Until 2016, worldwide obesity has nearly tripled since 1975, 39% of adults were overweight and 13% were obese.[1] The increasing prevalence of obesity means that associated metabolic comorbidities and complications pose a serious health and economic burden to societies around the world. In genomics research, numerous genome-wide association studies (GWAS) have discovered a large number of gene loci[2] linked to obesity. The single-nucleotide polymorphisms (SNPs) in particular significantly affect the occurrence and development of obesity and related metabolic diseases. However, the results from recent studies on obesity-related SNPs have varied considerably, even with similar participants and interventions.[3] These inconsistencies may be the result of gene– gene and gene–environment interactions, including the regulation of gene expression and epigenetic modification.
Dietary intervention is one of the most common strategies for traditional medical nutritional weight loss among obese patients. It contributes to changes in the composition of intestinal flora,[4] which is also an important environmental factor affecting the epigenetic regulation of genes. It is therefore important to understand how to integrate the influence of genes and environmental factors to predict the risk of obesity development, and thus choose the most effective dietary intervention strategies. One possible approach is the application of precision nutrition in the field of medical weight loss.
The concept of precision nutrition was first proposed during the 9th Congress of the International Society of Nutrigenetics/ Nutrigenomics.[5] It was defined as a medical model integrating phenotype, genotype, and social, environmental, and other factors to provide individualized nutrition interventions. In the field of medical weight loss,[6] precision nutrition is interpreted as formulating a customized medical weight management program based on genotype or phenotype, adjusting the total daily energy intake or consumption following individual metabolic or anthropometric responses. Compared with the previous weight loss programs solely based on the basic metabolism of the individual, the application of genomics, metabolomics, and other technologies makes the formulation of the program more personalized and precise.
Numerous GWAS studies have identified SNPs related to dietary intervention.[7] Participants who carry these alleles tend to lose more weight on specific diets. These SNPs are mainly located in the key regulatory genes involved in transportation and utilization of glucose or lipids. For example, participants carrying the GG genotype (wild-type) population of the gene encoding adipocytokine resistin (RETN) rs1862513[8,9] and adiponectin (ADIPOQ) rs1501299[10,11] appear to benefit the most from low-fat and high-monounsaturated fat diets. Meanwhile, high-protein and low-carbohydrate diets may be the optimal dietary choice for people with wild-type variants in β-3-adrenergic receptor (β3-AR) gene rs4994[12] and uncoupling protein 3 (UCP3) gene rs1800849,[13] because the proteins encoded by these genes are important in the development of insulin resistance.
However, dietary interventions for obesity have shown significant interactions with genetic effects. These interactions include epigenetic modifications induced by foods with antioxidant properties[14] and direct regulation of gene expression by the metabolites of intestinal flora.[15] In one study, the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial, the researchers provided carriers of the minor alleles of PCSK7 gene rs236918[16] and FGF21 gene rs838147[17] with a high-carbohydrate diet. They found significant reduction in waist circumference, total body fat mass, insulin, and HOMA-IR levels, which was contrary to the consensus prediction on the metabolic benefits of low-carbohydrate diets for weight loss. These unusual changes may be because gene expression is affected by carbohydrates with a low glycemic index. Wang et al.[15] presented a prediction model including genes (FFAR2, FFAR3, ANGPTL4, CD36, SLC16A1, SLC16A3, SLC16A4, SLC5A8, and TLR4) that played an important role in the transportation and recognition of metabolites of intestinal flora. The calculated weighted genetic risk score (wGRS) was significantly related to the variation in body mass index. These results solidified the knowledge that interactions between host genes and gut microbiota influenced predisposition to obesity.
Participants with the same SNP genotype may show different intervention outcomes, sometimes because the studies are conducted in different districts. These discrepancies are also one of the foci of precision nutrition. Two clinical trials involving the same intervention of a low-fat diet for participants with the same genotype in melatonin receptor 1B (MTNR1B) rs10830963 showed opposite results for anthropometric and metabolic responses, one in Valladolid, Spain,[18] and the other in Boston, Massachusetts, and Baton Rouge, Louisiana.[19] To minimize the confounding effect of race specificity, Dastani et al.[20] used the MANTRA (meta-analysis of trans-ethnic association studies) software to analyze GWAS data from different races. This meta-analysis of multiple GWAS gene loci can control the impact of study duration, race, and other environmental factors on genetic polymorphisms using heterogeneity analysis and help us explore the effect of gene–environment interactions.
Calculation of the genetic risk score (GRS)[21] is a polygenic approach that can evaluate the cumulative effect of high-risk genes at multiple SNP loci, and thereby help to predict the risk of diseases. The GRS calculated by Itziar[22] on the single-nucleotide variants derived from 25 obesity-related genes (including BDNF, CADM2, and FANCL) showed the predictive effect on the weight loss trajectory following intervention. Analysis of the interaction between diet and the GRS for BMI-related SNPs[23] can help to evaluate the effect of dietary interventions. Integrating genetic and environmental factors could minimize the interference of gene–gene and gene–environment interactions. The use of a wGRS[24] integrates even more genetic, phenotype, and environmental information to predict the effect of different dietary regimens on weight loss. This is therefore helpful in the development of personalized weight management programs.
The most common models used in the analysis of gene– environment interactions are general linear models[25] and linear mixed models.[26] However, the introduction of machine learning models can compensate for researchers’ lack of understanding of nonlinear high-dimensional interactions. This, in turn, allows more gene–environmental factors to be included in the predictive model for obesity and metabolic diseases. For example, the support vector machine algorithm used to solve the multiclass problem was used in the screening of obesity-related SNPs and effective attribution in the metabolic syndrome prediction model.[27] The addition of the random forest algorithm[28] can effectively avoid the over-fitting of the model and improve the accuracy and stability of predictions. Eun-Kyung[29] constructed a prediction model for metabolic syndrome with five kinds of machine learning methods, including naïve Bayes, random forest, support vector machine, multilayer perceptron, and decision trees. This model comprehensively considered the effect of clinical features (for example, age, gender, body mass index, smoking, drinking, and exercise) and genetic information (10 SNPs), providing an innovative and comprehensive way to analyze the impact of gene–environment interactions in medical weight loss (Figure 1).
Figure 1.
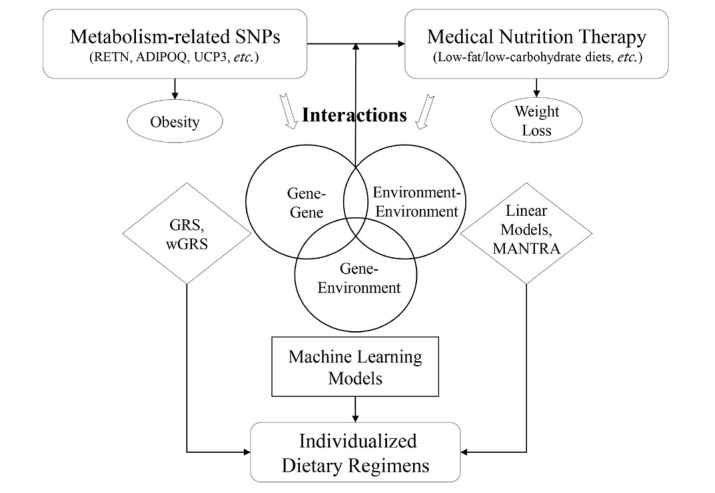
The relationship between SNPs and medical nutrition weight loss. SNPs: single-nucleotide polymorphisms; RETN: gene encoding adipocytokine resistin, rs1862513; ADIPOQ: gene encoding adiponectin, rs1501299; UCP3: gene encoding uncoupling protein 3, rs1800849; GRS: genetic risk score; wGRS: weighted genetic risk score; MANTRA: meta-analysis of trans-ethnic association studies.
To minimize the effect of gene–environment interactions, our group established a knowledge map of medical weight loss with deep-learning models. The issues, approaches, and recommendations in the knowledge map were based on the evidence-based guidelines on nutritional management of overweight/obesity, as well as the real-world evidence obtained from the 8000 patients’ database established by us. To build up this machine learning models, we extracted the characteristics and clinical indicators of various genotypes and metabolic types for evaluation and classification. With this knowledge map, we are committed to creating an intelligent weight management system that applies medical nutritional weight loss programs. This system can formulate precise nutritional weight loss regimens based on personalized data and provide users with artificial intelligence-assisted follow-up tailored to their needs. With implementation and application of such deep-learning technology into weight loss strategies, the majority of obese patients will be provided an efficient and convenient method for whole-process weight management based on individualized characteristics. In addition, the artificial intelligence-assisted follow-up platform can also reap the benefits in improving patients’ adherence to health behavior change, achieving weight loss maintenance, and implementing an efficient conduct of group-based weight management programs.
In summary, GWAS have found many SNP loci that are significantly correlated with variation in response to dietary interventions for weight loss. This information can be used to guide the formulation of personalized weight management programs. However, the relationships between SNP variants and the changes in metabolism and body weight after weight loss interventions are also affected by gene–gene and gene–environment interactions. These complex multifactor effects mean that when SNPs are used to indicate individualized dietary interventions, the actual responses might be affected by diet composition, metabolites of gut flora, race, and other factors. The use of state-of-the-art technologies can integrate genetic and environmental factors in a more effective and accurate way, including GWAS in-depth analysis methods such as MANTRA studies, GRS calculation, and machine learning models such as support vector machines and random forest algorithms. These technologies and strategies for precision nutrition therefore provide a promising future for medical weight loss. They may potentially enable responses to different weight loss regimens to be predicted accurately on an individual basis and individualized dietary intervention to be achieved.
Footnotes
Source of Funding
This work was supported by the Grants from Beijing Municipal Science & Technology Commission (No. Z191100008619006).
Conflict of Interest
None declared.
References
- 1.Fact sheet: obesity and overweight. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. World Health Organization. Available at. Accessed August 14, 2021.
- 2.Bray MS, Loos RJF, McCaffery JM, Ling C, Franks PW, Weinstock GM. NIH working group report—using genomic information to guide weight management: From universal to precision treatment. Obesity. 2016;24:14–22. doi: 10.1002/oby.21381. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang L, Wu H, Pan A, Patel B, Xiang G, Qi L. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103:1162–70. doi: 10.3945/ajcn.115.123448. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim MY, You HJ, Yoon HS, Kwon B, Lee JY, Lee S. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. 2017;66:1031–8. doi: 10.1136/gutjnl-2015-311326. et al. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson LR, De Caterina R, Görman U, Allayee H, Kohlmeier M, Prasad C, et al. Guide and Position of the International Society of Nutrigenetics/ Nutrigenomics on Personalised Nutrition: Part 1 - Fields of Precision Nutrition. Lifestyle Genomics. 2016;9:12–27. doi: 10.1159/000445350. [DOI] [PubMed] [Google Scholar]
- 6.Bray MS, Loos RJF, McCaffery JM, Ling C, Franks PW, Weinstock GM. NIH working group report—using genomic information to guide weight management: From universal to precision treatment. Obesity. 2016;24:14–22. doi: 10.1002/oby.21381. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Luis DA, Izaola O, Primo D, de la Fuente B, Mulero I, Aller R. The rs1862513 Variant in Resistin Gene-Modified Insulin Resistance and Insulin Levels after Weight Loss Secondary to Hypocaloric Diet. Ann Nutr Metab. 2017;69:256–62. doi: 10.1159/000453676. [DOI] [PubMed] [Google Scholar]
- 9.de Luis DA, Izaola O, Primo D, Aller R. Effect of the rs1862513 variant of resistin gene on insulin resistance and resistin levels after two hypocaloric diets with different fat distribution in subjects with obesity. Eur Rev Med Pharmaco. 2018;22:3865–72. doi: 10.26355/eurrev_201806_15271. [DOI] [PubMed] [Google Scholar]
- 10.de Luis D, Izaola O, Primo D, Aller R. Role of rs670 variant of APOA1 gene on metabolic response after a high fat vs. a low fat hypocaloric diets in obese human subjects. J Diabetes Complicat. 2019;33:249–54. doi: 10.1016/j.jdiacomp.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 11.de Luis DA, Izaola O, Primo D, Aller R. Role of rs670 variant of APOA1 gene on lipid profile, insulin resistance and adipokine levels in obese subjects after weight loss with a dietary intervention. Diabetes Res Clin Pract. 2018;142:139–45. doi: 10.1016/j.diabres.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 12.de Luis DA, Aller R, Izaola O, de la Fuente B, Romero E. Genetic variation in the beta-3-adrenoreceptor gene (Trp64arg polymorphism) and their influence on anthropometric parameters and insulin resistance after a high protein/low carbohydrate versus a standard hypocaloric diet. Nutr Hosp. 2015;32:487–93. doi: 10.3305/nh.2015.32.2.9293. [DOI] [PubMed] [Google Scholar]
- 13.de Luis DA, Aller R, Izaola O, Romero E. Effect of -55CT Polymorphism of UCP3 on Insulin Resistance and Cardiovascular Risk Factors after a High Protein/Low Carbohydrate versus a Standard Hypocaloric Diet. Ann Nutr Metab. 2016;68:157–63. doi: 10.1159/000444150. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Lacarte M, Milagro FI, Zulet MA, Martinez JA, Mansego ML. LINE-1 methylation levels, a biomarker of weight loss in obese subjects, are influenced by dietary antioxidant capacity. Redox Rep. 2016;21:67–74. doi: 10.1179/1351000215Y.0000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang AA, Harrison K, Musaad S, Donovan SM, Teran Garcia M. Genetic risk scores demonstrate the cumulative association of single nucleotide polymorphisms in gut microbiome‐related genes with obesity phenotypes in preschool age children. Pediatr Obes. 2019;14:e12530. doi: 10.1111/ijpo.12530. [DOI] [PubMed] [Google Scholar]
- 16.Huang T, Huang J, Qi Q, Li Y, Bray GA, Rood J. PCSK7 genotype modifies effect of a weight-loss diet on 2-year changes of insulin resistance: the POUNDS LOST trial. Diabetes Care. 2015;38:439–44. doi: 10.2337/dc14-0473. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heianza Y, Ma W, Huang T, Wang T, Zheng Y, Smith SR. Macronutrient Intake-Associated FGF21 Genotype Modifies Effects of Weight-Loss Diets on 2-Year Changes of Central Adiposity and Body Composition: The POUNDS Lost Trial. Diabetes Care. 2016;39:1909–14. doi: 10.2337/dc16-1111. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Luis DA, Izaola O, Primo D, Aller R. Association of the rs10830963 polymorphism in melatonin receptor type 1B (MTNR1B) with metabolic response after weight loss secondary to a hypocaloric diet based in Mediterranean style. Clin Nutr. 2018;37:1563–8. doi: 10.1016/j.clnu.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Goni L, Sun D, Heianza Y, Wang T, Huang T, Martínez JA. A circadian rhythm-related MTNR1B genetic variant modulates the effect of weight-loss diets on changes in adiposity and body composition: the POUNDS Lost trial. Eur J Nutr. 2019;58:1381–1389. doi: 10.1007/s00394-018-1660-y. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. Plos Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Zhao JH, Luan J, Luben RN, Rodwell SA, Khaw K. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am J Clin Nutr. 2010;91:184–90. doi: 10.3945/ajcn.2009.28403. et al. [DOI] [PubMed] [Google Scholar]
- 22.Lamiquiz-Moneo I, Mateo-Gallego R, Bea AM, Dehesa-García B, Pérez-Calahorra S, Marco-Benedí V, et al. Genetic predictors of weight loss in overweight and obese subjects. Sci Rep-Uk. 2019;9:10770. doi: 10.1038/s41598-019-47283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casas-Agustench P, Arnett DK, Smith CE, Lai C, Parnell LD, Borecki IB, et al. Saturated Fat Intake Modulates the Association between an Obesity Genetic Risk Score and Body Mass Index in Two US Populations. J Acad Nutr Diet. 2014;114:1954–66. doi: 10.1016/j.jand.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos-Lopez O, Cuervo M, Goni L, Milagro FI, Riezu-Boj JI, Martinez JA. Modeling of an integrative prototype based on genetic, phenotypic, and environmental information for personalized prescription of energy-restricted diets in overweight/obese subjects. Am J Clin Nutr. 2020;111:459–70. doi: 10.1093/ajcn/nqz286. [DOI] [PubMed] [Google Scholar]
- 25.Qi Q, Zheng Y, Huang T, Rood J, Bray GA, Sacks FM. Vitamin D metabolism-related genetic variants, dietary protein intake and improvement of insulin resistance in a 2 year weight-loss trial: POUNDS Lost. Diabetologia. 2015;58:2791–9. doi: 10.1007/s00125-015-3750-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin Receptor Substrate 1 Gene Variation Modifies Insulin Resistance Response to Weight-Loss Diets in a 2-Year Randomized Trial. Circulation. 2011;124:563–71. doi: 10.1161/CIRCULATIONAHA.111.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Chang S, Lin W, Chen C, Chiang S, Huang K. Machine Learning-Based Method for Obesity Risk Evaluation Using Single-Nucleotide Polymorphisms Derived from Next-Generation Sequencing. J Comput Biol. 2018;25:1347–60. doi: 10.1089/cmb.2018.0002. et al. [DOI] [PubMed] [Google Scholar]
- 28.López B, Torrent-Fontbona F, Viñas R, Fernández-Real JM. Single Nucleotide Polymorphism relevance learning with Random Forests for Type 2 diabetes risk prediction. Artif Intell Med. 2018;85:43–9. doi: 10.1016/j.artmed.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Choe EK, Rhee H, Lee S, Shin E, Oh S, Lee J. Metabolic Syndrome Prediction Using Machine Learning Models with Genetic and Clinical Information from a Nonobese Healthy Population. Genomics Inform. 2018;16:e31. doi: 10.5808/GI.2018.16.4.e31. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


