Abstract
The COVID-19 pandemic has affected millions of people worldwide. While coronaviruses typically have low rates of neurotropic effects, the massive transmission of SARS-CoV-2 suggests that a substantial population will suffer from potential SARS-CoV-2-related neurological disorders. The rapid and recent emergence of SARS-CoV-2 means little research exists on its potential neurological effects. Here we analyze the effects of similar viruses to provide insight into the potential effects of SARS-CoV-2 on the nervous system and beyond. Seven coronavirus strains (HCoV-OC43, HCoV-HKU1, HCoV-229E, HCoV-NL63, SARS-CoV, MERS-CoV, SARS-CoV-2) can infect humans. Many of these strains cause neurological effects, such as headaches, dizziness, strokes, seizures, and critical illness polyneuropathy/myopathy. Certain studies have also linked coronaviruses with multiple sclerosis and extensive central nervous system injuries. Reviewing these studies provides insight into the anticipated effects for patients with SARS-CoV-2. This review will first describe the effects of other coronaviruses that have caused severe disease (SARS-CoV, MERS-CoV) on the nervous system, as well as their proposed origins, non-neurological effects, and neurological infection mechanisms. It will then discuss what is known about SARS-CoV-2 in these areas with reference to the aforementioned viruses, with the goal of providing a holistic picture of SARS-CoV-2.
Keywords: central nervous system, infection, viruses
Introduction
The SARS-CoV-2 pandemic has affected millions of people globally since it appeared in December 2019 when multiple citizens in Wuhan, China developed pneumonia of unknown origins (WHO 2020a, 2020f). The virus then quickly began spreading throughout China and into other countries, including Thailand, Japan, and South Korea. This spread resulted in Asia being the epicenter of the outbreak (Khachfe et al. 2020, WHO 2020f). Next, it was transmitted to North America and Europe, where it initially reached France and Germany before quickly spreading throughout the other European countries (WHO 2020d, 2020e). It was around this time that The World Health Organization (WHO) declared SARS-CoV-2 a public health emergency on January 30th, 2020 (Rafiq et al. 2020). The WHO termed COVID-19 (the respiratory illness caused by SARS-CoV-2) a pandemic on March 11th, 2020 (Rafiq et al. 2020). It was the first time they had declared a pandemic since 2009s H1N1 outbreak. As of December 27, 2020, there are over 72,000,000 confirmed cases and over 1,700,000 deaths due to SARS-CoV-2 (WHO 2020b).
Viruses can be classified according to their morphology, chemical composition, or mode of replication. Coronaviruses, in the family Coronaviridae, are enveloped, single-stranded, positive-sense RNA viruses with seven strains that can infect humans (Fung et al. 2020; Huang et al. 2020; Zimmermann and Curtis 2020). Their morphology consists of a sphere with spike proteins protruding from their surface that attack the host cells to cause infection, shown in Figure 1 (Abdulamir et al. 2020; Alsaadi and Jones 2019; Rafiq et al., 2020). Four coronavirus strains cause common cold-like symptoms (HCoV-OC43, HCoV-HKU1, HCoV-229E, HCoV-NL63) whereas there are three coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2) that have more serious effects (Fung et al. 2020). Reported symptoms for COVID-19 vary between studies and patients, but the most common clinical symptoms are fever, cough and dyspnea (Francone et al. 2020; Wang et al. 2020). Other symptoms seen in patients include chills, sputum production, coughing blood, muscle aches, headache, diarrhea, sore throat, nasal congestion, vomiting, runny nose, and abdominal pain (Huang et al. 2020; Wang et al. 2020). The most severe complication of COVID-19 is respiratory failure. While many deaths caused by COVID-19 are related to type 1 respiratory failure (impaired gas exchange resulting in inadequate oxygen without the buildup of carbon dioxide), some authors suggest that other variations of respiratory failure bear partial responsibility for the death toll. A study completed in Wuhan, China, found that 12.6% of patients displayed symptoms of severe respiratory disease (n = 214) (Mao et al. 2020). SARS-CoV-2 has a median incubation period of approximately 5.5 days, ranging from 0 to 14 days (Repici et al. 2020). This median incubation period is similar to those of SARS-CoV and MERS-CoV, which are 4 and 5.2 days respectively (Zhu et al. 2020).
Figure 1:
A graphic of the SARS CoV-2 particle.
The outside is covered with the membrane binding spike proteins which give the family their distinctive morphology (Elfiky 2020). This figure is reprinted under the terms of the Creative Commons Attribution license.
Coronaviruses show some unique and puzzling characteristics in comparison to other well-known viruses such as influenza. First, SARS-CoV-2 has a lower rate of infection in children compared to adults – potentially due to a lower rate of diagnosis as children are more likely to be asymptomatic (Huang et al. 2020; Zimmermann and Curtis 2020). This property is unique to coronaviruses since children are typically found to have more severe infections and effects from most respiratory viruses than the adult population (Abdulamir et al. 2020). Additionally, while most common human coronaviruses typically affect the upper respiratory tract (Abdulamir et al. 2020), SARS-CoV-2 differs as it attacks the lower parts of the human respiratory system (bronchioles and alveoli) which results in severe pneumonia in 15–20% of cases (Abdulamir et al. 2020). An increased effect on the lower respiratory system is also seen with SARS-CoV and MERS-CoV (Memish et al. 2014).
Studies have also linked coronaviruses to neurological effects, ranging from mild to serious. The most common mild effects seen are headaches and dizziness (Whittaker et al. 2020). Other more severe, but less common, neural manifestations caused by coronaviruses include stroke, seizure, Guillain–Barré syndrome (GBS) and critical illness polyneuropathy/myopathy (Whittaker et al. 2020). Some studies have linked coronaviruses with the possibility of neural effects such as multiple sclerosis (MS) and extensive central nervous system (CNS) injury (Arbour et al. 2000; Whittaker et al. 2020). As the neurotropism of SARS-CoV-2 is currently being studied, it cannot be said for certain that brain tissue is susceptible to infection and ultimately destruction. Previous research into other coronavirus variants has revealed that they are capable of infecting neural tissues, particularly the brainstem (Li et al. 2020). Some authors suggest that infection of the brainstem results in respiratory failure and can partially explain the disproportionate number of patients classified as having severe infections exhibiting neurological symptoms (Hartung and Aktas 2020; Li et al. 2020; Mannan Baig 2020).
While coronaviruses have low rates of neurotropic effects, the high transmission rate and number of infections caused by the SARS-CoV-2 pandemic means there will be a substantial population that will suffer from SARS-CoV-2-related neurological disorders; as high as 33% of patients hospitalized for COVID-19 reported a loss of smell (Jarrahi et al. 2020). Due to the sudden emergence and spread of SARS-CoV-2, as well as the lack of data associated with its effects on brain tissue, the effects of other similar viruses must be analyzed to understand the potential effects that SARS-CoV-2 could have on the nervous system. The aim of this paper is to provide a holistic review on the neurological effects of similar coronaviruses that have caused substantial disease in the past. This review will first describe the effects of SARS-CoV and MERS-CoV on the nervous system and then examine what is known about the effects of SARS-CoV-2. We also provide information about each virus’ origin, typical non-neurological symptoms, and mechanism of neurological infection to give additional context and highlight their similarities. This information will help researchers and clinicians make inferences regarding treatments applicable to symptoms seen in previous instances, and inform the direction of research to uncover what remains unknown about SARS-CoV-2 and COVID-19.
Previously characterized coronaviruses
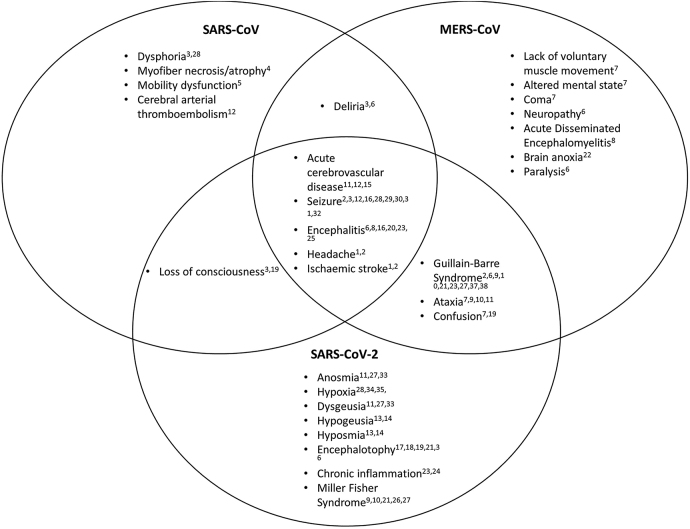
Some coronaviruses, such as HCoV-229E and HCoV-OC43, are typically less dangerous and are associated with the common cold; other coronavirus variants such as SARS-CoV and MERS-CoV typically cause more serious effects. SARS-CoV causes the respiratory illness Severe Acute Respiratory Syndrome (SARS) and MERS-CoV causes Middle Eastern Respiratory Syndrome (MERS). The novel coronavirus SARS-CoV-2 interacts with the cellular receptor angiotensin-converting-enzyme 2 (ACE2) like SARS-CoV, and both typically cause severe respiratory disease in humans (Wu et al. 2020). As such, SARS-CoV presents an important research opportunity for understanding the novel virus causing the current global pandemic. Furthermore, MERS-CoV is a coronavirus that has also caused significant outbreaks and is thus a useful tool to study SARS-CoV-2. Further research into the mechanisms by which SARS-CoV and MERS-CoV enter the nervous system may provide key insights into similar functions in SARS-CoV-2. A high-level overview of the neurological symptoms of each coronavirus discussed in this paper and their overlap can be seen in Figure 2.
Figure 2:
Venn diagram showing intersection of neurological symptoms for SARS-CoV, MERS-CoV, and SARS-CoV-2.
1(Antonio et al. 2003), 2(Whittaker et al. 2020), 3(Xu et al. 2020), 4(Leung et al. 2005), 5(Stainsby et al. 2011), 6(Kim et al. 2017), 7(Zegarra-Valdiria et al. 2020), 8(Arabi et al. 2015), 9(Esposito et al. 2017), 10(Yuki et al. 2012), 11(Mao et al. 2020), 12(Umapathi et al. 2004), 13(Nouchi et al. 2020), 14(Benezit et al. 2020), 15(Goldberg et al. 2020), 16(Saad et al. 2014), 17(Poyiadji et al. 2020), 18(Varatharaj et al. 2020), 19(Chen et al. 2020), 20(Ye et al. 2020), 21(Ellul et al. 2020), 22(Al-Hameed et al. 2017), 23(Wu et al. 2020), 24(Koralnik et al. 2020), 25(Li et al. 2017), 26(Sood et al. 2015), 27(Gutierrez-Ortiz et al. 2020), 28(Lau et al. 2004), 29(Emami et al. 2020), 30(Lu et al. 2020), 31(Anand et al. 2020), 32(Hung et al. 2003), 33(Giacomelli et al. 2020), 34(Nauen et al. 2021), 35(Solomon et al. 2020), 36(Helms et al. 2020), 37(Mannan Baig 2020), 38(Singh et al. 2020).
Severe acute respiratory syndrome (SARS-CoV)
The following subsections discuss SARS-CoV’s origins, non-neurological symptoms, proposed mechanisms of neurological invasion, and neurological manifestations.
Virus origins
In 2005, SARS-CoV found within Chinese horseshoe bats had a high degree of nucleotide similarity to that of the SARS-CoV in infected humans, suggesting that Chinese horseshoe bats could have been the primary mammalian host of SARS-CoV: palm civets are suspected to have been an intermediate host (Lau et al. 2005; Zhu et al. 2020). However, it remains uncertain as to whether another mammalian host was involved before human transmission. Globally, 774 people died from the disease as it spread during the spring of 2003 (Antonio et al. 2003; CDC 2004).
Non-neurological effects
SARS most commonly presents with a fever and non-productive cough, and primarily affects the lungs (Booth et al. 2003; Lau et al. 2004). Most infected patients presented an influenza-like prodrome stage, with symptoms such as fever, myalgias, headache, and diarrhea. Fevers were found to vary from low to high grade (CDC 2003b). After the prodrome stage, the respiratory stage begins and is associated with a dry cough, rigor, shortness of breath, and hypoxemia (Antonio et al. 2003; CDC 2003a). However, non-respiratory symptoms such as diarrhea, impaired liver function, and acute kidney injury suggest that SARS can also affect multiple organ systems, as described in a case report (Lau et al. 2004; Xu et al. 2005).
Proposed mechanisms of neurological infection
In mice transgenic for the human ACE2 receptor, the virus spread through neural tissue primarily through the olfactory bulb when infected intranasally, and then spread transneuronally to other brain regions (Netland et al. 2008). Past research into another coronavirus, mouse hepatitis virus, indicated that ablation of the olfactory bulb prevented viral transmission beyond the lesion site (Perlman et al. 1990). SARS-CoV was found to infect neural cell lines in vitro, specifically cancer cell lines; the human-oligodendroglioma-derived cell line (OL) and a rat-glioma-derived cell line (C6). It was found that both cell lines expressed undetectable levels of ACE2 and further proved the replication of the virus (Yamashita et al. 2005). Furthermore, elevated levels of monokines induced by interferon-γ (Mig) in the blood of diseased patients suggest that monokines may be involved in the immunopathology of SARS in the brain (Xu et al. 2005). This varies from infection in lung tissue, where interferon-inducible protein-10 (IP-10) was highly elevated. Mig and IP-10 both act as chemoattractant factors in the immunopathological process and it is hypothesized that the SARS-CoV infection of brain tissue uses a similar mechanism to that of the elevated IP-10, involved in its infection of lung tissue (Jiang et al. 2005; Xu et al. 2005). The results of these studies can be taken to indicate that SARS-CoV, and likely other coronaviruses, are capable of infecting the CNS through infection of the olfactory bulb and subsequent transmission through other parts of the brain anatomically connected to it.
Neurological effects
While SARS was primarily isolated as a respiratory virus, some patients experienced neurological symptoms such as dysphoria, deliria, tonic-clonic seizures, and loss of consciousness (Lau et al. 2004; Xu et al. 2005). In one such patient, the brain tissue obtained during autopsy was found to be infected by SARS-CoV, as neurocytes and gliocytes stained positive for the viral antigen (Xu et al. 2005). Accordingly, certain neurotropic coronaviruses, including SARS-CoV, can enter through neural cells, and spread to the cortex, basal ganglia, and midbrain, causing neuronal death (Netland et al. 2008; Perlman et al. 1990). Additionally, this phenomenon was found in numerous mouse models (Netland et al. 2008; Perlman et al. 1990).
In one study consisting of 183 children diagnosed with encephalitis, SARS-CoV was detected in 22 of the patients via testing for anti-CoV IgM antibodies (Li et al. 2017). While not definitive proof of the neuroinvasive potential of SARS-CoV and coronaviruses more generally, it certainly provides evidence for the case that these viruses may have associated neurological effects. Additionally, a study found five cases of acute cerebrovascular disease within 206 SARS patients (Umapathi et al. 2004). These patients were described to have low prior risk factors, but suffered from strokes. While the authors acknowledge the element of coincidence and other factors in this outcome, they also hypothesize that hypercoagulability may be associated with SARS due to additional thrombotic complications in other severely ill patients managed in the same center (Umapathi et al. 2004). Though seizure is not described as a common clinical symptom by many, some case studies indicate a concerning link between SARS and seizures. One case in particular described persistent seizures in a SARS patient despite no sign of relevant comorbidities (stroke, intracranial lesions, or cerebral edema). A lumbar puncture performed within a day of their first seizure showed that their cerebrospinal fluid and serum contained SARS-CoV RNA (Hung et al. 2003).
Furthermore, SARS causes significant effects in the peripheral nervous system (PNS), specifically in the neuromusculoskeletal junctions. Over 30% of patients presented with elevated levels of creatine kinase, an indicator of muscle damage or decay (Lee et al. 2003). Post-mortem studies show that even patients whose creatine kinase levels never exceeded the typical range had myofiber necrosis and/or myofiber atrophy (Leung et al. 2005). These acute effects on the musculoskeletal system could limit the ability of researchers to use muscular testing as a metric for long-term nerve dysfunction in SARS patients. Within the scope of this review paper, no studies on the effects in vitro of SARS-CoV on muscle fibers were found; research into this effect might provide key insight into the neuromuscular effects of SARS-CoV.
Middle East respiratory syndrome (MERS-CoV)
Virus origins
The Middle East respiratory syndrome coronavirus (MERS-CoV), of the genus betacoronavirus, was first spread to humans from other mammals (Mohd et al. 2016). The virus was initially isolated in June 2012 in Jeddah, Saudi Arabia, from a patient who presented with acute pneumonia but later succumbed to renal failure and death (Zaki et al. 2012). Since that incident, there have been outbreaks in various regions of Saudi Arabia, bringing the total number of confirmed cases globally to 2519 (fatality rate: 34.3%) by the end of January 2020 (WHO 2020c).
Non-neurological effects
Symptoms of MERS-CoV infection range from mild to severe pneumonia leading to respiratory failure, multiorgan failure, and often death. Previous studies indicated that approximately 98% of patients present with fever, 83% with fever and a cough, and 72% with difficulty breathing (Al-Hameed 2017). Based on the results of a 70-patient study, MERS-CoV mostly affects older adults (median 62 years) with comorbidities (Saad et al. 2014). It mainly affects the lower and upper respiratory tract, where it can progress to acute respiratory distress syndrome (ARDS) in which fluid builds up in the alveoli of the lungs (Algahtani et al. 2016). Treatment often requires patients to be intubated and placed on a ventilator for assistance in breathing (Algahtani et al. 2016; Teman et al. 2015).
Proposed mechanisms of neurological infection
Several paths for MERS-CoV neural infiltration have been theorized based on research done on similar coronaviruses (Dubé et al. 2018; Hosking and Lane 2010). Theories suggest that as MERS-CoV infects vascular and lymphatic endothelial cells, it enters the bloodstream and spreads to the CNS. It crosses the blood-brain barrier and infects glial cells and neurons, causing degeneration and loss of function of neural cells (Zegarra-Valdivia and Chino-Vilca 2020). Another possible mechanism for CNS infection is by retrograde axonal transport from the respiratory organs or enteric nervous system, directly infecting neurons, impairing their maintenance and function, and causing neurodegeneration (Zegarra-Valdivia and Chino-Vilca 2020).
Neurological effects
CNS infections resulting from MERS-CoV have shown neurological manifestations, both severe and facile. Neurological symptoms include ataxia, lack of voluntary muscle movement, and altered consciousness (Zegarra-Valdivia and Chino-Vilca 2020). Neurological issues similar to those seen in COVID-19 patients have also been reported during past MERS outbreaks, including coagulopathy and neuropathy (Algahtani et al. 2016; Kim et al. 2017; Kwong et al. 2020). A case with a 42-year old female showed a complete loss of gray and white matter differentiation of both cerebral hemispheres (Al-Hameed 2017). She was found to have a large frontal hematoma, complete brain anoxia, and lack of intracranial flow. Similarly, some studies have found patients displaying a disturbance of consciousness, paralysis, and GBS (Kim et al. 2017; Wu et al. 2020). GBS, a neurological autoimmune disorder, occurs when the immune system attacks the PNS, resulting in acute flaccid paralysis. GBS was also accompanied by Bickerstaff encephalitis, yet these neurological conditions did not appear until some time after the patients first exhibited symptoms (Kim et al. 2017; Wu et al. 2020). A previous study indicates that 25.7% of the infected population experienced confusion and 8.6% experienced seizures – most likely caused by encephalitis (Saad et al. 2014). Several MRI scans of three MERS infected patients exhibiting neurological symptoms revealed bilateral hyperintense lesions on scans of subcortical areas of the frontal, temporal, and parietal lobes, as well as other areas (Arabi et al. 2015). Specifically, a 57-year old male’s MRI showed signal abnormality in those regions, revealing an acute infarction demonstrated (Arabi et al. 2015). Furthermore, a 45-year-old male had complications of severe shock, acute kidney injury (AKI), and severe acute respiratory distress syndrome (ARDS). The patient subsequently developed encephalitis (Arabi et al. 2015). Within the same study, a 74-year-old male with multiple comorbidities presented with a three-day history of ataxia and confusion. The physical examination revealed dysmetria, decreased motor power on the left side, and his imaging was consistent with acute disseminated encephalomyelitis (ADEM). All of this information taken together appears to indicate the MERS-CoV can manifest with severe neurological symptoms. Knowledge obtained from these studies can be used to inform treatment of the neurological effects brought on by COVID-19, and predict ways in which it may manifest as the pandemic continues. The following diagram summarizes the various neurological effects that SARS-CoV, MERS-CoV, and SARS-CoV-2 can have on humans, as well as their overlap.
SARS-CoV-2
Virus origins
SARS-CoV-2 originated in Wuhan, China in late 2019 and quickly spread to neighboring countries, including Japan, China, Thailand and the Republic of Korea (WHO 2020a, 2020f). The WHO reported that one of the earliest SARS-CoV-2 outbreaks occurred at a seafood market in Wuhan, China (WHO 2020a, 2020f). The most probable origin of the virus itself is from an animal host, though it is unknown if it evolved into its current state in animal hosts prior to human infection, or if it covertly passed to humans hosts before assuming its current form (Andersen et al. 2020).
Non-neurological effects
As mentioned previously, SARS-CoV-2 causes the respiratory illness COVID-19, as well as an abundance of coinciding symptoms. These commonly include fever, coughing, difficulty breathing, myalgia, and fatigue, and less commonly diarrhea, headache, and more (Huang et al. 2020). COVID-19 has been found to target and have the highest mortality rates in older populations (>65). Aside from these symptoms there is also evidence of neurological effects (Mueller et al. 2020).
Proposed mechanisms of neurological infection
The neurotropism of SARS-CoV-2 has been tentatively demonstrated experimentally using brain organoids, mouse models, and human pathology results (Song et al. 2021). Clinical evidence relating to SARS-CoV-2, and experimental data described in previous sections on other coronaviruses, strengthen these findings (Baig et al. 2020; Chen et al. 2020; Mao et al. 2020; Whittaker et al. 2020). There are several mechanisms of neural invasion through the blood brain barrier currently proposed in the literature, similar to those discussed in sections on MERS and SARS-CoV. The ACE2 receptor, present in the cells of multiple organ systems including the lungs, vascular endothelium, kidneys, and intestines, is the main mechanism of infection (Li et al. 2020). First, the virus infects the cells through ACE2 receptors in the nasal mucosa, after which point the virus passes into the olfactory bulb across the cribriform plate (Whittaker et al. 2020). In addition, capillary endothelium ACE2 receptors play a part in neuronal transmission by binding to the virus following invasion of the systemic circulation (Whittaker et al. 2020). Other suggested pathways are hematogenous, and neuronal retrograde transport, potentially through trans-synaptic transmission occurring in the peripheral nerves (Mannan Baig 2020; Sepehrinezhad et al. 2020). Further research is necessary to confirm the mechanisms of invasion, though there are undeniably neurological symptoms associated with SARS-CoV-2, supporting the hypothesis that it is neurotropic.
Neurological effects
Anosmia and dysgeusia, loss or decrease in the sense of taste or smell respectively, are seemingly innocuous symptoms of COVID-19 that have been reported in several studies (Giacomelli et al. 2020; Mao et al. 2020). The percentage of patients suffering from these symptoms is estimated to range from 8.9% up to 33.9% (Giacomelli et al. 2020; Mao et al. 2020). Similar to SARS-CoV and HCoV-NL63, SARS-CoV-2 primarily targets ACE2 receptors, which are reported to exist in high density in epithelial cells of the oral mucosa, among other organ systems (Xu et al. 2020). The proximity of these mucosa to the cribriform plate and thus the olfactory bulb may indicate why neurologic manifestations of the virus can begin with these symptoms, which affect the regional senses (Baig et al. 2020). While these symptoms are seemingly benign, they may be the initial signs of neural infection; from these neural structures, the virus may be capable of spreading. A cross-sectional study was conducted in three adult COVID-19 patient groups, within the La Pitié-Salpêtrière Hospital, Paris, France. It found one-third of its patients were diagnosed with hyposmia and/or hypogeusia, occurring more frequently in non-severe outpatients (Nouchi et al. 2021). Furthermore, in another study on 252 patients positive for SARS-CoV-2, a combination of hypogeusia and hyposmia was found in patients. Even though other respiratory viruses are known to cause post-viral olfactory dysfunction, the sudden onset of hyposmia and hypogeusia seems therefore quite specific to COVID-19 in the current context (Bénézit et al. 2020).
It has been theorized that the introduction of SARS-CoV-2 into the CNS may lead to chronic inflammation due to both the activation of immune cells in the brain, and inflammatory factors discharged by the glial cells (Wu et al. 2020). Chronic inflammation has also been determined to be a result of continuous synthesis of serum IL-6, which was negatively correlated to the number of decreased T-cells among 522 patients infected with SARS-CoV-2 (Koralnik and Tyler 2020). This kind of chronic inflammation can lead to permanent brain damage, as well as multi-organ dysfunction, when the inflammation is pulmonary or systemic in nature (Wu et al. 2020). Further, cerebrovascular disorders such as stroke can be a result of proinflammatory cytokines which can be the cause of a hypercoagulative state or the destruction of endothelial cells (Hartung and Aktas 2020). Acute cerebrovascular disease was noted to occur in 3% of patients with neurological manifestations in a study from Wuhan, China. These 3% of patients were part of the larger group of 36% of patients who experienced neurological symptoms: it is noteworthy that acute cerebrovascular disease was significantly more prevalent in severe COVID-19 cases than in non-severe (6 vs. 1%) (Goldberg et al. 2020; Mao et al. 2020). While the mechanism behind this is not completely understood, authors suggest that COVID-19 may result in a hypercoagulable state due to the presence of ACE2 receptors in the vascular endothelium (Goldberg et al. 2020). A recent study (n = 5) also found megakaryocytes in brain vessels from COVID positive patients not present in the brains of deceased individuals with hypoxic brain injuries, which the authors suspect may be another cerebrovascular factor in the development of neurological symptoms (Nauen et al. 2021). Acute cerebrovascular disease also affected some of those with MERS-CoV and SARS-CoV, as mentioned in previous sections.
As with the coronaviruses mentioned in the previous sections, seizure has been documented as a rare symptom of COVID-19. In a study of 6,147 COVID-19 patients, only five (0.08%) had seizures. In the majority of them, seizures were a presenting symptom (Emami et al. 2020). While infection by SARS-CoV-2 could trigger seizures in patients with a history of epilepsy, some COVID-19 patients presented with seizure-like symptoms suggesting acute stress reaction and hypocalcaemia may be the cause (Anand et al. 2020; Lu et al. 2020).
An early clinical study from Wuhan, China studied 113 patients who died as a direct result of SARS-CoV-2 and 161 who recovered, out of a cohort of 799 patients. The remaining patients in the cohort were still displaying symptoms of the virus. Of those who died, 22% experienced disorders of consciousness, where either awareness or wakefulness is affected, such as confusion, whereas only 1% of those who went on to recover had this symptom (Chen et al. 2020). Altered mental states were also seen in patients with MERS-CoV and SARS-CoV infections as reported in previous sections. It is unclear whether these effects are the result of direct viral infection of neural tissue or a consequence of the other processes occurring in critically ill patients who fail to recover. However, evidence has been published that lends credence to the latter hypothesis (Ye et al. 2020). A male patient testing positive for SARS-CoV-2 presented to a Wuhan hospital with shortness of breath and myalgia, which progressed to confusion and disordered consciousness (Ye et al. 2020). A lumbar puncture that was assessed for presence of the virus was negative despite diagnosis of encephalitis after CT imaging. The authors suspect that the cause of encephalitis was the SARS-CoV-2 immune response leading to significant swelling and edema which affected the patient’s consciousness. As described in previous sections, encephalitis was also reported for some individuals infected with the previous coronaviruses, MERS-CoV and SARS-CoV.
A study conducted included 114 patients with neurological complications of COVID-19, of whom 16 (41%) reported to have encephalopathy (Varatharaj et al. 2020). Furthermore, Chen et al.’s retrospective case series on 113 COVID-19 patients reported that 24 of them developed hypoxic encephalopathy: 23 died, while one recovered (Chen et al. 2020). Other studies have suggested similar onsets of encephalopathy due to SARS-CoV-2 infection (Ellul et al. 2020; Poyiadji et al. 2020). A one-month long study was conducted in March 2020; results state that 69% of patients showed signs of encephalopathy, specifically agitation (n = 58) (Ellul et al. 2020; Helms et al. 2020). In an additional case study, a SARS-CoV-2 positive woman in her late 50s presented with a three-day history of fever, cough, and altered consciousness (Poyiadji et al. 2020). While a lumbar puncture was performed, it was not analyzed for the presence of SARS-CoV-2. Imaging revealed acute necrotizing hemorrhagic encephalopathy, which the authors suspect is not due to direct viral invasion, but instead to intercranial cytokine storms or other immune responses.
As of writing this review, there is limited evidence that SARS-CoV-2 infection can lead to ataxia, as in a sample size of 214 patients in one study, only a single individual expressed this symptom (Mao et al. 2020). A case study of an individual who presented with gait ataxia and a concomitant COVID-19 infection, where the gait ataxia spontaneously resolved after a period of 18 days, has been reported, though a causal link has yet to be established (Abdelnour et al. 2020).
A clinical study completed in a two-week span in April 2020 studied the autopsy reports of 18 deceased patients who had contracted SARS-CoV-2. The study concluded that evidence of acute hypoxic injury was found in both the cerebrum and cerebellum of all patients in the study. Neuronal degeneration was observed in the cerebral cortex, hippocampus, and cerebellar purkinje cell layer. Although hypoxic injury was prevalent, the virus was detected at low levels in regions of the brain in only five of the patients; the remaining patients did not exhibit neuroinvasion of SARS-CoV-2 in the tested regions of the brain (Solomon et al. 2020). The authors do not conclude any direct link between the presence of the virus in the brain and the brain damage present. Additional research also detected viral presence at low levels in the brain tissue of eight of the 22 patient cadavers studied (Puelles et al. 2020). This lack of correlation between viral presence in brain tissue and severity of neurological symptoms has been demonstrated in an early study, which discovered that neuropathology findings were relatively consistent across the studied patient population (n = 23), and independent of the severity of COVID symptoms (Matschke et al. 2020).
Since the spread of SARS-CoV-2 began, there have been an increased number of cases of GBS with a possible connection to the virus recorded by various studies conducted in 2020 (Ellul et al. 2020; Mannan Baig 2020; Singh et al. 2020; Whittaker et al. 2020). A study published in July 2020 stated that 19 cases of GBS and its variants to date have been reported (Ellul et al. 2020). Most patients recover from GBS, but the potential exists for chronic effects (Yuki and Hartung 2012). As the cause of GBS is unknown, it remains difficult to speculate on the pathogenic mechanism that contributes to the manifestation of GBS in COVID-19 patients. Potentially due to the autoimmune nature of the disorder, GBS is known to manifest in patients that have suffered respiratory or gastrointestinal infections which have caused the immune system to target the PNS (Esposito and Longo 2017; Yuki and Hartung 2012). As mentioned in a previous section, GBS cases have been associated with MERS-CoV as well.
A variant of GBS – Miller–Fisher Syndrome – is characterized by ophthalmoplegia, ataxia, and areflexia, and is found to strongly affect only the cranial nerves (Esposito and Longo 2017; Yuki and Hartung 2012). In association with SARS-CoV-2, there have been two recorded cases of the Miller–Fisher Syndrome to date, as well as one case of another variant of GBS, polyneuritis cranialis (Ellul et al. 2020; Gutiérrez-Ortiz et al. 2020; Sood et al. 2015). The onset of these syndromes may be attributed to an aberrant immune response caused by SARS-CoV-2, which may involve damage caused by high levels of proinflammatory cytokines in plasma (Sood et al. 2015). Of the two recorded cases of Miller–Fisher Syndrome, one patient recovered from neurological deficits entirely, whereas the other had ongoing anosmia and ageusia (Gutiérrez-Ortiz et al. 2020). As with GBS, it remains unclear whether there exists a connection between other types of dysimmune neuropathies and SARS-CoV-2 (Hartung and Aktas 2020).
Multiple neurodegenerative disorders have been discussed in connection to SARS-CoV-2 entering the CNS. Loss of neurons, likely attributable to neuronal degeneration and hypoxic injury previously discussed, were observed in all patients of a pathology study conducted on deceased patients who had contracted SARS-CoV-2 (n = 18) (Solomon et al. 2020). A study using 47 patients exhibiting varying severities of SARS-CoV-2 infections measured the neurofilament light chain protein and flail fibrillary acidic protein by single molecule array and found evidence of astrocytic activation and injury in patients exhibiting moderate and severe cases of SARS-CoV-2. Neuronal injury was found in only the most severe cases of the virus (Kanberg et al. 2020). Coronaviruses other than SARS-CoV, MERS-CoV, and SARS-CoV-2 have suspected neuroinvasive behavior as well: neuronal degeneration, as well as decreased locomotor activity, was found in mice infected with HCoV-OC43. A post-mortem study on human brain tissue concluded that HCoV-OC43 was found more prominently in the brain tissue and cerebrospinal fluid of MS patients (De Felice et al. 2020; Sepehrinezhad et al. 2020). Similar to SARS-CoV-2, HCoV-OC43 is classified as a betacoronavirus (Lau et al. 2011; Singh et al. 2020). Furthermore, HCoV-OC43 was found in the cerebrospinal fluid of patients with Parkinson’s disease (De Felice et al. 2020). More research must be done before we are able to draw conclusive findings on the connections between SARS-CoV-2 and HCoV-OC43 and whether similar neurodegenerative occurrences will be observed in patients infected with SARS-CoV-2.
Conclusion
Overall, the current literature reflects the potential for SARS-CoV-2 to have acute and long-term effects on the nervous system. SARS-CoV and MERS-CoV can be used as models to determine the neurological effects of SARS-CoV-2 due to their similarities to this novel virus, as demonstrated in this review. Additional case studies and research on the neurological effects of SARS-CoV-2 are needed in order confirm which acute and chronic neurological effects are directly related to SARS-CoV-2. A greater understanding of the effects of the virus on the nervous system, developed by research on previous coronavirus outbreaks and the current pandemic, may help inform treatment of those suffering from neurological symptoms associated with COVID-19, and provide direction for the research necessary to comprehend what lies ahead.
Acknowledgments
We would like to acknowledge Daniel Wallis for his critical reading of this manuscript.
Footnotes
Author contributions: AK, CS, CD, KA, KT, RK, IF, LA contributed to the writing of the initial draft. IF, LA, EA, SMW, CD, RK, AK, CS edited and formatted the draft for submission.
Research funding: This work was funded through the support of TECHNATION and Innovate BC student funding programs. Dr. Willerth receives support from the Canada Research Chairs program and the NSERC Discovery Grant Program.
Conflict of interest statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdelnour L., Eltahir Abdalla M., Babiker S. COVID 19 infection presenting as motor peripheral neuropathy. J. Formos. Med. Assoc. 2020;119:1119–1120. doi: 10.1016/j.jfma.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulamir A.S., Hafidh R., Hafidh R.R. Phytochemicals anticancer molecular mechanism view project the possible immunological pathways for the variable immunopathogenesis of COVID-19 infections among healthy adults, elderly and children. Electron. J. Gen. Med. 2020;2020:2516–3507. [Google Scholar]
- Al-Hameed F.M. Spontaneous intracranial hemorrhage in a patient with Middle East respiratory syndrome corona virus. Saudi Med. J. 2017;38:196–200. doi: 10.15537/smj.2017.2.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algahtani H., Subahi A., Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep. Neurol. Med. 2016:1–6. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P., Al-Faraj A., Sader E., Dashkoff J., Abdennadher M., Murugesan R., Cervantes-Arslanian A.M., Daneshmand A. Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 2020;112:1–5. doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio G.E., Wong K.T., Hui D.S.C., Lee N., Yuen E.H.Y., Wu A., Chung S.S.C., Sung J.J.Y., Ahuja A.T. Imaging of severe acute respiratory syndrome in Hong Kong. Am. J. Roentgenol. 2003;181:11–17. doi: 10.2214/ajr.181.1.1810011. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., Saeed B.T., Wahbi A., Saedy A., AlDabbagh T., et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74:8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Bénézit F., Turnier P. Le, Declerck C., Paillé C., Revest M., Dubée V., Tattevin P., Arvieux C., Baldeyrou M., Chapplain J.M., et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020;20:1014–1015. doi: 10.1016/s1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J. Am. Med. Assoc. 2003;289:2801–2809. doi: 10.1001/jama.289.21.joc30885. [DOI] [PubMed] [Google Scholar]
- CDC . Preliminary clinical description of severe acute respiratory syndrome . Morbidity and Mortality Weekly; 2003a. [PubMed] [Google Scholar]
- CDC . Preliminary clinical description of severe acute respiratory syndrome . Morbidity and Mortality Weekly; 2003b. [PubMed] [Google Scholar]
- CDC . Frequently asked questions about SARS . 2004. [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M., Coupanec A. Le, Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92:1–21. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. SARS-CoV-2 spike-heat shock protein A5 (GRP78) recognition may be related to the immersed human coronaviruses. Front. Pharmacol. 2020;11:42–44. doi: 10.3389/fphar.2020.577467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/s1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A., Fadakar N., Akbari A., Lotfi M., Farazdaghi M., Javanmardi F., Rezaei T., Asadi-Pooya A.A. Seizure in patients with COVID-19. Neurol. Sci. 2020;41:3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S., Longo M.R. Guillain–Barré syndrome. Autoimmun. Rev. 2017;16:96–101. doi: 10.1016/j.autrev.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Felice F.G. De, Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L., Panebianco V., Andreoli C., Colaiacomo M.C., Zingaropoli M.A., et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur. Radiol. 2020;30:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S.-Y., Yuen K.-S., Ye Z.-W., Chan C.-P., Jin D.-Y.A. Tug-of-War between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M.F., Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular disease in COVID-19. Am. J. Neuroradiol. 2020;41:1170–1172. doi: 10.3174/ajnr.a6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., Aragón-Gómez F.de, Benito-León J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5. doi: 10.1212/wnl.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Hartung H.P., Aktas O. COVID-19 and management of neuroimmunological disorders. Nat. Rev. Neurol. 2020;16:347–348. doi: 10.1038/s41582-020-0368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/nejmc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking M.P., Lane T.E. The pathogenesis of murine coronavirus infection of the central nervous system. Crit. Rev. Immunol. 2010;30:119–130. doi: 10.1615/critrevimmunol.v30.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung E.C.W., Chim S.S.C., Chan P.K.S., Tong Y.K., Ng E.K.O., Chiu R.W.K., Leung C.-B., Sung J.J.Y., Tam J.S., Lo Y.M.D. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003;49:2107–2108. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Alsaadi E.A., Jones I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019;14:275–286. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrahi A., Ahluwalia M., Khodadadi H., Silva Lopes Salles E. Da, Kolhe R., Hess D.C., Vale F., Kumar M., Baban B., Vaibhav K., et al. Neurological consequences of COVID-19: what have we learned and where do we go from here? J. Neuroinflammation. 2020;17 doi: 10.1186/s12974-020-01957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857oc. [DOI] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–59. doi: 10.1212/wnl.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Khachfe H.H., Chahrour M., Sammouri J., Salhab H.A., Makki B.E., Fares M.Y. An epidemiological study on COVID-19: a rapidly spreading disease. Cureus. 2020;12:e7313. doi: 10.7759/cureus.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I.J., Tyler K.L. COVID‐19: a global threat to the nervous system. Ann. Neurol. 2020;88:1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K., Yu W., Chu C., Lau S., Sheng B. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10:2–4. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Lee P., Tsang A.K.L., Yip C.C.Y., Tse H., Lee R.A., So L.-Y., Lau Y.-L., Chan K.-H., Woo P.C.Y., et al. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J. Virol. 2011;85:11325–11337. doi: 10.1128/jvi.05512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Woo P.C.Y., Li K.S.M., Huang Y., Tsoi H.W., Wong B.H.L., Wong S.S.Y., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Sc B., Leung C.B., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/nejmoa030685. [DOI] [PubMed] [Google Scholar]
- Leung T.W., Wong K.S., Hui A.C., To K.F., Lai S.T., Ng W.F., Ng H.K. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch. Neurol. 2005;62:1113–1117. doi: 10.1001/archneur.62.7.1113. [DOI] [PubMed] [Google Scholar]
- Li Y.‐C., Bai W., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li H., Fan R., Wen B., Zhang J., Cao X., Wang C., Song Z., Li S., Li X., et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2017;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., Mu J., Guo J., Li W., Wang G., et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61:e49–53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan Baig A. Updates on what ACS reported: emerging evidences of COVID-19 with nervous system involvement. ACS Chem. Neurosci. 2020;11:1204–1205. doi: 10.1021/acschemneuro.0c00181. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/s1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Al-Tawfiq J.A., Makhdoom H.Q., Assiri A., Alhakeem R.F., Albarrak A., Alsubaie S., Al-Rabeeah A.A., Hajomar W.H., Hussain R., et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J. Infect. Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016;13 doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A.L., Mcnamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people ? Aging (Albany, NY) 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen D.W., Hooper J.E., Stewart C.M., Solomon I.H. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78:760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/jvi.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Kee Kwong K.C., Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: a neurological perspective. J. Clin. Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi A., Chastang J., Miyara M., Lejeune J., Soares A., Ibanez G., Saadoun D., Morélot-Panzini C., Similowski T., Amoura Z., et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:691–697. doi: 10.1007/s10096-020-04056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Evans G., Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J. Exp. Med. 1990;172:1127–1132. doi: 10.1084/jem.172.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/nejmc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq D., Batool A., Bazaz M.A. Three months of COVID-19: a systematic review and meta-analysis. Rev. Med. Virol. 2020;30:e2113. doi: 10.1002/rmv.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repici A., Maselli R., Colombo M., Gabbiadini R., Spadaccini M., Anderloni A., Carrara S., Fugazza A., Leo M. Di, Galtieri P.A., et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest. Endosc. 2020;92:192–197. doi: 10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., Selim M.A.A., Mutairi M. Al, Nakhli D. Al, Aidaroos A.Y. Al, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrinezhad A., Shahbazi A., Sajad, Negah S. COVID-19 virus may have neuroinvasive potential and cause neurological complications: a perspective review. J. Neurovirol. 2020;26:324–329. doi: 10.1007/s13365-020-00851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Bhushan B., Maurya A., Mishra G., Santosh, Singh K., Awasthi R., Pradesh U., Noida U., Pradesh I. Novel coronavirus disease 2019 (COVID-19) and neurodegenerative disorders. Dermatol. Ther. 2020;33:e13591. doi: 10.1111/dth.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383:989–992. doi: 10.1056/nejmc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O. El, Liu F., Dai Y., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood I., Kasundra G., Bhargava A., Bhushan B., Shubhakaran K. Polyneuritis cranialis with generalized hyperreflexia as a presenting manifestation of thyrotoxicosis. Ann. Indian Acad. Neurol. 2015;18:240. doi: 10.4103/0972-2327.150625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teman N.R., Thomas J., Bryner B.S., Haas C.F., Haft J.W., Park P.K., Lowell M.J., Napolitano L.M. Inhaled nitric oxide to improve oxygenation for safe critical care transport of adults with severe hypoxemia. Am. J. Crit. Care. 2015;24:110–117. doi: 10.4037/ajcc2015570. [DOI] [PubMed] [Google Scholar]
- Umapathi T., Kor A.C., Venketasubramanian N., Lim C.C.T., Pang B.C., Yeo T.T., Lee C.C., Lim P.L., Ponnudurai K., Chuah K.L., et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J. Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7 doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R., Ge L., Wang F., Chen Q., Zhang Y., et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin. Microbiol. Infect. 2020;6:1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A., Anson M., Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol. Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus disease (COVID-19) situation report 141 2020a [Google Scholar]
- WHO COVID-19 weekly epidemiological update (dec 27 2020) 2020b [Google Scholar]
- WHO MERS situation update 2020c [Google Scholar]
- WHO Novel coronavirus (2019-NCoV) situation report – 7 2020d [Google Scholar]
- WHO Novel coronavirus (2019-NCoV) situation report – 9 2020e [Google Scholar]
- WHO Novel coronavirus (2019-NCoV) situtation report 1 2020f [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;3:1–8. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-NCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Yamate M., Li G.M., Ikuta K. Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus. Biochem. Biophys. Res. Commun. 2005;334:79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki N., Hartung H.P. Guillain-Barré syndrome. N. Engl. J. Med. 2012;366:2294–2304. doi: 10.1056/nejmra1114525. [DOI] [PubMed] [Google Scholar]
- Zaki A.M., Boheemen S.van, Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/nejmoa1211721. [DOI] [PubMed] [Google Scholar]
- Zegarra-Valdivia J.A., Chino-Vilca B.N. Neurological component in coronaviruses induced disease: review of the literature related to SARS-CoV, MERS-CoV, and SARS-CoV-2. Neurol. Res. Int. 2020;2020:2020. doi: 10.1155/2020/6587875. [DOI] [Google Scholar]
- Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020;39:355–368. doi: 10.1097/inf.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]