Abstract
Primary aldosteronism is considered the commonest cause of secondary hypertension. In affected individuals, aldosterone is produced in an at least partially autonomous fashion in adrenal lesions (adenomas, [micro]nodules or diffuse hyperplasia). Over the past decade, next-generation sequencing studies have led to the insight that primary aldosteronism is largely a genetic disorder. Sporadic cases are due to somatic mutations, mostly in ion channels and pumps, and rare cases of familial hyperaldosteronism are caused by germline mutations in an overlapping set of genes. More than 90% of aldosterone-producing adenomas carry somatic mutations in K+ channel Kir3.4 (KCNJ5), Ca2+ channel CaV1.3 (CACNA1D), alpha-1 subunit of the Na+/K+ ATPase (ATP1A1), plasma membrane Ca2+ transporting ATPase 3 (ATP2B3), Ca2+ channel CaV3.2 (CACNA1H), Cl− channel ClC-2 (CLCN2), β-catenin (CTNNB1), and/or G-protein subunits alpha q/11 (GNAQ/11). Mutations in some of these genes have also been identified in aldosterone-producing (micro)nodules, suggesting a disease continuum from a single cell, acquiring a somatic mutation, via a nodule to adenoma formation, and from a healthy state to subclinical to overt primary aldosteronism. Individual glands can have multiple such lesions, and they can occur on both glands in bilateral disease. Familial hyperaldosteronism, typically with early onset, is caused by germline mutations in steroid 11-beta hydroxylase/ aldosterone synthase (CYP11B1/2), CLCN2, KCNJ5, CACNA1H, and CACNA1D.
Keywords: adenoma, aldosterone, hyperaldosteronism, mutation
Aldosterone, the main mineralocorticoid hormone, is physiologically produced in the zona glomerulosa of the adrenal cortex. By binding to the mineralocorticoid receptor, it activates signaling cascades leading to increased renal salt and water reabsorption, as well as increased potassium and proton secretion. The production of aldosterone is normally tightly regulated. Angiotensin II (the main product of the renin-angiotensin system) and elevated serum potassium levels are the main stimuli of aldosterone production; adrenocorticotropic hormone can also temporarily increase aldosterone levels.1 In primary aldosteronism (PA), levels of aldosterone are inappropriate for salt, volume, and/or potassium status. This excess production causes variable degrees of hypertension, possible hypokalemia, and disproportionately high levels of cardiovascular disease.2 PA is considered the most important cause of secondary hypertension. An Italian study of 1672 primary care patients with hypertension, following the Endocrine Society guidelines for diagnosis, reported an overall prevalence of 5.9%, ranging from 3.9 in stage 1 hypertension to 11.8% in stage 3 hypertenson.3 Recently, using urinary aldosterone for diagnosis, even higher prevalence estimates for biochemically overt PA were reported, from 11.3% in normotension to 22.0% in stage 3 hypertension.4
Traditionally, several subforms of PA were distinguished: bilateral adrenal hyperplasia, also known as idiopathic hyperaldosteronism (about 60% of cases), aldosterone-producing adenomas (APAs; about 30% of cases), unilateral hyperplasia (less common), malignancy, and familial hyperaldosteronism (FH; both very rare).5 Recent histological and genetic studies have challenged this concept and will be discussed in this review. The diagnosis of PA is complicated, based upon the aldosterone/renin ratio as screening parameter and subsequent confirmatory testing. Of clinical importance is the distinction between unilateral and bilateral forms because unilateral forms are amenable to potentially curative surgery, whereas bilateral forms are treated with mineralocorticoid receptor antagonists.5
Until 2011, the molecular mechanisms underlying autonomous aldosterone production in PA were largely unknown. Discoveries made over the last decade, their potential future impact on clinical PA management and open questions in the field of PA genetics will be covered in this review.
Somatic Mutations in APAs
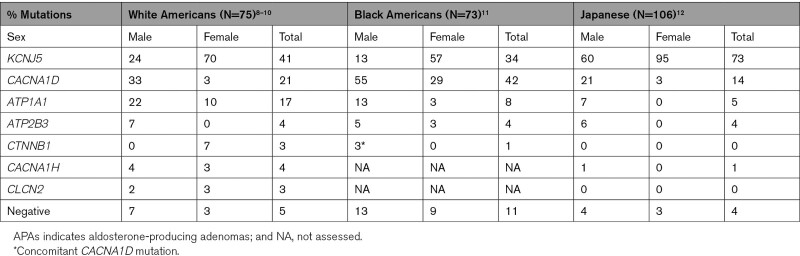
Because APAs are typically treated by unilateral adrenalectomy, tumor tissue is available for genetic studies. Advances in sequencing technology enabled the discovery of somatic (tumor specific) mutations in APAs. The initial study sequenced the exomes of 4 APAs and corresponding normal tissue. Two tumors carried heterozygous mutations in the KCNJ5 gene, which encodes the inward rectifier potassium channel Kir3.4. One mutation, G151R, was located within the selectivity filter of the channel, which enables K+ to pass through the channel and blocks the passage of Na+, and the second mutation, L168R, was close by. Both mutations led to abnormal sodium permeability and depolarization of the cell,6 which results in opening of voltage-gated calcium channels, calcium influx and increased aldosterone production.7 Sanger sequencing identified 6 additional somatic KCNJ5 mutations, all G151R and L168R, in 18 other APAs.6 Follow-up studies showed that G151R and L168R are recurrently mutated in APAs and account for >40% of APAs (Table 1). Other KCNJ5 mutations are rare.9,13 Among rare mutations, T158A has been functionally studied, demonstrating activation of transcriptional regulators NURR1 and ATF2 downstream of calcium signaling and upstream of CYP11B2.14,15 Further exome sequencing studies of KCNJ5 (K+ channel Kir3.4)-negative tumors revealed heterozygous mutations in CACNA1D, which encodes the L-type calcium channel CaV1.3.16,17 CACNA1D is large, and mutations cluster much less than in KCNJ5, which is why early Sanger sequencing may have missed mutations; recent studies (albeit with rather small case numbers) show frequencies between 14 and 42% (Table 1).9,11,18 Another frequently mutated gene with heterozygous somatic mutations is ATP1A1,17,19 which encodes the α-1 subunit of the Na+/K+-ATPase. Somatic mutations cause abnormal permeability to Na+ or H+ and loss of pump function, leading to depolarization, aldosterone production and proliferation, likely via increased intracellular calcium and cellular acidification.17,18,20,21 Less frequently, plasma membrane Ca2+ ATPase (ATP2B3) mutations cause abnormal Na+ and potentially Ca2+ permeability, along with reduced Ca2+ transport capacity, all resulting in elevated intracellular Ca2+ levels.19,22 Heterozygous somatic gain-of-function mutations in CACNA1H, encoding the T-type calcium channel CaV3.2, are also infrequent.10 These mutations confer gain-of-function, with increased calcium influx, like CACNA1D mutations. Similarly rare are mutations in the gene CLCN2, encoding the ClC-2 chloride channel.23,24
Table 1.
Somatic Mutation Frequencies in APAs From Different Ethnicities and Sexes8
Whereas the mutations discussed above affect ion channels or pumps and either directly or indirectly cause increased intracellular calcium levels (Figure 1), somatic mutations in CTNNB1, encoding β-catenin, point to a different mechanism. β-Catenin is part of the Wnt signaling pathway. Somatic CTNNB1 mutations are present in several tumor types,25 including benign and malignant, hormone-producing and nonproducing tumors of the adrenal gland, among them APAs.26–28 One study suggested an association of CTNNB1 mutations in APAs with pregnancy,29 which, however, was doubted30; such mutations were also found in males.31,32 Interestingly, unlike the mutations described above, CTNNB1 mutations have been described to frequently cooccur with other aldosterone-driver mutations, such as a CACNA1D mutation11 or mutations in the GNA11 and GNAQ genes, encoding G-protein α subunits G11 and Gq, respectively,33 downstream of the AT1R (angiotensin 1 receptor). Mutations in GNA11 and GNAQ have only been found in conjunction with CTNNB1 mutations and are likely not sufficient for APA formation alone. Binding of angiotensin II to the AT1R results in exchange of guanosine diphophate for guanosine triphosphate, liberation of Gβγ and activation of downstream phospholipase Cβ, which then cleaves phosphatidylinositol 4,5-bisphosphate into diacyl glycerol and inositol trisphosphate. Inositol trisphosphate causes Ca2+ release from intracellular stores (Figure 1).1 Somatic mutations prevent the hydrolysis of GTP that usually terminates signaling, causing constitutive activity and aldosterone production. Concurrent mutations in CTNNB1 and GNA11/Q were associated with increased expression of luteinizing hormone/choriogonadotropin receptor (LHCGR), potentially explaining an association with pregnancy.33 In mice, expression of a Ctnnb1 gain-of-function allele to the zona glomerulosa leads to a block of transdifferentiation of zona glomerulosa cells into zona fasciculata cells, resulting in progressive hyperplastic expansion of the zona glomerulosa and increased aldosterone levels.34 This observation suggests that CTNNB1 mutations may act primarily by increasing the number of aldosterone-producing cells, which may be sufficient to cause PA once tumors are large enough. Additional somatic mutations may cause increased aldosterone production on the individual cell level, aggravating the phenotype. GNA11 mutations may precede CTNNB1 mutations based on the finding of GNA11 single mutations in hyperplastic areas outside of double-mutant APAs.33 Last, PRKACA mutations, common in cortisol-producing adenomas, have been described in 2 PA cases, however, their role in causing PA is doubtful.35
Figure 1.
Somatic and germline mutations in primary aldosteronism (PA). In familial hyperaldosteronism (FH)-I, the CYP11B1/2 hybrid gene (1) is activated by adrenocorticotropic hormone (ACTH) via the MC2R (melanocortin 2 receptor) and cAMP signaling. KCNJ5 mutations (2) in aldosterone-producing adenomas (APAs) and in FH-III lead to abnormal Na+ influx, CLCN2 mutations (3) in APAs and in FH-II to higher Cl− efflux, and ATP1A1 (4) and ATP2B3 (5) mutations in APAs to channel like-permeabilities for Na+, H+, and Ca2+, as well as impaired pump function. These effects cause membrane depolarization, activation of voltage-gated calcium channels, calcium influx and increased calcium signaling, stimulating CYP11B2 expression and aldosterone production. Acidification may also play a role in ATP1A1 pathophysiology (not shown). Mutations in CACNA1D (6) and CACNA1H (7) directly increase calcium permeability. GNA11 and GNAQ mutations (8) in APAs prevent termination of G-protein signaling downstream of the AT1R (angiotensin 1 receptor), leading to increased calcium release from intracellular stores. They cooccur with CTNNB1 mutations (9) that prevent β-catenin degradation with increased signaling via the TCF/LEF (T-cell factor/lymphoid enhancer factor) family. Created with BioRender.com. DAG indicates diacyl glycerol; PLC, phospholipase C; IP3, inositol trisphosphate; and PIP2, phosphatidylinositol 4,5-bisphosphate.
Sex and Ethnicity Aspects in APA Somatic Mutation Frequencies
Silent adrenal masses (incidentalomas, Figure 2) are common, especially in the elderly,37 and routine histopathology cannot distinguish between nonproducing and aldosterone-producing lesions. The most reliable data on mutations frequencies are from recent studies that identify the culprit lesion by immunohistochemistry for aldosterone synthase (Figure 2) and perform highly sensitive panel sequencing for mutation identification. When all known genes were sequenced, mutations were found in at least 95% of APAs. As noted for KCNJ5 in earlier studies,13 mutation frequencies differ between sexes and ethnicities8 (Table 1). KCNJ5 mutations are more frequent in women, whereas CACNA1D and ATP1A1 mutations are more frequent in men. KCNJ5 mutations have the highest prevalence in Asian populations,38–40 followed by those of European origin9 and African ancestry.11 In contrast, CACNA1D mutations are more frequent in blacks, outnumbering KCNJ5 mutations.11 Differences in diagnostic algorithms, salt or phytoestrogen ingestion cannot be excluded; however, biological differences among sexes and ethnicities, such as those in circulating hormone levels, could well underlie these effects.8,41 Of note, female mice show a 3-fold higher adrenocortical tissue turnover than males. Female mice also use a stem cell/progenitor cell compartment in the adrenal capsule for renewal that is unused by males.42 Higher turnover and thus a higher probability of acquiring somatic mutations may thus underlie the higher frequency of adrenocortical tumors in women.43 It is also tempting to speculate that the mutational profile of APAs depends on their cells of origin, which may differ between sexes and, possibly, ethnicities.
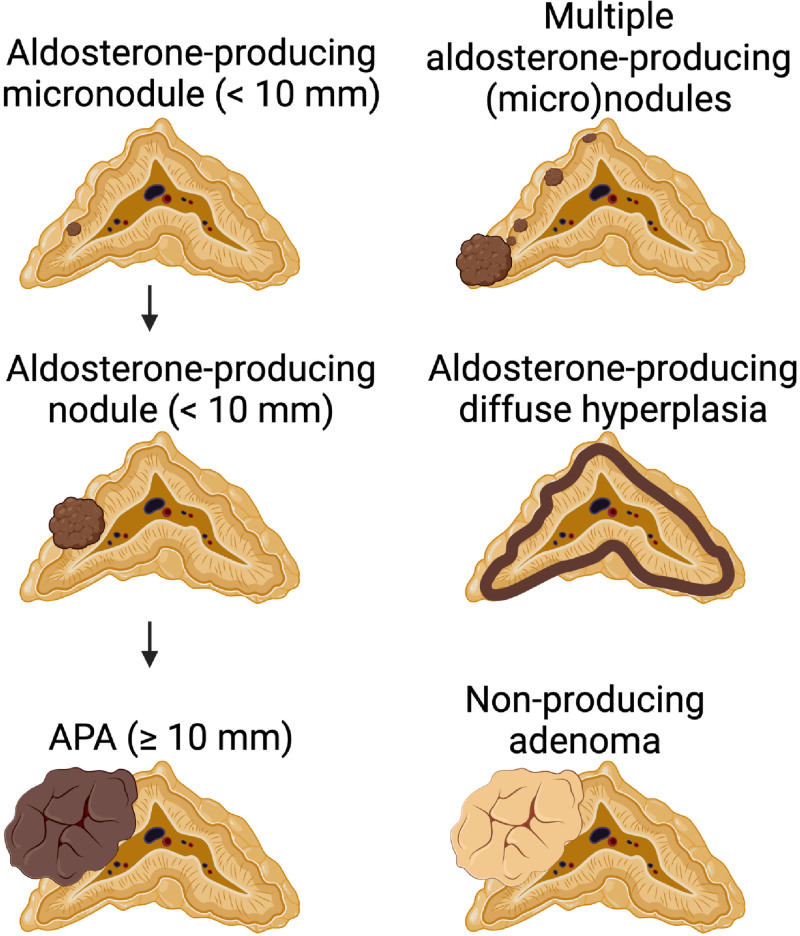
Figure 2.
Adrenal lesions.36 Aldosterone-producing micronodules (formerly aldosterone-producing cell clusters [APCCs]) are not recognizable by hematoxylin and eosin staining but stain positive for aldosterone synthase expression. Aldosterone-producing nodules are visible on hematoxylin and eosin staining and are distinguished from aldosterone-producing adenoma (APA) by size. Multiple (micro)nodules can cooccur within a single gland. Aldosterone-producing diffuse hyperplasia shows a broad, uninterrupted strip of aldosterone synthase-positive cells. Nonproducing adenomas stain negative for aldosterone synthase. Brown color indicates aldosterone synthase positivity on immunohistochemistry. Created with BioRendor.com.
Somatic Mutations in Aldosterone-Producing (Micro)nodules and in Bilateral Disease
Small nodules of cells with increased expression of aldosterone synthase that protrude beyond the zona glomerulosa into the zona fasciculata occur in healthy individuals and PA patients. They were first described as aldosterone-producing cell clusters (APCCs) in 201044 and are now, according to a recently published international consensus, referred to as aldosterone-producing micronodules (Figure 2).36 Using panel sequencing of APA disease genes, Nishimoto et al45 in 2015 discovered that APCCs from healthy kidney donors carried somatic mutations in CACNA1D (6/23) and ATP1A1 (2/23).45 Nanba et al46 expanded on these findings by demonstrating that the APCC area increases with age in healthy kidney donors, contrary to normal zona glomerulosa CYP11B2 expression. Interestingly, in a separate clinical study, they detected an increase of the aldosterone/renin ratio with age on a high-sodium diet, whereas aldosterone stimulation on a low-sodium diet was blunted with age, suggesting that autonomous aldosterone production may increase with age due to the development of aldosterone-producing (micro)nodules, whereas normal zona glomerulosa recedes.46 Micronodules resembling those in normal individuals were also found in adrenals from patients with unilateral PA without adenoma; they carried somatic mutations in CACNA1D, KCNJ5, ATP1A1, and ATP2B3, whereas no such mutations were found in nonnodular diffuse hyperplasia.47 Furthermore, 15 cases with bilateral disease who, as an unusual measure, underwent unilateral adrenalectomy, showed more and larger APCCs than normotensive controls. Sequencing of aldosterone-producing lesions revealed mutations in CACNA1D in 58% of APCCs, and of KCNJ5 in 1% (in a lesion considered by the authors as micro-APA),48 suggesting that somatic mutations in aldosterone-producing (micro)nodules may underlie bilateral disease. Interestingly, adrenal hyperplasia, including (micro)nodules, can also occur in adrenals carrying a circumscribed APA. De Sousa et al18 investigated such adrenal glands using CYP11B2 immunohistochemistry and next-generation sequencing. Sequencing of 57 APCCs adjacent to an APA revealed mutations in known APA driver genes in 15, distinct from the mutation in the APA, with different mutations among different APCCs within the same gland. Besides mutations in CACNA1D and ATP1A1, interestingly, mutations in KCNJ5, previously considered rare or absent in APCCs, but also mutations in CACNA1H, PRKACA, and CTNNB1 were observed.18 Taken together, these results suggest that somatic mutations causing autonomous aldosterone production frequently occur in human adrenal glands. They contribute to variable degrees of autonomous aldosterone secretion.
Impact of Somatic Mutations on Proliferation and Second-Hit Hypothesis
A controversy in the field has been whether mutations in aldosterone-driver genes are sufficient only to cause increased/at least partially autonomous aldosterone production or also increased proliferation. In the APCC model, a somatic mutation in a single cell is assumed to cause increased aldosterone production and proliferation, leading to an aldosterone-producing micronodule, aldosterone-producing nodule, and eventually APA. In the alternative concept of a second-hit hypothesis, genetic or environmental factors cause proliferation, with additional somatic mutations leading to APA formation.49 Whether KCNJ5 mutations cause proliferation has been debated. The overexpression of mutant KCNJ5 in cultured cells increases lethality rather than proliferation, likely due to massive Na+ influx.50,51 In vivo, however, KCNJ5-positive tumors show particularly low KCNJ5 expression.52 Evidence from germline KCNJ5 mutations (see below) and, specifically individuals with mosaicism for KCNJ5 mutations suggest that these mutations either increase proliferation or block transdifferentiation, leading to hyperplasia in vivo.50,53,54 APA-associated ATPA1A1 mutations would likely be lethal if present in the germline. However, a recent study provided evidence for proliferative effects of an ATPA1A mutation in the HAC15 adrenocortical cancer cell line in vitro, an effect that was serum dependent.21 Evidence for the second-hit hypothesis includes an individual with bilateral macronodular adrenal hyperplasia due to familial adenomatous polyposis with APC mutation and KCNJ5-positive APA.55 Zona glomerulosa hyperplasia but also increased nodulation and decreased vascularity and expression of stem/progenitor cell markers in areas surrounding APAs has also been suggested to support the second-hit hypothesis,56,57 however, such lesions typically carry independent somatic mutations.18
Germline Mutations in FH
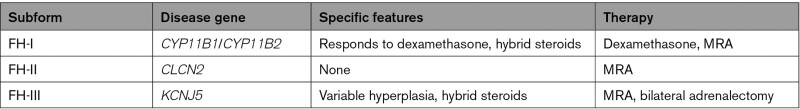
Genetic testing for FH subtypes (Table 2) is recommended in affected individuals with early onset and a positive family history of PA.58 Familial aggregation of PA was first described in 1966 in a father and a son whose phenotype was relieved by administration of dexamethasone (glucocorticoid-remediable aldosteronism, later also FH-I).59 This autosomal-dominant disorder is characterized by early-onset hypertension, often with a positive family history; hemorrhagic stroke and ruptured intracranial aneurysm are common in individuals without appropriate therapy.60 Incomplete penetrance or mild forms have been reported.61,62 In one study of 300 consecutive PA patients, 2 had FH-I. FH-I is caused by a hybrid gene that occurs due to a recombination event between CYP11B1 (11β-hydroxylase, involved in cortisol synthesis under the control of adrenocorticotropic hormone) and CYP11B2 (aldosterone synthase). This leads to ectopic expression of aldosterone synthase in the zona fasciculata under the control of adrenocorticotropic hormone and the formation of hybrid steroids 18-oxocortisol and 18-hydroxycortisol,63 indicating the abnormal colocalization of enzymes involved in aldosterone and cortisol synthesis. Treatment with glucocorticoids suppresses adrenocorticotropic hormone and, sometimes in combination with mineralocorticoid receptor antagonists, normalizes aldosterone and blood pressure. Unlike other FH genes, the chimeric CYP11B1/2 gene has not been found in APAs.64
Table 2.
FH Subforms
FH-II initially referred to all non-FH-I cases of FH.65 An often-quoted prevalence of 6% in PA66 is likely a considerable overestimate due to chance associations of sporadic PA cases in families. Because a heterozygous R172Q CLCN2 mutation was discovered in a family from the original report,65 FH-II now denotes individuals with germline mutations in the CLCN2.67 Mutations were found in 7 additional families, among them 2 de novo. FH-II patients typically had early-onset hypertension with elevated aldosterone/renin ratios, with age at diagnosis typically before age 20 years, although, as in FH-I, incomplete penetrance was reported.67 Hypertension responded to therapy with mineralocorticoid receptor antagonists or other antihypertensive agents. Another de novo germline N-terminal mutation was reported in a patient diagnosed with PA at age 9 years.68 The affected girl showed severe hypertension and persistent hypokalemia and responded to medical therapy. Due to a positive dexamethasone suppression test, she was initially suspected to have glucocorticoid-remediable aldosteronism, but genetic analysis for the chimeric CYP11B1/2 gene was negative. ClC-2, the chloride channel encoded by CLCN2, mediates net efflux of chloride in the zona glomerulosa.67 CLCN2 mutations lead to increased chloride efflux, cellular depolarization, calcium influx, and aldosterone production (Figure 1).67,68
FH-III refers to patients with heterozygous germline mutations in the KCNJ5 gene. The initially published kindred showed severe therapy-resistant PA in childhood. Hybrid steroids were even higher than in FH-I, but treatment with dexamethasone failed to normalize blood pressure or serum aldosterone.69 Severely enlarged adrenal glands were found upon bilateral adrenalectomy, with a large zone of lipid-laden cells that expressed aldosterone synthase and enzymes involved in cortisol synthesis, explaining hybrid steroid synthesis.69,70 Genetic analysis identified a KCNJ5 T158A mutation.6 Additional kindreds, often with bilateral adrenal hyperplasia, were subsequently identified, for example, patients with G151R mutation that is common as somatic mutation in APAs.50 Interestingly, patients with another mutation, G151E, do not show massive hyperplasia and can be treated medically50,71; this mutation appears to cause extreme Na+ influx that may interfere with cellular survival. This mutation does not occur in APAs, but, interestingly, has been identified in an APCC.18
FH-IV denotes patients with heterozygous germline mutations in the CACNA1H gene. One recurrent identical mutation, M1549V, was identified in 5 families with early-onset PA without other remarkable characteristics.72 An additional early-onset case with de novo M1549I mutation had PA and multiplex developmental disorder. The pathogenicity of additional variants is less certain.73,74 CACNA1H mutations lead to gain of channel function, with increased calcium influx and aldosterone production.72,75
A complex syndrome comprising PA, seizures, and neurological abnormalities was described in 2 individuals with de novo heterozygous gain-of-function CACNA1D mutations.16 Both were diagnosed with cerebral palsy. Variable associated abnormalities included transient hypoglycemia and cardiac defects. Expression of the channel in several other organs, including brain, pancreas, and heart, likely accounts for associated abnormalities; PA is variable.76–78 Several individuals with de novo CACNA1D mutations have been diagnosed with autism spectrum disorder without major endocrine abnormalities.79–81
Finally, any roles of ARMC5 and phosphodiesterase germline variants in PA remain to be confirmed.82,83 ATPase mutations have not been reported in FH, suggesting incompatibility of such germline mutations with survival.
Genetic Mouse Models of PA
Several mouse models of human PA have been generated. A mouse model with transgenic expression of human CYP11B2 under the control of the human CYP11B1 promoter (resembling FH-I), shows elevated aldosterone levels and hypertension on a high-salt diet.84 A knockin mouse (Clcn2R180Q/+) models the commonest FH-II mutation, with normal adrenal weight and morphology, mildly elevated aldosterone levels and mildly elevated blood pressure. Intracellular Ca2+ oscillatory activity in the adrenal zona glomerulosa is elevated.85 A second model carries a gain-of-function N-terminal deletion of 8 amino acids. In the homozygous state, the zona glomerulosa cells in this model are depolarized, with increased intracellular Ca2+ levels, elevated aldosterone and decreased renin, elevated blood pressure, hypokalemia, and moderate albuminuria.86 Kcnj5 is not expressed in rodent adrenal glomerulosa.87 A mouse model expressing wild type or mutant human KCNJ5 under the Akr1b7 promoter, reported only as an abstract, appears to lack adrenal hypertrophy.88 A knockin mouse with Cacna1hM1560V/+ mutation (FH-IV model) shows normal adrenal morphology, elevated adrenal Cyp11b2 expression and elevated blood pressure. Adrenals from these animals have elevated baseline and peak intracellular Ca2+ levels.89 Last, a transgenic mouse with adrenocortical expression of a Gq-coupled designer receptor develops disorganization of adrenal zonation and hyperaldosteronism,90 as in GNAQ mutations in APAs. Additional models with mutations in KCNK3 and KCNK9 potassium channels or cryptochrome genes have been reviewed elsewhere.91
Diagnostic and Therapeutic Advances Based on Genetic Discoveries
Of particular interest regarding clinical diagnosis are APAs with KCNJ5 mutations. Beyond their high prevalence, they are associated with early diagnosis, high aldosterone levels, large tumors,13 high cure rates,92 and lower precontrast Hounsfield units on computed tomography93 due to lipid-rich fasciculata-like tumor cells.17,93,94 Like FH-III patients, patients with KCNJ5-positive APAs have elevated concentrations of hybrid steroids,95 allowing prediction of unilateral disease based on steroid profiling.92 This, together with imaging, may in the future help to bypass adrenal venous sampling in individuals with KCNJ5 mutations.
In addition, blockers of mutant KCNJ5 channels could serve as diagnostic or therapeutic tools in PA. A drop in blood pressure or aldosterone in response to short-term treatment may help to identify KCNJ5-positive tumors. Long-term therapy might lead to tumor shrinkage.96 Sensitivity of mutant KCNJ5 channels to blockers of Na+ and Ca2+ transporting proteins such as verapamil (for G151R and L168R) and amiloride (only L168R analyzed) has been reported.7 In a high-throughput screen, macrolide antibiotics such as roxithromycin and clarithromycin were identified as specific blockers of both G151R and L168R, but not WT KCNJ5. Similarly, the nonantibiotic macrolide motilin receptor agonist idremcinal and synthesized macrolide derivatives without antibiotic or motilide activity specifically inhibited mutant KCNJ5 channels.96 Macrolide compounds decrease the excessive aldosterone production associated with expression of mutant KCNJ5 channels in an aldosterone-producing cell line in vitro96 and in APA cells carrying KCNJ5 mutations ex vivo.97 Results of proof-of-concept studies in humans evaluating macrolides for the diagnosis of PA are pending.98
The finding that intracellular calcium is the key signal for aldosterone also raises the question whether calcium channel blockers could be used to inhibit aldosterone production. Therapeutic use of calcium channel blockers in PA was considered long before the discovery of calcium channel mutations99 but approved compounds target vascular calcium channels, and their antihypertensive effect is mostly aldosterone-independent.5 Normal aldosterone values in Cacna1h knockout mice89,100 and the phenotype of Cacna1d knockout mice (deafness and sinoatrial node dysfunction with bradycardia and arrhythmia)101 argue against these channels as therapeutic targets.
Finally, the finding of a likely genetic continuum between hyperplastic lesions and APAs, combined with the availability of highly specific CYP11B2 antibodies, has led to a new histopathology consensus for the description of unilateral disease36 (Figure 2).
Summary and Future Perspectives
Genetic studies, published over the last decade, have—perhaps unexpectedly—established PA as a largely genetic disorder. The initial discovery that APAs are due to somatic mutations, mostly in ion channels and pumps, was followed by the finding that similar mutations are highly prevalent in what are now called aldosterone-producing (micro)nodules, particularly in bilateral disease. There is probably a biologic continuum between somatic mutations in single cells in otherwise healthy individuals, (micro)nodule formation and eventually APA development,102 making cutoffs for diagnosis of PA somewhat arbitrary. Indeed, there is clinical evidence that subclinical PA is present in normotensive individuals and is associated with an increased risk for the development of hypertension.103 One study, based on urinary aldosterone and oral sodium suppression tests, found a continuum of renin-independent aldosterone production, paralleling the severity of hypertension.4 Small lesions could be the histopathologic correlate of subclinical PA with low renin,103 in particular in individuals with resistant hypertension, as suggested by the good response to spironolactone in the PATHWAY-2 study.104
The recognition of this biological continuum also to some extent questions the distinction between unilateral and bilateral disease; hyperplastic or nodular areas are common in APA patients and can lead to contralateral recurrence.105 In addition, asymmetrical aldosterone production with bilateral lesions can prevent cure after adrenalectomy.106
Unanswered questions regarding the genetics and pathophysiology of PA include:
In which cell(s) does the initial somatic mutation occur—stem/progenitor cells107 and/or glomerulosa cells? What are the mechanisms underlying proliferation of aldosterone-producing lesions? Are there additional mutations to discover? Additional disease genes will explain only small fractions of APAs, but it is tempting to speculate that aldosterone-producing micronodules could carry mutations without major effects on proliferation, similar to GNA11/Q mutations discussed above, and that more FH cases will be solved. Last, how these discoveries will translate into clinical application is a major unresolved question. The major challenges in PA clinical care are low rates of screening and diagnosis,108 which, beyond a lack of awareness, are due to complex, expensive and often invasive screening procedures5 that are unavailable outside tertiary care centers. Furthermore, medical treatment options are scarce and include spironolactone, which is associated with severe side effects at higher doses, and eplerenone, which is not approved for the therapy of hypertension in many countries. Research discoveries have contributed to raised PA awareness in the academic world, but improved care will require less complicated diagnostic algorithms and new treatment options. Steroid profiling and drugs targeting mutant ion channels as discussed above are first steps. It is also conceivable that tracers could in the future recognize mutant surface proteins in imaging, but this again would likely be limited to large centers. The insight that PA, clinical or subclinical, occurs across normotension and all stages of hypertension suggests that simple screening tests, such as that for plasma renin should be performed in most hypertensive patients.
Article Information
Sources of Funding
This work was funded by the Stiftung Charité (BIH_PRO_406) and the German Research Foundation (DFG, CRC 1453 (Project-ID 431984000) and CRC 1365).
Disclosures
Rockefeller University holds a patent Compositions and methods for diagnosing and treating diseases and disorders associated with mutant KCNJ5 listing U.I. Scholl as an inventor.
Nonstandard Abbreviations and Acronyms
- APA
- aldosterone-producing adenoma
- APCC
- aldosterone-producing cell cluster
- AT1R
- angiotensin 1 receptor
- FH
- familial hyperaldosteronism
- KCNJ5
- K+ channel Kir3.4
- PA
- primary aldosteronism
- TCF/LEF
- T-cell factor/lymphoid enhancer factor
For Sources of Funding and Disclosures, see page 894.
References
- 1.Spät A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003 [DOI] [PubMed] [Google Scholar]
- 2.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 3.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052 [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF, Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 6.Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, Beuschlein F, Reincke M, Barhanin J, Bandulik S, et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155:1353–1362. doi: 10.1210/en.2013-1944 [DOI] [PubMed] [Google Scholar]
- 8.Nanba K, Rainey WE. Genetics in endocrinology: impact of race and sex on genetic causes of aldosterone-producing adenomas. Eur J Endocrinol. 2021;185:R1–R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, Miller BS, Giordano TJ, Tomlins SA, Rainey WE. Targeted molecular characterization of aldosterone-producing adenomas in white americans. J Clin Endocrinol Metab. 2018;103:3869–3876. doi: 10.1210/jc.2018-01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanba K, Blinder AR, Rege J, Hattangady NG, Else T, Liu CJ, Tomlins SA, Vats P, Kumar-Sinha C, Giordano TJ, et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75:645–649. doi: 10.1161/HYPERTENSIONAHA.119.14349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73:885–892. doi: 10.1161/HYPERTENSIONAHA.118.12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanba K, Yamazaki Y, Bick N, Onodera K, Tezuka Y, Omata K, Ono Y, Blinder AR, Tomlins SA, Rainey WE, et al. Prevalence of somatic mutations in aldosterone-producing adenomas in Japanese patients. J Clin Endocrinol Metab. 2020;105:dgaa595. doi: 10.1210/clinem/dgaa595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic kcnj5 k channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100:E1089–95. doi: 10.1210/jc.2015-2149 [DOI] [PubMed] [Google Scholar]
- 14.Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol. 2016;57:1–11. doi: 10.1530/JME-15-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782. doi: 10.1210/en.2011-1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716 [DOI] [PubMed] [Google Scholar]
- 18.De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, Rocha A, Giscos-Douriez I, Meatchi T, Amar L, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75:1034–1044. doi: 10.1161/HYPERTENSIONAHA.119.14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–4, 444e1. doi: 10.1038/ng.2550 [DOI] [PubMed] [Google Scholar]
- 20.Stindl J, Tauber P, Sterner C, Tegtmeier I, Warth R, Bandulik S. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na(+)/K(+)-ATPase. Endocrinology. 2015;156:4582–4591. doi: 10.1210/en.2015-1466 [DOI] [PubMed] [Google Scholar]
- 21.Kobuke K, Oki K, Gomez-Sanchez CE, Gomez-Sanchez EP, Itcho K, Ohno H, Nagano G, Yoshii Y, Baba R, Kodama T, et al. ATP1A1 mutant in aldosterone-producing adenoma leads to cell proliferation. Int J Mol Sci. 2021;22:10981. doi: 10.3390/ijms222010981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauber P, Aichinger B, Christ C, Stindl J, Rhayem Y, Beuschlein F, Warth R, Bandulik S. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca(2+)-ATPase ATP2B3. Endocrinology. 2016;157:2489–2499. doi: 10.1210/en.2015-2029 [DOI] [PubMed] [Google Scholar]
- 23.Dutta RK, Arnesen T, Heie A, Walz M, Alesina P, Söderkvist P, Gimm O. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181:K37–K41. doi: 10.1530/EJE-19-0377 [DOI] [PubMed] [Google Scholar]
- 24.Rege J, Nanba K, Blinder AR, Plaska S, Udager AM, Vats P, Kumar-Sinha C, Giordano TJ, Rainey WE, Else T. Identification of somatic mutations in CLCN2 in aldosterone-producing adenomas. J Endocr Soc. 2020;4:bvaa123. doi: 10.1210/jendso/bvaa123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Jeong S. Mutation hotspots in the beta-catenin gene: lessons from the human cancer genome databases. Mol Cells. 2019;42:8–16. doi: 10.14348/molcells.2018.0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf). 2008;68:264–270. doi: 10.1111/j.1365-2265.2007.03033.x [DOI] [PubMed] [Google Scholar]
- 27.Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, René-Corail F, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46:607–612. doi: 10.1038/ng.2953 [DOI] [PubMed] [Google Scholar]
- 28.Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, Ragazzon B, et al. Wnt/beta-catenin pathway activation in adrenocortical adenomas is frequently due to somatic ctnnb1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96:E419–426. doi: 10.1210/jc.2010-1885 [DOI] [PubMed] [Google Scholar]
- 29.Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA, et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. 2015;373:1429–1436. doi: 10.1056/NEJMoa1504869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murtha TD, Carling T, Scholl UI. Pregnancy, primary aldosteronism, and somatic CTNNB1 mutations. N Engl J Med. 2016;374:1492–1493. doi: 10.1056/NEJMc1514508 [DOI] [PubMed] [Google Scholar]
- 31.Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, Wang JJ, Connolly R, Hu YH, Gomez-Sanchez CE, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017;7:39121. doi: 10.1038/srep39121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. doi: 10.1038/srep19546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Azizan EAB, Cabrera CP, Fernandes-Rosa FL, Boulkroun S, Argentesi G, Cottrell E, Amar L, Wu X, O’Toole S, et al. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat Genet. 2021;53:1360–1372. doi: 10.1038/s41588-021-00906-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pignatti E, Leng S, Yuchi Y, Borges KS, Guagliardo NA, Shah MS, Ruiz-Babot G, Kariyawasam D, Taketo MM, Miao J, et al. Beta-Catenin causes adrenal hyperplasia by blocking zonal transdifferentiation. Cell Rep. 2020;31:107524. doi: 10.1016/j.celrep.2020.107524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhayem Y, Perez-Rivas LG, Dietz A, Bathon K, Gebhard C, Riester A, Mauracher B, Gomez-Sanchez C, Eisenhofer G, Schwarzmayr T, et al. PRKACA somatic mutations are rare findings in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101:3010–3017. doi: 10.1210/jc.2016-1700 [DOI] [PubMed] [Google Scholar]
- 36.Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, Mete O, Yamazaki Y, Zerbini MCN, Beuschlein F, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106:42–54. doi: 10.1210/clinem/dgaa484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149:273–285. doi: 10.1530/eje.0.1490273 [DOI] [PubMed] [Google Scholar]
- 38.Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Okada S, Rokutanda N, Takata D, et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. 2012;97:1311–1319. doi: 10.1210/jc.2011-2885 [DOI] [PubMed] [Google Scholar]
- 39.Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65:622–628. doi: 10.1161/HYPERTENSIONAHA.114.03346 [DOI] [PubMed] [Google Scholar]
- 40.Cheng CJ, Sung CC, Wu ST, Lin YC, Sytwu HK, Huang CL, Lin SH. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J Clin Endocrinol Metab. 2015;100:E155–E163. doi: 10.1210/jc.2014-3009 [DOI] [PubMed] [Google Scholar]
- 41.Lyraki R, Schedl A. The sexually dimorphic adrenal cortex: implications for adrenal disease. Int J Mol Sci. 2021;22:4889. doi: 10.3390/ijms22094889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grabek A, Dolfi B, Klein B, Jian-Motamedi F, Chaboissier MC, Schedl A. The adult adrenal cortex undergoes rapid tissue renewal in a sex-specific manner. Cell Stem Cell. 2019;25:290–296.e2. doi: 10.1016/j.stem.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 43.Audenet F, Méjean A, Chartier-Kastler E, Rouprêt M. Adrenal tumours are more predominant in females regardless of their histological subtype: a review. World J Urol. 2013;31:1037–1043. doi: 10.1007/s00345-012-1011-1 [DOI] [PubMed] [Google Scholar]
- 44.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010 [DOI] [PubMed] [Google Scholar]
- 45.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112:E4591–E4599. doi: 10.1073/pnas.1505529112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136:347–355. doi: 10.1161/CIRCULATIONAHA.117.028201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102:1182–1192. doi: 10.1210/jc.2016-2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, Anand SK, Guo Z, Stowasser M, Sasano H, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72:874–880. doi: 10.1161/HYPERTENSIONAHA.118.11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Genetic and genomic mechanisms of primary aldosteronism. Trends Mol Med. 2020;26:819–832. doi: 10.1016/j.molmed.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 50.Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A. 2012;109:2533–2538. doi: 10.1073/pnas.1121407109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Gomez-Sanchez CE, Jaquin D, Aristizabal Prada ET, Meyer LS, Knösel T, Schneider H, Beuschlein F, Reincke M, Williams TA. Primary aldosteronism: KCNJ5 mutations and adrenocortical cell growth. Hypertension. 2019;74:809–816. doi: 10.1161/HYPERTENSIONAHA.119.13476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandes-Rosa FL, Amar L, Tissier F, Bertherat J, Meatchi T, Zennaro MC, Boulkroun S. Functional histopathological markers of aldosterone producing adenoma and somatic KCNJ5 mutations. Mol Cell Endocrinol. 2015;408:220–226. doi: 10.1016/j.mce.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 53.Tamura A, Nishimoto K, Seki T, Matsuzawa Y, Saito J, Omura M, Gomez-Sanchez CE, Makita K, Matsui S, Moriya N, et al. Somatic KCNJ5 mutation occurring early in adrenal development may cause a novel form of juvenile primary aldosteronism. Mol Cell Endocrinol. 2017;441:134–139. doi: 10.1016/j.mce.2016.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maria AG, Suzuki M, Berthon A, Kamilaris C, Demidowich A, Lack J, Zilbermint M, Hannah-Shmouni F, Faucz FR, Stratakis CA. Mosaicism for KCNJ5 causing early-onset primary aldosteronism due to bilateral adrenocortical hyperplasia. Am J Hypertens. 2020;33:124–130. doi: 10.1093/ajh/hpz172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vouillarmet J, Fernandes-Rosa F, Graeppi-Dulac J, Lantelme P, Decaussin-Petrucci M, Thivolet C, Peix JL, Boulkroun S, Clauser E, Zennaro MC. Aldosterone-producing adenoma with a somatic KCNJ5 mutation revealing APC-dependent familial adenomatous polyposis. J Clin Endocrinol Metab. 2016;101:3874–3878. doi: 10.1210/jc.2016-1874 [DOI] [PubMed] [Google Scholar]
- 56.Boulkroun S, Samson-Couterie B, Golib-Dzib JF, Amar L, Plouin PF, Sibony M, Lefebvre H, Louiset E, Jeunemaitre X, Meatchi T, et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205 [DOI] [PubMed] [Google Scholar]
- 57.Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A, et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56:885–892. doi: 10.1161/HYPERTENSIONAHA.110.158543 [DOI] [PubMed] [Google Scholar]
- 58.Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, Beuschlein F, Rossi GP, Nishikawa T, Morganti A, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens. 2020;38:1919–1928. doi: 10.1097/HJH.0000000000002510 [DOI] [PubMed] [Google Scholar]
- 59.Sutherland DJ, Ruse JL, Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J. 1966;95:1109–1119. [PMC free article] [PubMed] [Google Scholar]
- 60.Litchfield WR, Anderson BF, Weiss RJ, Lifton RP, Dluhy RG. Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension. 1998;31(1 Pt 2):445–450. doi: 10.1161/01.hyp.31.1.445 [DOI] [PubMed] [Google Scholar]
- 61.Mulatero P, di Cella SM, Williams TA, Milan A, Mengozzi G, Chiandussi L, Gomez-Sanchez CE, Veglio F. Glucocorticoid remediable aldosteronism: low morbidity and mortality in a four-generation italian pedigree. J Clin Endocrinol Metab. 2002;87:3187–3191. doi: 10.1210/jcem.87.7.8647 [DOI] [PubMed] [Google Scholar]
- 62.Stowasser M, Bachmann AW, Jonsson JR, Tunny TJ, Klemm SA, Gordon RD. Clinical, biochemical and genetic approaches to the detection of familial hyperaldosteronism type I. J Hypertens. 1995;13(12 Pt 2):1610–1613. [PubMed] [Google Scholar]
- 63.Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0 [DOI] [PubMed] [Google Scholar]
- 64.Carroll J, Dluhy R, Fallo F, Pistorello M, Bradwin G, Gomez-Sanchez CE, Mortensen R. Aldosterone-producing adenomas do not contain glucocorticoid-remediable aldosteronism chimeric gene duplications. J Clin Endocrinol Metab. 1996;81:4310–4312. doi: 10.1210/jcem.81.12.8954032 [DOI] [PubMed] [Google Scholar]
- 65.Stowasser M, Gordon RD, Tunny TJ, Klemm SA, Finn WL, Krek AL. Familial hyperaldosteronism type II: five families with a new variety of primary aldosteronism. Clin Exp Pharmacol Physiol. 1992;19:319–322. doi: 10.1111/j.1440-1681.1992.tb00462.x [DOI] [PubMed] [Google Scholar]
- 66.Mulatero P, Tizzani D, Viola A, Bertello C, Monticone S, Mengozzi G, Schiavone D, Williams TA, Einaudi S, La Grotta A, et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms). Hypertension. 2011;58:797–803. doi: 10.1161/HYPERTENSIONAHA.111.175083 [DOI] [PubMed] [Google Scholar]
- 67.Scholl UI, Stölting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50:349–354. doi: 10.1038/s41588-018-0048-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandes-Rosa FL, Daniil G, Orozco IJ, Göppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50:355–361. doi: 10.1038/s41588-018-0053-8 [DOI] [PubMed] [Google Scholar]
- 69.Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123. doi: 10.1210/jc.2008-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez-Sanchez CE, Gomez-Sanchez EP, Nishimoto K. Immunohistochemistry of the human adrenal CYP11B2 in normal individuals and in patients with primary aldosteronism. Horm Metab Res. 2020;52:421–426. doi: 10.1055/a-1139-2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulatero P, Tauber P, Zennaro MC, Monticone S, Lang K, Beuschlein F, Fischer E, Tizzani D, Pallauf A, Viola A, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59:235–240. doi: 10.1161/HYPERTENSIONAHA.111.183996 [DOI] [PubMed] [Google Scholar]
- 72.Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. doi: 10.7554/eLife.06315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, et al. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine. 2016;13:225–236. doi: 10.1016/j.ebiom.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wulczyn K, Perez-Reyes E, Nussbaum RL, Park M. Primary aldosteronism associated with a germline variant in CACNA1H. BMJ Case Rep. 2019;12:e229031. doi: 10.1136/bcr-2018-229031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reimer EN, Walenda G, Seidel E, Scholl UI. Cacna1h mutant calcium channel causes autonomous aldosterone production in hac15 cells and is inhibited by mibefradil. Endocrinology. 2016;157:3016–22. doi: 10.1210/en.2016-1170 [DOI] [PubMed] [Google Scholar]
- 76.Semenova NA, Ryzhkova OR, Strokova TV, Taran NN. [The third case report a patient with primary aldosteronism, seizures, and neurologic abnormalities (PASNA) syndrome de novo variant mutations in the CACNA1D gene]. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118:49–52. doi: 10.17116/jnevro201811812149 [DOI] [PubMed] [Google Scholar]
- 77.De Mingo Alemany MC, Mifsud Grau L, Moreno Macián F, Ferrer Lorente B, León Cariñena S. A de novo CACNA1D missense mutation in a patient with congenital hyperinsulinism, primary hyperaldosteronism and hypotonia. Channels (Austin). 2020;14:175–180. doi: 10.1080/19336950.2020.1761171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flanagan SE, Vairo F, Johnson MB, Caswell R, Laver TW, Lango Allen H, Hussain K, Ellard S. A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes. 2017;18:320–323. doi: 10.1111/pedi.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortner NJ, Kaserer T, Copeland JN, Striessnig J. De novo CACNA1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism. Pflugers Arch. 2020;472:755–773. doi: 10.1007/s00424-020-02418-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry. 2015;77:816–822. doi: 10.1016/j.biopsych.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zilbermint M, Xekouki P, Faucz FR, Berthon A, Gkourogianni A, Schernthaner-Reiter MH, Batsis M, Sinaii N, Quezado MM, Merino M, et al. Primary aldosteronism and ARMC5 variants. J Clin Endocrinol Metab. 2015;100:E900–E909. doi: 10.1210/jc.2014-4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rassi-Cruz M, Maria AG, Faucz FR, London E, Vilela LAP, Santana LS, Benedetti AFF, Goldbaum TS, Tanno FY, Srougi V, et al. Phosphodiesterase 2A and 3B variants are associated with primary aldosteronism. Endocr Relat Cancer. 2021;28:1–13. doi: 10.1530/ERC-20-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gu H, Ma Z, Wang J, Zhu T, Du N, Shatara A, Yi X, Kowala MC, Du Y. Salt-dependent blood pressure in human aldosterone synthase-transgenic mice. Sci Rep. 2017;7:492. doi: 10.1038/s41598-017-00461-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schewe J, Seidel E, Forslund S, Marko L, Peters J, Muller DN, Fahlke C, Stölting G, Scholl U. Elevated aldosterone and blood pressure in a mouse model of familial hyperaldosteronism with ClC-2 mutation. Nat Commun. 2019;10:5155. doi: 10.1038/s41467-019-13033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Göppner C, Orozco IJ, Hoegg-Beiler MB, Soria AH, Hübner CA, Fernandes-Rosa FL, Boulkroun S, Zennaro MC, Jentsch TJ. Pathogenesis of hypertension in a mouse model for human CLCN2 related hyperaldosteronism. Nat Commun. 2019;10:4678. doi: 10.1038/s41467-019-12113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen AX, Nishimoto K, Nanba K, Rainey WE. Potassium channels related to primary aldosteronism: expression similarities and differences between human and rat adrenals. Mol Cell Endocrinol. 2015;417:141–148. doi: 10.1016/j.mce.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lichtenauer U, Schmid PL, Oßwald A, Renner-Müller I, Reincke M, Warth R, Wolf E, Beuschlein F. Establishment of an in vivo model for KCNJ5 dependent hyperaldosteronism. Exp Clin Endocrinol Diabetes. 2015;123:P09_25. doi: 10.1055/s-0035-1547718 [Google Scholar]
- 89.Seidel E, Schewe J, Zhang J, Dinh HA, Forslund SK, Marko L, Hellmig N, Peters J, Muller DN, Lifton RP, et al. Enhanced Ca(2+) signaling, mild primary aldosteronism, and hypertension in a familial hyperaldosteronism mouse model (cacna1h (m1560v/+)). Proc Natl Acad Sci U. S. A. 2021;118:e2014876118. doi: 0.1073/pnas.2014876118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor MJ, Ullenbruch MR, Frucci EC, Rege J, Ansorge MS, Gomez-Sanchez CE, Begum S, Laufer E, Breault DT, Rainey WE. Chemogenetic activation of adrenocortical Gq signaling causes hyperaldosteronism and disrupts functional zonation. J Clin Invest. 2020;130:83–93. doi: 10.1172/JCI127429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seidel E, Schewe J, Scholl UI. Genetic causes of primary aldosteronism. Exp Mol Med. 2019;51:1–12. doi: 10.1038/s12276-019-0337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eisenhofer G, Durán C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, Burrello J, Buffolo F, Prejbisz A, Beuschlein F, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open. 2020;3:e2016209. doi: 10.1001/jamanetworkopen.2020.16209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015;83:779–789. doi: 10.1111/cen.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ono Y, Yamazaki Y, Omata K, Else T, Tomlins SA, Rhayem Y, Williams TA, Reincke M, Carling T, Monticone S, et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab. 2020;105:e282–e289. doi: 10.1210/clinem/dgz235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67:139–145. doi: 10.1161/HYPERTENSIONAHA.115.06186 [DOI] [PubMed] [Google Scholar]
- 96.Scholl UI, Abriola L, Zhang C, Reimer EN, Plummer M, Kazmierczak BI, Zhang J, Hoyer D, Merkel JS, Wang W, et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest. 2017;127:2739–2750. doi: 10.1172/JCI91733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caroccia B, Prisco S, Seccia TM, Piazza M, Maiolino G, Rossi GP. Macrolides blunt aldosterone biosynthesis: a proof-of-concept study in KCNJ5 mutated adenoma cells ex vivo. Hypertension. 2017;70:1238–1242. doi: 10.1161/HYPERTENSIONAHA.117.10226 [DOI] [PubMed] [Google Scholar]
- 98.Maiolino G, Ceolotto G, Battistel M, Barbiero G, Cesari M, Amar L, Caroccia B, Padrini R, Azizi M, Rossi GP. Macrolides for KCNJ5-mutated aldosterone-producing adenoma (MAPA): design of a study for personalized diagnosis of primary aldosteronism. Blood Press. 2018;27:200–205. doi: 10.1080/08037051.2018.1436961 [DOI] [PubMed] [Google Scholar]
- 99.Nadler JL, Hsueh W, Horton R. Therapeutic effect of calcium channel blockade in primary aldosteronism. J Clin Endocrinol Metab. 1985;60:896–899. doi: 10.1210/jcem-60-5-896 [DOI] [PubMed] [Google Scholar]
- 100.Thuesen AD, Finsen SH, Rasmussen LL, Andersen DC, Jensen BL, Hansen PBL. Deficiency of t-type ca(2+) channels cav3.1 and cav3.2 has no effect on angiotensin ii-induced hypertension but differential effect on plasma aldosterone in mice. Am J Physiol Renal Physiol. 2019;317:F254–F263. doi: 10.1152/ajprenal.00121.2018 [DOI] [PubMed] [Google Scholar]
- 101.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1 [DOI] [PubMed] [Google Scholar]
- 102.Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, Shibata H, Kosaka T, Oya M, Suematsu M, et al. Case Report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J Clin Endocrinol Metab. 2016;101:6–9. doi: 10.1210/jc.2015-3285 [DOI] [PubMed] [Google Scholar]
- 103.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167:630–641. doi: 10.7326/M17-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, et al. ; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kong L, Zhang J, Dong L, Xu J, Gao PJ, Wang JG, Zhu L. Recurrence of primary aldosteronism 10 years after left adrenalectomy for aldosterone-producing adenoma: a case report. Front Endocrinol (Lausanne). 2021;12:728595. doi: 10.3389/fendo.2021.728595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hacini I, De Sousa K, Boulkroun S, Meatchi T, Amar L, Zennaro MC, Fernandes-Rosa FL. Somatic mutations in adrenals from patients with primary aldosteronism not cured after adrenalectomy suggest common pathogenic mechanisms between unilateral and bilateral disease. Eur J Endocrinol. 2021;185:405–412. doi: 10.1530/EJE-21-0338 [DOI] [PubMed] [Google Scholar]
- 107.Lyraki R, Schedl A. Adrenal cortex renewal in health and disease. Nat Rev Endocrinol. 2021;17:421–434. doi: 10.1038/s41574-021-00491-4 [DOI] [PubMed] [Google Scholar]
- 108.Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, Chertow GM, Leppert JT, Bhalla V. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75:650–659. doi: 10.1161/HYPERTENSIONAHA.119.14359 [DOI] [PubMed] [Google Scholar]