Abstract
Background:
Deprescribing of antihypertensive medications for older patients with normal blood pressure is recommended by some clinical guidelines, where the potential harms of treatment may outweigh the benefits. This study aimed to assess the cost-effectiveness of this approach.
Methods:
A Markov patient-level simulation was undertaken to model the effect of withdrawing one antihypertensive compared with usual care, over a life-time horizon. Model population characteristics were estimated using data from the OPTiMISE antihypertensive deprescribing trial, and the effects of blood pressure changes on outcomes were derived from the literature. Health-related quality of life was modeled in Quality-Adjusted Life Years (QALYs) and presented as costs per QALY gained.
Results:
In the base-case analysis, medication reduction resulted in lower costs than usual care (mean difference £185), but also lower QALYs (mean difference 0.062) per patient over a life-time horizon. Usual care was cost-effective at £2975 per QALY gained (more costly, but more effective). Medication reduction resulted more heart failure and stroke/TIA events but fewer adverse events. Medication reduction may be the preferred strategy at a willingness-to-pay of £20 000/QALY, where the baseline absolute risk of serious drug-related adverse events was ≥7.7% a year (compared with 1.7% in the base-case).
Conclusions:
Although there was uncertainty around many of the assumptions underpinning this model, these findings suggest that antihypertensive medication reduction should not be attempted in many older patients with controlled systolic blood pressure. For populations at high risk of adverse effects, deprescribing may be beneficial, but a targeted approach would be required in routine practice.
Keywords: aged, blood pressure, cardiovascular diseases, drug-related side effects and adverse reactions, hypertension, primary health care, quality of life
Novelty and Relevance.
What Is New?
This is the first study to examine the cost-effectiveness of antihypertensive medication reduction in older adults.
This analysis found that reducing antihypertensive medication in older adults was cost saving, but resulted in fewer quality adjusted life years gained when compared with usual care.
Medication reduction was found to be the preferred strategy at a willingness-to-pay of £20 000/quality adjusted life years only where the baseline absolute risk of serious drug-related adverse events was high (7.7% a year or greater).
What Is Relevant?
For most older patients with controlled systolic blood pressure, antihypertensive medication reduction was not a cost-effective treatment strategy.
In some specific populations at high risk of adverse events, antihypertensive medication reduction may carry potential benefits, so a targeted approach may be needed if this strategy is to be adopted in routine clinical practice.
Clinical/Pathophysiological Implications?
Despite some uncertainty regarding model inputs, due to a lack of evidence in this older population, these findings suggest that antihypertensive medication reduction should not be attempted in most older patients with controlled systolic blood pressure. Further research is required to understand the risks and benefit of antihypertensive medication reduction in older people at high risk of adverse effects from blood pressure lowering.
Hypertension is the leading risk factor for cardiovascular disease,1 the commonest cause of morbidity and mortality worldwide.2 Antihypertensive treatment has been shown to be very effective at preventing cardiovascular disease (CVD) across many different populations, including those with advancing age.3,4 However, most randomized controlled trials focusing on older people5,6 do not include those patients with significant frailty and multi-morbidity who are prescribed many medications to treat their conditions.7 As a result, clinical guidelines8,9 recommend caution when prescribing antihypertensive treatment in these older adults, due to a lack of evidence on efficacy and concerns about the potential for drug related harm.10
Increasingly, deprescribing of antihypertensive medications is being encouraged in patients with controlled blood pressure,11,12 where the potential harms of treatment10 may outweigh the benefits. It is also seen as a mechanism to reduce polypharmacy, since the most common comorbidity in older people is hypertension13 and most patients will need multiple antihypertensive medications to control their blood pressure.14 Indeed, it has been suggested that deprescribing treatment prescriptions which no longer provide benefit could be cost-saving for health care providers.15 However, there is very little evidence to support the practice of deprescribing antihypertensives.16
The OPTiMISE (Optimising Treatment for Mild Systolic Hypertension in the Elderly) trial sought to address this evidence gap through a randomized, open label, noninferiority trial of antihypertensive deprescribing (withdrawal of one antihypertensive) versus usual care.17 In 569 participants aged 80 years or older, antihypertensive deprescribing was shown to be possible with no difference in the proportion of participants with controlled systolic blood pressure (<150 mm Hg) between groups at 12-week follow-up. There were also no differences in serious adverse events or health-related quality of life, although blood pressure did increase modestly (3/2 mm Hg) in the deprescribing group.17 While this trial suggested that antihypertensive deprescribing was safe in the short-term, the long-term impacts on clinical outcomes remain unknown, as do the cost implications of this strategy if it were to be adopted in routine clinical practice.
The present study aimed to extrapolate results from the OPTiMISE trial to assess the longer-term cost-effectiveness of antihypertensive deprescribing from a National Health Service/Personal Social Services perspective, using a Markov model with individual patient level simulation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
A Markov patient-level simulation was undertaken in TreeAge 2019 (TreeAge Software, Inc, Williamstown, MA) to model the 2 treatment strategies (usual care and withdrawal of one antihypertensive agent). This type of Markov model tracks the costs and consequences of individual patients passing through the model, with characteristics (taken from OPTiMISE patient-level data)17 free to vary between patients. The model was run over a life-time (maximum of 20 years) time horizon to capture all relevant long-term costs and consequences, with a 3 month time cycle.
Patient Level Data Collection
Full details of the OPTiMISE trial have been published elsewhere.17,18 Briefly, this was a randomized controlled trial assessing a strategy of antihypertensive medication reduction (withdrawal of one drug) compared with usual care where no medication changes were mandated. Eligible patients were aged ≥80 years with systolic blood pressure <150 mm Hg and receiving ≥2 antihypertensive medications, whose primary care physician considered them appropriate for medication reduction due to increasing frailty or multi-morbidity.
The primary outcome of the trial was to determine whether a reduction in medication could be achieved with a proportion of participants maintaining clinically safe blood pressure levels (defined as a systolic blood pressure <150 mm Hg) that was noninferior to that achieved by the usual care group, over 12-weeks follow-up. Data were collected on prescribed antihypertensives, quality of life (EQ-5D-5 L), number of cardiovascular comorbidities and all variables required for the calculation of 10-year cardiovascular risk using the QRisk2 algorithm.19
Study Population
Patients in the model had characteristics (age, sex, cardiovascular risk) created by randomly sampling the trial patient-level data by means of a uniform distribution. These characteristics affected their probability of subsequent model events. The model was run with a large number of simulated patients (100 000) to account for interpatient variability and to adequately model a representative clinical population.
Model Comparators and Costs
In keeping with the original trial intervention, patients receiving the medication reduction strategy had a 4-week follow-up safety appointment and treatment was reinstated if systolic blood pressure was found to be above 150 mm Hg for more than one week, adverse events occurred or signs of accelerated hypertension developed. Both strategies included the cost of ongoing primary care consultations (assumed to be an average of 0.8 per 3 months included regardless of whether or not they were related to hypertension management)20 and antihypertensive prescriptions (Table S1). The medication reduction strategy also included the cost of the 4-week safety appointment, and an additional visit if treatment was reinstated. Costs of modelled clinical events (detailed in the Model Structure) including initial acute care costs and long-term care were obtained from previously published work, expert opinion, and standard reference costs (Table S1).21–31 Costs are reported in 2017/2018 prices (reflecting the trial timeframe) and inflated where applicable using the New Health Services Index.32
Model Structure and Assumptions
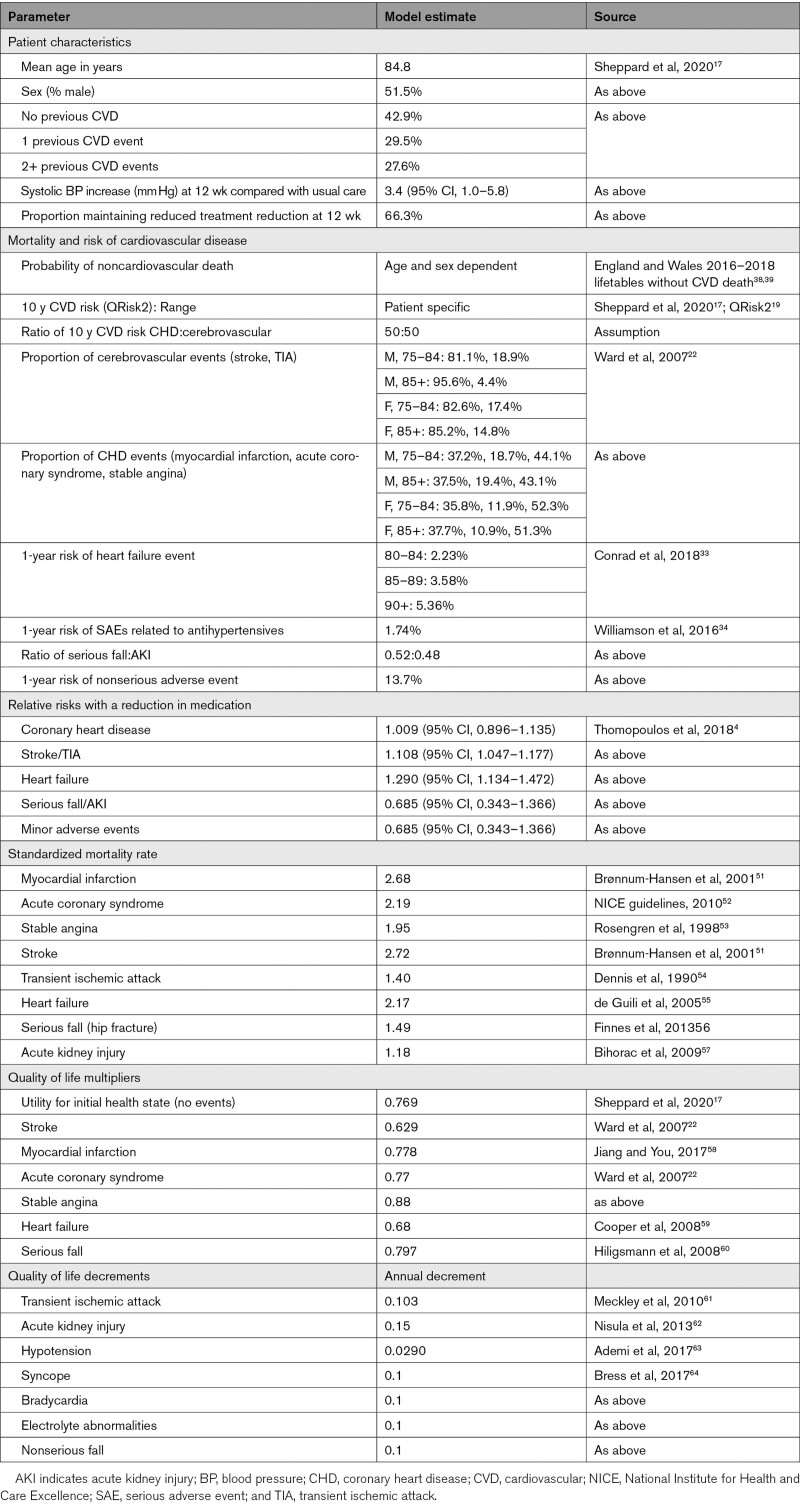
Within each 3-month time cycle, a patient had a risk of suffering a cardiovascular event, an antihypertensive-related serious or minor adverse event, or death (Figure S1). Possible cardiovascular events were coronary heart disease (stable angina, acute coronary syndrome, myocardial infarction), heart failure, stroke, and transient ischemic attack. Antihypertensive-related adverse events were acute kidney injury, hospitalized and nonhospitalized falls, hypotension, syncope, bradycardia, and electrolyte imbalance. Ten-year cardiovascular risk was calculated for each individual patient using the QRisk2 algorithm.19 In the absence of robust published estimates in this older population, an assumption of greater CVD risk was applied to those with CVD conditions by applying a multiplier of 1.5, based on expert clinical opinion. The distribution of coronary heart disease and stroke/transient ischemic attack events was dependent on age and gender,22 and heart failure risk was dependent on age.33 The risk of minor and serious adverse events (serious falls, acute kidney injury) from antihypertensive treatment were obtained from SPRINT (Systolic blood Pressure Intervention Trial) data in those aged 75 and over (Table 1).34
Table 1.
Model Parameters
Patients who suffered a nonfatal cardiovascular event or serious antihypertensive-related adverse event transitioned to a postevent health state with an adjusted mortality risk. Additional clinical events or medication changes were not modeled.
The impact of changes in blood pressure was taken from a meta-analysis of blood pressure lowering trials, focussing on patients aged over 80 (Table 1).4 These were applied as a relative risk, taking into account the mean difference in systolic blood pressure observed in the OPTiMISE trial (3.4 mm Hg higher in the intervention group),17 using log-linear interpolation. In the base-case analysis, it was assumed that the 12 week differences were maintained over the patient life-time. A half-cycle correction was applied to model costs and outcomes. Future costs and outcomes were discounted at an annual rate of 3.5% as recommended by National Institute for Health and Care Excellence.35 All model assumptions are summarized in Table S2.
Model Outcomes
Health-related quality of life outcomes were modelled in Quality-Adjusted Life Years (QALYs), taking into account quality of life and survival. Utility scores for health states are detailed in Table 1. Initial quality of life was estimated as the overall mean EQ-5D-5 L index36 at baseline taken from the OPTiMISE trial,17 calculated using the National Institute for Health and Care Excellence-recommended crosswalk algorithm.37 Utility values for long-term CVD events and serious adverse effects of treatment were applied multiplicatively to baseline utility scores. Disutilities for transient ischemic attack and minor side effects were assumed to last for one month and were subtracted from utility scores for one time cycle. Utility decrements for acute kidney injury were applied every 3 months for life. Gender-specific life tables were used to determine the probability of death at different ages, with adjustment to avoid double counting of circulatory deaths.38,39
Analysis
A cost-utility analysis from an National Health Service/Personal Social Services perspective was undertaken to estimate Incremental Cost-Effectiveness Ratios (ICERs). An ICER was calculated as the difference in costs divided by the difference in QALYs of 2 strategies, with results presented as cost per QALY gained. The cost-effectiveness of an intervention was considered in relation to the lower National Institute for Health and Care Excellence threshold of £20 000 per QALY.40 Probabilistic Sensitivity Analysis was undertaken to assess parameter uncertainty.41 Beta distributions were attached to probabilities and utilities, and gamma distributions were attached to costs. Log normal distributions were used for the relative risks associated with the change in systolic blood pressure from the intervention and mortality. The model was run for 1000 iterations across 100 000 patients and the results are expressed as a Cost-Effectiveness Acceptability Curve.42 Additional analysis was undertaken to estimate the number of disease events in each category per 100 000 patients.
Deterministic Sensitivity Analyses
Analyses to evaluate the impact of changing model assumptions and values were undertaken to assess model robustness.41 Whilst all parameter values were tested, focus was placed on areas of greatest uncertainty (in the underlying data), which could have the largest impact on the study results. The following scenarios were explored:
- Threshold analyses examining:
- The minimum baseline risk of serious adverse events required for usual care to exceed the £20 000/QALY threshold for cost-effectiveness.
- The minimum additional utility required to result in quality of life improvements in those patients reducing medications.
- Sensitivity analyses examining:
- Alternative values for the relative risk of cardiovascular and medication-related adverse events (using the upper and lower 95% CIs [Table 1] or a relative risk of 1).
- The effect of halving the risk of all cardiovascular events.
- Using the lower 95% CI of the increase in systolic blood pressure with the intervention (1 mm Hg).
- The effect of reducing the length of time the difference in blood pressure is sustained (ranging from 1 year to 10 years).
- The effect of reducing the time horizon to 5 years.
Sub-group analyses examining the results by level of frailty43 (fit or frail) and number of CVD comorbidities at baseline (none, 1, 2+).
Results
Cost-Effectiveness of Medication Reduction
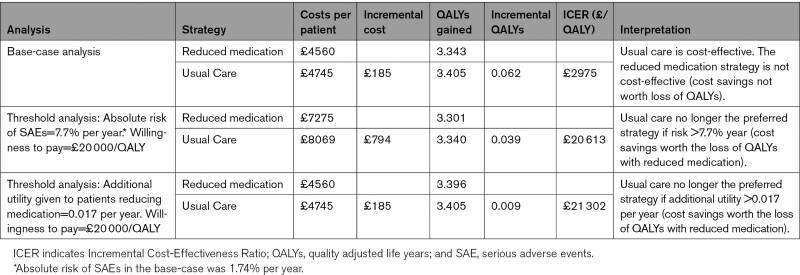
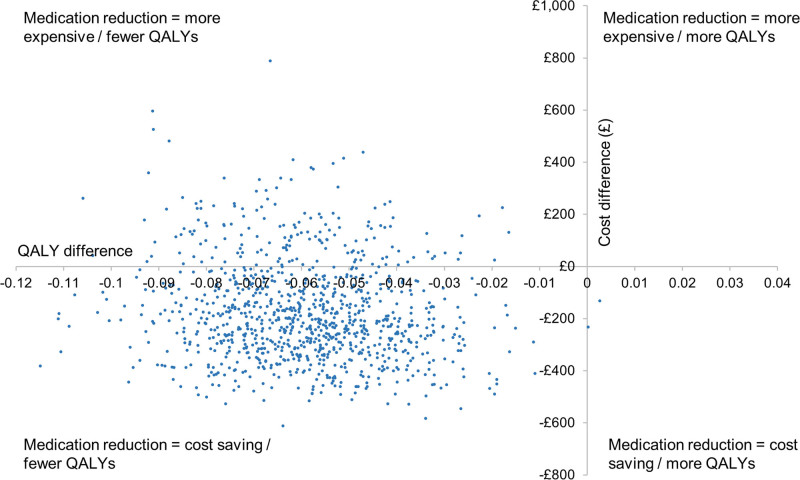
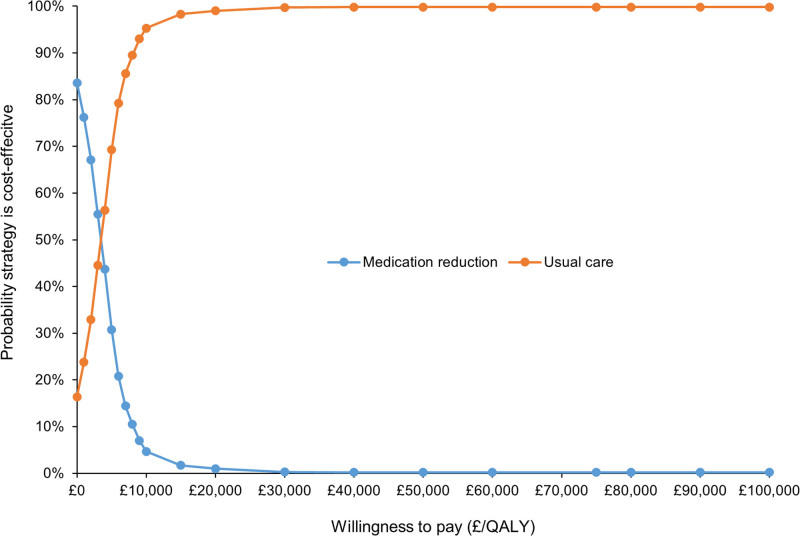
In the base-case analysis, medication reduction resulted in lower costs than usual care (mean difference £185) but also lower QALYs (mean difference 0.062) per patient over a life-time time horizon (Table 2). The ICER for usual care was £2975 per QALY gained (more costly, but more effective), meaning that usual care was highly cost-effective at the £20 000/QALY threshold. The probabilistic sensitivity analyses showed that usual care was the most cost-effective option in 99.0% of iterations at the £20 000/QALY threshold, and 99.7% at £30 000/QALY, with almost all replications of the model in the Western half of the plane (fewer QALYs for medication reduction; Figures 1 and 2).
Table 2.
Results of Base-Case and Threshold Cost-Effectiveness Analyses
Figure 1.
Cost-effectiveness plane for medication reduction vs usual care.
QALY indicates quality adjusted life years.
Figure 2.
Cost-effectiveness acceptability curve for medication reduction vs usual care.
Probability that usual care is cost effective at £20 000/quality adjusted life years (QALY)=99.0%.
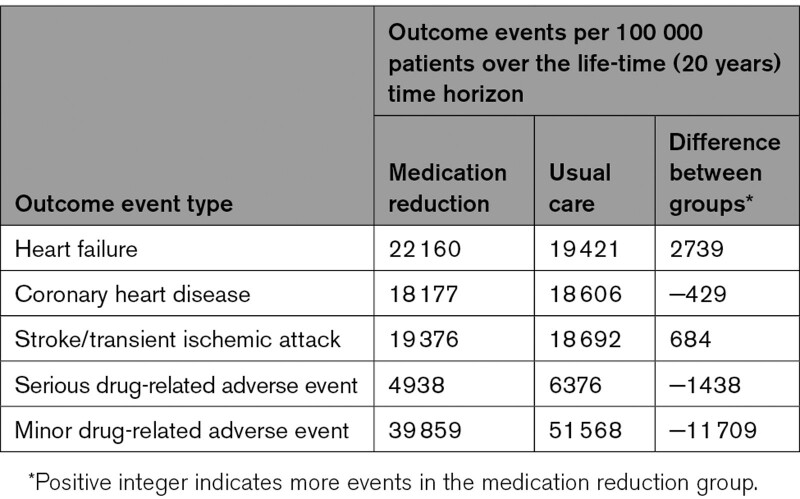
Medication reduction was estimated to result in an increase in the number of heart failure, stroke and transient ischemic attack events, with between 684 and 2739 events occurring per 100 000 population over the life-time (20 year) time horizon (Table 3). However, medication reduction was associated with fewer adverse events and coronary heart disease events (due to competing risks where patients were more likely to die before experiencing a coronary heart disease event; Table 3).
Table 3.
Estimated Incidence of Outcome Events in the Base-Case Analysis Over the Life-Time Time Horizon
Sensitivity Analyses
Using a willingness-to-pay of £20 000/QALY in the threshold analyses, medication reduction may be the preferred strategy (as the ICER for usual care exceeds £20 000/QALY), where the baseline absolute risk of serious drug-related adverse events was >7.7% a year for each individual in the model (compared with the base-case value of 1.7%; Table 2). Additional threshold analyses suggested that patients had to gain >0.017 of utility per year from having their medication reduced (compared with the base-case value of 0) for this intervention to become the preferred strategy (Table 2). Both analyses assume that decision makers are willing to forgo small QALY gains to reduce costs.
Assuming medication reduction conferred no additional risk (relative risk=1) for CVD simultaneously resulted in usual care no longer being cost-effective, with an ICER of £178 631 per QALY (Table S3). Usual care was still cost-effective when applying the upper and lower 95% CIs of the relative risks of cardiovascular events. Applying the same approach for the adverse events did not change the findings of the primary analysis and in some cases usual care became dominant (Table S3).
When the model time horizon was reduced to 5 years, maintaining antihypertensive prescription (usual care) remained cost-effective. The results were also robust when reducing the timeframe of the effect of the intervention (in terms of increased blood pressure) from life-time to 1 year through to 10 years, halving absolute cardiovascular risk, and when using the lower 95% CI of the observed systolic blood pressure change (Table S4). Usual care was also estimated to be cost-effective in subgroup analyses by frailty and number of cardiovascular conditions present at baseline (Table S5). Sensitivity analyses examining the remaining parameter values had no effect on the model findings.
Discussion
Main Findings
The primary finding of this study was that usual care, compared with antihypertensive deprescribing, was more expensive (due to higher medication costs) but results in more QALYs and has an ICER of £2975 per QALY. This indicates that usual care of continuation of antihypertensive drugs is highly cost-effective compared with deprescribing. The lower QALYs associated with the antihypertensive deprescribing strategy occurred due to a projected increase in cardiovascular events (particularly heart failure) caused by a modest sustained increase in systolic blood pressure. Antihypertensive deprescribing was only the preferred strategy when patients were assumed to have a high baseline risk of serious adverse events (eg, were at high risk of falling or experiencing acute kidney injury in the next year).
Many of the model inputs had considerable uncertainty or required assumptions to be made, due to a lack of evidence in this older population. Based on currently available data, these findings suggest that antihypertensive medication reduction should not be attempted in most older patients with controlled systolic blood pressure. In some specific populations at particularly high risk of adverse drug events, antihypertensive deprescribing may carry some benefits so a targeted approach may be needed if deprescribing is to be adopted in routine clinical practice.
Strengths and Weaknesses
The present analyses were informed by robust data from a pragmatic randomized controlled trial comparing antihypertensive deprescribing with usual care in a primary care setting. Participants recruited to this trial were representative of the general population aged 80 years and older registered at practices in primary care.17 This trial was limited to just 12 weeks of follow-up, meaning that the long-term effects of antihypertensive deprescribing had to be modeled on the basis of observed differences in blood pressure. For the base case analysis, such differences were assumed to be sustained over a lifetime which may not reflect experience in routine practice, although sensitivity analyses shortening the period in which a blood pressure difference existed from 1 to 10 years did not affect the primary findings of the analysis. This short period of follow-up in the trial meant that estimates of treatment safety and efficacy had to be taken from previous treatment intensification trials which are likely (and by design of OPTiMISE) to have recruited a different population to that considered for deprescribing.7,10 Estimates of CVD risk (which drove the observed differences in QALYs) were based on the best available cardiovascular risk score (QRisk2), which was not developed or validated for individuals aged 85 years or older.19 Also, while the OPTiMISE trial recruited a population of patients similar to the general older population in primary care,7 based on the sample size of the trial there may be some uncertainty around some of the parameters included in the model such as age and baseline cardiovascular risk. Changing these values in a sensitivity analysis did not alter the primary findings.
Ninety-eight percent of OPTiMISE trial participants were living with multiple long-term conditions which could carry competing risks eclipsing future CVD events. These could not be taken into account in the present analysis due to a lack of evidence. The present model was complex, requiring a number of assumptions related to the risk of CVD, and adverse events for which there is little evidence in this population. This meant it was not possible to add further complexity relating to treatment changes following cardiovascular events, terminal care costs or the impact of recurring events which often occur in real world practice. Such uncertainty, and reliance on data from antihypertensive intensification trials may have favored cost-effectiveness of the usual care strategy.
Findings in the Context of Existing Literature
To our knowledge, this is the first study to examine the cost-effectiveness of antihypertensive deprescribing in older adults aged 80 years and above. Indeed, few studies have examined the cost-effectiveness of deprescribing of any medication classes in routine clinical practice.44,45 Two analyses based on data from the D-PRESCRIBE (Developing Pharmacist-Led Research to Educate and Sensitize Community Residents to the Inappropriate Prescriptions Burden in the Elderly) trial46 examined the cost-effectiveness of NSAIDS44 and sedative deprescribing.45 In contrast to the present analyses, these studies found deprescribing of these medications to be a cost-effective intervention, both in terms of saving money and increasing health related quality of life. Although our analysis found antihypertensive deprescribing to be cost saving too, it is possible that the disutility from adverse events related to NSAID and sedative prescribing is higher than that from antihypertensives, resulting in fewer QALYs gained from stopping antihypertensive treatment. This was supported by sensitivity analyses which suggested that an increasing disutility associated with antihypertensive treatment prescription would have resulted in deprescribing becoming the preferred strategy. However, such a gain was not observed in the original trial over 3 months of follow-up.17 Indeed, there was no significant difference in EQ-5D-5L index between the 2 trial arms and a change of the magnitude modelled in this sensitivity analysis was outside the 95% CI for the observed difference.
Implications for Clinical Practice
Although based on data with some uncertainty, this study suggests that antihypertensive deprescribing may not be cost-effective in older patients aged 80 years and older, and therefore, should not be attempted in patients with controlled systolic blood pressure as a routine policy. This is important for guideline and policy makers, who are increasingly encouraging physicians to think about deprescribing chronic medications where the benefits of treatment no longer outweigh the harms.11,47,48 Sensitivity analyses conducted here were able to identify scenarios where this might occur, notably, in those with a high risk of medication related adverse events. However, it is currently difficult to determine who these patients might be in routine practice. For other treatments, such as anticoagulants, tools exist which can help physicians quantify an individual’s risk of bleeding which may be increased by treatment.49 Similar tools predicting adverse events related to antihypertensive treatment would help target deprescribing at those most likely to benefit, although this requires further research. In the interim, for physicians wishing to reduce antihypertensives prescriptions in older patients under their care, tools such as the electronic frailty index43 or QAdmissions score50 may be considered as a proxy to determine higher risk patients.
Perspectives
The present analysis found that deprescribing of antihypertensive medication in older adults was cost saving but resulted in fewer quality adjusted life years gained when compared with usual care. Although sensitivity analyses suggested that such a strategy may be preferred when targeted at individuals at high risk of adverse events, the lack of robust data regarding the underlying risk in this population, and the long-term effects of deprescribing preclude firm recommendations being drawn. While reducing polypharmacy in the elderly may still be a desirable policy, these data suggest that it may be better to attempt withdrawal of medications that do not reduce major clinical events.
Article Information
Acknowledgments
We acknowledge the OPTiMISE investigators (listed in the Supplemental Material) for their contributions to the original trial and thank the patients who participated in the trial.
Sources of Funding
This work received joint funding from the National Institute for Health Research (NIHR) Oxford Collaboration for Leadership in Applied Health Research and Care (CLAHRC) at Oxford Health NHS Foundation Trust (ref: P2-501) and the NIHR School for Primary Care Research (SPCR; ref 335). J.P. Sheppard now receives funding from the Wellcome Trust/Royal Society via a Sir Henry Dale Fellowship (ref: 211182/Z/18/Z). F.D.R. Hobbs reports personal fees from NOVARTIS and grants from Boehringer Ingelheim and Pfizer outside of the submitted work. R.J. McManus and J. Mant are NIHR Senior Investigators. J. Mant reports personal fees from BMS/Pfizer, outside the submitted work. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. This research was funded in part, by the Wellcome Trust [ref: 211182/Z/18/Z]. For the purpose of open access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CVD
- cardiovascular disease
- D-PRESCRIBE
- Developing Pharmacist-led Research to Educate and Sensitize Community Residents to the Inappropriate Prescriptions Burden in the Elderly
- ICERs
- Incremental Cost-Effectiveness Ratios
- OPTiMISE
- OPtimising Treatment for MIld Systolic hypertension in the Elderly
- QALYs
- Quality-Adjusted Life Years
- SPRINT
- Systolic blood Pressure Intervention Trial
R.J. McManus and J.P. Sheppard are joint senior authors.
A list of all OPTiMISE investigators is given in the Supplemental Material.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.18726.
For Sources of Funding and Disclosures, see page 1129.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 4.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment on cardiovascular outcomes and mortality: 13 - benefits and adverse events in older and younger patients with hypertension: overview, meta-analyses and meta-regression analyses of randomized trials. J Hypertens. 2018;36:1622–1636. doi: 10.1097/HJH.0000000000001787 [DOI] [PubMed] [Google Scholar]
- 5.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, et al. ; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 6.SPRINT Investigators. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheppard JP, Lown M, Burt J, Temple E, Lowe R, Ashby H, Todd O, Allen J, Ford GA, Fraser R, et al. Generalizability of blood pressure lowering trials to older patients: cross-sectional analysis. J Am Geriatr Soc. 2020;68:2508–2515. doi: 10.1111/jgs.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Guideline Centre. National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management [Nice Guideline 136]. Royal College of Physicians (UK); 2019. [Google Scholar]
- 9.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 10.Albasri A, Hattle M, Koshiaris C, Dunnigan A, Paxton B, Fox SE, Smith M, Archer L, Levis B, Payne RA, et al. ; STRATIFY investigators. Association between antihypertensive treatment and adverse events: systematic review and meta-analysis. BMJ. 2021;372:n189. doi: 10.1136/bmj.n189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benetos A, Bulpitt CJ, Petrovic M, Ungar A, Agabiti Rosei E, Cherubini A, Redon J, Grodzicki T, Dominiczak A, Strandberg T, et al. An expert opinion from the european society of hypertension-european union geriatric medicine society working group on the management of hypertension in very old, frail subjects. Hypertension. 2016;67:820–825. doi: 10.1161/HYPERTENSIONAHA.115.07020 [DOI] [PubMed] [Google Scholar]
- 12.Krishnaswami A, Steinman MA, Goyal P, Zullo AR, Anderson TS, Birtcher KK, Goodlin SJ, Maurer MS, Alexander KP, Rich MW, et al. ; Geriatric Cardiology Section Leadership Council, American College of Cardiology. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73:2584–2595. doi: 10.1016/j.jacc.2019.03.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 14.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 15.Kojima G, Bell C, Tamura B, Inaba M, Lubimir K, Blanchette PL, Iwasaki W, Masaki K. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012;13:818.e11–818.e15. doi: 10.1016/j.jamda.2012.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeve E, Jordan V, Thompson W, Sawan M, Todd A, Gammie TM, Hopper I, Hilmer SN, Gnjidic D. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst Rev. 2020;6:CD012572. doi: 10.1002/14651858.CD012572.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard JP, Burt J, Lown M, Temple E, Lowe R, Fraser R, Allen J, Ford GA, Heneghan C, Hobbs FDR, et al. ; OPTIMISE Investigators. Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA. 2020;323:2039–2051. doi: 10.1001/jama.2020.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheppard JP, Burt J, Lown M, Temple E, Benson J, Ford GA, Heneghan C, Hobbs FDR, Jowett S, Little P, et al. OPtimising Treatment for MIld Systolic hypertension in the Elderly (OPTiMISE): protocol for a randomised controlled non-inferiority trial. BMJ Open. 2018;8:e022930. doi: 10.1136/bmjopen-2018-022930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, Brindle P. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482. doi: 10.1136/bmj.39609.449676.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monahan M, Jowett S, Nickless A, Franssen M, Grant S, Greenfield S, Hobbs FDR, Hodgkinson J, Mant J, McManus RJ. Cost-Effectiveness of Telemonitoring and Self-Monitoring of Blood Pressure for Antihypertensive Titration in Primary Care (TASMINH4). Hypertension. 2019;73:1231–1239. doi: 10.1161/HYPERTENSIONAHA.118.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luengo-Fernandez R, Gray AM, Rothwell PM; Oxford Vascular Study. A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke. 2012;43:3343–3351. doi: 10.1161/STROKEAHA.112.667204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2007;11:1–144. doi: 10.3310/hta11400 [DOI] [PubMed] [Google Scholar]
- 23.Joint Formulary Committee. British National Formulary. BMJ Group and Pharmaceutical Press; 2018. [Google Scholar]
- 24.Palmer S Sculpher M Philips Z Robinson M Ginnelly L Bakhai A Packham C Abrams K Cooper N Alfikah K, et al. A cost-effectiveness model comparing alternative management strategies for the use of glycoprotein iib/iiia antagonists in non-st-elevation acute coronary syndrome. Technology Assessment Report. 2002. https://www.nice.org.uk/guidance/ta47/documents/costeffectiveness-model-glycoprotein-antagonists-2 [Google Scholar]
- 25.Taylor M, Scuffham PA, Chaplin S, Papo NL. An economic evaluation of valsartan for post-MI patients in the UK who are not suitable for treatment with ACE inhibitors. Value Health. 2009;12:459–465. doi: 10.1111/j.1524-4733.2008.00494.x [DOI] [PubMed] [Google Scholar]
- 26.Department of Health. National Schedule of Reference Costs - Year 2017-2018. National Health System United Kingdom; 2018. [Google Scholar]
- 27.National Institute for Health and Care Excellence. Management of Stable Angina. National Institute for Clinical Excellence; 2011. [Google Scholar]
- 28.Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U.K. National Health Service perspective. Heart. 2014;100:1031–1036. doi: 10.1136/heartjnl-2013-304598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence. Hip Fracture: Management. Clinical Guideline cg124. National Institute for Clinical Excellence; 2011 [Google Scholar]
- 30.Polinder S, Boyé ND, Mattace-Raso FU, Van der Velde N, Hartholt KA, De Vries OJ, Lips P, Van der Cammen TJ, Patka P, Van Beeck EF, et al. ; IMPROveFALL trial collaborators. Cost-utility of medication withdrawal in older fallers: results from the improving medication prescribing to reduce risk of FALLs (IMPROveFALL) trial. BMC Geriatr. 2016;16:179. doi: 10.1186/s12877-016-0354-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall PS, Mitchell ED, Smith AF, Cairns DA, Messenger M, Hutchinson M, Wright J, Vinall-Collier K, Corps C, Hamilton P, et al. The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation. Health Technol Assess. 2018;22:1–274. doi: 10.3310/hta22320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis L, Burns A. Unit Costs of Health and Social Care. Report number: 10.22024/UniKent/01.02.70995. University of Kent; 2018:201. [Google Scholar]
- 33.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–580. doi: 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. ; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence (NICE). Process and methods guides. Guide to the Methods of Technology Appraisal. National Institute for Health and Care Excellence; 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 [Google Scholar]
- 36.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15:708–715. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 38.Office for National Statistics. National Life Tables, England & Wales, 2016-2018. Office for National Statistics; 2018 [Google Scholar]
- 39.Office for National Statistics. Deaths Registered in England and wales: 2018. Office for National Statistics; 2018 [Google Scholar]
- 40.Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. BMJ. 2007;335:358–359. doi: 10.1136/bmj.39308.560069.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD; ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working Group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348 [DOI] [PubMed] [Google Scholar]
- 42.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787. doi: 10.1002/hec.635 [DOI] [PubMed] [Google Scholar]
- 43.Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, Mohammed MA, Parry J, Marshall T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanyal C, Turner JP, Martin P, Tannenbaum C. Cost-effectiveness of pharmacist-led deprescribing of NSAIDs in community-dwelling older adults. J Am Geriatr Soc. 2020;68:1090–1097. doi: 10.1111/jgs.16388 [DOI] [PubMed] [Google Scholar]
- 45.Turner JP, Sanyal C, Martin P, Tannenbaum C. Economic evaluation of sedative deprescribing in older adults by community pharmacists. J Gerontol A Biol Sci Med Sci. 2021;76:1061–1067. doi: 10.1093/gerona/glaa180 [DOI] [PubMed] [Google Scholar]
- 46.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320:1889–1898. doi: 10.1001/jama.2018.16131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Clinical Guideline Centre. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. NICE Guideline [NG5]. 2015. https://www.nice.org.uk/guidance/ng5 [PubMed] [Google Scholar]
- 48.National Guideline Centre. National institute for health and care excellence. Multimorbidity: Assessment, Prioritisation and Management of Care for People with Commonly Occurring Multimorbidity [Nice Guideline 56]. Royal College of Physicians (UK); 2016. [PubMed] [Google Scholar]
- 49.O’Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, Kowey PR, Mahaffey KW, Chang P, Fonarow GC, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36:3258–3264. doi: 10.1093/eurheartj/ehv476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hippisley-Cox J, Coupland C. Predicting risk of emergency admission to hospital using primary care data: derivation and validation of QAdmissions score. BMJ Open. 2013;3:e003482. doi: 10.1136/bmjopen-2013-003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brønnum-Hansen H, Jørgensen T, Davidsen M, Madsen M, Osler M, Gerdes LU, Schroll M. Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol. 2001;54:1244–1250. doi: 10.1016/s0895-4356(01)00405-x [DOI] [PubMed] [Google Scholar]
- 52.National Institute for Health and Clinical Excellence. Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events: review of nice technology appraisal guidance 90. 2010. [DOI] [PubMed]
- 53.Rosengren A, Wilhelmsen L, Hagman M, Wedel H. Natural history of myocardial infarction and angina pectoris in a general population sample of middle-aged men: a 16-year follow-up of the Primary Prevention Study, Göteborg, Sweden. J Intern Med. 1998;244:495–505. doi: 10.1111/j.1365-2796.1998.00394.x [DOI] [PubMed] [Google Scholar]
- 54.Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21:848–853. doi: 10.1161/01.str.21.6.848 [DOI] [PubMed] [Google Scholar]
- 55.de Giuli F, Khaw KT, Cowie MR, Sutton GC, Ferrari R, Poole-Wilson PA. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696,884 in the United Kingdom. Eur J Heart Fail. 2005;7:295–302. doi: 10.1016/j.ejheart.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 56.Finnes TE, Meyer HE, Falch JA, Medhus AW, Wentzel-Larsen T, Lofthus CM. Secular reduction of excess mortality in hip fracture patients >85 years. BMC Geriatr. 2013;13:25. doi: 10.1186/1471-2318-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b [DOI] [PubMed] [Google Scholar]
- 58.Jiang M, You JH. CYP2C19 LOF and GOF-Guided antiplatelet therapy in patients with acute coronary syndrome: a cost-effectiveness analysis. Cardiovasc Drugs Ther. 2017;31:39–49. doi: 10.1007/s10557-016-6705-y [DOI] [PubMed] [Google Scholar]
- 59.National Clinical Guideline Centre. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. National Institute for Health and Care Excellence (UK); 2008. [PubMed] [Google Scholar]
- 60.Hiligsmann M, Bruyère O, Ethgen O, Gathon HJ, Reginster JY. Lifetime absolute risk of hip and other osteoporotic fracture in Belgian women. Bone. 2008;43:991–994. doi: 10.1016/j.bone.2008.08.119 [DOI] [PubMed] [Google Scholar]
- 61.Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks and costs of warfarin pharmacogenomic testing. Pharmacoeconomics. 2010;28:61–74. doi: 10.2165/11318240-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 62.Nisula S, Vaara ST, Kaukonen KM, Reinikainen M, Koivisto SP, Inkinen O, Poukkanen M, Tiainen P, Pettilä V, Korhonen AM; FINNAKI-QOL Study Group. Six-month survival and quality of life of intensive care patients with acute kidney injury. Crit Care. 2013;17:R250. doi: 10.1186/cc13076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ademi Z, Pfeil AM, Hancock E, Trueman D, Haroun RH, Deschaseaux C, Schwenkglenks M. Cost-effectiveness of sacubitril/valsartan in chronic heart-failure patients with reduced ejection fraction. Swiss Med Wkly. 2017;147:w14533. doi: 10.4414/smw.2017.14533 [DOI] [PubMed] [Google Scholar]
- 64.Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, Berlowitz DR, Conroy MB, Fine L, Oparil S, et al. ; SPRINT Research Group. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377:745–755. doi: 10.1056/NEJMsa1616035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.