Abstract
Background
Accurate pediatric reference intervals (RIs) for laboratory tests determined in a healthy pediatric population are essential for correct laboratory test interpretation and clinical decision-making. In pediatrics, RIs require partitioning by age and/or sex; however, the need for partitioning based on ethnicity is unclear. Here, we assessed the influence of ethnicity on biomarker concentrations in the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort of healthy children and adolescents and compared the results with the National Health and Nutrition Examination Survey (NHANES).
Methods
A total of 52 biomarkers were measured in a multiethnic population of 846–1179 healthy children (aged 5 to <19 years) upon informed consent. Biomarker concentrations were retrospectively compared between four major ethnic groups (i.e. Black, Caucasian, East Asian, and South Asian, determined by parental ethnicity). Retrospective results were verified prospectively using an additional 500 healthy pediatric samples with equal sample size across ethnicities. Ethnic-specific differences were assessed based on statistical significance and biological and analytical variations. Appropriate age-, sex-, and ethnic-specific RIs were calculated.
Results
Ethnic-specific differences were not observed for 34 biomarkers examined in the retrospective analysis, while 18 demonstrated statistically significant ethnic differences. Among these, seven analytes demonstrated ethnic-specific differences in the prospective analysis: vitamin D, amylase, ferritin, follicle-stimulating hormone (FSH), immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM). Analysis of select NHANES data confirmed CALIPER findings.
Conclusions
This is the first comprehensive Canadian pediatric study examining ethnic-specific differences in common biomarkers. While the majority of biomarkers did not require ethnic partitioning, ethnic-specific RIs were established for seven biomarkers showing marked differences. Further studies in other populations are needed to confirm our findings.
Keywords: adolescents, CALIPER, children, ethnicity, pediatric, reference intervals
Introduction
In clinical laboratory medicine, patient test results are often interpreted by comparison to reference intervals (RIs), which are usually defined as the central 95% of laboratory test results obtained from a healthy reference population. The Clinical and Laboratory Standards Institute (CLSI) has established guidelines on defining, establishing, and verifying RIs [1]. Establishing accurate RIs is challenging, as it requires recruitment of a large healthy reference population. As a result, adult and pediatric RIs appropriately partitioned for key covariates that introduce inter-individual variation in population biomarker concentrations are lacking. Traditionally, RIs are partitioned based on age, sex, and/or Tanner Stage, while other important covariates such as ethnicity have been seldom examined [2], [3], [4], [5]. In fact, RIs have conventionally been calculated based on samples obtained from one ethnic group, particularly Caucasians [2], [3].
Many studies have noted ethnic differences in the levels of routine chemistry, fertility, and endocrine markers in pediatric and/or adult populations [2]. In pediatrics, Caucasians and South Asians were reported to exhibit higher alanine aminotransferase (ALT) levels than East Asians [3], while in both pediatrics and adults, Asians have higher amylase levels than Caucasians [3], [6]. One of the most notable differences in fertility markers is seen for follicle-stimulating hormone (FSH) in adults, where Caucasians have lower levels than Asians [7], [8]. Ethnic differences in thyroid hormones have also been observed in both pediatric and adult populations. Free thyroxine (FT4) levels are lower in Caucasian than in Asian children and adolescents [4] and higher in Caucasian adults compared to Black adults [9]. Conversely, pediatric total triiodothyronine (TT3) levels were reported to be higher in Caucasians than in Asians [4]. Evidence on the influence of ethnicity on many other biomarkers remains conflicting, potentially due to differences in environmental factors, age and sex of study participants, as well as the statistical analysis [2]. Furthermore, few studies have analyzed biomarker levels in East and South Asian populations [2]. Thus, our understanding of the influence of ethnicity on biomarker levels remains limited.
Only a few studies have established ethnic-specific RIs, most of which are based on adults [2]. For example, Lim et al. established adult ethnic-specific RIs for Asians, Blacks, Caucasians, and Hispanics living in the United States (US) using the National Health and Nutrition Examination Survey (NHANES) database [10]. Additionally, Ichihara et al. established adult ethnic-specific RIs using data from East and South East Asian countries [11], [12]. Expectedly, the lack of ethnic-specific RIs in the literature has the potential to lead to the misinterpretation of laboratory test results and contribute to misdiagnosis and clinical error. Therefore, understanding the influence of ethnicity on healthy biomarker concentrations is essential to improve the accuracy of laboratory test result interpretation and clinical decision-making.
Patients’ ethnicity is currently not considered in clinical interpretation of laboratory results. A robust investigation of the influence of ethnicity on biomarker concentrations is therefore essential to identify biomarkers with significant ethnic differences. The current study examined differences in pediatric biomarker concentrations between four major Canadian ethnic groups (i.e. Black, Caucasian, East Asian, and South Asian), categorized based on the 2016 Canadian census [13], and described in Supplementary Table 1. Ethnic-specific RIs were then calculated where necessary. This study utilized data from the Canadian Laboratory Initiative on Paediatric Reference Intervals (CALIPER) project, including those from published studies (retrospective) [3], [4], [5], as well as newly recruited participants from all four ethnic groups (prospective). NHANES data, containing US pediatric biomarker concentrations, were also analyzed to confirm results obtained using a Canadian population. This is the first extensive Canadian study that establishes ethnic differences and ethnic-specific RIs to improve the accuracy of laboratory test interpretation and clinical decision-making.
Materials and methods
Overview
This study was completed in three phases: (a) retrospective analysis of ethnic-specific differences using published CALIPER data, (b) prospective analysis of ethnic-specific differences using 500 additional samples, and (c) establishment of ethnic-specific RIs.
Participant recruitment, sample acquisition, and sample analysis
This study was approved by the Research Ethics Board at the Hospital for Sick Children (Toronto, ON). Informed consent was obtained from all participants and/or families. In summary, blood was collected from healthy community children and adolescents (aged 5 to <19 years) in serum separator tubes. Serum was separated, aliquoted within 4 h of phlebotomy, and frozen at −80 °C until analysis. Only samples from individuals belonging to one of the four major Canadian ethnicities (i.e. Black, Caucasian, East Asian, and South Asian), with the same self-identified maternal and paternal ethnic background, were included. Exclusion criteria for all parts of the study included the presence of acute illness, history of chronic or metabolic illness, pregnancy, and use of prescription medication within 2 weeks of phlebotomy.
Retrospective analysis
Pediatric biomarker concentrations tested from 2009 to 2013 were selected from three published CALIPER studies [3], [4], [5]. Additional samples tested from 2012 to 2016 using biochemical assays were added to further increase the statistical power of the analysis.
Biomarker concentrations for 35 biochemical assays (i.e. serum chemistry, enzymes, lipids, and proteins) measured on the Abbott ARCHITECT c8000 analyzer for a total of 1179 subjects (46 Blacks, 905 Caucasians, 103 East Asians, and 125 South Asians) from the study by Colantonio et al. [3] were used. The following biochemical assays were examined: albumin (bromocresol purple method), alkaline phosphatase (ALP), ALT, amylase, apolipoprotein A1 (apo A1), apolipoprotein B (apo B), antistreptolysin O (ASO), aspartate aminotransferase (AST), bilirubin (direct), bilirubin (total), complement component 3 (C3), complement component 4 (C4), calcium, cholinesterase, cholesterol (total), carbon dioxide, creatinine (enzymatic), high-sensitivity C-reactive protein (hsCRP), gamma-glutamyltransferase (GGT), haptoglobin, immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), iron, lipase, magnesium, phosphorus, prealbumin, rheumatoid factor, total protein, transferrin, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), urea, and uric acid.
Additionally, concentration data for 11 biochemical and endocrine markers from the study by Bailey et al. [4], and six fertility markers from Konforte et al. [5] for a total of 846 individuals (29 Blacks, 612 Caucasians, 79 East Asians, and 126 South Asians) measured using Abbott Architect i2000 analyzer, were examined. The 17 immunochemical assays examined include: α-fetoprotein (AFP), cobalamin, cortisol, estradiol, ferritin, folate, FSH, free triiodothyronine (FT3), FT4, luteinizing hormone (LH), progesterone, prolactin, sex hormone-binding globulin (SHBG), thyroid-stimulating hormone (TSH), TT3, total thyroxine (TT4), and 25(OH) vitamin D.
Prospective analysis
Eighteen biomarkers, including ALT, amylase, direct bilirubin, C3, C4, cholesterol (total), hsCRP, IgA, IgG, IgM, iron, transferrin, TG, uric acid, ferritin, FSH, SHBG, and 25(OH) vitamin D, that demonstrated significant ethnic differences in the retrospective analysis were selected to be retested in the prospective analysis. Five hundred samples (125 from each of the four ethnicities), uniformly encompassing ages 5 to <19 years, were selected for the prospective analysis and tested in 2017 using the same Abbott assays. Although 97 of these samples were also used in the retrospective analysis, they were retested in the prospective study for analytical consistency.
NHANES data analysis
Where available, NHANES data were analyzed to confirm the results of prospective findings. NHANES pediatric demographics, health, and laboratory data were downloaded for 25(OH) vitamin D (5 to <19 years), ALT (12 to <19 years), iron (12 to <19 years), TG (12 to <19 years), and total cholesterol (12 to <19 years) gathered in 2011–2016, as well as SHBG (13 to <19 years) gathered in 2013–2016 (www.cdc.gov/nchs/nhanes). 25(OH) vitamin D, including D2 and D3, was measured using ultra high performance liquid chromatography-tandem mass spectrometry. ALT, iron, total cholesterol, and TG were measured on Beckman UniCel® DxC800/660i Synchron. SHBG was tested on Roche/Hitachi cobas e411 analyzer. While 5- to <19-year-old data were available for vitamin D, data from <12 years were not available for ALT, iron, TG, and total cholesterol. Additionally, due to the large age-dependent changes in SHBG [5], and the large sample size of adolescents, only participants aged 13 to <19 years were included. Overall, only participants who were both interviewed and examined, as well as those belonging to one of the following four ethnic groups were included: Black (i.e. non-Hispanic Black), Caucasian (i.e. non-Hispanic White), Asian (i.e. East, South, and South East Asian), and Hispanic (i.e. Mexican American and Other Hispanic). Following the application of inclusion and exclusion criteria, 2958 participants were selected to be analyzed.
Calculation of ethnic-specific reference intervals
Ethnic-specific RIs were established for the seven biomarkers showing ethnic differences in both retrospective and prospective analysis: 25(OH) vitamin D, amylase, ferritin, FSH, IgA, IgG, and IgM. To calculate ethnic-specific RIs, results from 713 CALIPER samples were used, including data from the prospective analysis (n=497), analysis of new CALIPER samples (n=140), and retrospective data for ferritin and FSH (n=76), due to the analytical similarity of these two assays over time.
Statistical procedures
Statistical procedures for the retrospective analysis, prospective analysis, and the calculation of RIs are included in detail in the Supplementary Methods. All statistical analyses were conducted on R software (R Version 3.5.1) and Excel (Microsoft).
In summary, concentrations of biomarkers in the retrospective analysis (Supplementary Figure 1) were compared between ethnic groups, while adjusting for age, using analysis of covariance (ANCOVA). ANCOVA results were verified by analysis of variance (ANOVA). To assess potential clinical significance of the statistical findings, statistically significant ethnic differences for a given biomarker were compared to biomarker-specific reference change values (RCVs) previously determined by CALIPER or available in the literature [14], [15], [16], [17], [18]. RCV is a measure of biological (intra-individual) and analytical (assay imprecision) variations. Biomarkers showing statistically significant ethnic differences that were either greater than or marginally below RCVs were selected to be tested in the prospective analysis. In the prospective analysis (Supplementary Figure 2), values of biomarkers partitioned by age in previous CALIPER publications [3], [4], [5] were compared using ANCOVA with age adjustment. Otherwise, ANOVA was performed. Biomarkers showing statistically significant ethnic differences exceeding respective RCVs in the prospective analysis were selected to establish ethnic-specific RIs. NHANES data were analyzed the same way as the prospective analysis, except for the use of Cook’s distance to remove outliers. After dividing the data into ethnic partitions, based on the results of the prospective analysis, age and sex partitions were tested using the Harris and Boyd method [3], [4], [5]. Following this, age-, sex-, and ethnic-specific RIs were calculated using the nonparametric rank method (n≥120) or Horn and Pesce robust method (40≤n<120) (Supplementary Figure 3).
Results
Retrospective analysis
Out of the 35 biochemical assays examined, 17 demonstrated statistically significant differences in concentration between at least two ethnicities (Supplementary Tables 2 and 3): direct bilirubin, calcium, iron, magnesium, uric acid, ALT, amylase, total cholesterol, TG, albumin, C3, C4, hsCRP, IgA, IgG, IgM, and transferrin. Furthermore, eight out of 17 immunochemical assays showed statistically significant differences between at least two ethnicities (Supplementary Tables 2 and 3): 25(OH) vitamin D, ferritin, folate, FT3, FT4, FSH, progesterone, and SHBG. Among these biochemical and immunochemical assays, statistically significant differences observed for the following 14 biomarkers exceeded their respective RCVs and were selected to be verified in the prospective analysis: 25(OH) vitamin D, amylase, C3, C4, hsCRP, ferritin, FSH, IgA, IgG, IgM, SHBG, total cholesterol, transferrin, and uric acid. Four additional biomarkers showed large statistically significant differences, marginally below RCVs, between at least a pair of ethnicities, including: ALT, direct bilirubin, iron, and TG. These biomarkers were also verified in the prospective analysis.
Prospective analysis
To verify the retrospective analysis, a prospective study was conducted using samples from an equal number (i.e. 125) of participants in each of the four ethnic groups. The G*Power software was used to calculate the statistical power of the analysis: medium effect size (i.e. 0.25), an α of 0.05, and a total sample size of 500 participants generated a statistical power (i.e. β) of 0.9988.
Of the 18 biomarkers examined, 14 demonstrated statistically significant differences between at least two ethnicities. Concentrations of biomarkers showing statistically significant ethnic differences are shown in Figures 1–4 and Supplementary Figures 4–17. These differences were observed in both males and females, unless specifically indicated: ALT, amylase, 25(OH) vitamin D, C3, C4, ferritin (males only), FSH (males only), IgA, IgG, IgM (separately in males and females), SHBG, transferrin, TG, and uric acid. Seven of these biomarkers demonstrated statistically significant ethnic differences greater than their respective RCVs (Figures 1–4, Table 1, and Supplementary Table 4), including: 25(OH) vitamin D, amylase, ferritin (males only), FSH (males only), IgA, IgG, and IgM (separately in males and females). These seven biomarkers were identified as showing clear ethnic-specific differences.
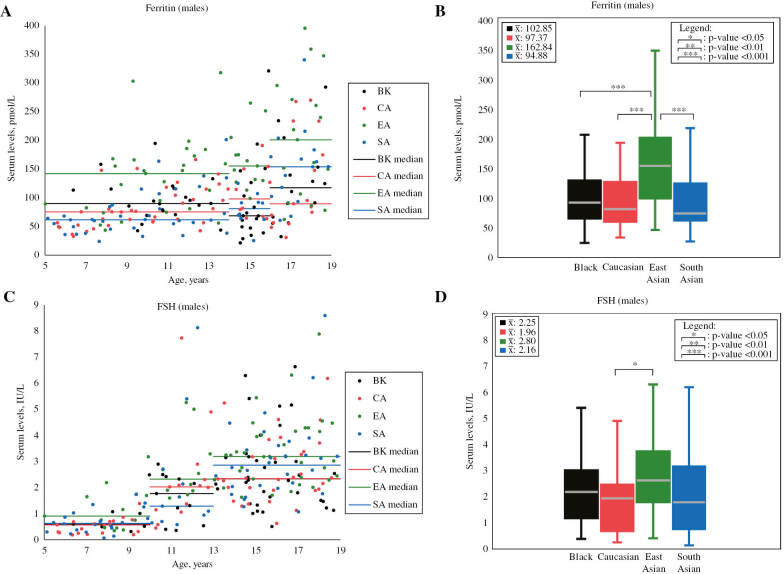
Figure 1:
Scatterplots and boxplots of male ferritin and FSH concentrations partitioned by ethnicity.
Ferritin scatterplot (A) and boxplot (B), as well as FSH scatterplot (C) and boxplot (D) compare serum concentrations between males of different ethnicities in the prospective analysis. Mean values (unadjusted for age) are shown in the top left corner of boxplots. p-Values shown in boxplots were calculated while adjusting for age, as age difference is indicated by previous CALIPER reference interval studies [4], [5]. Statistically significant ethnic differences were found to exceed RCVs (see Table 1). Note: outliers are not shown in scatterplots and boxplots. FSH, follicle-stimulating hormone. Values are in SI units.
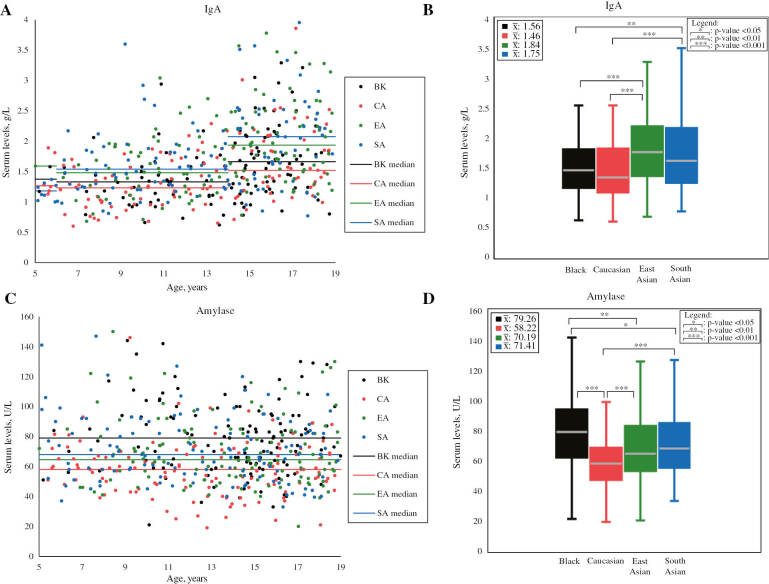
Figure 4:
Scatterplots and boxplots of IgA and amylase concentrations partitioned by ethnicity.
IgA scatterplot (A) and boxplot (B), as well as amylase scatterplot (C) and boxplot (D) compare serum concentrations between participants of different ethnicities in the prospective analysis. Mean values (unadjusted for age) are shown in the top left corner of boxplots. p-Values shown in boxplots were calculated for IgA while adjusting for age and for amylase without adjusting for age, as age difference is indicated for IgA, but not indicated for amylase by previous CALIPER reference interval studies [3]. Statistically significant ethnic differences were found to exceed RCVs (see Table 1). IgA, immunoglobulin A. Note: outliers are not shown in scatterplots and boxplots. IgA values are in SI units.
Table 1:
Biomarker concentrations in SI units and ethnic comparison of biomarkers tested in the prospective analysis.
| Biochemical marker, units | Sex | Black |
Caucasian |
East Asian |
South Asian |
Statistically significant ethnic differences (% difference) | RCVs | RCV reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean±SD | Age-adjusted mean | n | Mean±SD | Age-adjusted mean | n | Mean±SD | Age-adjusted mean | n | Mean±SD | Age-adjusted mean | |||||
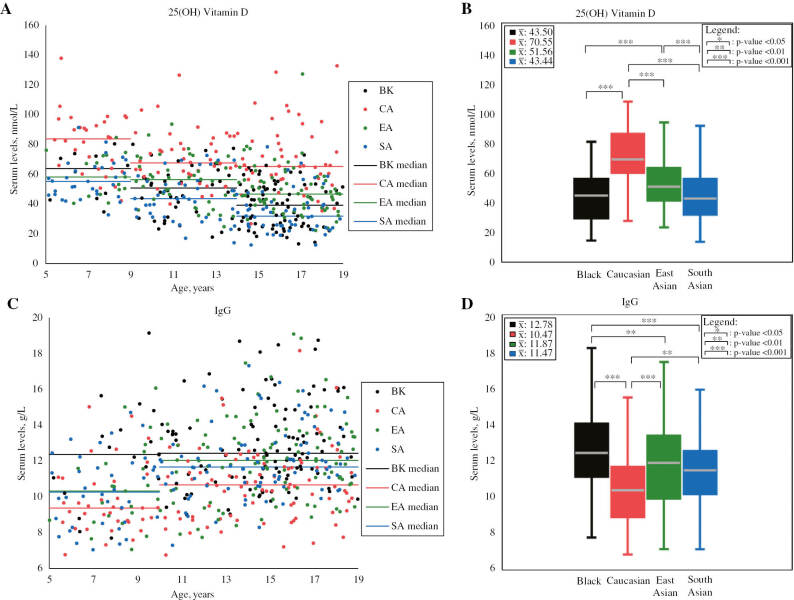
| 25(OH) vitamin D, nmol/L | M+F | 125 | 43.50±16.74 | 44.70 | 120 | 70.55±18.08 | 69.50 | 123 | 51.56±15.04 | 52.65 | 123 | 43.44±16.59 | 42.15 | BK<CA (43.43) EA<CA (27.59) SA<CA (48.99) SA<EA (22.15) |
18.0% | [15] |
| ALT, U/L | M | 54 | 17.26±8.35 | 15.72 | 61 | 17.43±5.96 | 16.59 | 56 | 15.63±7.13 | 14.43 | 61 | 17.24±6.22 | 16.29 | NS | 47.4% | [16] |
| F | 71 | 11.73±5.31 | 10.97 | 63 | 16.05±6.41 | 15.09 | 65 | 12.24±4.90 | 11.54 | 62 | 15.30±7.96 | 13.91 | BK<CA (31.62) EA<CA (26.66) BK<SA (23.63) |
|||
| Amylase, U/L | M+F | 123 | 79.26±24.03 | N/A | 125 | 58.22±18.28 | N/A | 124 | 70.19±22.81 | N/A | 123 | 71.41±22.18 | N/A | CA<BK (30.61) CA<EA (18.64) CA<SA (20.35) |
16.7% | [16] |
| C3, g/L | M+F | 125 | 1.21±0.19 | N/A | 123 | 1.19±0.18 | N/A | 124 | 1.10±0.19 | N/A | 123 | 1.25±0.19 | N/A | EA<BK (9.52) EA<CA (7.86) EA<SA (12.77) |
15.2% | [16] |
| C4, g/L | M+F | 124 | 0.24±0.06 | N/A | 124 | 0.21±0.06 | N/A | 121 | 0.21±0.06 | N/A | 123 | 0.25±0.07 | N/A | CA<BK (13.33) EA<BK (13.33) CA<SA (17.39) EA<SA (17.39) |
18.0% | [16] |
| Direct bilirubin, μmol/L | M | 52 | 4.69±2.49 | 4.02 | 58 | 4.40±2.22 | 4.08 | 58 | 4.44±2.00 | 3.99 | 58 | 4.23±2.21 | 3.91 | NS | N/A | N/A |
| F | 70 | 3.68±1.36 | 3.40 | 63 | 4.27±2.30 | 3.93 | 66 | 3.98±1.40 | 3.66 | 61 | 3.87±1.88 | 3.64 | NS | |||
| Ferritin, pmol/L | M | 54 | 102.85±61.80 | 86.45 | 62 | 97.37±55.82 | 85.89 | 57 | 162.84±82.20 | 142.39 | 61 | 94.88±57.91 | 82.08 | SA<EA (53.74) CA<EA (49.50) BK<EA (48.89) |
46.2% | [17] |
| F | 71 | 80.92±62.42 | 63.14 | 63 | 79.20±37.03 | 72.43 | 66 | 98.24±59.08 | 81.44 | 62 | 80.31±61.75 | 65.58 | NS | |||
| FSH, IU/L | M | 54 | 2.25±1.49 | 1.60 | 62 | 1.96±1.59 | 1.46 | 58 | 2.80±1.51 | 2.19 | 61 | 2.16±1.81 | 1.63 | CA<EA (40.00) | 27.0% | [17] |
| F | 69 | 3.71±1.98 | 2.77 | 61 | 3.01±2.16 | 2.35 | 66 | 3.49±1.71 | 2.69 | 61 | 3.59±2.07 | 3.18 | NS | |||
| hs-CRP, mg/L | M+F | 124 | 0.82±1.41 | 0.29 | 122 | 0.86±1.57 | 0.31 | 124 | 0.72±1.31 | 0.23 | 123 | 0.83±1.35 | 0.29 | NS | N/A | N/A |
| IgA, g/L | M+F | 122 | 1.56±0.56 | 1.52 | 122 | 1.46±0.52 | 1.50 | 124 | 1.84±0.65 | 1.79 | 123 | 1.75±0.64 | 1.79 | BK<EA (16.31) CA<EA (17.63) BK<SA (16.31) CA<SA (17.63) |
12.9% | [16] |
| IgG, g/L | M+F | 123 | 12.78±2.43 | 12.67 | 123 | 10.47±2.03 | 10.57 | 123 | 11.87±2.39 | 11.75 | 122 | 11.47±2.11 | 11.60 | CA<SA (9.29) CA<EA (10.57) CA<BK (18.07) SA<BK (8.82) |
7.8% | [16] |
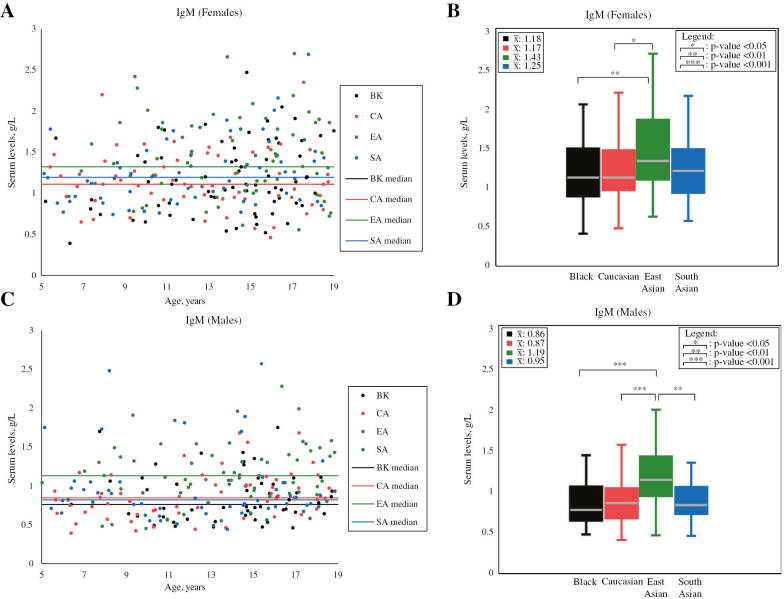
| IgM, g/L | M | 53 | 0.86±0.32 | N/A | 62 | 0.87±0.29 | N/A | 57 | 1.19±0.43 | N/A | 61 | 0.95±0.41 | N/A | BK<EA (32.20) CA<EA (31.07) SA<EA (22.43) |
12.3% | [16] |
| F | 70 | 1.18±0.43 | N/A | 63 | 1.17±0.36 | N/A | 64 | 1.43±0.48 | N/A | 62 | 1.25±0.41 | N/A | BK<EA (19.16) CA<EA (20.00) |
|||
| Iron, μmol/L | M | 54 | 15.56±4.28 | 14.80 | 62 | 16.54±5.51 | 15.74 | 57 | 17.71±6.37 | 16.44 | 61 | 15.64±6.19 | 14.61 | NS | N/A | N/A |
| F | 71 | 14.46±4.82 | 13.48 | 63 | 16.50±5.42 | 15.76 | 66 | 15.95±5.57 | 14.69 | 62 | 14.09±4.86 | 13.28 | NS | |||
| SHBG, nmol/L | M | 54 | 51.03±28.97 | 47.84 | 60 | 68.16±43.19 | 52.61 | 58 | 50.27±34.83 | 42.68 | 61 | 58.70±38.03 | 44.34 | NS | 37.5% | [17] |
| F | 70 | 64.64±30.97 | 60.39 | 61 | 81.36±41.30 | 66.09 | 65 | 51.44±24.03 | 48.90 | 62 | 60.79±37.66 | 46.50 | SA<BK (25.99) SA<CA (34.80) EA<CA (29.90) |
|||
| Total cholesterol, mmol/L | M+F | 125 | 4.06±0.75 | N/A | 125 | 4.11±0.64 | N/A | 122 | 4.08±0.62 | N/A | 122 | 4.12±0.71 | N/A | NS | 7.9% | [16] |
| Transferrin, g/L | M+F | 125 | 2.76±0.34 | N/A | 125 | 2.78±0.27 | N/A | 123 | 2.70±0.32 | N/A | 123 | 2.93±0.29 | N/A | BK<SA (5.98) CA<SA (5.25) EA<SA (8.17) |
9.8% | [16] |
| Triglycerides, mmol/L | M+F | 124 | 0.89±0.45 | N/A | 122 | 1.05±0.57 | N/A | 123 | 1.23±0.67 | N/A | 123 | 1.24±0.67 | N/A | BK<EA (32.08) BK<SA (32.86) CA<SA (16.59) |
74.9% | [16] |
| Uric acid, μmol/L | M | 54 | 287.50±67.52 | 273.06 | 61 | 284.90±69.81 | 282.22 | 57 | 312.46±71.02 | 299.63 | 60 | 288.43±89.48 | 281.89 | NS | 19.2% | [16] |
| F | 71 | 228.64±50.57 | 219.18 | 61 | 253.84±53.29 | 252.06 | 66 | 270.17±55.70 | 258.39 | 62 | 246.64±52.17 | 246.64 | BK<CA (13.95) BK<EA (16.42) BK<SA (11.79) |
|||
ALT, alanine aminotransferase; C3, complement C3; C4, complement C4; BK, Black; CA, Caucasian; EA, East Asian; F, female; FSH, follicle-stimulating hormone; hs-CRP, high-sensitivity C-reactive protein; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; M, male; M+F, combined male and female values; N/A, not applicable; NS, not significant; RCV, reference change value; SA, South Asian; SD, standard deviation; SHBG, sex hormone-binding globulin. Mean and SD were calculated without adjusting for age for all biomarkers. Statistical tests were conducted and age-adjusted means were calculated if the biomarker demonstrated age differences in previous CALIPER studies [3], [4], [5]. Statistically significant differences refer to ANCOVA or ANOVA p-values<0.05. There was no significant interaction between the covariate (i.e. age) and factors (i.e. sex and ethnicity) for any of the biomarkers; thus, multiple regression was never performed (see Supplementary Figure bib2). Values are reported in conventional units in Supplementary Table 4.
Figure 2:
Scatterplots and boxplots of 25(OH) vitamin D and IgG concentrations partitioned by ethnicity.
25(OH) vitamin D scatterplot (A) and boxplot (B), as well as IgG scatterplot (C) and boxplot (D) compare serum concentrations between participants of different ethnicities in the prospective analysis. Mean values (unadjusted for age) are shown in the top left corner of boxplots. p-Values shown in boxplots were calculated while adjusting for age, as age difference is indicated by previous CALIPER reference interval studies [3], [4]. Statistically significant ethnic differences were found to exceed RCVs (see Table 1). Note: outliers are not shown in scatterplots and boxplots. IgG, immunoglobulin G. Values are in SI units.
Figure 3:
Scatterplots and boxplots of female and male IgM concentrations partitioned by ethnicity.
Scatterplots and boxplots compare IgM male (A–B) and female (C–D) concentrations between participants of different ethnicities in the prospective analysis. Mean values (unadjusted for age) are shown in the top left corner of boxplots. p-Values shown in boxplots were calculated without adjusting for age, as age difference is not indicated by a previous CALIPER reference interval study [3]. Statistically significant ethnic differences were found to exceed RCVs (see Table 1). IgM, immunoglobulin M. Note: outliers are not shown in scatterplots and boxplots. Values are in SI units.
NHANES data analysis
NHANES data were used to verify the results of the prospective analysis. The G*Power software was used to calculate the statistical power of the analysis: medium effect size (i.e. 0.25), an α of 0.05, and a minimum sample size of 872 participants (i.e. SHBG) generated a statistical power (i.e. β) of 1.0000.
Statistically significant ethnic differences were observed in the levels of ALT, iron, total cholesterol, TG, and SHBG, but none of these differences were greater than their respective RCVs. Vitamin D levels were higher in Caucasians than in Blacks, Asians, and Hispanics. These differences were statistically significant, exceeded the RCV, and were consistent with those observed in the prospective study. Thus, NHANES data confirmed prospective analysis results by showing clear ethnic-specific differences in the levels of 25(OH) vitamin D, but not ALT, iron, total cholesterol, and TG (Supplementary Table 5). NHANES biomarker concentrations showing statistically significant ethnic differences are shown in Supplementary Figures 18–35.
Calculation of ethnic-specific reference intervals
For the seven biochemical markers showing statistically significant differences exceeding RCV, data from the prospective and retrospective analysis, as well as additional sample analyses, were used to calculate ethnic-specific RIs, further partitioned by age and sex (Table 2 and Supplementary Table 6). Biomarker concentration data used to calculate ethnic-specific RIs are shown in Supplementary Figures 36–43.
Table 2:
Ethnic-specific reference intervals in SI units for the four major Canadian ethnic groups partitioned further by age and sex.
| Biomarker name | Ethnic partition | Age/sex partition | Lower limit | Upper limit | Number of samples | Lower limit 90% confidence interval | Upper limit 90% confidence interval |
|---|---|---|---|---|---|---|---|
| 25(OH) vitamin D, nmol/L | CA | 5 to <19 MF | 40 | 127 | 139 | 35–44 | 106–138 |
| EA | 5 to <9 MFa | 40 | 97 | 30 | N/A | N/A | |
| 9 to <15 MF | 27 | 84 | 82 | 22–31 | 80–89 | ||
| 15 to <19 MF | 25 | 71 | 52 | 22–28 | 66–76 | ||
| BK+SA | 5 to <9 MF | 30 | 86 | 50 | 26–34 | 81–93 | |
| 9 to <14 M | 23 | 80 | 38 | N/A | N/A | ||
| 9 to <14 F | 14 | 74 | 44 | 10–18 | 67–81 | ||
| 14 to <19 MF | 13 | 67 | 125 | 12–19 | 62–78 | ||
| Amylase, U/L | BK | 5 to <19 MF | 39 | 135 | 122 | 33–45 | 120–144 |
| EA+SA | 40 | 126 | 246 | 37–41 | 120–147 | ||
| CA | 5 to <19 M | 33 | 96 | 59 | 28–37 | 90–103 | |
| 5 to <19 F | 27 | 82 | 61 | 22–31 | 76–87 | ||
| Ferritin, pmol/L | EA | 5 to <14 M | 34 | 275 | 73 | 28–42 | 246–304 |
| 14 to <16 M | 86 | 415 | 39 | N/A | N/A | ||
| 16 to <19 M | 54 | 451 | 47 | 36–75 | 404–504 | ||
| CA+SA+BK | 5 to <16 M | 24 | 193 | 172 | 21–33 | 154–201 | |
| 16 to <19 M | 27 | 299 | 57 | 19–38 | 268–336 | ||
| FSH, IU/L | EA | 5 to <10 M | 0.4 | 3.2 | 29 | N/A | N/A |
| 10 to <13 M | 0.8 | 5.3 | 32 | N/A | N/A | ||
| 13 to <19 M | 1.3 | 5.7 | 68 | 1.1–1.6 | 5.3–6.2 | ||
| CA+SA+BK | 5 to <10 M | 0.1 | 1.7 | 60 | 0.1–0.1 | 1.4–2.0 | |
| 10 to <13 M | 0.3 | 4.3 | 51 | 0.2–0.5 | 3.7–5.0 | ||
| 13 to <19 M | 1.1 | 6.2 | 120 | 1.0–1.1 | 5.2–6.6 | ||
| IgA, g/L | EA+SA | 5 to <14 MF | 0.8 | 2.7 | 127 | 0.7–1.0 | 2.5–3.0 |
| 14 to <19 MF | 0.9 | 3.6 | 118 | 0.8–1.0 | 3.4–3.8 | ||
| BK+CA | 5 to <14 MF | 0.6 | 2.2 | 123 | 0.4–0.7 | 2.0–2.3 | |
| 14 to <19 MF | 0.8 | 3.2 | 121 | 0.7–1.0 | 2.8–3.9 | ||
| IgG, g/L | BK | 5 to <19 MF | 9.3 | 18.7 | 142 | 8.8–9.8 | 16.9–20.0 |
| EA+SA | 5 to <12 MF | 6.9 | 14.9 | 92 | 6.5–7.4 | 14.3–15.5 | |
| 12 to <19 MF | 8.7 | 17.3 | 153 | 8.3–9.2 | 16.2–19.1 | ||
| CA | 5 to <10 MF | 7.3 | 11.2 | 42 | 6.9–7.6 | 10.7–11.6 | |
| 10 to <19 MF | 7.3 | 14.6 | 85 | 6.9–7.7 | 14.0–15.3 | ||
| IgM, g/L | EA | 5 to <19 Mb | 0.5 | 2.0 | 56 | 0.4–0.6 | 1.8–2.2 |
| BK+CA+SA | 0.4 | 1.8 | 175 | 0.4–0.5 | 1.7–1.9 | ||
| EA | 0.7 | 2.8 | 66 | 0.6–0.8 | 2.5–3.0 | ||
| SA | 5 to <19 Fc | 0.6 | 2.2 | 62 | 0.6–0.7 | 2.0–2.4 | |
| CA+BK | 0.5 | 2.2 | 133 | 0.4–0.6 | 1.8–2.5 |
To calculate ethnic-specific RIs, reference value data were gathered from the prospective analysis, analysis of new CALIPER samples, as well as the retrospective analysis for ferritin and FSH, due to the analytical similarity of these two assays over time. The “+” sign indicates ethnicities being combined together within a single ethnic partition due to the similarity of their values. BK, Black; CA, Caucasian; EA, East Asian; F, female partition; FSH, follicle-stimulating hormone; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; M, male partition; MF, combined male and female partition (i.e. no sex difference), and SA, South Asian. For partitions with sample sizes <40, minimum and maximum values were selected for lower and upper limits, respectively, and confidence intervals were not calculated. Values are reported in conventional units in Supplementary Table 6. aSex difference was indicated, but sex-specific reference interval was not calculated due to insufficient sample size. bAge difference was found between East Asian values below and above the age of 13 years, but age partitioning was not possible due to insufficient sample size. cAge difference was found between South Asian values below and above the age of 11 years, but age partitioning was not possible due to insufficient sample size.
Discussion
This is the first comprehensive study to assess the influence of ethnicity on biomarker concentrations in a cohort of multi-ethnic Canadian pediatric participants and establish ethnic-specific RIs. Population concentrations from the CALIPER cohort of healthy children and adolescents were used to retrospectively analyze the influence of ethnicity on pediatric biomarker concentrations between four major Canadian ethnic groups (i.e. Caucasian, East Asian, South Asian, and Black). Among the 52 markers analyzed, the majority (i.e. 34) showed no statistically significant differences between children of different ethnicities. Those with clear ethnic-specific differences were verified by a prospective analysis, which identified seven biomarkers (i.e. 25(OH) vitamin D, amylase, ferritin, FSH, IgA, IgG, and IgM) with large ethnic differences that were both statistically significant and exceeded corresponding RCVs. US pediatric data (i.e. NHANES) were used to confirm the findings in the Canadian pediatric population for six of the original 52 studied biomarkers. Thus, while preliminary findings suggest high concordance between cohorts, no strong conclusions can be made regarding the application of CALIPER data to the US population for all 52 biomarkers. Age-, sex-, and ethnic-specific RIs were calculated for the seven biomarkers. Unlike many studies in the literature that use solely statistical methods to establish ethnic differences, the current study uses statistical, analytical, and biological criteria to identify such differences.
Both CALIPER and NHANES data show that 25(OH) vitamin D levels are higher in Caucasians than in Asians and Blacks. Similarly, other pediatric and adult studies have reported higher 25(OH) vitamin D levels in Caucasians compared to Blacks [2] and South Asians [19]. Such ethnic differences may be explained by diet, sun exposure, and skin pigmentation [2]. Despite lower 25(OH) vitamin D levels in Blacks, compensatory physiological mechanisms may be present to prevent bone loss. These include higher skeletal resistance to PTH and lower renal calcium excretion, explaining their higher bone quality and lower bone fracture rate compared to Caucasians [2]. Additionally, genetic differences in vitamin D-binding protein (e.g. rs7041 gene) between Caucasians and Blacks have been found to influence its avidity for 25(OH) vitamin D and, despite lower total 25(OH) vitamin D levels, result in higher bioavailability in Blacks [20].
With the exception of 13- to <19-year upper limit, East Asian males exhibited higher FSH levels compared to males of other ethnicities in our study. Similarly, Wang et al. reported higher adult FSH levels in Asian men compared to Caucasian men [7]. This difference may result from slight ethnic variation in the hypothalamic-pituitary-gonadal axis. Lower East Asian testicular volume (i.e. lower number of Sertoli cells) results in reduced inhibin levels compared to Caucasians [7], [8]. As inhibin suppresses FSH synthesis in the anterior pituitary [21], reduced levels may explain higher FSH levels in East Asians compared to other ethnicities, highlighting the importance of ethnic-specific FSH RIs. Furthermore, compared to our findings, other studies reported higher FSH reference limits in East Asians. Ichihara et al. reported adult East Asian and South East Asian FSH RI as 2–14 IU/L [12]. Yu et al. calculated FSH RIs as 1.4–8.9 IU/L and 1.3–9.1 IU/L using parametric and nonparametric methods, respectively, for Chinese adult men [22]. Interestingly, Yu et al. also reported increasing FSH reference limits with age in adults. In addition to potential analytical and environmental differences, age-dependent increases in FSH levels may explain the higher FSH reference limits in the two adult studies compared to those established in the current pediatric study.
Previous pediatric and adult studies reported similar ethnic differences for amylase (i.e. lower in Caucasians and higher in Blacks than in Asians) [3], [6], ferritin (i.e. higher in East Asians than in Caucasians and South Asians) [4], [23], [24], and IgG (i.e. lower in Caucasians than in Asians and Blacks) [3], [25]. Conversely, inconsistent findings have been reported for some biomarkers, including IgA and IgM, particularly between Blacks and Caucasians [26], [27], [25]. Additional studies are needed to confirm the ethnic differences observed in the current study, and identify the underlying genetic and/or environmental factors responsible for these differences.
Our findings highlight the need for careful examination of the influence of ethnicity on pediatric RIs for various disease biomarkers to identify those of clinical relevance. If ethnic differences are not considered during clinical evaluation of laboratory results, this could result in patient error. For instance, a pediatric East Asian male who presents with a ferritin level within the Caucasian RI, but below the lower reference limit of the East Asian RI, might have his iron deficiency status go unnoticed for years if outdated RIs (i.e. generally calculated based on Caucasians) are used. Alternatively, if his ferritin level is above the Caucasian upper reference limit, but within the East Asian RI, the outdated RI might incorrectly suggest hemochromatosis. This may result in unnecessary treatments, psychological distress, and financial costs. Thus, the clinical implementation of ethnic differences and ethnic-specific RIs could contribute to physical, psychological, and social benefits to patients and their families.
Data presented in the CALIPER cohort indicate that the majority of routine biomarkers (i.e. 45 out of 52 [86.5%]) are not strongly influenced by ethnicity to the extent that could alter clinical decision-making. While these data consisted of the four major ethnic groups in Canada, these groups also represent the largest ethnic populations around the world. Thus, we postulate that due to the lack of ethnic differences among these diverse ethnic groups, ethnicity may not be a major covariate for the majority of biomarkers. Although there are difficulties in the adoption of multi-covariate RIs for all tests in the laboratory information system, our finding that only a minority of biomarkers require ethnic-specific RIs would allow for a relatively easier implementation of ethnic-specific RIs. Furthermore, knowledge of the biomarkers that do not show ethnic differences would result in a more efficient clinical assessment of laboratory results, as clinicians do not need to consider ethnicity as a factor.
Regardless of the strengths of the current study, there are also certain limitations. Firstly, due to the lack of RCVs established for some Abbott assays (e.g. FSH), RCVs established on other analytical platforms (e.g. Siemens Dimension Vista) were used. Due to the limited sample size, results were neither assessed nor adjusted for various environmental factors (e.g. seasonal differences in 25(OH) vitamin D levels). Furthermore, CALIPER does not collect information on socioeconomic factors, which have been reported to contribute to observed ethnic differences in some biomarkers. For example, eosinophil counts have been reported to be higher in Blacks relative to Caucasians; however, these ethnic-specific differences were not determined in Blacks and Caucasians living in the same area [2]. The inclusion of socioeconomic and regional factors in study analysis has the potential to distinguish socioeconomic and/or environmental from genetic causes of ethnic differences and provide further insight into whether these differences are solely due to ethnicity. Lastly, although RCVs were used to assess the clinical relevance of ethnic differences, this is only one approach to check clinical applicability. Further large-scale multi-center longitudinal outcome studies are required. Such studies can verify the clinical applicability of our findings and determine the underlying physiological reasons behind the observed ethnic differences. Ideally, each country or region should determine population concentrations and assess potential ethnic differences within their own populations.
In summary, seven biomarkers showed substantial ethnic differences in the current study, requiring ethnic stratification for their RIs. The majority of assays examined in our CALIPER cohort did not show appreciable differences between the four major ethnic groups examined. These findings will allow for a more accurate and efficient laboratory test interpretation and clinical decision-making, thus contributing to the advancement of the quality of pediatric patient care. Further studies are needed to substantiate these findings in larger cohorts, and in other ethnic populations, including those of mixed ethnicities.
Supplementary Material
Supplementary material
Acknowledgments
We thank all study participants and their families, without whom this study would not have been possible.
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/cclm-2019-0876).
Funding Statement
This research was supported by the Canadian Institutes of Health Research (funder Id: http://dx.doi.org/10.13039/501100000024, Grant Number: 353989).
Footnotes
Author contributions: KA and HT conceptualized the research question and designed the study plan. AH, AC, RT, MKB, and JM were involved in participant recruitment and sample collection. VT and SA contributed to retrospective and prospective data analysis, in addition to HT. All authors contributed to drafting and revising the final manuscript. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline-Third Edition. CLSI document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 2.Tahmasebi H, Trajcevski K, Higgins V, Adeli K. Influence of ethnicity on population reference values for biochemical markers. Crit Rev Clin Lab Sci. 2018;55:359–75. doi: 10.1080/10408363.2018.1476455. [DOI] [PubMed] [Google Scholar]
- 3.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA. et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58:854–68. doi: 10.1373/clinchem.2011.177741. [DOI] [PubMed] [Google Scholar]
- 4.Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D. et al. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 2013;59:1393–405. doi: 10.1373/clinchem.2013.204222. [DOI] [PubMed] [Google Scholar]
- 5.Konforte D, Shea JL, Kyriakopoulou L, Colantonio D, Cohen AH, Shaw J. et al. Complex biological pattern of fertility hormones in children and adolescents: a study of healthy children from the CALIPER cohort and establishment of pediatric reference intervals. Clin Chem. 2013;59:1215–27. doi: 10.1373/clinchem.2013.204123. [DOI] [PubMed] [Google Scholar]
- 6.Tsianos EB, Jalali MT, Gowenlock AH, Braganza JM. Ethnic “hyperamylasaemia”: clarification by isoamylase analysis. Clin Chim Acta Int J Clin Chem. 1982;124:13–21. doi: 10.1016/0009-8981(82)90314-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Berman NG, Veldhuis JD, Der T, McDonald V, Steiner B. et al. Graded testosterone infusions distinguish gonadotropin negative-feedback responsiveness in Asian and white —men – a Clinical Research Center study. J Clin Endocrinol Metab. 1998;83:870–6. doi: 10.1210/jcem.83.3.4625. [DOI] [PubMed] [Google Scholar]
- 8.van Houten ME, Gooren LJ. Differences in reproductive endocrinology between Asian men and Caucasian men – a literature review. Asian J Androl. 2000;2:13–20. [PubMed] [Google Scholar]
- 9.Boucai L, Surks MI. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin Endocrinol (Oxf) 2009;70:788–93. doi: 10.1111/j.1365-2265.2008.03390.x. [DOI] [PubMed] [Google Scholar]
- 10.Lim E, Miyamura J, Chen JJ. Racial/ethnic-specific reference intervals for common laboratory tests: a comparison among Asians, Blacks, Hispanics, and White. Hawaii J Med Public Health J Asia Pac Med Public Health. 2015;74:302–10. [PMC free article] [PubMed] [Google Scholar]
- 11.Ichihara K, Ceriotti F, Tam TH, Sueyoshi S, Poon PM, Thong ML. et al. The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin Chem Lab Med. 2013;51:1429–42. doi: 10.1515/cclm-2012-0421. [DOI] [PubMed] [Google Scholar]
- 12.Ichihara K, Ceriotti F, Kazuo M, Huang Y-Y, Shimizu Y, Suzuki H. et al. The Asian project for collaborative derivation of reference intervals: (2) results of non-standardized analytes and transference of reference intervals to the participating laboratories on the basis of cross-comparison of test results. Clin Chem Lab Med. 2013;51:1443–57. doi: 10.1515/cclm-2012-0422. [DOI] [PubMed] [Google Scholar]
- 13.Statistics Canada. Census Profile, 2016 Census [Internet] [cited 2018 Aug 30]. Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=35&Geo2=&Code2=&Data=Count&SearchText=Ontario&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=35 . [Google Scholar]
- 14.Bugdayci G, Oguzman H, Arattan HY, Sasmaz G. The use of reference change values in clinical laboratories. Clin Lab. 2015;61:251–7. doi: 10.7754/clin.lab.2014.140906. [DOI] [PubMed] [Google Scholar]
- 15.Brescia V, Tampoia M, Cardinali R. Biological variability of serum 25-hydroxyvitamin D and other biomarkers in healthy subjects. Lab Med. 2013;44:20–4. [Google Scholar]
- 16.Bailey D, Bevilacqua V, Colantonio DA, Pasic MD, Perumal N, Chan MK. et al. Pediatric within-day biological variation and quality specifications for 38 biochemical markers in the CALIPER cohort. Clin Chem. 2014;60:518–29. doi: 10.1373/clinchem.2013.214312. [DOI] [PubMed] [Google Scholar]
- 17.Ricós C, Cava F, García-Lario JV, Hernández A, Iglesias N, Jiménez CV. et al. The reference change value: a proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest. 2004;64:175–84. doi: 10.1080/00365510410004885. [DOI] [PubMed] [Google Scholar]
- 18.Willeman T, Casez O, Faure P, Gauchez AS. Evaluation of biotin interference on immunoassays: new data for troponin I, digoxin, NT-Pro-BNP, and progesterone. Clin Chem Lab Med. 2017;55:e226–9. doi: 10.1515/cclm-2016-0980. [DOI] [PubMed] [Google Scholar]
- 19.Lowe NM, Bhojani I. Special considerations for vitamin D in the south Asian population in the UK. Ther Adv Musculoskelet Dis. 2017;9:137–44. doi: 10.1177/1759720X17704430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer-Gould A, Lucas RM, Xiang AH, Wu J, Chen LH, Gonzales E. et al. Vitamin D-binding protein polymorphisms, 25-hydroxyvitamin D, sunshine and multiple sclerosis. Nutrients. 2018;10:184. doi: 10.3390/nu10020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makanji Y, Harrison CA, Robertson DM. Feedback regulation by inhibins A and B of the pituitary secretion of follicle-stimulating hormone. Vitam Horm. 2011;85:299–321. doi: 10.1016/B978-0-12-385961-7.00014-7. [DOI] [PubMed] [Google Scholar]
- 22.Yu S, Qiu L, Liu M, Li S, Tao Z, Zhang Q. et al. Establishing reference intervals for sex hormones and SHBG in apparently healthy Chinese adult men based on a multicenter study. Clin Chem Lab Med. 2018;56:1152–60. doi: 10.1515/cclm-2017-0749. [DOI] [PubMed] [Google Scholar]
- 23.Harris EL, McLaren CE, Reboussin DM, Gordeuk VR, Barton JC, Acton RT. et al. Serum ferritin and transferrin saturation in Asians and Pacific Islanders. Arch Intern Med. 2007;167:722–6. doi: 10.1001/archinte.167.7.722. [DOI] [PubMed] [Google Scholar]
- 24.Yenson PR, Yoshida EM, Li CH, Chung HV, Tsang PW. Hyperferritinemia in the Chinese and Asian community: a retrospective review of the University of British Columbia experience. Can J Gastroenterol. 2008;22:37–40. doi: 10.1155/2008/245096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckley CE, Dorsey FC. Serum immunoglobulin levels throughout the life-span of healthy man. Ann Intern Med. 1971;75:673–82. doi: 10.7326/0003-4819-75-5-673. [DOI] [PubMed] [Google Scholar]
- 26.Tollerud DJ, Brown LM, Blattner WA, Weiss ST, Maloney EM, Kurman CC. et al. Racial differences in serum immunoglobulin levels: relationship to cigarette smoking, T-cell subsets, and soluble interleukin-2 receptors. J Clin Lab Anal. 1995;9:37–41. doi: 10.1002/jcla.1860090107. [DOI] [PubMed] [Google Scholar]
- 27.Satoh T, Brown LM, Blattner WA, Maloney EM, Kurman CC, Nelson DL. et al. Serum neopterin, beta2-microglobulin, soluble interleukin-2 receptors, and immunoglobulin levels in healthy adolescents. Clin Immunol Immunopathol. 1998;88:176–82. doi: 10.1006/clin.1998.4568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material