Summary
Respiration changes intrathoracic pressure and lung volumes in a cyclic manner, which affect cardiac function. Invasive ventricular pressure-volume (PV) loops can be recorded during ongoing mechanical ventilation or in transient apnea. No consensus exists considering ventilatory mode during PV loop recording. The objective of this study was to investigate the magnitude of any systematic difference of bi-ventricular PV loop variables recorded during mechanical ventilation versus apnea. PV loops were recorded simultaneously from the right ventricle and left ventricle in a closed chest porcine model during mechanical ventilation and in transient apnea (n=72). Variables were compared by regression analyses. Mechanical ventilation versus apnea affected regression coefficients for important PV variables including right ventricular stroke volume (1.22, 95% CI [1.08–1.36], p=0.003), right ventricular ejection fraction (0.90, 95% CI [0.81–1.00], p=0.043) and right ventricular arterial elastance (0.61, 95%CI [0.55–0.68], p<0.0001). Right ventricular pressures and volumes were parallelly shifted with Y-intercepts different from 0. Few left ventricular variables were affected, mainly first derivatives of pressure (dP/dt(max): 0.96, 95% CI [0.92–0.99], p=0.016, and dP/dt(min): 0.92, 95% CI [0.86–0.99], p=0.026), which might be due to decreased heart rate in apnea (Y-intercept −6.88, 95% CI [−12.22; −1.54], p=0.012). We conclude, that right ventricular stroke volume, ejection fraction and arterial elastance were mostly affected by apnea compared to mechanical ventilation. The results motivate future standardization of respiratory modality when measuring PV relationships.
Keywords: Right ventricular function, Left ventricular function, Animal model Positive pressure ventilation, Respiration
Introduction
Pressure-volume (PV) loop recording provides the most comprehensive evaluation of ventricular function with both load-dependent and -independent variables [1]. The use of PV recording is therefore recommended in preclinical research [2,3].
Ventilation causes cyclic changes in intrathoracic pressure and lung volumes that affect ventricular function, especially right ventricular (RV) function. Increased intrathoracic pressure can affect venous return and thereby RV preload and volumes whereas transpulmonary pressure gradient can affect RV outflow. Due to the higher pressures in the left ventricle (LV), the respiratory influence is presumably smaller [4–6]. Previous studies have mostly investigated the cardiac effects from different phases of the ventilatory cycle (i.e. inspiration versus expiration) but not focused on the entire respiratory cycle as a whole [6,7]. PV loops can be recorded in apnea or during ongoing ventilation, and, for the latter, analysis would include an average of the entire respiratory cycle. However, there is no established standard for setting of ventilation during recording of PV data despite a plausible systematic difference.
The lack of consensus is evident in the literature. Examples exist of animal studies with both RV and left ventricular (LV) PV loops recorded in apnea [8–11] as well as examples of studies using an average of respiratory cycles [12–14]. Unfortunately, papers often fail to provide ventilatory information during PV recordings [15–17]. The same inconsistency exists in human PV studies [18–20]. The magnitude of a potential systematic difference between mechanical ventilation versus apnea has not been investigated, but would be important in preclinical cardiovascular research to ensure reproducibility, validity and translational impact.
The aim of the present study was to correlate bi-ventricular PV recordings measured during ongoing mechanical ventilation versus transient apnea. We aimed to establish which PV variables were affected by ventilation versus apnea and the magnitude of any difference.
Materials and Methods
Ethical approval
The Danish Animal Research Inspectorate had approved the study (license No. 16-15-0201-00840). All animals were treated according to Danish law and standards of animal care. The study complies with the ARRIVE guidelines [21].
Animals
Danish, female slaughter pigs (crossbreed of Landrace, Yorkshire and Duroc) of 60±2 kg were used which corresponds to 4–5 months of age. No immune or genetic modification was used. Pigs were housed in pens with concrete floors. Snout contact was possible through fences but the animals were led out daily for free physical activation. The light-dark cycle was 12:12 h. The pigs received a conventional diet and free access to water. Each animal served as experimental unit. For further details, see [22].
Design
This was a secondary analysis of data recorded for a previous, prospective, interventional study [22]. The original study consisted of nine consecutive time points for each animal: a baseline measurement followed by measurements after acute pulmonary embolism induction and different degrees of RV afterload reduction. Only PV data recorded during ongoing ventilation were analyzed for the original study. However, at each of the nine timepoints of data collection, PV data were recorded both during ongoing mechanical ventilation as well as during transient apnea in end-expiration. Each pair of measurements (i.e. ventilation and apnea) from each of the nine time points were recorded with less than 30 s interval, under the same hemodynamic and interventional conditions with only the ventilation as difference. The PV data recorded in apnea are accordingly only used in the present study.
Inclusion criteria for the present study were timepoints where PV loops were recorded in both apnea and during ventilation from the group of animals (n=8) with different degrees of RV afterload reduction. With eight animals going through the nine timepoints of the study design, a total of n=72 PV recordings were to be compared in the present study. PV loops from both the RV and LV were recorded simultaneously and both included in the present study. For the PV loops recorded during ongoing ventilation, we used an average of three ventilatory cycles, i.e. the number of included PV loops for each timepoint would depend on heart rate. For the PV loops recorded during apnea, we used an average of as many PV loops as possible, typically 5–10 loops.
No animals died before experiments were concluded. The experiments were terminal and animals were euthanized by a lethal dose of pentobarbital.
Pressure-volume loop instrumentation and recordings
The procedures have been described in detail previously [13,22]. Sheaths were inserted guided by ultrasound to be minimally invasive. An 8F sheath (Terumo Radifocus Introducer II, Japan) was placed in the left carotid artery for LV access, and a 16F sheath (Check-Flo Performer, Cook Medical, USA) was placed in the left external jugular vein for RV access.
Bi-ventricular PV catheters (emka Technologies, Paris, France) were inserted using a closed chest approach guided by fluoroscopy. Catheters were connected to admittance control units (ADV500, Transonic Scisense, London, Canada) and PowerLab 8/35 (ADInstruments, Oxford, UK). Data were recorded in LabChart (ADInstruments). Calibration was performed per manufacturer instructions based on phase and magnitude signals. Stroke volume was calibrated by thermodilution using an average of three injections of 10 ml 5 % isotonic glucose of 5 °C.
Data was analyzed offline with the observer blinded to protocol timepoints. Data during ongoing ventilation was analyzed as an average of three respiratory cycles. Data from apnea was analyzed as an average of as many loops as possible only including PV loops without PV-shifting caused by mechanical ventilation (see Discussion).
For the present study, outcome measures were the following PV variables for both RV and LV; end-systolic volume (ESV), end-diastolic volume (EDV), end-systolic pressure (ESP), end-diastolic pressure (EDP), stroke volume (SV), stroke work (SW), ejection fraction (EF), arterial elastance (Ea), maximal and minimal first derivative of pressure (dP/dtmax and dP/dtmin) and tau. Those are mostly load-dependent variables.
Anesthesia and mechanical ventilation
Premedication for transportation consisted of Zoletil (0.1 ml·kg−1, mixture of tiletamine 25 mg·ml−1, zolazepam 25 mg·ml−1, butorphanol 10 mg·ml−1, ketamine 100 mg·ml−1 and xylazine 20 mg·ml−1). Animals were anesthetized with propofol (5 mg·kg−1·h−1) and fentanyl (12.5 μg·kg−1·h−1) [22]. We did not use neuromuscular blockers as it could mask insufficient anesthesia. However, the general anesthesia was sufficient to totally suppress spontaneous inspiratory efforts during apnea.
Animals were intubated (7.5 mm, Smith Medical ASD Inc, USA) by direct laryngoscopy. They were mechanically ventilated (Datex-Ohmeda S/5, Avance, GE Helathcare, USA) with the PCV-VG setting (pressure control ventilation – volume guaranteed); a setting delivering a preset tidal volume but with a decelerating flow at the lowest possible peak inspiratory pressure with a preset respiratory rate (RR) and during a preset inspiratory time. Tidal volume was set to 500 ml (8 ml·kg−1). No positive end-expiratory pressure (PEEP) was added, and the adjustable pressure limiting (APL) valve was set to minimum yielding an internal PEEP of 2–3 cmH2O. Inspiration-to-expiration ratio was 1:2. RR was adjusted prior to baseline evaluation targeting a normal end-tidal CO2 of 5.0–5.5 kPa. Apnea was obtained by transient (<10 s) switch of the selector knob from ventilator mode to bag mode but without disconnecting the animal from the ventilator.
Statistics
Sample size calculation is described in the original study [22]. Data was evaluated for normal distribution by Shapiro-Wilks test and QQ plots. Data is presented as mean (SD) or with 95 % confidence intervals (95% CI). Under the null-hypothesis of no difference between ventilation and apnea, PV data recorded in each ventilatory mode would correlate on a straight line with slope=1 and Y-intercept=0. Accordingly, we correlated each PV variable by least squares methods fitting a straight line by nonlinear regression under the assumption of no batch effect. Slopes and Y-intercepts including 95% CI were calculated as well as r2 values for goodness of fit, and we tested slopes versus a hypothetical value of 1 and Y-intercepts against a hypothetical value of 0. A p-value<0.05 was considered statistically significant. We used Prism 9.0.0 (GraphPad Software, LCC, USA) for analyses.
Results
A total of n=72 timepoints were included. Animals were ventilated with an RR of 14.6 (1.2) min−1. Ventilatory peak pressure was 20.9 (2.0) cm H2O.
Heart rate (HR) correlated well between ventilation and apnea (slope 1.04, 95% CI 0.95–1.13, p=0.374, r2=0.89), but its Y-intercept was decreased to −6.88 (95% CI [−12.22; −1.54], p=0.012).
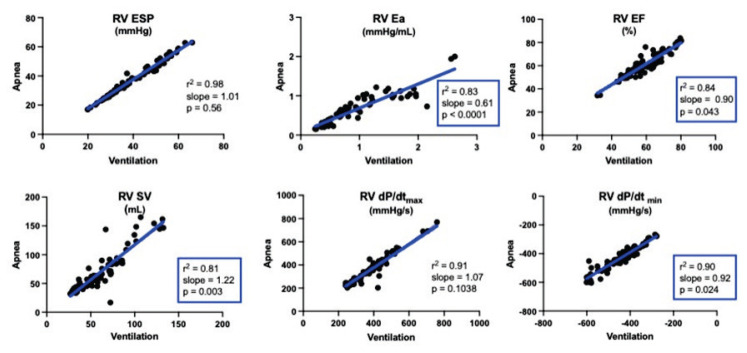
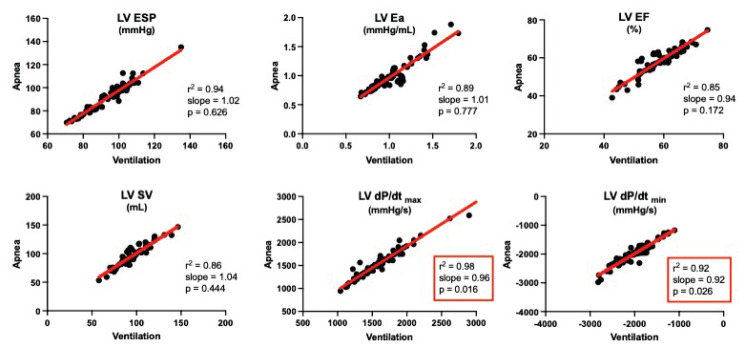
We observed a high degree of goodness of fit with r2-values over 0.80 for practically all PV variables from both ventricles. A majority of PV variables correlated well with slopes not significantly different from 1. But, the slopes of certain variables like RV EF, RV SV, and RV Ea were different from 1 (Table 1 and Fig. 1). For LV, this was the case for LV EDP and dP/dt (Table 2 and Fig. 2).
Table 1.
Right ventricular pressure-volume variables.
| Variable | Slope | 95% CI | p-value vs. 1 | r2 | Y intercept | 95% CI | p-value vs. 0 |
|---|---|---|---|---|---|---|---|
| RV ESP | 1.01 | 0.98–1.04 | 0.563 | 0.98 | ÷2.79 | ÷4.13;÷1.44 | <0.0001 |
| RV EDP | 1.01 | 0.92–1.09 | 0.911 | 0.88 | ÷1.06 | ÷1.47;÷0.64 | <0.0001 |
| RV ESV | 1.02 | 0.97–1.07 | 0.493 | 0.96 | 3.05 | 1.15–4.94 | 0.002 |
| RV EDV | 0.98 | 0.86–1.09 | 0.664 | 0.81 | 14.57 | ÷0.82–29.96 | 0.063 |
| RV SW | 1.05 | 0.95–1.16 | 0.302 | 0.86 | 63.49 | ÷146.30–273.30 | 0.548 |
| RV SV | 1.22 | 1.08–1.36 | 0.003 | 0.81 | ÷4.64 | ÷14.11–4.83 | 0.332 |
| RV EF | 0.90 | 0.81–1.00 | 0.043 | 0.84 | 7.51 | 1.73–13.28 | 0.012 |
| RV Ea | 0.61 | 0.55–0.68 | <0.0001 | 0.83 | 0.07 | 0.00–0.14 | 0.049 |
| RV dP/dt (max) | 1.07 | 0.99–1.15 | 0.104 | 0.91 | ÷50.36 | ÷83.55; ÷17.16 | 0.004 |
| RV dP/dt (min) | 0.92 | 0.85–0.99 | 0.024 | 0.90 | ÷23.53 | ÷56.10–9.05 | 0.154 |
| RV tau | 0.53 | 0.41–0.64 | <0.0001 | 0.53 | 9.94 | 4.84–15.05 | 0.0002 |
Slopes and intercepts with 95 % confidence intervals (95% CI) from linear fits of each variable during mechanical ventilation versus apnea. n=72 for all. RV, right ventricular; ESP, end-systolic pressure; EDP, end-diastolic pressure; ESV, end-systolic volume; EDV, end-diastolic volume; SW, stroke work; SV, stroke volume; EF, ejection fraction; Ea, arterial elastance; dP/dt, first derivative of pressure.
Fig. 1.
Correlation during ventilation versus apnea for right ventricular (RV) end-systolic pressure (ESP), arterial elastance (Ea), ejection fraction (EF), stroke volume (SV), and maximal and minimal first derivative of pressure (dP/dtmax and dP/dtmin). Compare to Figure 2 for LV variables. n=72 for all.
Table 2.
Left ventricular pressure-volume variables.
| Variable | Slope | 95% CI | p-value vs. 1 | r2 | Y intercept | 95% CI | p-value vs. 0 |
|---|---|---|---|---|---|---|---|
| LV ESP | 1.02 | 0.95–1.08 | 0.626 | 0.94 | ÷3.82 | ÷9.86–2.22 | 0.211 |
| LV EDP | 0.87 | 0.78–0.96 | 0.005 | 0.85 | 0.02 | ÷0.81–0.86 | 0.957 |
| LV ESV | 1.02 | 0.94–1.10 | 0.694 | 0.90 | 0.70 | ÷4.55–5.94 | 0.792 |
| LV EDV | 1.01 | 0.95–1.06 | 0.744 | 0.95 | ÷1.77 | ÷11.58–8.04 | 0.720 |
| LV SW | 1.05 | 0.97–1.12 | 0.254 | 0.91 | ÷463.6 | ÷1115.0–188.1 | 0.160 |
| LV SV | 1.04 | 0.94–1.14 | 0.444 | 0.86 | 2.80 | ÷12.22–6.61 | 0.554 |
| LV EF | 0.94 | 0.84–1.03 | 0.172 | 0.85 | 3.42 | ÷2.09–8.93 | 0.220 |
| LV Ea | 1.01 | 0.93–1.10 | 0.777 | 0.89 | ÷0.04 | ÷0.13–0.05 | 0.321 |
| LV dP/dt (max) | 0.96 | 0.92–0.99 | 0.016 | 0.98 | 9.59 | ÷49.97–69.15 | 0.749 |
| LV dP/dt (min) | 0.92 | 0.86–0.99 | 0.026 | 0.91 | ÷139.5 | ÷276.2; ÷2.9 | 0.045 |
| LV tau | 1.01 | 0.90–1.12 | 0.899 | 0.82 | ÷3.52 | ÷7.79–0.75 | 0.104 |
Slopes and intercepts with 95 % confidence intervals (95%CI) from linear fits of each variable during mechanical ventilation versus apnea. n=72 for all. LV, left ventricular; ESP, end-systolic pressure; EDP, end-diastolic pressure; ESV, end-systolic volume; EDV, end-diastolic volume; SW, stroke work; SV, stroke volume; EF, ejection fraction; Ea, arterial elastance; dP/dt, first derivative of pressure.
Fig. 2.
Correlation during ventilation versus apnea for left ventricular (LV) end-systolic pressure (ESP), arterial elastance (Ea), ejection fraction (EF), stroke volume (SV), and maximal and minimal first derivative of pressure (dP/dtmax and dP/dtmin). Compare to Figure 1 for RV variables. n=72 for all.
RV volumes and pressures were parallelly shifted evident by Y-intercepts different from 0. The same applied to RV EF and Ea (Table 1). Of the LV variables, only dP/dtmin was parallelly shifted with a Y-intercept different from 0 (Table 2).
Discussion
In the present study, we correlated frequently reported PV-variables recorded during ongoing ventilation versus transient apnea and observed significant magnitudes of difference for key PV variables. Apnea compared to ongoing ventilation affected RV ejection fraction, stroke volume, arterial elastance, dP/dtmin, and tau, and RV volumes and pressure were parallelly shifted. Only LV dP/dt and end-diastolic pressure were affected by mechanical ventilation.
These findings are important since no recommendation or consensus exists on how to record PV data, whereas thorough guidelines detailing best practice in other fields of preclinical cardiovascular research have been published [23,24]. Similar recommendations on PV measurements including ventilation versus apnea would be of interest. On one side, PV recordings during apnea can avoid pressure-volume changes from mechanical ventilation [10], while recordings during ongoing ventilation mimic intrathoracic cyclic changes from normal spontaneous respiration [5]. Nevertheless, mechanical ventilation differs from spontaneous respiration by being positive pressure ventilation reducing venous return during inspiration, which is opposite from spontaneous respiration.
We showed a systematic difference on several PV variables why an established standard setting of ventilation would be of relevance. Otherwise, reproducibility and validity are reduced and comparability between different studies of different ventilatory methodology made difficult. As long as a consensus does not exist, researchers ought to state ventilatory information in the papers. Furthermore, the magnitudes of difference are of relevance as they may outweigh interventional effects. For example, we show a parallel shift of RV EF of 7.5 %-points just from apnea compared to ventilation, but a similar effect from a pharmacological intervention would be of scientific and clinical interest.
Ventilation and right ventricular function
We found that several variables on RV function were affected by apnea compared to mechanical ventilation with RV SV, EF, arterial elastance, and tau as the variables affected to the greatest extent.
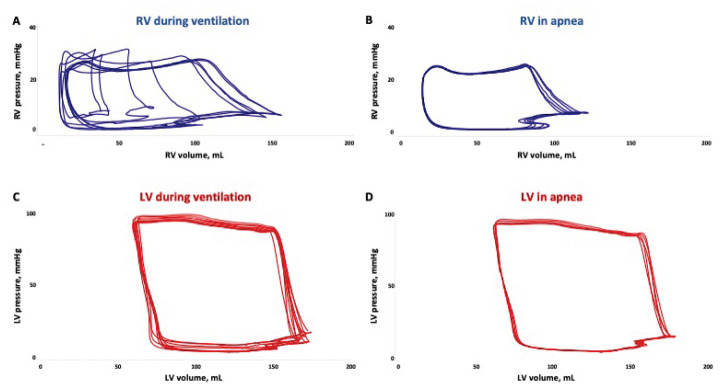
RV pressures were parallel shifted downwards and volumes upwards which affected RV EF and SV correlation coefficients. RV function is greatly dependent on preload [25] which changes in a cyclic manner with ventilation [26]. It cannot be expected that inspiratory and expiratory effects on cardiac function would directly counteract each other since the duration of the phases are different and since airway pressure and flow waveforms are not necessarily opposite in the two phases of respiration. Accordingly, it is rational to compare the average of a respiratory cycle to apnea as the purpose of the present study. Figure 3A–B shows RV PV loops from ventilation and apnea and illustrates the high impact of ventilation on RV function. Note how RV PV loops in Figure 3A are shifted back and forth on the volume axis by ventilation. This emphasizes the high degree of preload dependence of the RV as it easily adapts to preload changes exploiting the Starling forces. On the contrary, the RV loops are not moving during apnea as illustrated in Figure 3B.
Fig. 3.
Representative pressure-volume loops from right ventricle (RV) with ongoing mechanical ventilation (A) and in transient apnea (B). Note how RV volumes changes substantially through the respiratory cycle. Similar loops from the left ventricle (LV) with ongoing ventilation (C) and in apnea (D). The differences between ventilatory mode are less pronounced for the LV than the RV. Both (A) and (C) shows loops from two consecutive respiratory cycles.
Mechanical ventilation increases intrathoracic pressure and reduces RV preload [4,26,27] like we observed reduced RV EDP. However, apnea-induced bradycardia allowed longer diastolic filling time with increased RV volumes and SV. Our observations of reduced HR and increased SV corresponds to human data where cardiac output was similar between mechanical ventilation and apnea [28].
RV arterial elastance (Ea) was numerically highly affected and was underestimated in ventilation compared to apnea. This finding is relevant as Ea is a measure of ventricular afterload and is part of the ventriculo-arterial coupling. The coupling describes ventricular function to its faced afterload and is key in PV interpretation [1]. Positive pressure ventilation occurs in mechanical inspiration, and the increased intrathoracic pressure and lung volume increase pulmonary vascular resistance [4,6,26,27], which is another estimate of RV afterload. The opposite will happen in expiration, i.e. decrease pulmonary vascular resistance. In the present study, expiration was of longer duration than inspiration which might explain the RV Ea decrease that we observed. Furthermore, Ea can be estimated as ESP/SV. The correlation coefficient of RV SV was affected by apnea compared to mechanical ventilation, whereas RV ESP was not. The increased stroke volume for an unchanged pressure also yields a lower elastance.
RV dP/dtmin and tau are two frequently used parameters of RV diastolic function and both were changed by apnea in the present study. Intrathoracic pressures from mechanical ventilation are transferred to pleural and pericardial pressures which can affect diastolic filling [27,29].
Ventilation and left ventricular function
Few variables on LV function were susceptible to mechanical ventilation compared to the RV and the magnitudes were smaller. This is illustrated in Figure 3C–D where the LV PV loops are very similar.
Changes in LV systolic function were only observed in LV dP/dtmax, which is frequently reported as a contractility estimate. Mechanical ventilation is not known to affect LV contractility [26], and we interpret the observed decrease in LV dP/dtmax to be caused by the reduced heart rate. LV volumes and SV may change through the respiratory cycle from ventricular interaction and blood pulmonary transit time [4,7] but in the present study we averaged the entire respiratory cycle and found no changes.
LV inflow is, however, altered by mechanical ventilation with increased intrathoracic pressure and lung volumes, which can increase pulmonary vascular resistance and decrease lung blood volume and LV preload [6,26,27]. This is comparable to our observations on LV EDP and LV dP/dtmin.
Limitations
The present study has some limitations to consider. First, mechanical ventilation includes cyclic changes in intrathoracic pressure and volume but the conditions are still different from spontaneous respiration. Ventilatory apnea will similarly differ from voluntary end-expiration breath hold. However, as most preclinical research include mechanically ventilated animals, our results are externally valid in such circumstances, whereas translation into human, normal physiology must be done with caution. Similarly, in the setting of positive intrathoracic pressure, the transmural pressures will be different from the direct intracardiac measurements. The oscillation in transmural pressure was not directly measured, but we aimed to minimize such effects by the respiratory settings without adding PEEP and APL on minimum. Secondly, the retrospective analyses included an animal model of increased RV afterload and afterload-reducing interventions. Accordingly, the spectrum of RV measurements is wider than for the LV. Furthermore, we only performed transient apnea and LV affection might be delayed due to the ventricles’ serial connection. Longer apnea duration may have affected LV function further but would have increased risks of cardiovascular reflexes to affect data. Third, PV offers load-independent variables as well, but such are always obtained during apnea and preload reduction why inclusion of load-independent data was not possible in the present study. First derivative of pressure and tau originates from the pressure signal and are therefore not strictly a loop component, but the variables are included in the study as they are often recorded and reported in studies of invasive pressure measurements. Lastly, we did not include PEEP in the present study. PEEP-induced cardiovascular changes have been investigated previously [6,29,30] and were beyond the scope of this paper.
Conclusions
We showed that many pressure-volume loop variables were affected by transient apnea compared to ongoing mechanical ventilation. Right ventricular variables were more often affected than left ventricular variables. The results emphasize the need for future standardization of respiratory conditions when measuring PV relationships.
Acknowledgements
No funding was received for the present, secondary analysis. The primary study [22] was funded by Aarhus University, the Laerdal Foundation for Acute Medicine (3374), Knud and Edith Eriksens Memorial Foundation, Holger and Ruth Hesse’s Memorial Foundation, Director Emil C. Hertz Foundation, King Christian the 10th’s Foundation, Novo Nordisk Foundation (NNF16OC0023244, NFF17CO0024868), and Alfred Benzons Foundation. No foundations had any influence on any aspect of the study. Thanks to Simon Grund Soerensen for analytical support.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 2.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Venteuolo CE, Vieillard-Baron A, Voelkel NF, Vonk-Noordegraaf A, Hassoun PM. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am J Resp Crit Care. 2018;198:e15–43. doi: 10.1164/rccm.201806-1160st. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lara-Pezzi E, Menasché P, Trouvin J-H, Badimón L, Ioannidis JPA, Wu JC, Hill JA, Koch WJ, Felice AFD, Waele PD, Steenwinckel V, Hajjar RJ, Zeiher AM. Guidelines for Translational Research in Heart Failure. J Cardiovasc Transl. 2015;8:3–22. doi: 10.1007/s12265-015-9606-8. [DOI] [PubMed] [Google Scholar]
- 4.Michard F, Teboul J-L. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care. 2000;4:282–289. doi: 10.1186/cc710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinsky MR. Cardiopulmonary interactions: Physiologic basis and clinical applications. Ann Am Thorac Soc. 2018;15(Suppl 1):S45–S48. doi: 10.1513/annalsats.201704-339fr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, Marini JJ. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intens Care Med. 2016;42:739–749. doi: 10.1007/s00134-016-4326-3. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell JR, Whitelaw WA, Sas R, Smith ER, Tyberg JV, Belenkie I. RV filling modulates LV function by direct ventricular interaction during mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289:H549–H557. doi: 10.1152/ajpheart.01180.2004. [DOI] [PubMed] [Google Scholar]
- 8.Bove T, Bouchez S, Hert SD, Wouters P, Somer FD, Devos D, Somers P, Nooten GV. Acute and chronic effects of dysfunction of right ventricular outflow tract components on right ventricular performance in a porcine model implications for primary repair of tetralogy of fallot. J Am Coll Cardiol. 2012;60:64–71. doi: 10.1016/j.jacc.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Gorcsan J, Strum DP, Mandarino WA, Gulati VK, Pinsky MR. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography: Comparison with sonomicrometry and pressure-volume relations. Circulation. 1997;95:2423–2433. doi: 10.1161/01.cir.95.10.2423. [DOI] [PubMed] [Google Scholar]
- 10.De Jong R, Van Hout GPJ, Houtgraaf JH, Takashima S, Pasterkamp G, Hoefer I, Duckers HJ. Cardiac function in a long-term follow-up study of moderate and severe porcine model of chronic myocardial infarction. Biomed Res Int. 2015;2015:1–11. doi: 10.1155/2015/209315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Missant C, Rex S, Segers P, Wouters PF. Levosimendan improves right ventriculovascular coupling in a porcine model of right ventricular dysfunction. Crit Care Med. 2007;35:707–715. doi: 10.1097/01.ccm.0000257326.96342.57. [DOI] [PubMed] [Google Scholar]
- 12.Alogna A, Manninger M, Schwarzl M, Zirngast B, Steendijk P, Verderber J, Zweiker D, Maechler H, Pieske BM, Post H. Inotropic effects of experimental hyperthermia and hypothermia on left ventricular function in pigs-comparison with dobutamine. Crit Care Med. 2016;44:e158–e167. doi: 10.1097/ccm.0000000000001358. [DOI] [PubMed] [Google Scholar]
- 13.Lyhne MD, Schultz JG, Kramer A, Mortensen CS, Nielsen-Kudsk JE, Andersen A. Right ventricular adaptation in the critical phase after acute intermediate-risk pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2021;10:243–249. doi: 10.1177/2048872620925253. [DOI] [PubMed] [Google Scholar]
- 14.Roosens CD, Ama R, Leather HA, Segers P, Sorbara C, Wouters PF, Poelaert JI. Hemodynamic effects of different lung-protective ventilation strategies in closed-chest pigs with normal lungs. Crit Care Med. 2006;34:2990–996. doi: 10.1097/01.ccm.0000242758.37427.16. [DOI] [PubMed] [Google Scholar]
- 15.Boulate D, Ataam JA, Connolly AJ, Giraldeau G, Amsallem M, Decante B, Lamrani L, Fadel E, Dorfmuller P, Perros F, Haddad F, Mercier O. Early development of right ventricular ischemic lesions in a novel large animal model of acute right heart failure in chronic thromboembolic pulmonary hypertension. J Card Fail. 2017;23:876–886. doi: 10.1016/j.cardfail.2017.08.447. [DOI] [PubMed] [Google Scholar]
- 16.Gufler H, Wagner S, Niefeldt S, Klopsch C, Brill R, Wohlgemuth WA, Yerebakan C. Levels of agreement between cardiac magnetic resonance and conductance catheter measurements of right ventricular volumes after pulmonary artery banding. Acta Radiol. 2019;61:894–902. doi: 10.1177/0284185119886318. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa K, Aguero J, Oh JG, Hammoudi N, Fish LA, Leonardson L, Picatose B, Santos-Gallego CG, Fish KM, Hajjar RJ. Increased stiffness is the major early abnormality in a pig model of severe aortic stenosis and predisposes to congestive heart failure in the absence of systolic dysfunction. J Am Heart Assoc. 2015;4:e001925. doi: 10.1161/jaha.115.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axell RG, Messer SJ, White PA, McCabe C, Priest A, Statopoulou T, Drozdzynska M, Viscasillas J, Hinchy EC, Hampton-Till J, Alibhai HI, Morrell N, Pepke-Zaba J, Large SR, Hoole SP. Ventriculo-arterial coupling detects occult RV dysfunction in chronic thromboembolic pulmonary vascular disease. Physiol Rep. 2017;5:e13227. doi: 10.14814/phy2.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornwell WK, Tran T, Cerbin L, Coe G, Muralidhar A, Hunter K, Altman N, Ambardekar AV, Tompkins C, Zipse M, Schulte M, O’Gean K, Ostertag M, Hoffman J, Pal JD, Lawley JS, Levine BD, Wolfel E, Kohrt WM, Buttrick P. New insights into resting and exertional right ventricular performance in the healthy heart through real-time pressure-volume analysis. J Physiol. 2020;598:2575–2587. doi: 10.1113/jp279759. [DOI] [PubMed] [Google Scholar]
- 20.Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: A pressure-volume loop analysis. J Am Coll Cardiol. 2010;55:1701–1710. doi: 10.1016/j.jacc.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 21.Du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol. 2020;598:3793–3801. doi: 10.1113/jp280389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyhne MD, Hansen JV, Dragsbaek SJ, Mortensen CS, Nielsen-Kudsk JE, Andersen A. Oxygen therapy lowers right ventricular afterload in experimental acute pulmonary embolism. Crit Care Med. 2021;49:e891–e901. doi: 10.1097/ccm.0000000000005057. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol. 2018;314:H733–H752. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenceslau CF, McCarthy CG, Earley S, England SK, Filosa JA, Goulopoulou S, Gutterman DD, Isakson BE, Kanagy NL, Martinez-Lemus LA, Sonkusare SK, Thakore P, Trask AJ, Watts SW, Webb RC. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am J Physiol Heart Circ Physiol. 2021;321:H77–H111. doi: 10.1152/ajpheart.01021.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e578–e622. doi: 10.1161/cir.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 26.Pinsky MR. The hemodynamic consequences of mechanical ventilation: an evolving story. Intens Care Med. 1997;23:493–503. doi: 10.1007/s001340050364. [DOI] [PubMed] [Google Scholar]
- 27.Alviar CL, Miller PE, McAreavey D, Katz JN, Lee B, Moriyama B, Soble J, Diepen SV, Solomon MA, Morrow DA. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018;72:1532–1553. doi: 10.1016/j.jacc.2018.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castor G, Klocke RK, Stoll M, Helms J, Niedermark I. Simultaneous measurement of cardiac output by thermodilution, thoracic electrical bioimpedance and Doppler ultrasound. Br J Anaesth. 1994;72:133–138. doi: 10.1093/bja/72.1.133. [DOI] [PubMed] [Google Scholar]
- 29.Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intens Care Med. 2003;29:1426–1434. doi: 10.1007/s00134-003-1873-1. [DOI] [PubMed] [Google Scholar]
- 30.Luecke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care. 2005;9:607621. doi: 10.1186/cc3877. [DOI] [PMC free article] [PubMed] [Google Scholar]