppression by histidine. Pharmacol Biochem Behav. 1985;23:721–726. doi: 10.1016/0091-3057(85)90061-9. [DOI] [PubMed] [Google Scholar]
- 99.Yoshimatsu H, Chiba S, Tajima D, Akehi Y, Sakata T. Histidine suppresses food intake through its conversion into neuronal histamine. Exp Biol Med (Maywood) 2002;227:63–68. doi: 10.1177/153537020222700111. [DOI] [PubMed] [Google Scholar]
- 100.Holeček M. Influence of histidine administration on ammonia and amino acid metabolism: A review. Physiol Res. 2020;69:555–564. doi: 10.33549/physiolres.934449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solomon JK, Geison RL. Effect of excess dietary L-histidine on plasma cholesterol levels in weanling rats. J Nutr. 1978;108:936–943. doi: 10.1093/jn/108.6.936. [DOI] [PubMed] [Google Scholar]
- 102.Harvey PW, Hunsaker HA, Allen KG. Dietary L-histidine-induced hypercholesterolemia and hypocupremia in the rat. J Nutr. 1981;111:639–647. doi: 10.1093/jn/111.4.639. [DOI] [PubMed] [Google Scholar]
- 103.Hitomi-Ohmura E, Amano N, Aoyama Y, Yoshida A. The effect of a histidine-excess diet on cholesterol synthesis and degradation in rats. Lipids. 1992;27:755–760. doi: 10.1007/BF02535845. [DOI] [PubMed] [Google Scholar]
- 104.Holeček M, Vodeničarovová V. Effects of histidine supplementation on amino acid metabolism in rats. Physiol Res. 2020;69:99–111. doi: 10.33549/physiolres.934296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017;8:529–541. doi: 10.1002/jcsm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsieh LC, Chow CJ, Chang WC, Liu TH, Chang CK. Effect of beta-hydroxy-beta-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia Pac J Clin Nutr. 2010;19:200–208. [PubMed] [Google Scholar]
- 107.Vukovich MD, Stubbs NB, Bohlken RM. Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J Nutr. 2001;131:2049–2052. doi: 10.1093/jn/131.7.2049. [DOI] [PubMed] [Google Scholar]
- 108.Singh SS, Kumar A, Welch N, Sekar J, Mishra S, Bellar A, Gangadhariah M, Attaway A, Al Khafaji H, Wu X, Pathak V, Agrawal V, McMullen MR, Hornberger TA, Nagy LE, Davuluri G, Dasarathy S. Multiomics-identified intervention to restore ethanol-induced dysregulated proteostasis and secondary sarcopenia in alcoholic liver disease. Cell Physiol Biochem. 2021;55:91–116. doi: 10.33594/000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC., Jr Beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130:1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- 110.Rathmacher JA, Nissen S, Panton L, Clark RH, Eubanks May P, Barber AE, D’Olimpio J, Abumrad NN. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enteral Nutr. 2004;28:65–75. doi: 10.1177/014860710402800265. [DOI] [PubMed] [Google Scholar]
- 111.Holecek M, Muthny T, Kovarik M, Sispera L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol. 2009;47:255–259. doi: 10.1016/j.fct.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 112.Holeček M, Vodeničarovová M, Fingrová R. Dual effects of beta-hydroxy-beta-methylbutyrate (HMB) on amino acid, energy, and protein metabolism in the liver and muscles of rats with streptozotocin-induced type 1 diabetes. Biomolecules. 2020;10:1475. doi: 10.3390/biom10111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005;135(6 Suppl):1553S–1556S. doi: 10.1093/jn/135.6.1553S. [DOI] [PubMed] [Google Scholar]
- 114.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 115.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond) 2010;7:57. doi: 10.1186/1743-7075-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–79. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- 119.Xu ZR, Tan ZJ, Zhang Q, Gui QF, Yang YM. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis. Br J Nutr. 2015;113:25–34. doi: 10.1017/S0007114514002475. [DOI] [PubMed] [Google Scholar]
- 120.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- 121.Verhoeven S, Vanschoonbeek K, Verdijk LB, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 122.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. 2011;141:1070–076. doi: 10.3945/jn.111.138495. [DOI] [PubMed] [Google Scholar]
- 123.Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–580. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- 124.May RC, Piepenbrock N, Kelly RA, Mitch WE. Leucine-induced amino acid antagonism in rats: muscle valine metabolism and growth impairment. J Nutr. 1991;121:293–301. doi: 10.1093/jn/121.3.293. [DOI] [PubMed] [Google Scholar]
- 125.Block KP, Harper AE. Valine metabolism in vivo: effects of high dietary levels of leucine and isoleucine. Metabolism. 1984;33:559–566. doi: 10.1016/0026-0495(84)90012-x. [DOI] [PubMed] [Google Scholar]
- 126.Aftring RP, Block KP, Buse MG. Leucine and isoleucine activate skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1986;250:E599–E604. doi: 10.1152/ajpendo.1986.250.5.E599. [DOI] [PubMed] [Google Scholar]
- 127.Jones SM, Yeaman SJ. Phosphorylation of branched-chain 2-oxo acid dehydrogenase complex in isolated adipocytes. Effects of 2-oxo acids. Biochem J. 1986;236:209–213. doi: 10.1042/bj2360209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frick GP, Tai LR, Blinder L, Goodman HM. L-Leucine activates branched chain alpha-keto acid dehydrogenase in rat adipose tissue. J Biol Chem. 1981;256:2618–2620. [PubMed] [Google Scholar]
- 129.Hertzman PA, Blevins WL, Mayer J, Greenfield B, Ting M, Gleich GJ. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N Engl J Med. 1990;322:869–873. doi: 10.1056/NEJM199003293221301. [DOI] [PubMed] [Google Scholar]
- 130.Fernstrom JD. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J Nutr. 2012;142:2236S–2244S. doi: 10.3945/jn.111.157065. [DOI] [PubMed] [Google Scholar]
- 131.Scotton WJ, Hill LJ, Williams AC, Barnes NM. Serotonin syndrome: Pathophysiology, clinical features, management, and potential future directions. Int J Tryptophan Res. 2019;12:1178646919873925. doi: 10.1177/1178646919873925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xue P, Fu J, Zhou Y. The aryl hydrocarbon receptor and tumor immunity. Front Immunol. 2018;9:286. doi: 10.3389/fimmu.2018.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valente-Silva P, Cervenka I, Ferreira DMS, Correia JC, Edman S, Horwath O, Heng B, Chow S, Jacobs KR, Guillemin GJ, Blomstrand E, Ruas JL. Effects of tryptophan supplementation and exercise on the fate of kynurenine metabolites in mice and humans. Metabolites. 2021;11:508. doi: 10.3390/metabo11080508. [DOI] [PMC free article] [PubMed] [Google Scholar]