Abstract
Objective
Hepatic arterial infusion chemotherapy (HAIC) is an effective treatment for advanced unresectable colorectal cancer liver metastases (CRLM). This study was conducted to predict the efficacy of HAIC in patients with unresectable CRLM by radiomics methods based on pretreatment computed tomography (CT) examinations and clinical data.
Materials and Methods
A total of 63 patients were included in this study (41 in the training group and 22 in the validation group). All these patients underwent CT examination before HAIC. During the follow-up period, CT scans and laboratory examinations were performed regularly. Eighty-five radiological features were extracted from the regions of interest (ROIs) of CT images using the PyRadiomics program. The t-test and correlation were applied to select features. These features were analyzed using LASSO-Cox regression, and a linear model was developed to predict overall survival (OS).
Results
After reducing features by t-test and correlation test, seven features remained. After LASSO-Cox cross-validation, four features remained at λ = 0.232. They were gray level co-occurrence matrix (GLCM), gray level run length matrix (GLRLM), neighborhood gray tone difference matrix (NGTDM), and the location of the primary tumor. The C-index was 0.758 in the training group and 0.743 in the test group. Nomograms predicting 1-, 2-, and 3-year survival were established.
Conclusion
Our study demonstrates that a radiomics approach based on pretreatment CT texture analysis has the ability to predict early the outcome of HAIC in patients with advanced unresectable colorectal cancer with a high degree of accuracy and feasibility.
Key words: colorectal liver metastases, hepatic arterial infusion chemotherapy, radiomics, computed tomography, overall survival
Introduction
Colorectal cancer (CRC) is the third most common diagnosed cancer and more than 50% of patients will develop liver metastases during the course of the disease, which is the leading cause of death in patients with CRC.[1, 2] Complete surgical resection of all metastases is the only potentially curative treatment for colorectal cancer liver metastases (CRLM) patients, with a 5-year survival rate of 40%–50%.[3, 4, 5] However, no more than 20% of patients are available for hepatic resection at the time of diagnosis.[6] Chemotherapy combined with targeted therapy is the main therapeutic option for the vast majority of patients presenting with unresectable CRLM. For those patients who have failed systemic chemotherapy, hepatic arterial infusion chemotherapy (HAIC), a treatment in which chemotherapy drugs are continuously infused directly through a catheter inserted into the hepatic artery, has shown promising results. Compared with systemic chemotherapy, HAIC delivers chemotherapeutic agents to the tumor area with higher concentrations, resulting in more effective tumor shrinkage and reduction of systemic toxicity of chemotherapeutic agents,[5,7, 8, 9] better disease control rate (DCR), better objective response rate (ORR), better disease-free survival (DFS), and longer overall survival (OS).[10, 11]
The Response Evaluation Criteria in Solid Tumors (RECIST) based on computed tomography (CT) are the most widely used criteria for evaluating therapeutic efficacy to HAIC.[12] However, determining the chemotherapy response relying on tumor size changes takes too long for patients with advanced CRLM because several months are needed to detect the change. The prediction of therapeutic effect and prognosis before HAIC treatment are especially critical for these patients.
Recently, radiomics, which can extract an increased number of quantitative features from medical images, has attracted great attention. It is widely used in prediction of disease stage, histological grade, response to therapy, and survival in various tumors including CRLM.[13, 14, 15] For example, Dohan et al.[16] proposed a radiomic nomogram to predict early response in CRLM patients treated with FOLFIRI and bevacizumab through baseline and 2-month evaluation CT, which showed that the patients with a SPECTRA score >0.02 had a lower OS in the training and validation cohort. In other words, the SPECTRA score was able to stratify survival outcomes at 2 months with the same accuracy as RECIST at 6 months.
Up to now, there are no studies on the application of radiomics to predict the prognosis of CRLM treated with HAIC. This study is aimed to develop and validate a radiomics-based model for predicting the OS in unresectable CRLM patients after receiving HAIC treatment.
Materials and methods
This retrospective study was approved by the local ethics committee (202KT01). Informed written consent requirement was waived.
Patients
Between January 2007 and December 2016, a total of 213 patients with progressive CRLM were treated with standardized HAIC in our single center.
Inclusion criteria were:
those patients who progressed after receiving first- or second-line systemic therapy with unresectable liver metastases occupying less than 70% of the liver;
those who received standardized HAIC treatment and had complete pre- and postoperative imaging review; and
those patients who had complete follow-up information.
The exclusion criteria were no standardized CT scan before HAIC treatment, incomplete imaging data, or no follow-up records.
Finally, 150 patients were excluded from this study (Figure 1), and our sample size of this study ended up being 63, which was randomly divided into a training group (n = 41) and a test group (n = 22).
Figure 1.
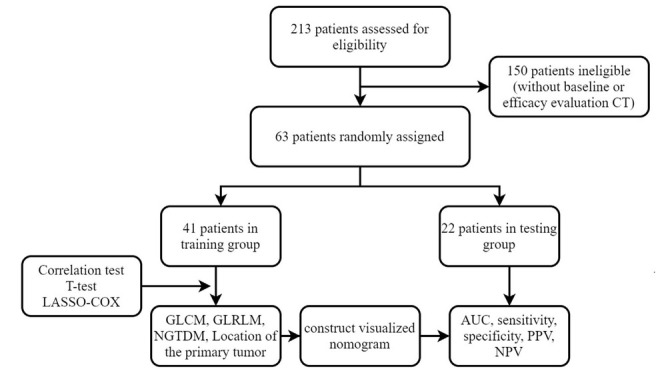
Flowchart of study inclusion and exclusion. AUC: area under the curve; CT: computed tomography; GLCM: gray level co-occurrence matrix; GLRLM: gray level run length matrix; NGTDM: neighborhood gray tone difference matrix; NPV: negative predictive value; PPV: positive predictive value.
Interventional approach and dosing regimen
In standardized HAIC treatment, patients were required to undergo abdominal artery, common hepatic artery, and superior mesenteric artery angiography to understand the number of liver tumors, blood supply, anatomical location, and associated tumor arteries. Also, HAIC was performed every 4 weeks using a coaxial microcatheter, leaving the catheter in the innominate hepatic artery and closing the collateral blood supply arteries, with the following protocol. Oxaliplatin 85 mg/m2 was continuously pumped through the hepatic artery for 4 h and 5-fluorouracil (5-Fu) 2 g/m2 through the hepatic artery for 44 h. The catheter and arterial sheath were removed after each cycle of drug infusion. Also, the patient was discharged from bed after 12 h with a compression device or bandage.
Computed tomography
All patients underwent abdominal CT scan with 64-MDCT scanners 2 weeks before chemotherapy (Lightspeed VCT; GE Healthcare, Milwaukee, WI, USA). The parameters listed were as follows: slice thickness: 5 mm, slice interval: 5 mm, tube voltage: 120 kVp, automatic tube current modulation, and a tube rotation time of 0.50–0.75 s. The scanning ranged from the diaphragmatic dome to the symphysis pubis. The patients were scanned before and after intravenous administration of contrast agent (Iopromide, Ultravist 300; Schering, Berlin, Germany) through the elbow vein with 600 mg iodine/kg body weight injected at 3.0 mL/s, followed by a flush with 20 mL of saline solution with a power injector. Multiphase abdominal CT scan was performed in the hepatic arterial phase at 25–30 s, portal venous phase at 70–80 s, and equilibrium phase at 150 s.
The CT images of the portal phase were exported through the picture archiving and communication system (PACS), and the liver metastases were outlined on 3D-slicer software (version 4.11, www.slicer.org). Regions of interest (ROIs) were manually outlined by a radiologist with 5 years of experience and repeated by another radiologist with 10 years of experience (Figure 2). Reproducibility of features was analyzed by interobserver intraclass correlation coefficient (ICC).
Figure 2.
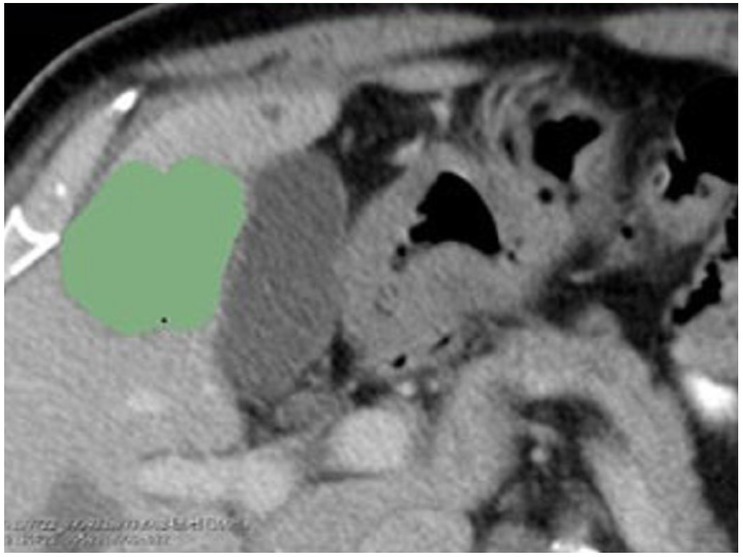
Manually delineated tumor area in the portal phase of the pre-treatment CT image, the green area in the figure was the example of ROIs. CT:computed tomography; ROI: region of interest.
Feature extraction
Eight hundred and fifty-one radiological features were extracted from the ROI in CT images by using PyRadiomics program. The original features included 14 shape features, 18 first-order features, and 75 second-order (texture) features (24 gray level co-occurrence matrix [GLCM] features, 14 gray level dependence matrix features, 16 gray level run length matrix [GLRLM] features, 16 gray level size zone matrix features, 5 neighborhood gray tone difference matrix [NGTDM] features). The wavelet features included eight types of transform by using high-pass (H) and low-pass (L) filters along three directions (HHH, HHL, HLH, HLL, LHH, LHL, LLH, and LLL). Therefore, the total number of radiological features was 851 [(18 + 75)× 8 + 18 + 75 + 14 = 851].
The clinical features collected in this study included the basic characteristics of patients, the position of the primary tumor, surgically resected status of the primary tumor, time of appearance of liver metastases, pathological grading, KARS gene, combination status of extrahepatic metastases, and preoperative carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9).
Modeling
The t-test and correlation were applied for feature selection. First, median OS was used to divide the subjects in the training group into two subgroups. The t-test was used to calculate the difference in each feature between the two groups. If the P-value was larger than 0.05, the feature was removed. Second, the correlation coefficient was calculated between each of two remaining features. If the absolute value of the correlation coefficient was larger than 0.5, the feature with larger P-value in the first step was removed.
LASSO-Cox regression was employed both for further selection of selected features and establishment of a linear predicting model for the OS. A hyperparameter λ was introduced for further feature selection. The λ was optimized by threefold cross-validation in the training group to maximize the average Harrell’s C-index. After the determination of λ, the training group was repeatedly trained to obtain the final model. Also, the model was evaluated by the test group. Probability of 1-, 2-, and 3-year survival was predicted with nomogram. All the calculations were performed by R (version 4.1.1) with “glmnet,” “rms,” “survival,” and “survminer” packages.
Statistical analysis
OS was defined as the date from diagnosis to death for any cause. Tumor response to treatment was evaluated by imaging analysis according to RECIST1.1. Patients underwent CT scan before each cycle of HAIC. During the follow-up period, CT scan was performed every 8 weeks. Patient characteristics were compared between the training group and the test group by t-test for continuous variables and chi-square for categorical variables. C-index was introduced to evaluate the prediction of OS. Receiver operating characteristic (ROC) curves were plotted for the prediction of 1-, 2-, and 3-year survival, and the area under the curve (AUC) was calculated. The largest Youden index was used to determine the cutoff value for the calculation of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results
Table 1 shows the characteristics of the patients included in this study. There were no significant difference in age, gender, position, metastases type, extrahepatic metastases, pathological diagnosis, pre-HAIC CEA, pre-HAIC CA19-9, and OS between the training group and the test group.
Table 1.
Characteristics of patients
| Characteristics | Training group | Test group | Test value | P value |
|---|---|---|---|---|
| Age (years) | 59.54 ± 9.70 | 60.20 ± 10.84 | 0.247 | 0.806 |
| Gender | — | — | 0.333 | 0.564 |
| Male | 29 (70.7%) | 14 (63.6%) | — | — |
| Female | 12 (29.3%) | 8 (36.4%) | — | — |
| Position | 0.929 | 0.335 | ||
| Left hemicolon | 29 (70.7%) | 18 (81.8%) | ||
| Right hemicolon | 12 (29.3%) | 4 (18.2%) | ||
| Metastases type | 0.01 | 0.97 | ||
| Synchronous | 30 (73.2%) | 16 (72.7%) | ||
| Metachronous | 11 (26.8%) | 6 (27.3) | ||
| Extrahepatic metastases | 0.894 | 0.344 | ||
| Present | 21 (51.2%) | 14 (63.6%) | ||
| Absent | 20 (48.8%) | 8 (36.4%) | ||
| Primary tumor excised | 0.224 | 0.636 | ||
| Yes | 32 (78.0%) | 16 (72.7%) | ||
| No | 9 (22.0%) | 6 (27.3%) | ||
| KRAS | 2.323 | 0.313 | ||
| Mutation | 6 (14.6%) | 6 (59.1%) | ||
| Wild type | 11 (26.8%) | 3 (13.6%) | ||
| Unknown | 24 (58.5%) | 13 (27.3%) | ||
| Pathological grade | 7.117 | 0.028 | ||
| Low | 6 (14.6%) | 4 (18.2%) | ||
| Middle/high | 33 (80.5%) | 12 (54.5%) | ||
| Unknown | 2 (4.9%) | 6 (27.3%) | ||
| Pre-HAIC CEA | 1076 ± 2318 | 1665 ± 3133 | 0.464 | 0.644 |
| Pre-HAIC CA19-9 | 806 ± 1993 | 514 ± 880 | 2.195 | 0.033 |
| OS (months) | 26.99 ± 16.10 | 30.24 ± 25.76 | 0.616 | 0.540 |
HAIC: hepatic arterial infusion chemotherapy; OS: overall survival; CA19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen.
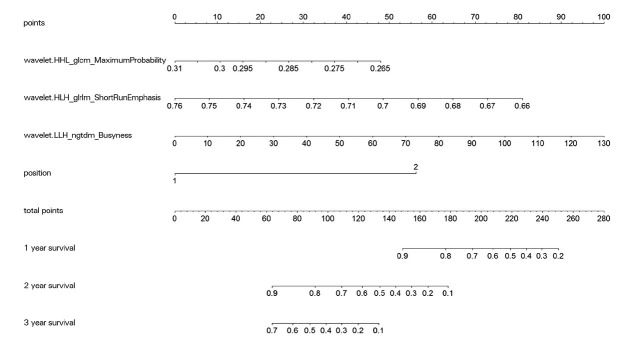
After features filtration by t-test and correlation test, seven features remained. Also, four features emerged at λ = 0.232 after cross-validation by LASSO-Cox. They were maximum probability of GLCM, short-run emphasis of GLRLM, busyness of NGTDM, and location of the primary tumor (1 was left colon and 2 was right). The C-index was 0.758 for the training group and 0.743 for the test group.
Figure 3 shows the nomogram versus 1-, 2-, and 3-year survival. The corresponding ROC curves are plotted in Figure 4. Table 2 shows the AUC value, sensitivity, specificity, PPV, and NPV. The nomogram helps us to estimate survival in an easier way based on clinical data and radiomics features. With the nomogram, our prediction model makes it easier for us to evaluate patients.
Figure 3.
Nomogram for the prediction of 1-, 2-, and 3-year survival.
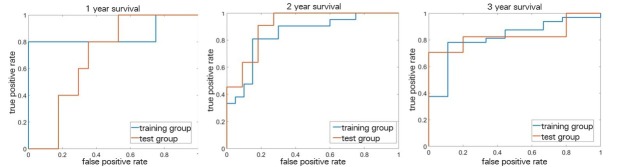
Figure 4.
Receiver operating characteristic curves of the prediction.
Table 2.
Prediction of survival in the training group and the test group
| Survival | Statistics | Training group (n = 41) | Test group (n = 22) |
|---|---|---|---|
| 1-year survival | AUC | 0.850 (0.555–1.000) | 0.694 (0.469–0.919) |
| Sensitivity | 80.0 (28.4–99.5) | 100.0 (47.8–100.0) | |
| Specificity | 100.0 (90.3–100.0) | 47.1 (23.0-72.2) | |
| PPV | 100.0 (39.8–100.0) | 35.7 (12.8–64.9) | |
| NPV | 97.3 (85.8–99.9) | 100.0 (63.1–100.0) | |
| 2-year survival | AUC | 0.845 (0.722–0.968) | 0.909 (0.784–1.000) |
| Sensitivity | 81.0 (58.1–94.6) | 100.0 (71.5–100.0) | |
| Specificity | 85.0 (62.1–96.8) | 72.7 (39.0–94.0) | |
| PPV | 85.0 (62.1–96.8) | 78.6 (49.2–95.3) | |
| NPV | 81.0 (58.1–94.6) | 100.0 (63.1–100.0) | |
| 3-year survival | AUC | 0.819 (0.670–0.969) | 0.835 (0.661–1.000) |
| Sensitivity | 78.1 (60.0–90.7) | 70.6 (44.0–89.7) | |
| Specificity | 88.9 (51.8–99.7) | 100.0 (47.8–100.0) | |
| PPV | 96.2 (80.4–99.9) | 100.0 (73.5–100.0) | |
| NPV | 53.3 (26.6–78.7) | 50.0 (18.7–81.3) |
AUC: area under the curve; NPV: negative predictive value; PPV: positive predictive value.
Table 2 shows the AUC, sensitivity, specificity, PPV, and NPV for the prediction of 1-, 2-, and 3-year survival in the training group (n = 41) and test group (n = 22) with their 95% confidential intervals (CIs).
Discussion
For patients with advanced CRC, effective treatment of liver metastases ensures their long-term survival. With the development and wide application of minimally invasive interventional techniques, transhepatic arterial interventional therapy represented by HAIC has become the recommended treatment for unresectable CRC liver metastases, which has been written as the recommended treatment in the Chinese Guidelines for Diagnosis and Comprehensive Treatment of CRC Liver Metastases[17] and included in the National Comprehensive Cancer Network (NCCN) 2021 edition as Class 2B recommendation. A retrospective analysis of clinical data from 11 studies of 1514 unresectable CRC patients with liver metastases who were treated with HAIC showed a median survival of 24 months (95% CI, 13–36 months); cumulative survival rates at 1, 2, 3, and 5 years were 76%, 51%, 29%, and 11%, respectively.[18] HAIC combined with systemic chemotherapy can better control local tumor progression, with response rates at 76%–92% and conversion resection rates up to 47%.[19, 20]
However, in clinical practice, how to predict efficacy before treatment or at an early stage has become an important issue with HAIC treatment. Tumor efficacy evaluation commonly uses RECIST criteria, which often require a treatment period of 3–6 months for accurate assessment. HAIC is often used as second- or third-line treatment for patients with advanced CRC liver metastases, who may be in worse health and have a worse prognosis, and this period may be too long for them. Moreover, HAIC treatment is accompanied by embolization therapy, and the post-treatment lesions often do not show significant changes in tumor size, but show significant decrease in density or calcification within the tumor,[20] which further increases the difficulty of evaluating the efficacy by RECIST criteria. Additionally, abdominal CT has limitations as the most used clinical tool for the detection and evaluation of the efficacy of liver metastases. Its results are based on morphological evaluation, which weakens the effectiveness of evaluation and objectivity. In the meanwhile, except for objective measurements such as diameter and size, all other evaluation criteria are subjective and may cause certain evaluation biases.[21]
Investigating the data based on clinical features, laboratory tests, and routine imaging to find out the internal characteristics of diseases explains the changes in the body at the tissue, cellular, and genetic levels, which will have a significant positive impact on clinical treatment. According to this theory, imaging-omics was born. It extracts high-throughput features from medical images to quantify major diseases such as tumors, showing great advantages in tumor phenotyping, treatment plan selection, and prognosis analysis, and is a popular research topic in the fields of clinical medicine and biomedical engineering. But most of the studies on intra-arterial therapies focused on patients with hepatocellular carcinoma undergoing transarterial chemoembolization (TACE).[22, 23, 24] Some studies on HAIC and CRLM have been developed for predicting tumor response or survival with machine learning. Abajian et al.[22] used 36 patients’ imaging and clinical data and therapeutic features (conventional TACE or not and systemic chemotherapy or not) to create a framework model for predicting the outcomes of TACE. The model predicted the treatment response with 78% accuracy, 62.5% sensitivity, and 82.1% specificity. A similar approach was used by Mahringer et al.,[23] who trained the artificial neural network (ANN) with clinical, laboratory, and imaging parameters, which are used in current risk scores to predict 1-year survival after TACE. With an AUC of 0.77, this ANN performed better than traditional scoring systems in predicting 1-year survival after TACE.
Our retrospective study focused on patients with CRLM who were treated with HAIC; we collected pretreatment CT images of patients to construct a radiomics model, collected clinical information as much as possible, performed RECIST evaluation and follow-up of patients, and built a clinical-omics model to predict patients’ outcomes. This study suggests that machine learning based on selected texture analysis features may be a useful tool to predict treatment response based on pretreatment CT images of patients with liver metastases treated with HAIC. This texture analysis tool is an open-source developed software to improve the reliability and reproducibility of feature computation. The tool has been employed and validated in several radiomics studies under different imaging modalities and medical conditions.[25, 26] The study shows that the model built by combining clinical data imaging histological features has potential to be a predictor of the outcome of patients with advanced CRLM who received HAIC.
We applied LASSO-Cox regression to select variables in the filtered radiological sign models and clinical variable models to develop the combined model. The factors involved in the radiological sign score and clinical score models may be correlated, and combining them without considering the potential correlation between these correlations may lead to overfitting of the data. The LASSO method identifies the variables that are most orthogonal to each other. Therefore, we performed additional LASSO steps and t-tests to correct for potential correlations between the variables from the first and second models. We ended up with four features, three from the radiological scoring model and one from the clinical model.
The feature from a clinical point of view is the location of the primary focus. A review of the literature[27] shows that right and left hemicolectomized colon cancers differ not only in location, but also in histocytogenesis, microbiome, and chromosomal and molecular features, and the pathology of right hemicolectomized colon cancer is mostly mucinous adenocarcinoma with MSI-H, hypermutation status, and a poorer overall prognosis, which is consistent with previous studies. As shown in Table 1, we collected data on gene, CEA, CA19-9, and so on in our study. However, after statistical analysis, these three results did not show positive significance. Data on the prognostic value of CEA, CA19-9, and other common laboratory parameters in assessing CRLM are scarce, with some studies being inconclusive or concluding otherwise. Recent literature[28] suggests that CEA may have some value in identifying patients with worse prognosis after CRLM resection. Regarding the absence of positive results for , we consider that it may be caused by the insufficient number of patients we collected.
The three imaging histological features were maximum probability of GLCM, short run emphasis of GLRLM, and busyness of NGTDM. Among them, GLCM has also been mentioned to be associated with tumor heterogeneity in some literature reports on primary and metastatic hepatocellular carcinoma. GLCM-based features can quantify textural information and are useful for quantifying intratumoral heterogeneity in various cancer types, including liver cancer.[29] Four GLCM-based features (GLCM energy, GLCM informational measure of correlation, GLCM maximum probability, GLCM contrast, and GLCM sum average) have been reported in the literature, and they all reflect different aspects of textural heterogeneity. Tumors with high textural heterogeneity tend to have a poor prognosis, and therefore have a negative impact on survival.[30] In contrast, GLRLM, NGTDM, and liver tumor correlations have been reported less frequently. Conventional evaluation of imaging can only interpret morphology, while texture analysis features are abstract numbers. Moreover, artificial intelligence can determine treatment decisions for tumor patients with objective digital imaging data. Extracted texture analysis features show differences between patients with and without treatment, so ANNs trained based on extracted texture analysis features and corresponding treatment responses can be used for new data sets for outcome prediction.[31, 32]
Based on the data and results of our study, we created a nomogram to show our results. This nomogram can clearly show the relationship between the four features in our study and the 1-, 2-, and 3-year survival rates of patients, which helps us to make easy clinical estimates of the efficacy and survival of patients with CRLM treated with HAIC. In a retrospective study[33] by Guo et al., CRC patients with synchronous liver metastases were screened from the SEER database as development set, while patients in Harbin (China) were enrolled as validation set. OS was used as an endpoint. Variables were screened by LASSO-Cox regression, and then OS nomograms were constructed by Cox regression at 1, 3, and 5 years. Also, 1347 and 112 patients were included in the development set and validation set, respectively. The C-index values of the OS nomogram in the development and validation groups were 0.701 and 0.670, respectively, which were lower than the values of the present study. In addition, Guo’s study was developed based on the fact that CRC patients with synchronous liver metastases can be treated by synchronous surgery and may not be suitable for patients treated by other modalities such as HAIC.
In conclusion, our study shows that imaging-omics–based machine learning has potential utilization in predicting outcomes and survival of CRLM patients with liver metastases, with pretreatment imaging information and clinical data with a certain level of accuracy. Obviously, as a retrospective study, the limitation is significant, which is stability of the image feature values is limited by the relatively small sample size. Further studies with larger samples are required to fully validate our findings in the future.
Funding Statement
This work was supported by Beijing Hospitals Authority Ascent Plan (Code: 20191103), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201803), Beijing Natural Science Foundation (Z200015), and PKU-Baidu Fund (No. 2020BD027).
Footnotes
Conflict of Interest
All author declared no conflicts of interest.
Ethics Approval and Consent to Participate
This retrospective study was approved by the ethics committee of Peking University Cancer Hospital (202KT01). Informed written consent requirement was waived.
Reference
- 1.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–35. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83. doi: 10.1200/JCO.2008.20.5278. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–52. doi: 10.1016/j.jamcollsurg.2009.12.040. et al. 752-5. [DOI] [PubMed] [Google Scholar]
- 5.Boige V, Malka D, Elias D, Castaing M, De Baere T, Goere D. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15:219–26. doi: 10.1245/s10434-007-9581-7. et al. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506. doi: 10.1200/JCO.2012.42.8201. et al. [DOI] [PubMed] [Google Scholar]
- 7.Goéré D, Deshaies I, de Baere T, Boige V, Malka D, Dumont F. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Ann Surg. 2010;251:686–91. doi: 10.1097/SLA.0b013e3181d35983. et al. [DOI] [PubMed] [Google Scholar]
- 8.Nishiofuku H, Tanaka T, Aramaki T, Boku N, Inaba Y, Sato Y. Hepatic arterial infusion of 5-fluorouracil for patients with liver metastases from colorectal cancer refractory to standard systemic chemotherapy: a multicenter, retrospective analysis. Clin Colorectal Cancer. 2010;9:305–10. doi: 10.3816/CCC.2010.n.044. et al. [DOI] [PubMed] [Google Scholar]
- 9.Lim A, Le Sourd S, Senellart H, Luet D, Douane F, Perret C. Hepatic Arterial Infusion Chemotherapy for Unresectable Liver Metastases of Colorectal Cancer: A Multicenter Retrospective Study. Clin Colorectal Cancer. 2017;16:308–15. doi: 10.1016/j.clcc.2017.03.003. et al. [DOI] [PubMed] [Google Scholar]
- 10.Boerner T, Zambirinis C, Gagnière J, Chou JF, Gonen M, Kemeny NE. et al. Early liver metastases after “failure” of adjuvant chemotherapy for stage III colorectal cancer: is there a role for additional adjuvant therapy? HPB (Oxford) 2021;23:601–8. doi: 10.1016/j.hpb.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang K, Yang T, Cao Y, Liang W, Yang X. Meta-Analysis of Hepatic Arterial Infusion for Liver Metastases From Colorectal Cancer. Front Oncol. 2021;11:628558. doi: 10.3389/fonc.2021.628558. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. et al. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi R, Oki E, Hasuda H, Sano E, Miyashita Y, Sakai A. Radiomics Texture Analysis for the Identification of Colorectal Liver Metastases Sensitive to First-Line Oxaliplatin-Based Chemotherapy. Ann Surg Oncol. 2021;28:2975–85. doi: 10.1245/s10434-020-09581-5. et al. [DOI] [PubMed] [Google Scholar]
- 14.Mühlberg A, Holch JW, Heinemann V, Huber T, Moltz J, Maurus S. The relevance of CT-based geometric and radiomics analysis of whole liver tumor burden to predict survival of patients with metastatic colorectal cancer. Eur Radiol. 2021;31:834–46. doi: 10.1007/s00330-020-07192-y. et al. [DOI] [PubMed] [Google Scholar]
- 15.Zhu HB, Xu D, Ye M, Sun L, Zhang XY, Li XT. Deep learning-assisted magnetic resonance imaging prediction of tumor response to chemotherapy in patients with colorectal liver metastases. Int J Cancer. 2021;148:1717–30. doi: 10.1002/ijc.33427. et al. [DOI] [PubMed] [Google Scholar]
- 16.Dohan A, Gallix B, Guiu B, Le Malicot K, Reinhold C, Soyer P. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2020;69:531–9. doi: 10.1136/gutjnl-2018-316407. et al. [DOI] [PubMed] [Google Scholar]
- 17.Surgeons Branch of Chinese Medical Doctor Association, Gastrointestinal Surgery Team of Surgery Branch of Chinese Medical Association, Colorectal Cancer Surgery Team of Surgery Branch of Chinese Medical Association. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (version 2020) Chin J Clin Med. 2021;28:129–44. et al. [Google Scholar]
- 18.Chan DL, Alzahrani NA, Morris DL, Chua TC. Systematic review and meta-analysis of hepatic arterial infusion chemotherapy as bridging therapy for colorectal liver metastases. Surg Oncol. 2015;24:162–71. doi: 10.1016/j.suronc.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Kemeny NE, Melendez FDH, Capanu M, Paty PB, Fong Y, Schwartz LH. Conversion to Resectability Using Hepatic Artery Infusion Plus Systemic Chemotherapy for the Treatment of Unresectable Liver Metastases From Colorectal Carcinoma. J Clin Oncol. 2009;27:3465–71. doi: 10.1200/JCO.2008.20.1301. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Angelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF. Phase II Trial of Hepatic Artery Infusional and Systemic Chemotherapy for Patients With Unresectable Hepatic Metastases From Colorectal Cancer Conversion to Resection and Long-term Outcomes. Ann Surg. 2015;261:353–60. doi: 10.1097/SLA.0000000000000614. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalchandani UR, Sahai V, Hersberger K, Francis IR, Wasnik AP. A Radiologist’s Guide to Response Evaluation Criteria in Solid Tumors. Curr Probl in Diagn Radiol. 2019;48:576–85. doi: 10.1067/j.cpradiol.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS. Predicting treatment response to intra-arterial therapies for hepatocellular carcinoma with the use of supervised machine learning-an artificial intelligence concept. J Vasc Interv Radiol. 2018;29:850–7. doi: 10.1016/j.jvir.2018.01.769. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mähringer-Kunz A, Wagner F, Hahn F, Weinmann A, Brodehl S, Schotten S. Predicting survival after transarterial chemoembolization for hepatocellular carcinoma using a neural network: a Pilot Study. Liver Int. 2020;40:694–703. doi: 10.1111/liv.14380. et al. [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Kang S, Ning Z, Deng H, Shen J, Xu Y. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur Radiol. 2019;30:413–24. doi: 10.1007/s00330-019-06318-1. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannil M., von Spiczak J., Manka R., Alkadhi H.. Texture analysis and machine learning for detecting myocardial infarction in Noncontrast low-dose computed tomography: unveiling the invisible. Invest Radiol. 2018;53:338–43. doi: 10.1097/RLI.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 26.Kambakamba P, Mannil M, Herrera PE, Müller PC, Kuemmerli C, Linecker M. The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: a proof-of-principle study. Surgery. 2020;167:448–54. doi: 10.1016/j.surg.2019.09.019. et al. [DOI] [PubMed] [Google Scholar]
- 27.Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. et al. [DOI] [PubMed] [Google Scholar]
- 28.Loosen SH, Roderburg C, Alizai PH, Roeth AA, Schmitz SM, Vucur M. Comparative Analysis of Circulating Biomarkers for Patients Undergoing Resection of Colorectal Liver Metastases. Diagnostics (Basel) 2021;11:1999. doi: 10.3390/diagnostics11111999. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Zhu Y, Liu Z, Liang C. Texture analysis of baseline multiphasic hepatic computed tomography images for the prognosis of single hepatocellular carcinoma after hepatectomy: a retrospective pilot study. Eur J Radiol. 2017;90:198–204. doi: 10.1016/j.ejrad.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 30.Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22:796–802. doi: 10.1007/s00330-011-2319-8. [DOI] [PubMed] [Google Scholar]
- 31.Sinha I, Aluthge DP, Chen ES, Sarkar IN, Ahn SH. Machine learning offers exciting potential for predicting postprocedural outcomes: a framework for developing random forest models in IR. J Vasc Interv Radiol. 2020;31:1018–24. doi: 10.1016/j.jvir.2019.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saba L, Biswas M, Kuppili V, Cuadrado Godia E, Suri HS, Edla DR. The present and future of deep learning in radiology. Eur J Radiol. 2019;114:14–24. doi: 10.1016/j.ejrad.2019.02.038. et al. [DOI] [PubMed] [Google Scholar]
- 33.Guo X, Liu Y, Liu LJ, Li J, Zhao L, Jin XR. et al. Development and validation of survival nomograms in colorectal cancer patients with synchronous liver metastases underwent simultaneous surgical treatment of primary and metastatic lesions. Am J Cancer Res. 2021;11:2654–69. [PMC free article] [PubMed] [Google Scholar]