Summary
The aim of this study was to evaluate therapeutic potential of edaravone in the murine model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE) and to expand the knowledge of its mechanism of action. Edaravone (6 mg/kg/day) was administered intraperitoneally from the onset of clinical symptoms until the end of the experiment (28 days). Disease progression was assessed daily using severity scores. At the peak of the disease, histological analyses, markers of oxidative stress (OS) and parameters of mitochondrial function in the brains and spinal cords (SC) of mice were determined. Gene expression of inducible nitric oxide synthase (iNOS), nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1) and peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha was determined at the end of the experiment. Edaravone treatment ameliorated EAE severity and attenuated inflammation in the SC of the EAE mice, as verified by histological analysis. Moreover, edaravone treatment decreased OS, increased the gene expression of the Nrf2 and HO-1, increased the activity of the mitochondrial complex II/III, reduced the activity of the mitochondrial complex IV and preserved ATP production in the SC of the EAE mice. In conclusion, findings in this study provide additional evidence of edaravone potential for the treatment of multiple sclerosis and expand our knowledge of the mechanism of action of edaravone in the EAE model.
Keywords: Experimental autoimmune encephalomyelitis, Edaravone, Mitochondrial dysfunction, Nrf2/HO-1 pathway, Oxidative stress
Introduction
Multiple sclerosis (MS) is an autoimmune neurological disease characterized by chronic inflammation of the central nervous system (CNS), resulting in a range of physical, mental, or even psychiatric symptoms [1]. Despite outstanding progress in the development of novel therapeutic agents for MS in recent years, we are still far from discovering an ultimate drug for MS. Current disease-modifying therapies aim to prevent the inflammatory damage to the CNS, but their severe adverse effects urge new, safe therapeutic approaches.
MS is characterized by auto-reactive IFN-gamma-producing Th1 and IL-17-producing Th17 effector cells and other immune cells (CD8+ T cells, B cells) that penetrate the CNS and damage the myelin sheath [2]. The inflammation in the CNS also results in activation of microglia, which produce pro-inflammatory mediators and elicit demyelination and axonal loss [3]. Another crucial feature of MS is oxidative stress (OS) emerging from the uncontrolled generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), in addition to the mitochondrial dysfunction and energy deficit [4]. Inflammation and OS are intricately linked processes; even a new term “OxInflammation” has been suggested to depict the vicious cycle of chronic inflammation and OS [5]. Pro-inflammatory mediators promote generation of ROS and RNS, but on the other side, reactive species also favor the pro-inflammatory response, creating a vicious cycle that is difficult to break [5]. Additionally, prolonged microglia activation impairs activation of peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α), a major regulator of mitochondrial biogenesis and energy metabolism [6]. PGC-1α downregulation has been linked to an augmented level of mitochondria – derived ROS, whereas its upregulation was associated with protection of neural cells against OS [4,7,8]. A plethora of players in this complex network of “OxInflammation” are mediated by the Kelch-like ECH – associated protein 1 – Nuclear factor erythroid 2 related factor 2 – Antioxidant response element (Keap1/Nrf2/ARE) signaling pathway, a master regulator of antioxidant and phase II detoxification genes [8]. Heme oxygenase-1 (HO-1) is a representative downstream enzyme of this pathway, which generates carbon monoxide, biliverdin, and iron ions, apart from removing toxic heme. HO-1 inducers exert favorable effects in MS, through the protection against OS, and inflammation and regulation of apoptosis [8].
Edaravone is an amphiphilic free radical scavenger, which is currently approved for the treatment of amyotrophic lateral sclerosis (ALS) in the USA and Japan and for the treatment of acute-phase cerebral infarction in Japan [9]. Mechanism of action of edaravone is not completely understood, but its neuroprotective effects have primarily been ascribed to ability to scavenge peroxynitrite [9]. Lately, the treatment with edaravone has been associated with the activation of Nrf2 pathway in various animal models of the CNS disorders [10–12]. Edaravone was also reported to ameliorate clinical severity in the model of experimental autoimmune encephalomyelitis (EAE), the most commonly used murine model of MS [13]. However, the Nrf2 pathway expression was not investigated in this study. Edaravone attenuated lymphocytes infiltration to the CNS and reduced expression of induced NO synthase (iNOS) in the microglia in spinal cords (SC) of EAE mice. Further research is needed to assess the potential of edaravone in MS treatment. The aim of this study was to evaluate the therapeutic potential of edaravone in the EAE model and to expand the knowledge of its mechanism of action. We hypothesized that the effect of edaravone on mitochondrial functions might be involved in its therapeutic effects.
Materials and Methods
Animals and induction of EAE
Mice were housed in the animal facility of Institute of Pharmacology, First Faculty of Medicine, Charles University in Prague. They had free access to a standard granulated diet and water ad libitum. The mice were housed in standard environmental conditions: light (12 h light and 12 h dark); temperature (22±2 °C); relative humidity (50±10 %). All experiments were approved by the Ministry of Education, Youth and Sports of the Czech Republic under the number MSMT-9445/2018-8.
The EAE was actively induced in conventional inbred female C57Bl/6J mice (9–13 weeks old) by immunization with myelin oligodendrocyte glycoprotein (MOG) 35–55 peptide (Prospec, Rehovot, Israel) and complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis H37Ra (Sigma-Aldrich, Prague, Czech Republic). The MOG/CFA emulsion was prepared by connecting two glass syringes to a 3-way connector and passing the solutions through the connector. The emulsion was injected subcutaneously in two 50 μl doses. In total, 100 μg of MOG peptide per mouse was administered. To facilitate the transfer of lymphocytes into the CNS, 300 ng of pertussis toxin (List Biologicals, Campbell, California, USA) in 200 μl of phosphate buffer saline (PBS) was injected intraperitoneally two hours and two days after the EAE induction [14].
The mice were monitored daily for the signs of EAE, which were scored as follows: 0 – no signs of clinical disease; 0.5 – partially limp tail; 1 – limp tail; 2 – loss in coordinated movement; hind limb paresis; 2.5 – one hind limb paralyzed; 3 – complete paralysis of hind legs; 3.5 – hind limbs paralyzed, weakness in forelimbs; 4 – paralysis of hind and fore legs; 5 – dead [15].
All mice were observed until they showed mild EAE symptoms, such as a partially limp tail (clinical score 0.5) and were then divided into ET (which received edaravone) and CG1 (which received the vehicle) groups until the end of the experiment.
Treatments
The mice were randomly allocated to one of the following groups:
Group evaluating therapeutic potential of edaravone (ET group): dose of 6 mg/kg/day intraperitoneally (this dose was previously shown to be the effective dose in the EAE model [13]);
Control group 1 (CG1 group): mice treated with a vehicle from the onset of EAE symptoms until the end of the experiment;
Control group 2 (CG2 group): mice treated with the vehicle, but not immunized with MOG/CFA.
Pilot study before the main experiments has indicated that the peak of the EAE disease in our laboratory is on the 6th day after the beginning of the clinical symptoms. Therefore, on day 6 after the onset of clinical symptoms, half of the mice were sacrificed by a rapid decapitation. SCs and brains were removed and subsequently used for histological analysis, qPCR for gene expression, determination of markers of OS and parameters of mitochondrial function. The rest of the mice were observed for clinical scores until day 28 after the immunization, when they were sacrificed. Brains and SCs were harvested and used for qPCR analysis for gene expression.
Histological analyses
Samples of the vertebral column with SC were collected and fixed with 4 % formaldehyde. Twenty-four hours later, and SC was carefully removed from the vertebral canal. Material was divided into three segments encompassing cervical, thoracic and lumbar part of the spinal cord and embedded into paraffin wax. Sections (7μm thick) were stained with hematoxylin-eosin staining. Images were captured at Leica DMLB microscope with MC170 HD camera (Leica Microsystems, Wetzlar, Germany) [16,17]. The inflammation was scored as previously described [16,18]. The inflammation extent was scored as follows: 0=no inflammation evident; 1=small number of inflammatory cells; 2=numerous infiltrating cells; 3=extension of perivascular cuffing into adjacent tissues (widespread infiltration) [16]. The final score for each experimental animal was obtained by evaluating each of the three segments on 4 sections separated from each other by approximately 100 μm (12 sections per animal).
qPCR for gene expression
SCs and brains were stored in RNAlaterTM Stabilization Solution at −20 °C for subsequent qPCR analysis. Total RNA was extracted using TRI reagent (Sigma-Aldrich, Prague, Czech Republic). cDNA was synthesized from RNA using an M-MLV Reverse Transcriptase (Top Bio, Prague, Czech Republic). cDNA served as a template for amplification of target genes, as well as the housekeeping gene β-actin (Actb gene, forward primer: CTGTCGAGTCGCGTCCACC, reverse primer: TCGTCATCCATGGCGAACTGG) by the quantitative real-time PCR with SsoAdvanced™ Universal SYBR® Green Supermix (Biorad, USA), using the manufacturer’s instructions. Target genes were Nrf2 (forward primer: CGCCAGC-TACTCCCAGGTTG, reverse primer: GGGGATATCCAGGGCAAGCG) and HO-1 (forward primer: GAGCCGTCTCGAGCATAGCC, reverse primer: ATCCTGGGG-CATGCTGTCGG), iNOS (forward primer: ATGGACCAGTATAAGGCAAGC; reverse primer: GCTCTGGATGAGCCTATATTG) and PGC1-α (forward primer: GGCTGGTTGCCTGCATGAGT, reverse primer: CCAACCAGAGCAGCACACTCT). The expression of target genes was calculated by comparing the relative levels after normalization to β-actin expression.
Determination of oxidative stress markers
The extent of lipid peroxidation (as Thiobarbituric Acid Reactive Substances (TBARS)) and conjugated dienes (CD) were assessed in the SCs, and brains homogenates as previously described [19]. All chemicals were purchased from Sigma-Aldrich (Prague, Czech Republic). SCs and brains homogenates (10 %) were prepared in the buffer (0.2 M Tris-HCl pH 7.4 + 0.002 M EDTA-Na2 + 0.025 M sucrose) and centrifuged at 4000 rpm.
Total protein assessment
The total amount of protein in all samples used in this study (for OS markers and mitochondria function parameters) was determined by the Bradford method [20] using the Bio-Rad protein assay (Bio-Rad, Prague, Czech Republic).
Mitochondrial functions
Isolated purified mitochondria were used for determination of the electron transport chain (ETC) complexes activity (I-IV), mitochondrial respiration linked to the ETC complexes I and II, as well as adenosine triphosphate (ATP) production. All chemicals were purchased from Sigma-Aldrich (Prague, Czech Republic).
Isolation of mitochondria from the brains and spinal cords
Preparation of mitochondria was performed as described previously [21]. Mitochondrial isolation buffer (pH 7.4) consisted of 200 mM mannitol, 75 mM sucrose, 5 mM HEPES, 0.1 % BSA, 1 mM EDTA. Freshly prepared mitochondria kept on ice were used for respirometry and ATP formation; the ETC complexes activity was measured with frozen mitochondria stored at −80 °C.
Activity of respiratory chain complexes
Isolated mitochondria were resuspended in the hypotonic buffer (25 mM potassium phosphate, 5 mM MgCl2, pH 7.2) and ultrasonicated three times to reach the maximum enzymatic activity. Each independent measurement had a corresponding control. Samples were measured in a total reaction volume of 3 ml at 30 °C. The activity of the ETC complexes and CS was measured spectrophotometrically using a GENESYS 180 UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Activity of the complexes I, II/III and IV was measured by the methods as previously described [22–24].
Mitochondrial respiration
The mitochondrial respiration medium (MiR05) consisted of 110 mM sucrose, 60 mM K+-lactobionate, 20 mM taurine, 3 mM MgCl2·6 H2O, 10 mM KH2PO4, 0.5 mM EGTA, 1 g/l, and 20 mM HEPES, and was adjusted to pH 7.1 with KOH.
The following stock solutions were used for respirometry: 2 mM malate, 5 mM pyruvate, 10 mM succinate, 1.25 mM ADP, 0.75 mM MgCl2, 2 μM rotenone in ethanol, and 2.5 μg/ml antimycin A in ethanol. Substrates and inhibitors were used at the final concentrations described previously [22]. Drug-induced changes in mitochondrial respiration were measured by high-resolution respirometry to detect changes in the oxygen consumption rate of isolated mitochondria as described previously using an Oxygraph-2k (Oroboros Instruments Corp, Innsbruck, Austria) and a TIP2k automatic titration-injection micropump [22].
ATP production
ATP production was measured by bioluminescence method as previously described [25]. Briefly, an ATP Bioluminescence Assay Kit CLS II with 5 mM malate, 5 mM pyruvate, 10 mM succinate, 5 mM glutamate and 1 mM ADP as substrates was used to determine ATP formation fluorometrically using FluoroMax3 (Jobin Yvon, Edison, New Jersey, USA). The reaction was initiated by the addition of luciferase reagent and luminescence was measured at 532 nm.
Statistical analysis
Normal distribution of the data was checked using the Shapiro-Wilk test. To compare the differences between the groups regarding parameters of mitochondrial function, markers of OS, and gene expression, ANOVA with post hoc Bonferroni test was used. To compare clinical scores, as well as inflammatory scores between the ET and CG1 groups, unpaired t-test was used. The results for the variables’ data are expressed as mean and standard deviation (SD) or mean and standard error of the mean (SEM). The differences were considered statistically significant when p<0.05. Statistical analyses and data visualization were performed using GraphPad Prism, version 8.0.0 for Windows, GraphPad Software (San Diego, California, USA).
Results
Edaravone ameliorated EAE severity
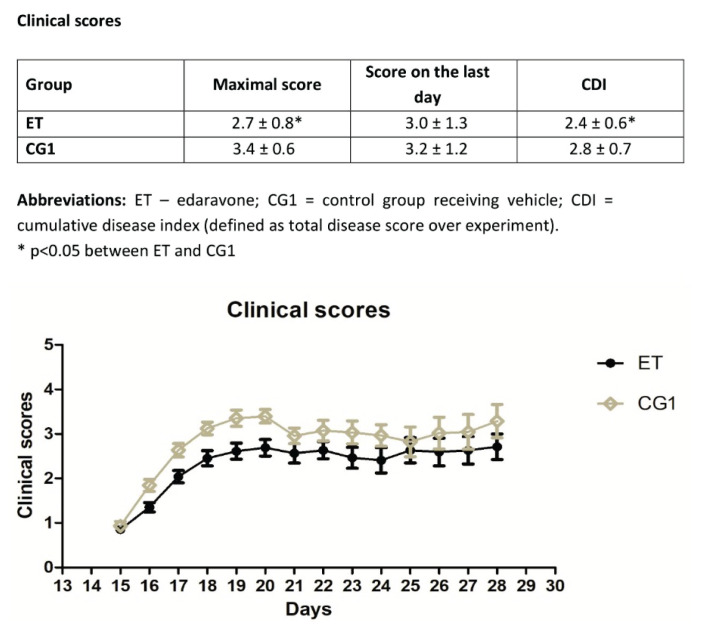
Treatment with edaravone ameliorated clinical scores over the course of the experiment (Fig. 1). There was a significant difference between the clinical scores in the ET and CG1 group at the peak of the disease, as well as in the cumulative disease index (defined as total disease score over experiment and calculated as a mean of daily scores of all mice in the group during the whole experiment). There was no significant difference in the clinical scores on the last day of the experiment (28th day after EAE induction).
Fig. 1.
Clinical scores in the ET and CG1 group during the experiment. Results are presented as mean ± SEM (n=28 samples per group).
Edaravone suppressed inflammation in the SC
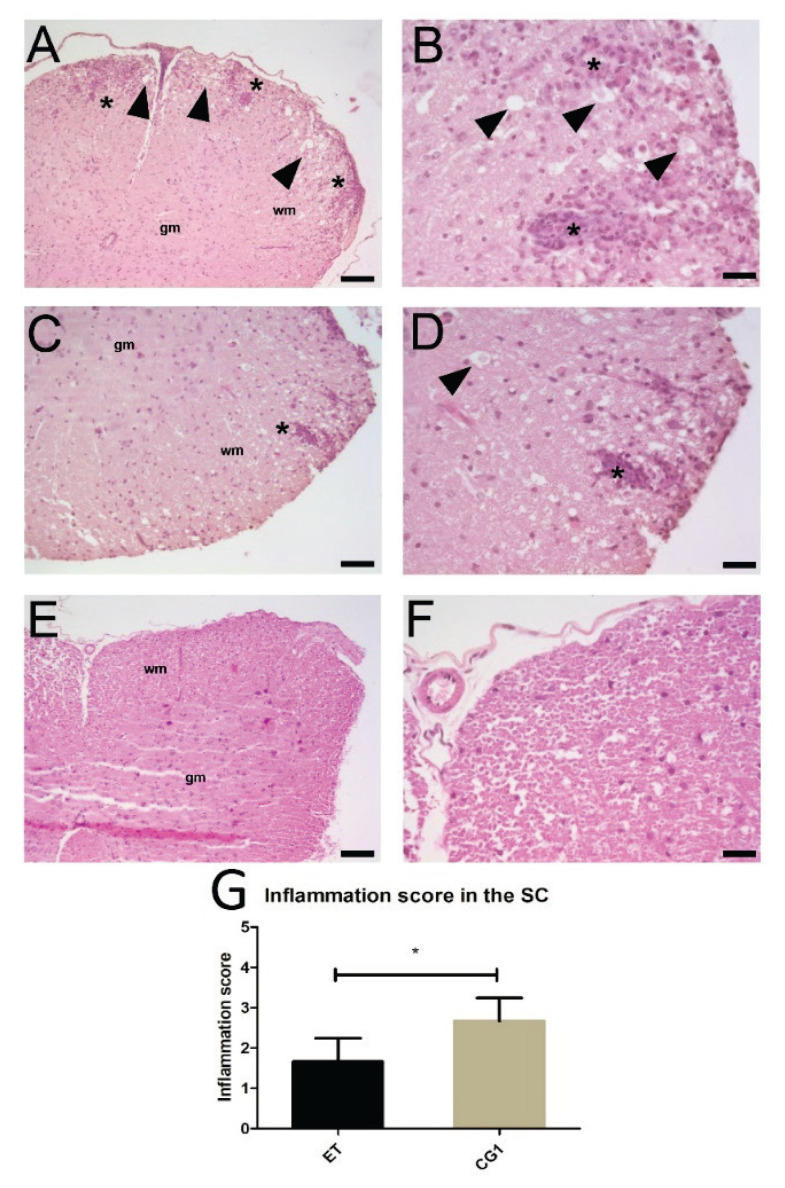
Hematoxylin-eosin staining was used to determine the extent of infiltration of mononuclear cells and perivascular cuffing in the SC (Fig. 2). In accordance with the clinical results, the CG1 group exerted massive infiltration of mononuclear cells into the SC with several foci of inflammation, whereas ET group showed significantly fewer infiltrating cells and perivascular cuffing, as verified by the inflammatory scores (p<0.05).
Fig. 2.
Morphology of the spinal cord in animals with EAE treated with edaravone. (A) Image of the spinal cord from an animal with EAE (CG1 group). Widespread infiltration extending from perivascular infiltrate into the surrounding white matter is visible (asterisks). In addition, numerous abnormally enlarged axons can be seen (arrowheads). Scale bar=200 μm. (B) High magnification view of white matter of the spinal cord shown in A. Two inflammatory foci are visible (asterisks) together with multiple swollen axons (arrowheads). Scale bar=50 μm. (C) Image of the spinal cord from an animal with EAE treated with edaravone (ET group). Isolated inflammatory focus is visible (asterisk). Swollen axons are rare. Scale bar=100 μm. (D) High magnification view of the white matter of the spinal cord shown in C. An inflammatory focus is visible (asterisk) as well as some swollen axons (arrowheads). Scale bar=50 μm. (E) Spinal cord from the control healthy animal (CG2 group). No inflammatory foci can be detected. Scale bar=200 μm. (F) Image of white matter of the spinal cord shown in E. Structure of the spinal cord is well-preserved, and no inflammatory infiltration is present. Scale bar=50 μm. Hematoxylin-eosin staining. Wm=white matter, gm=gray matter. (G) Inflammatory scores in the ET and CG1 groups. Results are presented as mean ± SD (n=4 samples per group), * p<0.05.
Edaravone attenuated OS in the SC and the brain
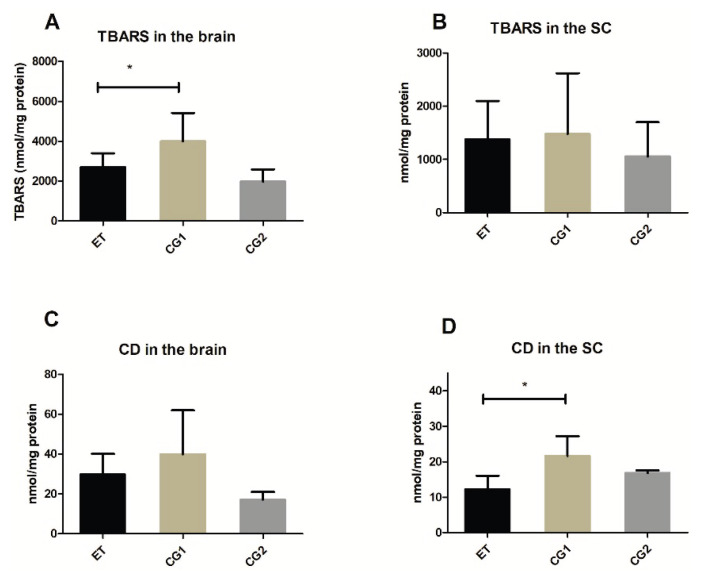
In the homogenates of the SCs, there was a significant difference between the ET and CG1 group, but no differences were observed for the level of TBARS. Conversely, in the brain homogenates, there was a significantly lower TBARS level in the ET group compared to the CG1 group, whereas no difference was observed for the CD level (Fig. 3).
Fig. 3.
Markers of oxidative stress in the brain and the spinal cord (SC) in the group treated with edaravone (ET), control group with EAE (CG1) and control group consisting of healthy mice (CG2). (A) Level of Thiobarbituric Acid Reactive Substances (TBARS) in the brain (B) Level of TBARS in the SC (C) Level of conjugated dienes (CD) in the brain (D) Level of CD in the SC. Results are presented as mean ± SD (n=12 samples per group). * p<0.05.
Edaravone changes genes expression in the SCs
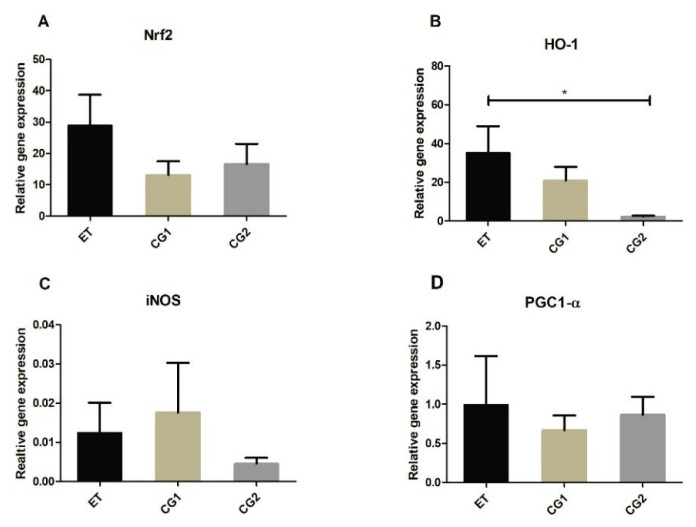
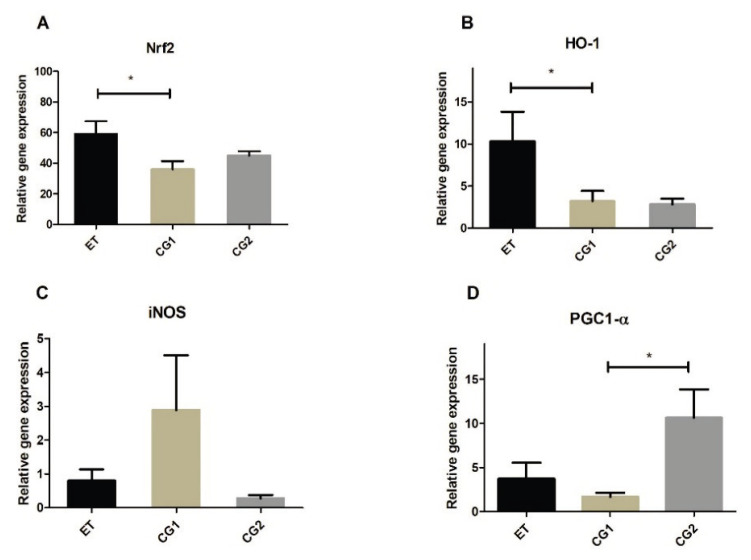
Gene expression of Nrf2, HO-1, PGC1-α and iNOS was determined in the brains and the SCs of mice at the peak of the disease (6th day after the symptoms onset) and at the end of the experiment (28th day after EAE induction). There were no significant differences in the brain samples for any gene neither at the peak of the disease, nor at the end of the experiment (data not shown). In the SCs, mRNA for HO-1 was significantly higher in the ET group compared to mRNA of HO-1 in the CG2 group at the peak of the disease (Fig. 4B). There were no significant differences in other genes expression between the ET, CG1 and CG2 groups (Fig. 4A, C and D). At the end of the experiment, mice in the ET group expressed higher levels of mRNA of Nrf2 and HO-1 compared to the mice in the CG2 group (Fig. 5A, B). Additionally, there was a difference in the PGC1-α mRNA expression between the CG1 and CG2 groups (Fig. 5D). No differences were found in the iNOS mRNA expression between the groups neither at the peak of the disease, nor at the end of the experiment.
Fig. 4.
Gene expression in the spinal cord (SC) in the group treated with edaravone (ET), control group with EAE (CG1) and control group consisting of healthy mice (CG2) at the peak of the EAE. (A) Relative gene expression of nuclear factor erythroid 2-related factor 2 (Nrf2) (B) Relative gene expression of heme oxygenase (HO-1) (C) Relative gene expression of induced NO synthase (iNOS) (D) Relative gene expression of peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1-α). Results are presented as mean ± SD (n=12 samples per group). * p<0.05.
Fig. 5.
Gene expression in the spinal cord (SC) in the group treated with edaravone (ET), control group with EAE (CG1) and control group consisting of healthy mice (CG2) at the 28th day after EAE induction. (A) Relative gene expression of nuclear factor erythroid 2-related factor 2 (Nrf2). (B) Relative gene expression of heme oxygenase (HO-1). (C) Relative gene expression of induced NO synthase (iNOS). (D) Relative gene expression of peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1-α). Results are presented as mean ± SD (n=6 samples per group). * p<0.05
Edaravone has a profound effect on the mitochondrial functions in the brain and the SC activity of respiratory chain complexes
No changes in the ETC complex I activity were detected in the SC and brain in the ET and CG1 groups compared with the CG2 group (Fig. 6C, D). However, edaravone treatment was associated with an increase in the activity of ETC complex II+III (Fig. 6E) in the SC. Similar observations were noted in the brain (Fig. 6F). Additionally, edaravone treatment caused inhibition of the ETC complex IV in the brain and SC, as activity of ETC complex IV was lower in the ET group compared to both CG1 and CG2 groups (Fig. 6G, H).
Fig. 6.
Mitochondrial functions in the spinal cord (SC) and the brain in the group treated with edaravone (ET), control group with EAE (CG1) and control group consisting of healthy mice (CG2) at the peak of the EAE. (A) Activity of mitochondrial complex I in the SC. (B) Activity of the complex I in the brain. (C) Activity of the complex II+III in the SC (D) Activity of the complex II+III in the brain. (E) Activity of the complex IV in the SC. (F) Activity of the complex IV in the brain. (G) Complex I-linked respiration in the SC. (H) Complex I-linked respiration in the brain (I) Complex II-linked respiration in the SC (J) Complex II-linked respiration in the brain. (K) ATP production in the spinal cord (SC). (L) ATP production in the brain. Results are presented as mean ± SD (n=3 samples per group). * p<0.05.
Mitochondrial respiration
Complex I-linked respiration was also not altered neither by EAE, nor by edaravone treatment (Fig. 6I, J). Complex II-linked respiration in the SC was increased in the ET group compared to the CG2 group (Fig. 6K). In the brain, respiration linked to the ETC complex II was significantly decreased in the ET group when compared to the CG1 group (Fig. 6L).
ATP production
Level of ATP production in the SC was significantly reduced in the CG1 group compared to the CG2 group (Fig. 6A), but this was not observed in the ET group. This indicates that treatment with edaravone preserves ATP production in the mitochondria in the SC. This effect was not seen in the brain (Fig. 6B).
Discussion
This study has shown that edaravone ameliorates disease severity in the EAE model, attenuates inflammation in the SC, reduces OS and his profound effects on the mitochondrial function in the brain and SC of the EAE mice. Additionally, edaravone has been found to increase gene expression ofNrf2 and HO-1. These findings provide additional evidence of edaravone potential for treatment in MS and expand our knowledge of the mechanisms of action of edaravone in the EAE model.
Edaravone is a potent antioxidant that is currently approved for the treatment of ALS and the management of neurological symptoms associated with acute ischemic stroke. In this study, edaravone treatment decreased levels of OS markers TBARS and CD in the homogenates of the brains and SCs, respectively. In addition to its anti-oxidative properties, edaravone has a role in attenuation of inflammation in the CNS, as well as in apoptotic cell death prevention, therefore it fits to the class of multi-target compounds [26]. We have shown that edaravone reduces clinical scores in the EAE model, and ameliorates inflammatory cellular infiltration into the SC of the EAE mice. These results are in accordance with the findings of an earlier study by Moriya et al. [13].
The antioxidative properties of edaravone have been mainly attributed to its ability to scavenge ROS. However, there are a growing number of studies demonstrating the ability of edaravone to act as an Nrf2/HO-1 pathway inducer. Edaravone was shown to increase Nrf2 and HO-1 mRNA expression in animal models of vascular dementia [11], cerebral infarction [10], and traumatic brain injury [12]. We have examined the expression of Nrf2/HO-1 pathway at the peak of the disease (5 days of edaravone treatment) and at the end of experiment (14 days of edaravone treatment), as the level of gene expression is time-dependent. At the peak of the disease, there were no significant changes between the groups regarding Nrf2, whereas expression of HO-1 mRNA was markedly augmented in the ET group in comparison to the CG2 group. At the end of the experiment, i.e. after 14 days of edaravone treatment, the mRNA expression of both Nrf2 and HO-1 was significantly increased in the ET group compared to the CG1 group.
Mitochondrial dysfunction is a critical event in the pathophysiology of MS which leads to impaired oxidative phosphorylation and consequent energy failure [4,8]. In addition to its antioxidant and anti-inflammatory properties, Nrf2 plays a role in maintaining mitochondrial homeostasis. There is an emerging body of evidence of the existence of the regulatory loop involving Nrf2 and PGC-1α [7]. PGC-1α, a transcriptional coactivator, is a major regulator of mitochondrial biogenesis and energy metabolism, which seems to be decreased in MS probably due to prolonged microglia activation [27]. PGC-1α downregulation has been linked to an augmented level of mitochondria – derived ROS, whereas its upregulation was associated with protection of neural cells against OS [28]. In this study, the level of mRNA expression of PGC-1α was lower in the ET and CG1 groups compared to the CG2, however, a significant difference was observed only between the CG1 and CG2 groups.
Reduced intracellular ATP level is an important indicator of mitochondrial dysfunction. Importantly, edaravone treatment preserved ATP level in the SCs of the EAE mice. Conversely, ATP production was significantly reduced in the CG1 group compared to the CG2 group. Previously, edaravone protected against hyperosmolarity-induced OS in primary human corneal epithelial cells by increasing the levels of ATP and mitochondrial membrane potential (MMP) [29]. Similarly, edaravone treatment improved kidney function in rats with ischemia-reperfusion injury by increasing ATP levels and MMP [30]. In this study, edaravone administration induced significant changes in the ETC complexes activity in the brain and the SCs of the mice. ETC complex II/III activity was increased in the ET group, whereas ETC complex IV activity was reduced in the ET group compared to the CG1 and CG2 groups. In accordance with this finding, complex II-linked respiration in the SC was also increased in the ET group. During complex I-linked respiration, ROS are produced from complexes I and III, whereas complex II-linked respiration leads to ROS production to some extent by complex III, but also through reverse electron flow to complex I. We can presume that reduced ROS production occurs when ETC complex II/III activity is increased and enhanced by edaravone. The reduction in the ETC complex IV activity might represent a compensatory mechanism of increased complex II/III activity. In a recent study, edaravone treatment completely restored activity of ETC complexes I-IV in the muscles of transgenic mice with impaired oxidative phosphorylation [31]. These effects were observed after a month of treatment with edaravone, whereas in this study ETC complexes activity were determined at the peak of the disease (5 days after edaravone treatment). Therefore, it is reasonable to assume that a longer treatment with edaravone is needed for a complete restoration of ETC complexes activity and this should be investigated in the future studies.
In the previous EAE study [13], edaravone reduced iNOS mRNA expression in the SC of the EAE mice. iNOS is responsible for the synthesis of NO, an important inflammatory player in the pathophysiology of MS. In our study, the iNOS mRNA expression in the SC in the CG1 group was higher compared to the CG2 and ET groups, however, these differences were not statistically significant. Discrepancies between the results are probably due to the fact that iNOS mRNA expression in the previous study was examined specifically in the microglia in the SC, whereas in this study it was assessed in the whole SC samples.
A limitation of the current study is that the brain tissue was not sufficiently explored. This was due to the fact that in the EAE model, inflammation is mainly limited to the SC, whereas the brain stem, the cerebellum and the forebrain are affected to a lesser extent [32]. Moreover, inflammation and demyelination are confined only to the specific regions of hippocampus, striatum, cerebellum, corpus callosum, and the cerebral cortex [33]. This could actually be a reason why we found no significant differences in the levels of mRNA expression of Nrf2, HO-1, PGC1-α and iNOS, as these analyses were performed in the whole brain samples, but not in the specific regions of the brain.
Conclusions
Edaravone treatment attenuates disease severity in the EAE model by reducing inflammation in the SC, diminishing OS, and improving mitochondrial function in the CNS of the EAE mice, and increases expression of Nrf2 and HO-1. Findings in this study provide additional evidence of edaravone potential for treatment in MS and expand our knowledge of the mechanisms of action of edaravone in the EAE model.
Acknowledgements
We thank our technician Jana Plačková for helping with the animals’ care and Dr. Jelena Kotur-Stevuljevic for help with determination of oxidative stress markers. This study was funded by the MH CZ-DRO (General University Hospital in Prague-VFN 00064165) and by the Charles University Project Progres Q25 and Progres Q27. The work was also funded by Czech Science Foundation (17-07332S and 20-09732S).
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 2.Diebold SS. Determination of T-cell fate by dendritic cells. Immunol Cell Biol. 2008;86:389–397. doi: 10.1038/icb.2008.26. [DOI] [PubMed] [Google Scholar]
- 3.Grigoriadis N, van Pesch V. A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015;22:3–13. doi: 10.1111/ene.12798. [DOI] [PubMed] [Google Scholar]
- 4.Michaličková D, Šíma M, Slanař O. New insights in the mechanisms of impaired redox signaling and its interplay with inflammation and immunity in multiple sclerosis. Physiol Res. 2020;69:1–19. doi: 10.33549/physiolres.934276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valacchi G, Virgili F, Cervellati C, Pecorelli A. OxInflammation: From subclinical condition to pathological biomarker. Front Physiol. 2018;9:858. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witte ME, Mahad DJ, Lassmann H, van Horssen J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol Med. 2014;20:179–187. doi: 10.1016/j.molmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaličková D, Hrnčíř T, Canová NK, Slanař O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur J Pharmacol. 2020;873:172973. doi: 10.1016/j.ejphar.2020.172973. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018:17–62. doi: 10.3164/jcbn.17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Jiang Y, Zhang G, Lin Z, Du S. Protective effect of edaravone on blood-brain barrier by affecting NRF-2/HO-1 signaling pathway. Exp Ther Med. 2019;18:2437–2442. doi: 10.3892/etm.2019.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Xiao Y, Lv P, Dong Y, Qi Q, Liu Z. Edaravone attenuates oxidative stress induced by chronic cerebral hypoperfusion injury: role of ERK/Nrf2/HO-1 signaling pathway. Neurol Res. 2018;40:1–10. doi: 10.1080/01616412.2017.1376457. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Teng CH, Wu FF, Ge L-Y, Xiao J, Zhang H-J, Chen D-Q. Edaravone attenuates traumatic brain injury through anti-inflammatory and anti-oxidative modulation. Exp Ther Med. 2019;18:467–474. doi: 10.3892/etm.2019.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriya M, Nakatsuji Y, Miyamoto K, Okuno T, Kinoshita M, Kumanogoh A, Kusunoki S, Sakoda S. Edaravone, a free radical scavenger, ameliorates experimental autoimmune encephalomyelitis. Neurosci Lett. 2008;440:323–326. doi: 10.1016/j.neulet.2008.05.110. [DOI] [PubMed] [Google Scholar]
- 14.Contarini G, Giusti P, Skaper SD. Active induction of experimental autoimmune encephalomyelitis in C57BL/6 mice. Methods Mol Biol. 2018;1727:353–360. doi: 10.1007/978-1-4939-7571-6_26. [DOI] [PubMed] [Google Scholar]
- 15.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 16.Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Soltan Dallal MM, Tabasi N, Khazaee M, Roozbeh Nasiraii L, Mahmoudi M. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed Pharmacother. 2017;95:1535–1548. doi: 10.1016/j.biopha.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 17.Sloka S, Metz LM, Hader W, Starreveld Y, Yong VW. Reduction of microglial activity in a model of multiple sclerosis by dipyridamole. J Neuroinflamm. 2013;10:1–11. doi: 10.1186/1742-2094-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horstmann L, Schmid H, Heinen AP, Kurschus FC, Dick HB, Joachim SC. Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J Neuroinflamm. 2013;10:1–12. doi: 10.1186/1742-2094-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farghali H, Černý D, Kameníková L, Martínek J, Horínek A, Kmonícková E, Zídek Z. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide. 2009;21:216–225. doi: 10.1016/j.niox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Fisar Z, Hroudova J. Pig brain mitochondria as a biological model for study of mitochondrial respiration. Folia Biol (Praha) 2016;62:15–25. doi: 10.14712/fb2016062010015. [DOI] [PubMed] [Google Scholar]
- 22.Folbergrová J, Ješina P, Haugvicová R, Lisý V, Houštěk J. Sustained deficiency of mitochondrial complex I activity during long periods of survival after seizures induced in immature rats by homocysteic acid. Neurochem Int. 2010;56:394–403. doi: 10.1016/j.neuint.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 24.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/S0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 25.Morciano G, Sarti AC, Marchi S, Missiroli S, Falzoni S, Raffaghello L, Pistoia V, Giorgi C, Di Virgilio F, Pinton P. Use of luciferase probes to measure ATP in living cells and animals. Nat Protoc. 2017;12:1542. doi: 10.1038/nprot.2017.052. [DOI] [PubMed] [Google Scholar]
- 26.Ismail H, Shakkour Z, Tabet M, Abdelhady S, Kobaisi A, Abedi R, Nasrallah L, Pintus G, Al-Dhaheri Y, Mondello S, El-Khoury R, Eid AH, Kobeissy F, Salameh J. Traumatic brain injury: oxidative stress and novel anti-oxidants such as mitoquinone and edaravone. Antioxidants. 2020;9:943. doi: 10.3390/antiox9100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijland PG, Witte ME, van het Hof B, van der Pol S, Bauer J, Lassmann H, van der Valk P, de Vries HE, van Horssen J. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: Implications for multiple sclerosis. Acta Neuropathol Commun. 2014;2:170. doi: 10.1186/s40478-014-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Liu H, Zeng W, Wei J. Edaravone protects against hyperosmolarity-induced oxidative stress and apoptosis in primary human corneal epithelial cells. PLoS One. 2017;12:e0174437. doi: 10.1371/journal.pone.0174437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Zhang E, Ren X, Bai X, Wang D, Bai L, Luo D, Guo Z, Wang Q, Yang J. Edaravone alleviates cell apoptosis and mitochondrial injury in ischemia-reperfusion-induced kidney injury via the JAK/STAT pathway. Biol Res. 2020;53:1–12. doi: 10.1186/s40659-019-0267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-González C, Nuevo-Tapioles C, Herrero Martín JC, Pereira MP, Serrano Sanz S, Ramírez de Molina A, Cuezva JM, Formentini L. Dysfunctional oxidative phosphorylation shunts branched-chain amino acid catabolism onto lipogenesis in skeletal muscle. EMBO J. 2020;39:e103812. doi: 10.15252/embj.2019103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133:223–244. doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton AM, Forkert ND, Yang R, Wu Y, Rogers JA, Yong VW, Dunn JF. Central nervous system targeted autoimmunity causes regional atrophy: a 9.4 T MRI study of the EAE mouse model of Multiple Sclerosis. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-44682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]