Abstract
Background:
This study aimed to determine the disease burden and strain distribution of rotavirus in children with diarrhea <5 years old in Suzhou, China.
Methods:
The study was conducted among children with diarrhea <5 years old at Suzhou University Affiliated Children’s Hospital from 2013 to 2019. Rotavirus antigen was detected in clinical laboratory and then sent to Suzhou Centers for Disease Control and Prevention for further molecular analysis. Group A rotavirus (RVA) was detected through enzyme-linked immunosorbent assays, and G-genotype and P-genotype of RVA were tested using reverse transcription-polymerase chain reaction.
Results:
Of a total of 198,130 children with diarrhea, 70,813 (35.7%) were positive for RVA; RVA-related diarrhea was detected in 7798 (20.7%, n = 7798/37,710) inpatients and 63,015 (39.3%, n = 63,015/160,420) outpatients. Most children (92.0%, n = 65,171/70,813) positive for RVA were found as children <3 years old. Children 12-35 months old were reported as the highest prevalence among all age groups. The seasonal peak of RVA was in the autumn and winter. Among all 673 RVA strains genotyped, the G9P[8] strain was reported to be persistently predominant in the pediatric population from 2013 to 2019.
Conclusions:
The burden of diarrhea disease due to rotavirus infection remains high in Suzhou.
Keywords: rotavirus infection, childhood diarrhea, prevalence, genotypes
Diarrhea has been found as the second leading cause of death in children <5 years of age, causing approximately 500,000 deaths annually worldwide.1,2 In such an age group of children, rotavirus (RV) has been reported as the single most common cause of severe diarrhea, which is estimated at 10 million severe cases and 118,000 to 183,000 deaths each year.1–3 Despite the decreasing number of deaths due to diarrhea, RV is still the major global cause of diarrhea-related morbidity and mortality in children <5 years of age.4 Group A rotavirus (RVA) is known as the most important pathogen that causes infective diarrhea in infants.5 RVAs fall into G and P types in accordance with the sequence diversity of the genes encoding the outer viral proteins VP7 (glycoprotein) and VP4 (protease-sensitive protein).6,7 From the global perspective, 5 strains, that is, G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8], have caused most of the severe diarrhea illness in children in most nations over the past few years.8
Most of RV deaths are reported in Asia and sub-Saharan Africa.3,9 In 2013, World Health Organization (WHO) Position Paper on Rotavirus Vaccines recommended that RV vaccines should be covered in all national immunization programs and considered a priority, particularly in nations with high RV gastroenteritis-related fatality rates (eg, south and south-eastern Asia and sub-Saharan Africa).10 Currently, there are 4 WHO prequalified RV vaccines. Besides the globally available RotaTeq (Merck & Co., Inc, West Point, PA, USA) and Rotarix (GlaxoSmithKline Biologicals SA, Rixensart, Belgium), Rotavac (Bharat Biotech, Hyderabad, India) and Rotasiil (Serum Institute of India, Pune, Maharashtra, India) have been licensed and manufactured in India, and prequalified by WHO in 2018.9,11 RV vaccines were introduced in 108 countries by the end of 2019, and global coverage was estimated at 39%.12 In China, one of the high-burden nations for diarrhea,13 however, RV vaccination has not been included into the national immunization program thus far. RotaTeq, one of international RV vaccines, was licensed for use in China in the middle of 2018. Moreover, there was a domestic vaccine (the Lanzhou lamb rotavirus vaccine [LLR], the Lanzhou Institute of Biological Products, Lanzhou, China) licensed in China in 2000, but this vaccine cannot be covered in the Expanded Program on Immunization due to the lack of reliable data of LLR protection on childhood RV infections. In Suzhou, consistent with the overall situation in China, oral RV vaccines have served as category II vaccine (other vaccines administered voluntarily by citizens at their own expense, compared with the first type vaccine provided free of charge by the government), and 2 RV vaccines (RotaTeq and LLR) are licensed for use.
In China, there has been a lack of studies on RV infectious diarrhea in children with large samples and accurate descriptions of the disease burden in this area. To inform policy makers on RV diarrhea prevention and promote the integration of medical treatment and prevention to reduce diarrhea attributed to RV in children, a retrospective analysis was conducted on long-time routine surveillance of children <5 years of age with RV infection in Suzhou University Affiliated Children’s Hospital (SCH). This study obtained the prevalence, seasonality and strain diversity of RV infection among diarrhea children <5 years of age in Suzhou using data generated by the hospital information system and electronic medical records of SCH from 2013 to 2019.
MATERIALS AND METHODS
Study Site and Population
Suzhou is known as a major city located in Eastern China. It has a population of approximately 10.7 million, with about 65% registered residents, and a Gross Domestic Product per capita up to 24,000 US dollars in 2017.14 SCH is a 1500-bed hospital with estimated 1.9 million outpatient and 70,000 inpatient visits annually.15 This hospital is the only tertiary hospital for children in Suzhou. This retrospective hospital-based observational study was conducted from January 2013 to December 2019 at SCH. The year-round diarrhea surveillance was conducted among children of inpatient, outpatient and emergency departments. Patients visiting the SCH were registered and then screened for the eligibility of inclusion by attending practitioners. WHO recommendations were used to define diarrhea as ≥3 passages of watery, loose, mucoid or bloody stools within a 24-hour period.16 Children consistent with the case definition of diarrhea were eligible for inclusion. The sample size of eligible patients for the whole hospital was nearly 30,000 per year. Multiple samples taken from the same patient were considered duplicates if collected in the same hospitalization or in case of a time-lapse of shorter than 28 days between 2 samples. Attending doctors in the hospital decided when, whom, in what hospital setting and how many children would be recruited. However, whether an eligible patient actually had a stool specimen that can be retained to detection ultimately determined whether the child was included. To account for the seasonal study, spring was defined as March to May, summer was defined as June to August, autumn was defined as September to November and winter was defined as December to February in Suzhou.
This study was approved by the Institutional Review Board of the School of Public Health, Fudan University.
Data and Specimen Collection
The data of inpatient and outpatient departments of diarrhea cases were identified from January 2013 to December 2019 using data from the hospital information system and the electronic medical record. The information collected primarily consisted of demographics (eg, sex, date of birth and address) and clinical information (eg, signs/symptoms, date of specimen collection and diagnosis of RV laboratory test result). For children diagnosed as diarrhea on an outpatient basis, samples were included and tested in accordance with the diagnosis and treatment needs of clinicians and the practical situation of the presence or absence of stool specimens of the children. After recruitment, whole stool was collected in a sterilized container without preservative and then stored at –20 °C till RV screening and strain characterization. RV antigen was detected in clinical laboratory of SCH, and further RV strain typing was performed by Suzhou Centers for Disease Control and Prevention (Suzhou CDC). Using the unified surveillance program, Suzhou CDC regularly detected specimens of children <5 years of age with diarrhea from SCH, which were distributed evenly every month. From June to September, no less than 20 specimens would be collected from hospitalized children every month. In other months, no less than 25 specimens would be collected per month, and at least 300 specimens of hospitalized children would be collected throughout every year. Outpatient/emergency department would collect 20 specimens of children per month and 240 specimens every year.
Laboratory Testing
The SCH has 2 locations in the city of Suzhou, each of which has outpatient, emergency and inpatient departments. In addition, each respective location has its own laboratory for RV testing. A standardized method and an operation procedure were adopted by all laboratories for RV testing and characterization of strains, which were validated before initiating surveillance. RVA were detected directly in stool samples through enzyme-linked immunosorbent assays (ProSpecTTM Rotavirus kit; Oxoid Ltd, Basingstoke, United Kingdom), according to the manufacturer’s instructions. Then further G- and P-genotyping of RVA strains were detected through multiplex reverse transcription-polymerase chain reaction for enzyme-linked immunosorbent assay-positive samples. Viral nucleic acid was extracted from the respective specimen with the Viral RNA/DNA Kit (Geneaid Biotech, Taipei, Taiwan) or the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA) following the manufacturers’ instructions. The primers employed in G- and P-genotyping polymerase chain reactions and detection procedures have been described extensively.17,18
Statistical Analysis
In this study, the data from children under 5 with diarrhea in SCH were analyzed. Prevalence of RV was calculated by dividing the number of children that detected positive for RV by the total number of children tested. To express the seasonal pattern of RV infection and provide a sense of when RV occurs relative to all-cause diarrhea, we accounted for the positive number and negative number of children who detected for RV infection in different months. We performed χ2 tests or Fisher exact tests for categorical variables, as well as Wilcoxon rank-sum or Kruskal-Wallis tests for continuous variables, as appropriate. The data analysis was conducted using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).19
RESULTS
Characteristics of Patients
From January 1, 2013, to December 31, 2019, 198,130 cases of diarrhea children <5 years of age were admitted in total to the SCH and included in our study. Among these cases, 160,420 (81.0%) were recruited at outpatient settings and 37,710 (19.0%) were recruited at inpatient settings. Fecal samples of hospitalized children with diarrhea were tested at a rate of over 95%. Almost all outpatients, indeed having a stool specimen for detection were recruited. Among children included, 118,405 (59.8%) patients were male and most children’s diarrhea mainly occurred under 2 years old (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/E653). Moreover, the majority of infant diarrhea occurred in the autumn and winter seasons.
Prevalence and Seasonality of Rotavirus Infection
Overall, RVA was detected in 70,813 children (35.7%). Children 24–35 months of age had the highest positive percentage in RVA tests compared with other age groups (41.3%, n = 11,416/27,674, P < 0.001). For pediatric outpatients with diarrhea, the highest prevalence of RV reached 45.3% in children 24–35 months of age. However, the prevalence of RV in hospitalized children was peaked at 23.8% in children 0–5 months old and decreased with age groups. RV also showed slightly higher prevalence rates in male children compared with female (35.9% vs. 35.5%; P < 0.05). Moreover, RVA was more frequently detected among children attending an outpatient department than children visiting an inpatient setting (39.3% vs. 20.7%; P < 0.001). The seasonal peak of RVA activity was in winter months (50.7%), and significant difference of prevalence between different seasons was observed (P < 0.001) (Table 1).
TABLE 1.
Frequency of Group A Rotavirus Detected in Children With Diarrhea in Suzhou, 2013–2019, Stratified by Departments
| Characteristics | All Patients | P | Outpatient | P | Inpatient | P | |||
|---|---|---|---|---|---|---|---|---|---|
| No. Detected | No. Positivity,(%) | No. Detected | No. Positivity,(%) | No. Detected | No. Positivity,(%) | ||||
| Total | 198,130 | 70,813 (35.7) | 160,420 | 63,015 (39.3) | 37,710 | 7798 (20.7) | |||
| Gender | 0.040 | 0.007 | 0.622 | ||||||
| Male | 118,405 | 42,534 (35.9) | 95,524 | 37,783 (39.6) | 22,881 | 4751 (20.8) | |||
| Female | 79,725 | 28,279 (35.5) | 64,896 | 25,232 (38.9) | 14,829 | 3047 (20.5) | |||
| Age groups (mo) | <0.001 | <0.001 | <0.001 | ||||||
| 0~5 | 48,618 | 16,842 (34.6) | 33,330 | 13,197 (39.6) | 15,288 | 3645 (23.8) | |||
| 6~11 | 49,562 | 16,704 (33.7) | 43,884 | 15,452 (35.2) | 5678 | 1252 (22.1) | |||
| 12~23 | 51,765 | 20,209 (39.0) | 44,660 | 18,762 (42.0) | 7105 | 1447 (20.4) | |||
| 24~35 | 27,674 | 11,416 (41.3) | 23,258 | 10,542 (45.3) | 4416 | 874 (19.8) | |||
| 36~47 | 10,707 | 3352 (31.3) | 8143 | 3007 (36.9) | 2564 | 345 (13.5) | |||
| 48~59 | 9804 | 2290 (23.4) | 7145 | 2055 (28.8) | 2659 | 235 (8.8) | |||
| Season | <0.001 | <0.001 | <0.001 | ||||||
| Spring | 36,974 | 12,339 (33.4) | 28,230 | 10,491 (37.2) | 8744 | 1848 (21.1) | |||
| Summer | 50,150 | 12,762 (25.4) | 42,120 | 11,613 (27.6) | 8030 | 1149 (14.3) | |||
| Autumn | 56,804 | 18,252 (32.1) | 47,225 | 16,444 (34.8) | 9579 | 1808 (18.9) | |||
| Winter | 54,202 | 27,460 (50.7) | 42,845 | 24,467 (57.1) | 11,357 | 2993 (26.4) | |||
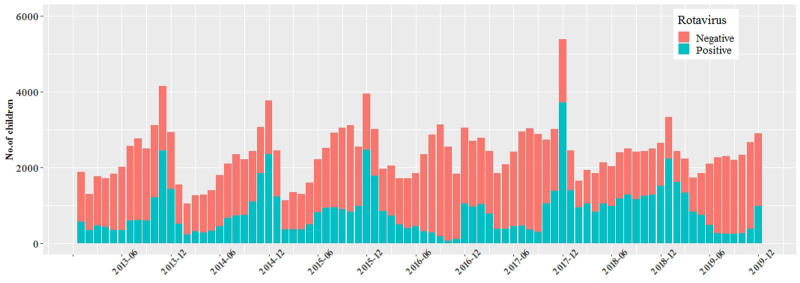
In this study, peak RV infection (68.9%) was reported in November 2017, and its prevalence showed obvious seasonality; the seasonal peak of RV diarrhea occurred from October to January of the following year, compared with the peak of all-cause diarrhea since June or July (Fig. 1). Despite the monthly infection peak is known to vary from year to year, and from region to region, RV peak is usually between September to February of the following year in China.
FIGURE 1.
Monthly distribution of rotavirus detection among children <5 with diarrhea.
Distribution of Rotavirus Strains
On the whole, 673 isolates were genotyped from 2013 to 2019 by Suzhou CDC. For the G types studied, 644 (95.7%) had a single G specificity, 17 (2.5%) achieved mixed G specificities and 12 (1.8%) were not be assigned any G-type specificity. Moreover, for P types, 664 (98.7%) isolates had a single P specificity, 5 (0.7%) had mixed P specificities and 4 (0.6%) were unable to assign any P-type specificity. Among the 644 isolates with a single G specificity, the most common G-type detected was the G9 strain (n = 566, 84.1%), while the remaining 11.6% (n = 78) of the single G-type detected isolates were constituted by the 4 common strains, G1, G2, G3 and G8. In the meantime, P[8] and P[4] together constituted single P-type detected isolates in this study. Among 664 isolates with a single P specificity, P[8] (n = 635, 94.5%) was the most common P-type. Regardless of mixed infections and nontypeable strains, the strain combination of G9P[8] accounted for 82.8%(n = 557/673) of all detected RV strain combinations (Table 2).
TABLE 2.
Genotype Distribution of Rotavirus Detection Among Children <5 in Suzhou (January 2013–December 2019)
| G-type | P-type | ||||
|---|---|---|---|---|---|
| P[4] | P[8] | P mix | P nt | Total (%) | |
| G1 | 3 | 28 | 0 | 0 | 31 (4.6) |
| G2 | 21 | 3 | 0 | 0 | 24 (3.6) |
| G3 | 0 | 22 | 0 | 0 | 22 (3.3) |
| G8 | 1 | 0 | 0 | 0 | 1 (0.2) |
| G9 | 3 | 557 | 3 | 3 | 566 (84.1) |
| G mix | 0 | 15 | 2 | 0 | 17 (2.5) |
| G nt | 0 | 11 | 0 | 1 | 12 (1.8) |
| Total (%) | 28 (4.2) | 635 (94.5) | 5 (0.7) | 4 (0.6) | 673 (100.0) |
nt indicates nontypeable.
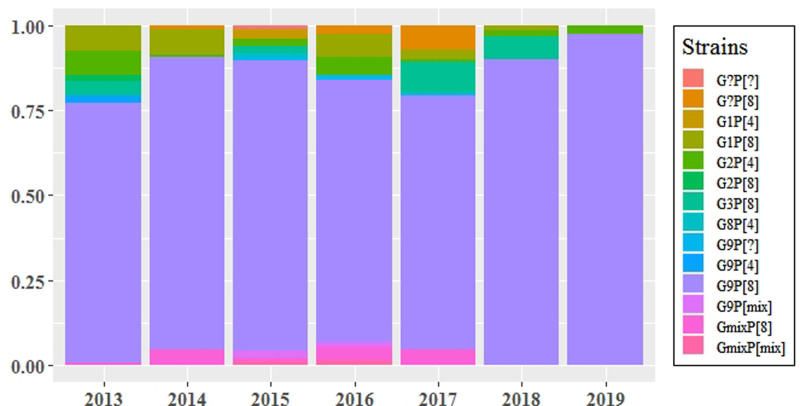
From 2013 to 2019, RVA strains circulating in Suzhou among children <5 years old changed from year to year, but G9P[8] was persistently predominant in the population of this study (Fig. 2).
FIGURE 2.
Rotavirus strains distribution among children <5 in Suzhou, 2013–2019.
DISCUSSION
With the use of large-scale, long-term clinical surveillance data, this study tested RV infection and strain diversity among children <5 years of age in Suzhou, stressing the importance of routine surveillance, as an attempt to more effectively identify priorities for intervention and predominant strains to guide relevant vaccination activity. This study had a large sample size and accurately described the disease burden of RV infection in children <5 years old in Suzhou.
As revealed by our monitoring results, in Suzhou, over one-third of childhood diarrhea was correlated with RV infection, and the prevalence of RV in outpatients was higher than that in inpatients, respectively, taking up 39.3% of outpatient visits and 20.7% of hospitalizations due to diarrhea in children under 5. The results above were different from national diarrhea surveillance data and other previous reports, demonstrating that the RV prevalence in inpatients was higher.20–22 This is likely because the outpatient settings included the general outpatient and emergency in our data, so the number of children with diarrhea in outpatient visits was significantly larger than that in hospitalization. Moreover, most children have mild symptoms with diarrhea, which probably accounted for why parents preferred visiting an outpatient clinic.23 Some children with severe diarrhea were treated with antivirals in the outpatient department and then hospitalized for further treatment, which resulted in a certain proportion of duplication of outpatient and inpatient cases. Because vaccine effectiveness against severe disease is generally higher than the vaccine effectiveness against less severe disease, the existing RV vaccination in Suzhou may have prevented more severe cases to a certain extent. As indicated by the Suzhou CDC, the RV vaccine coverage of children (the third dose in 5 rounds/the number of vaccines that should be received at 8 months of age) in Suzhou reached 4.77% in 2019. The overall percentage of RV infection of diarrhea cases detected in this study (35.7%) was slightly higher than that national data previously observed (30.0%),22 suggesting that the disease burden of diarrhea due to RV infection in Suzhou was consistent with or slightly higher than the national level. Of the total RV infections that occurred in children, 92% were detected in children ≤ 35 months, and children 24–35 months of age achieved the highest prevalence of RV among all age groups in this study. This result might be correlated with the increased cross-infection of older children in nurseries or care facilities. WHO recommended that the first dose of the RV vaccine (RotaTeq) should be administered at 6–12 weeks of age and the last dose at a maximum age of 32 weeks.10 In addition, the LLR vaccine is recommended for children 2–36 months old, and the first dose should be administrated in 12 weeks of age.24 This is favorable for the vaccine to confer full protection for young children before their risk of infection becomes high. On the other hand, slightly significant difference in the prevalence of RV infection between male and female children was observed. In other words, the prevalence of RV in male children was slightly higher than that in female, which is consistent with previous reports.22,25
The onset of diarrhea in children caused by RV infection had obvious seasonality.26 Hospitalizations for RV diarrhea after a cold or dry month tended to be more common than those after a warm or wet corresponding calendar month.27 RV infection showed a strong seasonal peak in colder, drier months, as seen in other Asian nations.28 In this study, the highest prevalence of RV infection occurred in November or December, and January, generally complying with the epidemic seasons of RV infection in China that varies slightly with regions, mainly in autumn and winter.29 However, the season of all-cause diarrhea was peaked from summer, so which one of various pathogens is the most responsible for disease is difficult to determine, since it varies with locations and time of years.30 As reported by existing studies, weather in different seasons can significantly drive diarrheal pathogen transmission.30 It is noteworthy noting that the seasonality of the results was indicated to vary significantly around 2016, which might be correlated with the establishment and completion of a new branch area of the SCH from 2015 to 2016. The new branch area of hospital would expand the range of new medical services to a certain extent. There were also differences in the types of epidemic strains in different nations. Globally common combinations of epidemic strains include G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8].9,31 On the whole, the prevalent strains in China are consistent with those around the world, whereas G9 became the predominant G-genotype after year of 2011.22 G9P[8] has been the most prevalent strain in China.32 This was also true in this study; although the RV strain circulating in the pediatric population changed unpredictably from year to year, G9P[8] is still the most dominant single strain of RV infection among children <5 years of age in Suzhou, with certain persistence from 2013 to 2019.33 The first emergence of G9P[8] was in the 1980s,7 and then G9P[8] has spread worldwide, which gained predominance in several nations (eg, Thailand, Ghana, Australia,25,34,35 as well as China36). The 2 globally licensed vaccines (ie, RotaTeq and Rotarix) contain the P[8] component,9 thereby making them demonstrate protective efficacy against G9P[8]. Furthermore, there are efficacy and effectiveness data of the mentioned 2 vaccines for G9P[8], thereby demonstrating that the vaccines above are highly effective against this strain.37,38 Researches further focusing on effective vaccines against circulating RV strains should be conducted subsequently.
This study has several limitations. First, this is a retrospective study and the stool samples of children with diarrhea were collected and investigated before our research and analysis, so our surveillance protocol was not developed in advance of the implementation of monitoring. Second, Suzhou’s only tertiary care children’s hospital was selected as the surveillance site to estimate prevalence of RV infection in children <5 years of age, while there are other general hospitals in Suzhou which provide inpatient and outpatient care to pediatric diarrhea patients. At the same time, population-based rates for RV-related diarrhea were not readily available, owing to the lack of population denominators in this study. Third, some factors in the process of data inclusion and collection can introduce unpredictable biases. For instance, different health-seeking behaviors of pediatric patients, subjective judgment of clinicians on when and whom to sample and a sampling scheme not predetermined could have yielded patterns in the data that do not represent the true burden and epidemiological features of RV. Fourth, since children with a stool specimen for detection were ultimately included, this study failed to show difference in severity or age between the children who had a stool collected and those without a stool collected, whereas both of the mentioned could greatly impact the positive proportion of RV. Nevertheless, the large sample size collected over a long time in this study might, to a certain extent, offer some relief to the concerns above. We were devoted to make great efforts to further improvement of the surveillance scheme attributes based on these aspects. Finally, the historical information of vaccine usage for the diarrhea children in this study was not collected, so the vaccine efficacy could not be assessed here.
In brief, the burden of diarrhea disease due to RV remains high in economically developed areas in China (eg, Suzhou). Children 12–35 months of age are more likely to suffer from diarrhea due to RV infection, and the peak of RV infection occurred in the autumn and winter. Our results support the inclusion of RV vaccine in the national immunization program to mitigate RV burden. Continuous surveillance of RV strain evolution is required to ensure that currently marketed vaccines have protective efficacy against current circulating and antigenically distinct strains in China and the global health community. Furthermore, more efforts should be made to facilitate the integration of medical treatment and prevention for reducing RV infection and promoting RV vaccination.
ACKNOWLEDGMENTS
The authors express our gratitude to all children and their parents or guardians who have participated in this study, as well as the staff of Suzhou University Affiliated Children’s Hospital and Suzhou Centers for Disease Control and Prevention involved in testing specimens. The authors appreciate Merck Sharp & Dohme Corp for supported in part by a research grant from Investigator-Initiated Studies Program of it.
Supplementary Material
Footnotes
The opinions presented in this study are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
This study was sponsored by Fudan University. Funding was provided by the Shanghai New Three-year Action Plan for Public Health (Grand No. GWV-10.1-XK16) and A Merck MISP Grant (No. 59169).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(suppl 2):S96–S105. [DOI] [PubMed] [Google Scholar]
- 4.Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global Bburden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthijnssens J, Otto PH, Ciarlet M, et al. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch Virol. 2012;157:1177–1182. [DOI] [PubMed] [Google Scholar]
- 6.Gentsch JR, Woods PA, Ramachandran M, et al. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J Infect Dis. 1996;174(suppl 1):S30–S36. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood CD. Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs. J Infect Dis. 2010;202(suppl):S43–S48. [DOI] [PubMed] [Google Scholar]
- 8.Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:847–856. [DOI] [PubMed] [Google Scholar]
- 9.Soares-Weiser K, Bergman H, Henschke N, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2019;11:CD008521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Rotavirus vaccines: WHO position paper-- July 2021. Wkly Epidemiol Rec. 2021;96:301–319. [Google Scholar]
- 11.Skansberg A, Sauer M, Tan M, et al. Product review of the rotavirus vaccines ROTASIIL, ROTAVAC, and Rotavin-M1. Hum Vaccin Immunother. 2021;17:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Rotavirus vaccines completed dose (RotaC) immunization coverage among 1-year-olds (%). Available at: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/rotavirus-vaccines-completed-dose-(rotac)-immunization-coverage-among-1-year-olds-(-). Accessed August 16, 2021.
- 13.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzhou Statistics Bureau. China Statistics Press (Press CS), ed. Suzhou Statistical Yearbook 2018. [Google Scholar]
- 15.Suzhou University Affiliated Children’s Hospital. 2021. Available at: http://www.sdfey.cn/yi-yuan-jie-shao.html. Accessed August 16, 2021.
- 16.Gidudu J, Sack DA, Pina M, et al. ; Brighton Collaboration Diarrhea Working Group. Diarrhea: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:1053–1071. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Li Z, Han D, et al. Viral agents associated with acute diarrhea among outpatient children in southeastern China. Pediatr Infect Dis J. 2013;32:e285–e290. [DOI] [PubMed] [Google Scholar]
- 18.Simmonds MK, Armah G, Asmah R, et al. New oligonucleotide primers for P-typing of rotavirus strains: strategies for typing previously untypeable strains. J Clin Virol. 2008;42:368–373. [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. R: A Language and Environment for Statistical Computing 2019. 2019. Available at: https://www.R-Project.org. Accessed August 16, 2021.
- 20.Wu D, Yen C, Yin ZD, et al. The public health burden of rotavirus disease in children younger than five years and considerations for rotavirus vaccine introduction in China. Pediatr Infect Dis J. 2016;35:e392–e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Xu Z, Li D, et al. Update on the disease burden and circulating strains of rotavirus in China: a systematic review and meta-analysis. Vaccine. 2014;32:4369–4375. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Lai S, Geng Q, et al. Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009-2015. J Infect. 2019;78:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassaw MW, Abebe AM, Kassie AM, et al. Evidence from 2016 ethiopian demographic and health survey data: maternal practice in managing childhood diarrhea at home. J Pediatr Nurs. 2020;55:e250–e256. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhang Y, Yang Y, et al. Effectiveness of Lanzhou lamb rotavirus vaccine in preventing gastroenteritis among children younger than 5 years of age. Vaccine. 2019;37:3611–3616. [DOI] [PubMed] [Google Scholar]
- 25.Damanka S, Adiku TK, Armah GE, et al. Rotavirus infection in children with diarrhea at Korle-Bu teaching hospital, Ghana. Jpn J Infect Dis. 2016;69:331–334. [DOI] [PubMed] [Google Scholar]
- 26.Patel MM, Pitzer VE, Alonso WJ, et al. Global seasonality of rotavirus disease. Pediatr Infect Dis J. 2013;32:e134–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandt CD, Kim HW, Rodriguez WJ, et al. Rotavirus gastroenteritis and weather. J Clin Microbiol. 1982;16:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lestari FB, Vongpunsawad S, Wanlapakorn N, et al. Rotavirus infection in children in Southeast Asia 2008-2018: disease burden, genotype distribution, seasonality, and vaccination. J Biomed Sci. 2020;27:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sai L, Sun J, Shao L, et al. Epidemiology and clinical features of rotavirus and norovirus infection among children in Ji’nan, China. Virol J. 2013;10:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao DL, Roose A, Roh M, et al. The seasonality of diarrheal pathogens: a retrospective study of seven sites over three years. PLoS Negl Trop Dis. 2019;13:e0007211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthijnssens J, Bilcke J, Ciarlet M, et al. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4:1303–1316. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Wang YH, Pang BB, et al. Surveillance of human rotavirus in Wuhan, China (2011-2019): predominance of G9P[8] and emergence of G12. Pathogens. 2020;9:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C, Fu J, Ai J, et al. Phylogenetic analysis of human G9P[8] rotavirus strains circulating in Jiangsu, China between 2010 and 2016. J Med Virol. 2018;90:1461–1470. [DOI] [PubMed] [Google Scholar]
- 34.Chan-It W, Chanta C. Emergence of G9P[8] rotaviruses in children with acute gastroenteritis in Thailand, 2015-2016. J Med Virol. 2018;90:477–484. [DOI] [PubMed] [Google Scholar]
- 35.Kirkwood C, Bogdanovic-Sakran N, Palombo E, et al. Genetic and antigenic characterization of rotavirus serotype G9 strains isolated in Australia between 1997 and 2001. J Clin Microbiol. 2003;41:3649–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Liu H, Jia L, et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children: Beijing Municipality and Gansu Province, China. Pediatr Infect Dis J. 2015;34:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ. 2013;346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Justino MC, Araújo EC, van Doorn LJ, et al. Oral live attenuated human rotavirus vaccine (Rotarix™) offers sustained high protection against severe G9P[8] rotavirus gastroenteritis during the first two years of life in Brazilian children. Mem Inst Oswaldo Cruz. 2012;107:846–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.