Abstract
Sordarin derivatives (Glaxo Wellcome) are a new class of compounds that selectively inhibit fungal protein synthesis and have a broad spectrum of activity. Systemic coccidioidomycosis was established in female CD-1 mice infected with Coccidioides immitis, and therapy was begun on day 4 with either GM193663, GM211676, GM237354, fluconazole, or no treatment; compounds were given twice daily orally for 19 days at 20 or 100 mg/kg/day. The serum pharmacokinetics of the compounds were studied in uninfected mice. The MICs of GM193663, GM211676, and GM237354 for C. immitis were 1.56, 0.39, and 0.39 μg/ml, respectively, and the minimum fungicidal concentrations were 6.25, 3.13, and 0.39 μg/ml, respectively. Peak serum levels (sampled at 1 to 2 h) after a single 50-mg/kg dose were 9.8 μg/ml for GM193663, 13 μg/ml for GM211676, and 6.0 μg/ml for GM237354. No accumulation occurred after 19 days of dosing, and peak levels were lower at 3.2 μg/ml for GM193663, 4.0 μg/ml for GM211676, and <2.5 μg/ml for GM237354. We estimate that the t1/2 for each compound in serum is <2 h. In vivo, all compounds showed dose-responsive efficacy, significantly prolonging survival over the control groups (100% lethal dose); 80 to 100% of the mice given the 100-mg/kg doses of fluconazole or a GM drug survived. All 100-mg/kg/day regimens were equivalent. At 20 mg/kg/day, GM211676 was equivalent to 100 mg of fluconazole/kg/day, indicating that GM211676 was ∼5-fold more efficacious. No mice surviving the 49 days of the experiment were free of infection. All drugs dose responsively reduced the fungal burden in the spleen, liver, and lungs, and GM237354 at 100 mg/kg/day was superior to all of the other regimens in the reduction of burden in all organs. C. immitis was susceptible both in vitro and in vivo to the GM compounds, which were found to be equivalent or superior to fluconazole. These results are encouraging, indicating that further testing in other models of fungal disease is warranted.
The discovery of new and more potent broad-spectrum antifungal drugs remains an important area of research with the continued increase in the number of severe fungal infections. Current therapies are limited, with treatment failures, relapses while on therapy, and development of resistance reducing the number of successful regimens. Efforts to improve antifungal agents have included molecular modifications of existing compounds, different forms of carrier delivery, and screening of new classes of compounds for activity.
Sordarins are a novel class of antifungal agents that are tetrahydropyran derivatives of a naturally occurring diterpene sordaricin (7, 8, 10). These molecules inhibit the fungal protein synthesis elongation cycle (7), with the specific target being translation elongation factor 2 (1, 7). The chemical structure of these compounds has been published previously (1, 7, 10, 12). Overall, these compounds have broad-spectrum activity in vitro against some species of Candida, including Candida albicans, and also Cryptococcus neoformans (7, 10, 12). However, reduced or minimal in vitro activity has been demonstrated by sordarins against yeasts such as C. parapsilosis or C. krusei (12). In addition, these compounds have been demonstrated to have activity against a variety of filamentous fungi that are considered to be emerging pathogens (12). These compounds have also shown activity in vivo against several types of fungi, including Histoplasma capsulatum (11), Pneumocystis carinii, and C. albicans, with possible limited activity against Aspergillus fumigatus (reviewed in reference 10).
The objective of our study was to evaluate the in vivo efficacies of three sordarin derivatives in a murine model of systemic coccidioidomycosis. Our results indicate that these compounds are active in vivo against Coccidioides immitis and warrant further study.
MATERIALS AND METHODS
In vivo model.
A model of systemic coccidioidomycosis was established in 6-week-old female CD-1 mice (mean weight, 24.4 g) by intravenous injection of 382 viable arthroconidia of C. immitis Silveira, which was similar to the models described previously (3–5). Therapy began 4 days postinfection, and groups of 10 mice received GM193663, GM211676, GM237354, fluconazole, or no treatment. All three GM compounds (Glaxo Wellcome S.A., Madrid, Spain) and fluconazole were given at 20 or 100 mg/kg/day in sterile water. All therapies were given by gavage twice daily (one-half of the daily dose at each time) in a 0.1-ml volume per dose on days 4 through 22 postinfection (19 days of therapy).
Deaths were tallied through 49 days postinfection. All surviving mice were euthanized for quantitation of residual burdens of C. immitis in the spleen, liver, and lungs. Organs were removed aseptically and homogenized in 5 ml of sterile saline. Serial dilutions of the homogenates were placed onto Mycosel agar (Difco Laboratories, Detroit, Mich.) and incubated at 37°C to determine the number of viable CFU remaining in each organ (2, 3, 5, 9). Fungal burdens were expressed as the log10 number of CFU per entire organ.
Statistical analyses.
Survival data were analyzed by day of death using a Wilcoxon rank sum test, and CFU burdens in the organs were analyzed by the Mann-Whitney U test (13) using GB-Stat version 6.0 (Dynamic Microsystems, Silver Spring, Md.) as described previously (3, 5, 9).
Pharmacokinetics.
The serum pharmacokinetics of the three GM compounds were studied in uninfected mice. One group was given 19 days of therapy with each GM compound at 100 mg/kg/day, and one group received a single dose of 50 mg/kg. The mice were exsanguinated at various times after the last dose, and the sera from two mice at each time point were pooled to determine drug levels by bioassay as described previously using Candida kefyr SA as the indicator organism (14). Assays were also done using C. albicans 5 as the indicator organism to determine whether the sensitivity of the assay would be improved.
In vitro susceptibility testing.
The in vitro activities of the sordarin derivatives GM193663, GM211676, and GM237354, as well as that of fluconazole, were tested against the same strain of C. immitis that had been used in the in vivo studies. The methods used for the determination of MIC and minimum fungicidal concentration (MFC) have been described previously (3).
RESULTS
In vitro antifungal activity.
The in vitro activity of the GM drugs against the infecting strain of C. immitis used, Silveira, was determined, and the infecting strain was found to be susceptible to each of the GM compounds. All three compounds have greater in vitro activity against C. immitis than fluconazole for the Silveira strain (Table 1).
TABLE 1.
In vitro susceptibility of C. immitis Silveira to fluconazole and sordarin derivatives
Serum pharmacokinetics of sordarin derivatives.
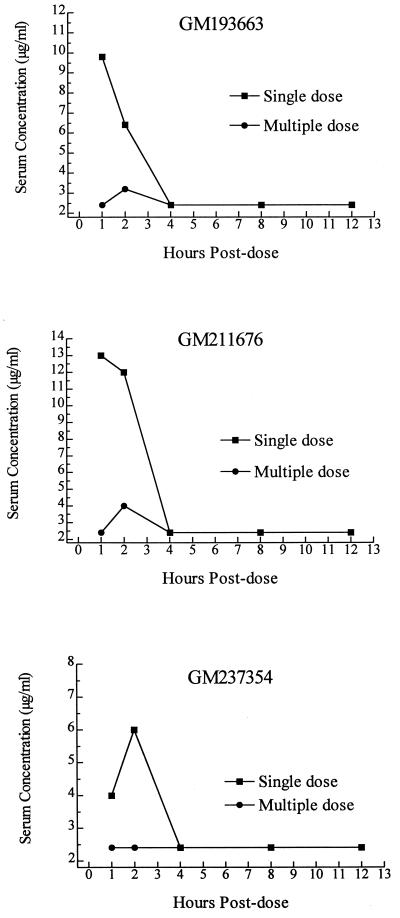
The serum pharmacokinetics of each GM compound were determined in uninfected mice (Fig. 1). For GM193663, after a single 50-mg/kg dose a peak of 9.8 μg/ml was reached at 1 h postdose, with a decline by 2 h postdose and no drug detectable by 4 h. Similarly, after a single 50-mg/kg dose of GM211676, a peak level of 13 μg/ml occurred at 1 h postdose, but no drug was detectable by 4 h postdose. GM237354 showed a lower peak serum concentration of 6.0 μg/ml at 2 h postdose, but it was also not detectable by 4 h.
FIG. 1.
Serum levels of GM193663 (top panel), GM211676 (middle panel), and GM237354 (bottom panel) in uninfected mice given either a single oral 50-mg/kg dose or 19 daily oral doses of 100 mg/kg/day. Serum levels were determined by bioassay, which had a lower limit of detectable drug concentration for each of these compounds of 2.5 μg/ml.
The levels obtained after 19 days of dosing at 100 mg/kg/day were much lower for each compound (Fig. 1). No accumulation occurred after chronic dosing. The data suggest accelerated metabolism. A peak level of 3.2 μg/ml was achieved by 2 h postdose for GM193663, and a peak level of 4.0 μg/ml was achieved by 2 h for GM211676. No GM237354 was detectable in the serum at any time point after the chronic dosing. These results were determined using two different bioassay indicator organisms, with little difference noted in the determined levels. However, it should be noted that the lower end of detectable drug concentration in each of these assay systems was 2.5 μg/ml. Thus, drug could still be present in the serum but not detectable by the assay conditions used for these studies.
In vivo antifungal activity of sordarin derivatives.
A murine model of systemic coccidioidomycosis was established to compare the therapeutic efficacy of three GM compounds with that of fluconazole. The results of the in vivo model clearly demonstrate that all three GM drugs and fluconazole showed dose-responsive efficacy against systemic coccidioidomycosis. All were well tolerated, with no overt toxicities observed either during the course of the experiment or upon necropsy.
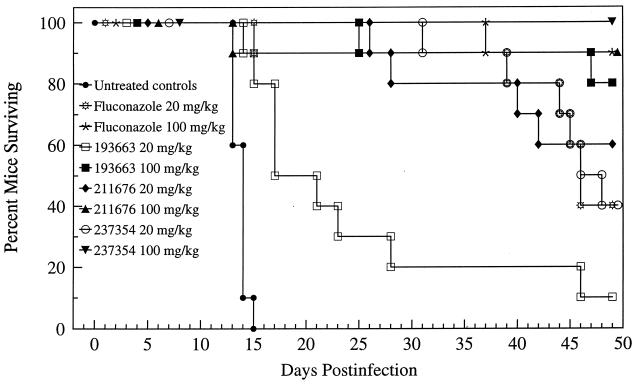
All drug regimens significantly prolonged survival versus the control group (P < 0.001); 80 to 100% of the mice given the 100-mg/kg doses of fluconazole or one of the GM drugs survived (Fig. 2). GM193663 at 20 mg/kg/day was the least efficacious regimen and was inferior to both fluconazole regimens (P < 0.05 and 0.001). All 100-mg/kg/day regimens were equivalent. At 20 mg/kg/day, GM211676 was equivalent to 100 mg of fluconazole/kg/day thus indicating that GM211676 was ∼5-fold more efficacious than fluconazole on the basis of milligrams per kilogram of body weight. Both GM193663 and GM237354 at 100 mg/kg/day were equivalent to 100 mg of fluconazole/kg/day with respect to the prolongation of survival.
FIG. 2.
Cumulative mortality of mice infected with C. immitis and given either no treatment or 19 days of therapy with fluconazole, GM193663, GM211676, or GM237354 at the indicated dosages. Therapy began on day 4 of infection.
Further comparisons of activity were made on the basis of recovery of C. immitis from the organs of surviving mice. No mice treated with fluconazole or with any of the GM compounds were free of infection (Table 2). Comparison of the residual burdens of organisms did show differences in efficacy. All drugs showed dose-responsive reduction of fungal burden in all three organs (Table 2). GM237354 at 100 mg/kg/day was superior to all other regimens in the reduction of burden in all organs (P < 0.01 to 0.001 depending on the comparison). The next most effective regimen was GM211676 at 100 mg/kg/day, which was more effective than 100 mg of fluconazole/kg/day in all three organs (P < 0.05). GM211676 was the only one of the four drugs that was efficacious at 20 mg/kg/day in comparison with controls, and it was so in all three organs (P < 0.05). Similarly, GM193663 at 100 mg/kg/day was effective, but it was superior to a 100-mg/kg/day dose of fluconazole only in the spleen (P < 0.05). No 20-mg/kg/day dose of a GM compound was superior to either dose of fluconazole in the reduction of infection burden.
TABLE 2.
Recovery of C. immitis from the organs of surviving mice treated with GM193663, GM211676, GM237354, or fluconazole
| Treatment group and dose (mg/kg) | No. of survivors/ no. of mice cured | Log10 geometric mean CFU in survivors (95% CI)a in:
|
||
|---|---|---|---|---|
| Spleen | Liver | Lung | ||
| Untreated | 0/0 | |||
| Fluconazole | ||||
| 20 | 4/0 | 5.05 (4.1–5.9) | 4.92 (4.1–5.7) | 5.99 (5.0–7.0) |
| 100 | 9/0 | 4.43 (4.0–4.8) | 4.20 (3.7–4.7) | 5.10 (4.7–5.5) |
| GM193663 | ||||
| 20 | 1/0 | 4.38 | 4.33 | 5.51 |
| 100 | 8/0 | 3.46 (2.9–4.0) | 3.72 (3.3–4.2) | 4.97 (4.7–5.3) |
| GM211676 | ||||
| 20 | 6/0 | 4.40 (3.3–5.5) | 3.75 (2.6–4.8) | 5.31 (4.3–6.3) |
| 100 | 9/0 | 3.49 (2.9–4.1) | 3.55 (3.3–3.8) | 4.50 (4.2–4.8) |
| GM237354 | ||||
| 20 | 4/0 | 4.34 (3.1–5.5) | 4.34 (3.9–4.8) | 5.42 (4.5–6.3) |
| 100 | 10/0 | 2.69 (2.2–3.1) | 3.06 (2.7–3.4) | 3.91 (3.6–4.3) |
CI, confidence interval. No survivors of the 49-day experiment were free of infection.
DISCUSSION
Coccidioidomycosis, especially when manifested as severe systemic or meningeal disease, requires therapeutic intervention. However, current therapies using azoles are often ineffective, with a high percentage of patients relapsing after the cessation of therapy (6). Thus, improved treatments are sorely needed. The sordarin compounds have a mechanism of action that is novel for antifungal compounds in that sordarin compounds inhibit protein synthesis (7, 8, 10).
The results of the present studies clearly demonstrate that sordarins show in vitro and in vivo activity against C. immitis. The in vitro results are similar to those previously reported for other fungi (12) and expand the spectrum of activity for this class of compound. In vitro, all three derivatives had MICs fourfold or more lower than that of fluconazole for the Silveira strain of C. immitis, which was used in the murine model. Unlike fluconazole, MFCs were obtainable for these compounds and were at least 16-fold or more lower. Among the new drugs tested, GM237354 showed greater activity, as demonstrated by the lowest MFC.
From the pharmacokinetics data we estimate that the t1/2 in serum for each of the GM compounds is <2 h after oral dosing. Similarly, GM193663 and GM237354 have been reported to have t1/2 in serum of <0.5 h after intravenous administration into mice (10). After a single 50-mg/kg dose, the serum levels were greater than the MIC by approximately 4- to 30-fold for at least 2 h after dosing. However, the serum levels of the GM compounds after chronic dosing indicated that drug levels may be only equivalent or up to 10-fold greater than the MIC for about 2 h after a dose is given. It should be noted that none of the GM compounds showed a steady-state accumulation with levels below those obtained after a single dosage. Metabolic studies on elimination remain to be done (10). Additional studies are required to obtain a better estimate of serum half-life and to determine more precise drug levels after oral administration.
In spite of the relatively short half-life of the GM compounds in serum, all were efficacious in the treatment of experimental systemic coccidioidomycosis. Each GM compound was at least equivalent or superior to fluconazole in efficacy. However, differences in efficacy among the GM compounds were noted. GM211676 was most active in the prolongation of survival, being about fivefold more active than fluconazole on the basis of milligrams per kilogram of body weight. Interestingly, this greater effectiveness was not demonstrated by GM211676 in reducing or clearing the animals of infectious burden in the target organs even at the 100-mg/kg dosage. GM237354 was found to be superior to all other treatments in the clearance of infectious burden from the organs. This difference in activity correlates well with the MFC value for each of the compounds, with GM237354 being the most active, GM211676 being less active, and GM193663 being the least active. Thus, it may be that the MFC for these compounds will indicate the in vivo efficacy.
In conclusion, C. immitis was susceptible both in vitro and in vivo to this new class of antifungal compound, the sordarins. In vivo, each of the three GM compounds tested were found to be equivalent or superior to fluconazole in the treatment of experimental systemic coccidioidomycosis. Although GM237354 at 100 mg/kg proved to be the most active of the drugs tested, modification of the dosing schedule to more frequent daily dosing (e.g., three times daily) to account for the short serum half-life might alter the comparative efficacies among the GM compounds as well as in comparison with fluconazole. Overall, these results are encouraging, indicating that further testing in other models of fungal disease is warranted.
ACKNOWLEDGMENT
This studies was funded in part by Glaxo S.A.
REFERENCES
- 1.Capa L, Mendoza A, Lavandera J L, Gomez De Las Heras F, Garcia-Bustos J F. Translation elongation factor 2 is part of the target for a new family of antifungals. Antimicrob Agents Chemother. 1998;42:2694–2699. doi: 10.1128/aac.42.10.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons K, Stevens D A. Comparative efficacy of amphotericin B colloidal dispersion and amphotericin B deoxycholate suspension in treatment of murine coccidioidomycosis. Antimicrob Agents Chemother. 1991;35:1829–1833. doi: 10.1128/aac.35.9.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemons K V, Hanson L H, Perlman A M, Stevens D A. Efficacy of SCH39304 and fluconazole in a murine model of disseminated coccidioidomycosis. Antimicrob Agents Chemother. 1990;34:928–930. doi: 10.1128/aac.34.5.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemons K V, Leathers C R, Lee K W. Systemic Coccidioides immitis infection in nude and beige mice. Infect Immun. 1985;47:814–821. doi: 10.1128/iai.47.3.814-821.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemons K V, Stevens D A. Efficacies of amphotericin B lipid complex (ABLC) and conventional amphotericin B against murine coccidioidomycosis. J Antimicrob Chemother. 1992;30:353–363. doi: 10.1093/jac/30.3.353. [DOI] [PubMed] [Google Scholar]
- 6.Dewsnup D H, Galgiani J N, Graybill J R, Diaz M, Rendon A, Cloud G A, Stevens D A. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med. 1996;124:305–310. doi: 10.7326/0003-4819-124-3-199602010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Domínguez J M, Kelly V A, Kinsman O S, Marriott M S, Gómez de las Heras F, Martín J J. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domínguez J M, Martín J J. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galgiani J N, Sun S H, Clemons K V, Stevens D A. Activity of cilofungin against Coccidioides immitis: differential in vitro effects on mycelia and spherules correlated with in vivo studies. J Infect Dis. 1990;162:944–948. doi: 10.1093/infdis/162.4.944. [DOI] [PubMed] [Google Scholar]
- 10.Gargallo-Viola D. Sordarins as antifungal compounds. Curr Opin Anti-infect Investig Drugs. 1999;1:297–305. [Google Scholar]
- 11.Graybill J R, Najvar L, Fothergill A, Bocanegra R, Gomez de las Heras F. Activities of sordarins in murine histoplasmosis. Antimicrob Agents Chemother. 1999;43:1716–1718. doi: 10.1128/aac.43.7.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herreros E, Martinez C M, Almela M J, Marriott M S, Gomez de las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivatives against pathogenic yeasts, Pneumocystis carinii, and filamentous fungi. Antimicrob Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokal R R, Rohlf F J. Biometry. 2nd ed. San Francisco, Calif: W. H. Freeman & Co.; 1981. [Google Scholar]
- 14.Tucker R M, Williams P L, Arathoon E G, Stevens D A. Treatment of mycoses with itraconazole. Ann N Y Acad Sci. 1988;544:451–470. doi: 10.1111/j.1749-6632.1988.tb40443.x. [DOI] [PubMed] [Google Scholar]