Abstract
Endoscopic submucosal dissection (ESD) is a curative treatment for superficial esophageal cancer with distinct advantages. However, esophageal stenosis after ESD remains a tough problem, especially after large circumferential proportion of esophageal mucosa is removed, which limits the wide use of ESD, especially in circumferential lesions. In this scenario, preventive procedures are highly recommended against post-ESD esophageal stenosis. However, the efficacy and safety of traditional prophylactic methods (steroids, metal and biodegradable stents, balloon dilation, radial incision, etc.) are not satisfactory and novel strategies need to be developed. Regenerative medicine has been showing enormous potential in the reconstruction of organs including the esophagus. In this review, we aimed to describe the current status of regenerative medicine in prevention of post-ESD esophageal stenosis. Cell injection, cell sheet transplantation, and extracellular matrix implantation have been proved effective. However, numerous obstacles still exist and further studies are necessary.
Key words: endoscopic submucosal dissection, esophageal stenosis, regenerative medicine
Introduction
Esophageal cancer is the seventh most frequent malignancy, which accounted for 544,000 deaths in 2020.[1] The role of endoscopic therapies (endoscopic mucosal resection [EMR], endoscopic submucosal dissection [ESD], etc.) has been widely recognized, since they have similar clinical outcomes with minimal complications and tissue damage.[2,3] Among the endoscopic procedures, ESD has evolved into a reliable treatment for early esophageal cancer due to excellent long-term outcomes, fewer adverse events, and better life quality,[4, 5, 6] while EMR could be considered only for small lesions less than 10 mm in size when the operator lacks experience with ESD.[7] The Japan Gastroenterological Endoscopy Society recommended ESD as potential curative therapy for esophageal lesions which are (1) cT1aN0M0-EP/ LPM non-circumferential lesions or clinical T1aN0M0-EP/LPM circumferential lesions with longitudinal length less than 5 cm and (2) cT1aN0M0-MM or cT1bN0M0-SM1 non-circumferential lesions.[8,9]
Unfortunately, however, esophageal stenosis remains a most annoying complication after ESD. The reported incidence of esophageal post-ESD stenosis is 7.1%–26.8%.[10, 11, 12, 13] Symptomatic esophageal stenosis and repeated endoscopic dilatation impair patients’ quality of life and predispose them to psychiatric problems, including mood disorder and suicidality.[14]
Risk factors for postoperative stenosis include larger size of esophageal lesions (longitudinal length and circumferential percentage), invasion into submucosa, failure of en bloc resection, and intraoperative muscular injury.[11, 12, 13, 15, 16, 17, 18, 19, 20, 21]
Numerous preventive and therapeutic approaches have been deployed against postoperative stenosis. Balloon dilation first showed promising effect, but the high risk of perforation was a major limitation.[22,23] Preemptive metal or self-degradable stent implantation was also applied, but was troubled with displacement, difficulty in removal, and disturbance of local surveillance of recurrence.[24, 25, 26, 27, 28] Radial incision has been reported to reduce treatment periods, but is also accompanied by high risk of perforation.[29,30] Currently, steroid therapy, including local application and systematic administration, is a widely used safe and effective protocol, while there is still concern regarding disseminated infection and deterioration of diabetes mellitus.[31, 32, 33, 34, 35, 36, 37]
Recently, regenerative medicine approaches are emerging as alternatives for the prevention of post-ESD esophageal stenosis. Regenerative medicine focuses on the “repair, replacement or regeneration of cells, tissues or organs … stimulate and support the body’s own self-healing capacity,”[38] and the central element of regenerative medicine is human cells.[39] When applied to this specific topic, regenerative medicine approaches might be defined as methods utilizing human cells or tissues to promote regeneration of esophageal mucosa and healing of iatrogenic wound to prevent postoperative esophageal stenosis. Here, we summarize studies in this field to shed light on these promising solutions (Figure 1).
Figure 1.
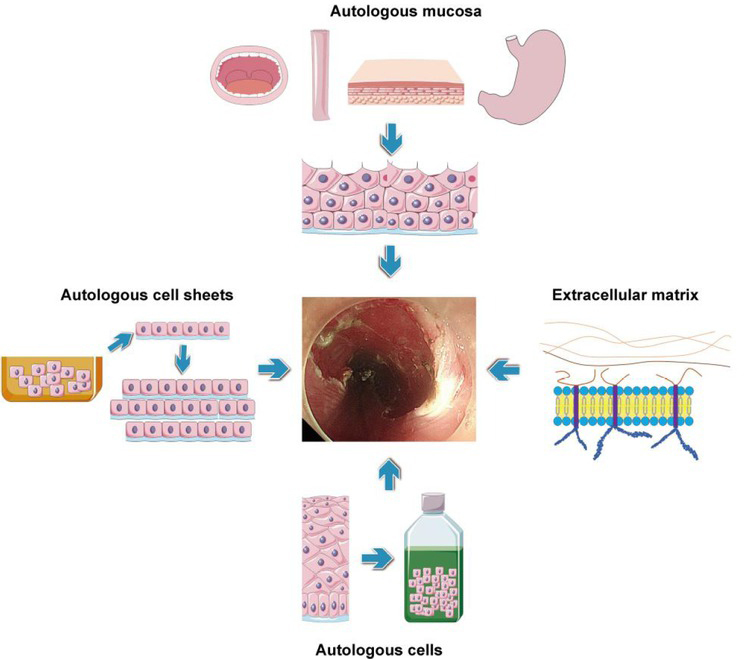
Regenerative medicine approaches in the prevention of post-ESD esophageal stenosis. ESD: endoscopic submucosal dissection.
Autologous cell implantation
There have been a few animal studies of cell therapy with different cell origins. However, there have been no reports of clinical trials of cell therapy in human beings so far.
Autologous keratinocyte implantation
Zuercher et al.[40] evaluated the feasibility of autologous keratinocytes to prevent esophageal stenosis in the sheep model with circumferential esophageal mucosal defect. Briefly, the keratinocytes were isolated from sheep skin, cultured in optimal conditions. A circumferential mucosal defect with over 6 cm longitudinal length was created, after which the keratinocyte suspension was injected into the mucosal defect at multiple sites. The procedure was safe with no serious complications (massive bleeding, perforation). None of the animals developed strictures. Microscopic analysis showed fibrosis in 10% (range 0–25%) of the circumferential muscularis propria interna layer and 7.2% (range 0–25%) of the muscularis propria externa layer, 6 months after the operation. No circumferential transmural fibrosis was identified.
Sakurai et al.[41] applied keratinocytes from buccal mucosa to the esophageal mucosal defect after ESD in four pigs. Ulcer, scar formation, and contraction were observed in the control sites, while no stricture was observed after injection of autologous buccal keratinocytes. They suggested that autologous keratinocyte injection accelerated re-epithelialization and prevented deep ulceration and inflammation.
Adipose tissue–derived stromal cell (ADSC) implantation
Perrod et al.[42] conducted a comparative study in a porcine model to evaluate the effectiveness of ADSC in preventing post-ESD esophageal stenosis. Firstly, the ADSC was isolated, cultured, coated with a poly-N-isopropylacrylamide membrane, and transferred onto paper support membrane to construct a double-layer structure. Twelve female pigs were treated with ESD, which resected 5 cm length of hemi-circumferential esophageal mucosa, and were randomized into two groups. The ADSC group (n = 6) received four double-cell sheets of allogenic ADSC, while the control group (n = 6) received four paper support membranes without ADSCs. Clinical and endoscopic follow-ups were performed at days 3, 14, and 28 after surgery. Pigs from the ADSC group showed less-frequent alimentary trouble (e.g., regurgitation, vomiting), and endoscopic evaluation showed that one out of six (17%) animals developed a severe esophageal stricture, which was much less compared with the control group (5/5). Upper gastrointestinal contrast imaging demonstrated a lower degree of stricture in the ADSC group on day 14 and day 28. Histological analysis revealed decreased fibrosis development in the ADSC group, both on the surface and in maximal depth. The transplantation of allogenic ADSCs, organized in double-cell sheets, after extended ESD was successful and strongly associated with a lower esophageal stricture rate.
Autologous cell sheets
Although the injection methods were effective, direct injection of cells into the host organ has some disadvantages of low viability and rapid diffusion. The cell sheets may help solve the problem of rapid loss. Application of autologous cells sheets has been widely explored and has shown some promising results in both animal studies and clinical practice. Studies in this field were categorized based on the origin of cell sheets.
Fabricated autologous epidermal cell sheets
Preemptive epidermal cell sheet (ECS) transplantation immediately after large-scale ESD has been shown to be safe and effective in the prevention of esophageal strictures. Kanai et al.[43] applied cultured autologous ECS in pigs to evaluate the efficacy to prevent severe esophageal stricture after circumferential ESD. ECS was isolated from lower abdomen skin and cultured on temperature-responsive inserts. After circumferential esophageal ESD, ECS sheets were applied to the mucosal defect. All pigs in the control group developed severe esophageal stenosis after 2 weeks. The mean degrees of constriction were 88% and 56% in the control and ECS groups, respectively. Early re-epithelialization and mild fibrosis in the muscularis were observed in the ECS transplanted group.
Another proof-of-concept study conducted by Kobayashi et al.[44] evaluated the efficacy of ECS transplantation plus endoscopic balloon dilation (EBD) in a porcine model. After circumferential esophageal ESD, two pigs received EBD plus ECS transplantation, two pigs received only EBD, and the other two pigs received only endoscopic evaluation. The stricture rates were 55 % and 60 %, respectively, in the ECS transplantation group, 92.2 % and 87.7 %, respectively, in the control group, and 71.7 % and 78.2 %, respectively, in the EBD group. Histological analysis showed lowest infiltration of inflammatory cells in the ECS transplantation group compared with the control and EBD-treated pigs. Additionally, inflammation of ulcer sites was weakened while atrophy and fibrosis of LPM. No adverse events were observed. The results showed the importance of protection of MP layer from inflammation at the ulcer sites.
Autologous oral mucosal epithelial cell sheets
The idea of utilization of autologous oral mucosal epithelial cell sheets was first proposed by Takagi et al.[45] in 2011 to evaluate their efficacy to treat iatrogenic ulcer after esophageal ESD. Human oral mucosal epithelial cell (hOMEC) was harvested and isolated from healthy volunteers and cultured into cell sheets. After hemi-circumferential ESD was performed, the cells sheets were deployed by endoscopy on the artificial ulcers. Microscopic observation revealed that hOMEC sheets were successfully attached to the ulcer surface, which proved the feasibility of hOMEC as a medical device that promotes repair of esophageal ulcers after ESD.
Ohki et al.[46] advanced the research of hOMEC into human trials. They collected small pieces of oral mucosa from nine patients who were diagnosed with superficial esophageal neoplasms and scheduled for esophageal ESD. After fabrication and incubation ex vivo for 16 days, these sheets were endoscopically implanted to the mucosal defect immediately after ESD. Weekly postoperative endoscopic examination revealed that complete re-epithelialization of the artificial occurred within 3.5 weeks (median time). No patients presented with symptoms of esophageal stricture, including dysphagia, stricture, or other complications.
Jonas et al.[47] conducted similar research with tissue-engineered oral cell sheets and concluded that cell sheet transplantation was safe and effective to protect the patients from post-ESD esophageal stenosis.
Autologous gastric mucosa transplantation
Hochberger et al.[48] first attempted to use autologous gastric mucosa to cover post-ESD mucosal defects in a 72-year-old man who suffered from circumferential high-grade intraepithelial neoplasia (HGIN) in the cervical esophagus. The esophageal lesion was removed by ESD en bloc, leaving a mucosal defect of 10 cm in size. The gastric mucosa was gathered by a second ESD of the anterior wall of gastric antrum and was then divided into three small pieces and attached to the esophageal mucosal defect and fixed with clips and an uncovered metal mesh stent. The stent was removed 20 days after the operation. Within 5 months after ESD and gastric mucosa transplantation, most segments of the circumferential mucosal defect healed with no obvious strictures. However, stenosis occurred in the uppermost 1 cm area which was not covered by gastric mucosa due to technical reasons. The patient was followed up for 32 months after surgery and there were no more complications.
Autologous esophageal mucosa transplantation
Liao et al.[49] reported their study using transplantation of autologous esophageal mucosa to prevent post-ESD stricture. Nine patients who underwent circumferential ESD for early esophageal cancer were enrolled. Autologous esophageal mucosa was harvested by EMR from esophageal sites away from the lesion and cut into small pieces. The patches were attached to the ulcer surface by hemoclips and were finally fixed with a covered metal mesh stent, which was removed 7 days after the procedure. Results showed rapid epithelialization with high graft survival rate. Unfortunately, however, strictures occurred in eight out of nine patients, which required repeated endoscopic balloon dilatation sessions. This study proved that autologous esophageal mucosa transplantation cannot fully avoid post-ESD stricture, but could reduce the severity as patients with transplantation required less balloon dilation sessions. A major limitation regarding this study was that harvesting normal esophageal mucosa may induce undesired stricture formation or extended hospitalization.[50]
Liu et al.[51] also reported the application of autologous esophageal mucosa transplantation to prevent esophageal stenosis after circumferential ESD. A total of 25 patients were enrolled and 14 of them did not develop stenosis, which suggested that autologous esophageal mucosa transplantation might be an effective solution to prevent post-ESD stenosis.
Autologous skin graft
Chai et al.[52] explored the application of autologous skin graft in preventing esophageal stenosis after circumferential endoscopic submucosal tunnel dissection (ESTD) of superficial esophageal lesions in eight patients. Before ESTD, patients’ skin was harvested from their thigh and shaped like oversleeve, which was then applied over the surface of a fully covered esophageal stent. After ESTD, the system was deployed at the site of dissection with help of an overtube. During a median follow-up of 7 months, only three patients developed stenosis, which was treated with balloon dilatation. This study proved the efficacy of autologous skin graft in preventing circumferential mucosal dissection and provided a feasible solution for implantation of similar materials.
Extracellular matrix scaffold
Extracellular matrix (ECM) is an indispensable branch of regenerative medicine, which refers to the remaining components after decellularization of tissues or organs.[53] ECM biomaterials have been proved effective in promoting angiogenesis, wound healing, nerve regeneration, and bone regeneration.[54, 55, 56, 57]
Nieponice et al.[58] started exploration using ECM as a preventive method against post-ESD esophageal stenosis. Briefly, porcine urinary bladder was harvested, trimmed, delaminated, decellularized, fabricated, and disinfected to produce sterile ECM tubular stents, which matched the shape of esophagus of dogs. Five dogs then underwent circumferential ESD at the cervical esophagus segment, and ECM stents were implanted with endoscope, positioned with a specific balloon, and fixed with degradable adhesive, while five other dogs underwent circumferential ESD without placement of ECM stents, which served as the control group. After 2 months of observation, three out of five dogs developed esophageal stricture in the control group, while no animals suffered from esophageal stenosis after ECM stents’ implantation. Microscopic observation of the tissues revealed that the mucosal structure was similar to normal esophageal tissue with intact mucosa and inflammation was at a minimum level. Submucosal layers were filled with well-organized connective tissue and blood vessels.
However, several reports indicated that deployment of ECM might not be satisfactory in preventing post-ESD stenosis. Schomisch et al.[59] compared the effects of stents covered with ECM, uncovered stents alone, acellular dermal matrix, and urinary bladder matrix. The conclusion was that commercially available ECM biomaterial did not prevent postoperative esophageal stenosis. Badylak et al.[60] attempted to use ECM scaffolds in a clinical scenario. They deployed ECM as well as a radially expanding stent in five patients who suffered from esophageal superficial cancer and underwent circumferential resection. Histological analysis showed that ECM was completely replaced by normal esophageal squamous epithelium 4 months after the operation. However, all five patients developed esophageal stricture, which was treated with balloon dilation.
Discussion
Postoperative esophageal stenosis has been a complicated adverse event since the introduction of ESD. Although numerous attempts have been made to solve the problem permanently,[61, 62, 63, 64, 65, 66] none of them is perfectly effective.[67]
In order to develop satisfactory methods to prevent post-ESD esophageal stenosis, we must try to unveil the healing process after ESD and the underlying mechanism of stenosis formation. Honda et al.[68] found that after mucosal resection, ulcer formation and inflammatory cell invasion began in the submucosa layer on day 2 and 4 after the operation, respectively. Formation of new vessels was noticed 7 days after resection of the mucosal layer. After 28 days, the ulcer was completely repaired by re-epithelialization, and notably, fibrosis of the LPM occurred almost at the same time. Nonaka et al.[69] compared the histological changes after esophageal ESD with and without steroid injection. In animals without steroid injection, myofibroblasts were arranged in a parallel fashion, which extended horizontally at the base of ulcer. Three weeks after ESD, luminal stenosis appeared with spindle-shaped myofibroblasts’ proliferation covered with regenerated epithelium. On the contrary, the stromal cells were randomly dispersed in the granulation tissue and no obvious stricture was noticed after steroid injection. These findings were confirmed by Kawamura et al.,[70] who also noticed that subepithelial fibrous tissue was much thicker in patients without steroid treatment. With these findings, we might deduce that the migration and aggregation of inflammatory cells, proliferation of spindle-shaped myofibroblasts, and hyperplasia of subepithelial fibrosis tissue play important roles in the formation of post-ESD esophageal stenosis.
Regenerative medicine has been widely applied in the research of liver diseases,[71] neurodegenerative diseases,[72] colorectal pathologies,[73] cardiac repair,[74] tendon healing,[75] and so on. The key concept of regenerative medicine is to stimulate and support the body’s own self-healing capacity by cell-based biomaterials (cell suspension, cell sheets, ECM scaffold, etc.). The intention is to induce cell proliferation, differentiation, re-epithelization, and formation of normal esophageal structure, while inhibiting inflammation and fibrosis process by applying these materials.[76] Notably, the efforts of regeneration of esophagus have never stopped since the year 2000.[77, 78, 79, 80, 81, 82, 83, 84, 85]
Autologous cell transplantation is easy to apply by endoscope, which only requires an injection needle. Keratinocytes could either proliferate and form the epithelial linings by themselves or stimulate the adjacent squamous cells to repair the epithelium defects.[40] ADSC brings anti-inflammatory effect and induces neovascularization and mesenchymal differentiation.[42] However, the material cannot be dispersed homogeneously over the defect, which may reduce its effect. Moreover, the cell injection cannot provide mechanical shield against adverse factors inside esophageal lumen, including gastric acid, food, and so on.
Autologous cell sheets and skin graft have been proved to be safe and effective in the prevention of post-ESD stenosis. Specifically, the advantages come from the convenience and safety of the harvesting process and absence of immunogenicity.[45,47,86] Although the transplantation process may be time consuming, a novel device has been developed to shorten the procedures.[87] The presence of sheets as cells’ carrier guarantees the even distribution and longer preservation over the surface of ulcers.
The preparation of ECM biomaterials is a process to remove the resident cells in the tissue, while keeping the proteins and other derivatives. After the decellularization process, immunogenicity was greatly reduced, endowing the material better histocompatibility.[53] Compared with artificial stents, ECM scaffolds exhibit better capacity to reduce inflammation and promote ulcer healing.[88]
Nevertheless, there are a few obstacles ahead. Firstly, a large proportion of the methods mentioned above are still considered experimental with evidence from only animal studies and the effect might be altered in human beings due to the difference in anatomy. Secondly, the feasibility of certain materials is still controversial (e.g., ECM). Thirdly, most of the biomaterials are not commercially available and require complicated processes of preparation. The lack of standardized protocols and quality control measures might lead to unstable prognosis of the patients. To settle these problems, clinical trials including larger population should be performed to evaluate the efficacy and safety of regenerative medicine therapies in human beings. Besides, standard operating procedures (SOPs) should be established as soon as possible to simplify the production and implantation process in order to achieve stable effect.
Conclusion
Regenerative medicine provides feasible and promising solutions for post-ESD esophageal stenosis, but more large-scale clinical trials are still necessary for evaluation of the safety and efficacy in human beings. To maximize the effort to ameliorate post-ESD stenosis, SOPs with personalized strategies should be arranged for each patient.
Footnotes
Conflict of Interest
None declared.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. et al. [DOI] [PubMed] [Google Scholar]
- 2.Wani S, Drahos J, Cook M, Rastogi A, Bansal A, Yen R. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc. 2014;79:224–32. doi: 10.1016/j.gie.2013.08.002. et al. e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das A, Singh V, Fleischer D, Sharma V. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340–5. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol. 2005;3:S74–6. doi: 10.1016/s1542-3565(05)00254-5. [DOI] [PubMed] [Google Scholar]
- 5.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. et al. [DOI] [PubMed] [Google Scholar]
- 6.Yeh JH, Huang RY, Lee CT, Lin CW, Hsu MH, Wu TC. Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2020;13:1756284820964316. doi: 10.1177/1756284820964316. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CH, Yang DH, Kim JW, Kim JH, Kim JH, Min YW. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin Endosc. 2020;53:142–66. doi: 10.5946/ce.2020.032. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara R, Arima M, Iizuka T, Oyama T, Katada C, Kato M. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020;32:452–93. doi: 10.1111/den.13654. et al. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara R. Indications of esophageal cancer for endoscopic submucosal dissection, curability, and future perspectives. Mini-invasive Surgery. 2021;5:36. [Google Scholar]
- 10.Yu X, Liu Y, Xue L, He S, Zhang Y, Dou L. Risk factors for complications after endoscopic treatment in Chinese patients with early esophageal cancer and precancerous lesions. Surg Endosc. 2020;35:214453. doi: 10.1007/s00464-020-07619-z. et al. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Dang Y, Ding C, Yang J, Si X, Zhang G. Lesion size and circumferential range identified as independent risk factors for esophageal stricture after endoscopic submucosal dissection. Surg Endosc. 2020;34:4065–71. doi: 10.1007/s00464-020-07368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funakawa K, Uto H, Sasaki F, Nasu Y, Mawatari S, Arima S. Effect of endoscopic submucosal dissection for superficial esophageal neoplasms and risk factors for postoperative stricture. Medicine (Baltimore) 2015;94:e373. doi: 10.1097/MD.0000000000000373. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775–83. doi: 10.1055/s-0034-1391844. et al. [DOI] [PubMed] [Google Scholar]
- 14.Anand N, Sharma A, Shah J, Kochhar R, Singh SM. Quality of life in patients of corrosive esophageal stricture treated with endoscopic dilatation. JGH Open. 2021;5:301–6. doi: 10.1002/jgh3.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:62631. doi: 10.1111/j.1442-2050.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagami Y, Ominami M, Shiba M, Sakai T, Fukunaga S, Sugimori S. Prediction of esophageal stricture in patients given locoregional triamcinolone injections immediately after endoscopic submucosal dissection. Dig Endosc. 2018;30:198–205. doi: 10.1111/den.12946. et al. [DOI] [PubMed] [Google Scholar]
- 17.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661–5. doi: 10.1055/s-0029-1214867. et al. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640–4. doi: 10.1055/s-0034-1365648. et al. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Fujiya M, Ueno N, Saito T, Sugiyama Y, Murakami Y. White coat status is a predictive marker for post-esophageal endoscopic submucosal dissection stricture: a retrospective study. Esophagus. 2019;16:258–63. doi: 10.1007/s10388-019-00659-y. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Liu Y, Xue L, He S, Zhang Y, Dou L. Risk factors for complications after endoscopic treatment in Chinese patients with early esophageal cancer and precancerous lesions. Surg Endosc. 2021;35:214453. doi: 10.1007/s00464-020-07619-z. et al. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Kong F, Li J, Liu F, Kong X, Li Z. Independent risk factors for esophageal refractory stricture after extensive endoscopic submucosal dissection. Surg Endosc. 2021;35:3618–27. doi: 10.1007/s00464-020-07840-w. [DOI] [PubMed] [Google Scholar]
- 22.Yoda Y, Yano T, Kaneko K, Tsuruta S, Oono Y, Kojima T. Endoscopic balloon dilatation for benign fibrotic strictures after curative nonsurgical treatment for esophageal cancer. Surg Endosc. 2012;26:2877–83. doi: 10.1007/s00464-012-2273-9. et al. [DOI] [PubMed] [Google Scholar]
- 23.Kishida Y, Kakushima N, Kawata N, Tanaka M, Takizawa K, Imai K. Complications of endoscopic dilation for esophageal stenosis after endoscopic submucosal dissection of superficial esophageal cancer. Surg Endosc. 2015;29:2953–9. doi: 10.1007/s00464-014-4028-2. et al. [DOI] [PubMed] [Google Scholar]
- 24.Hair CS, Devonshire DA. Severe hyperplastic tissue stenosis of a novel biodegradable esophageal stent and subsequent successful management with high-pressure balloon dilation. Endoscopy. 2010;42:E132–3. doi: 10.1055/s-0029-1244011. [DOI] [PubMed] [Google Scholar]
- 25.Orive-Calzada A, Alvarez-Rubio M, Romero-Izquierdo S, Cobo Martin M, Juanmartiñena JF, Ogueta-Fernández M. Severe epithelial hyperplasia as a complication of a novel biodegradable stent. Endoscopy. 2009;41:E137–8. doi: 10.1055/s-0029-1214634. et al. [DOI] [PubMed] [Google Scholar]
- 26.Wen J, Lu Z, Yang Y, Liu Q, Yang J, Wang S. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig Dis Sci. 2014;59:658–63. doi: 10.1007/s10620-013-2958-5. et al. [DOI] [PubMed] [Google Scholar]
- 27.Hirdes M, Siersema P, Vleggaar F. A new fully covered metal stent for the treatment of benign and malignant dysphagia: a prospective follow-up study. Gastrointest Endosc. 2012;75:712–8. doi: 10.1016/j.gie.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Talreja J, Eloubeidi M, Sauer B, Al-Awabdy B, Lopes T, Kahaleh M. Fully covered removable nitinol self-expandable metal stents (SEMS) in malignant strictures of the esophagus: a multicenter analysis. Surg Endosc. 2012;26:1664–9. doi: 10.1007/s00464-011-2089-z. et al. [DOI] [PubMed] [Google Scholar]
- 29.Muto M, Ezoe Y, Yano T, Aoyama I, Yoda Y, Minashi K. Usefulness of endoscopic radial incision and cutting method for refractory esophagogastric anastomotic stricture (with video) Gastrointest Endosc. 2012;75:965–72. doi: 10.1016/j.gie.2012.01.012. et al. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira JF, Moura EG, Bernardo WM, Ide E, Cheng S, Sulbaran M. Prevention of esophageal stricture after endoscopic submucosal dissection: a systematic review and meta-analysis. Surg Endosc. 2016;30:2779–91. doi: 10.1007/s00464-015-4551-9. et al. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:111521. doi: 10.1016/j.gie.2011.02.005. et al. [DOI] [PubMed] [Google Scholar]
- 32.Pih GY, Kim DH, Gong EJ, Na HK, Jung KW, Lee JH. Preventing esophageal strictures with steroids after endoscopic submucosal dissection in superficial esophageal neoplasm. J Dig Dis. 2019;20:609–16. doi: 10.1111/1751-2980.12819. et al. [DOI] [PubMed] [Google Scholar]
- 33.Kadota T, Yoda Y, Hori K, Shinmura K, Oono Y, Ikematsu H. Prophylactic steroid administration against strictures is not enough for mucosal defects involving the entire circumference of the esophageal lumen after esophageal endoscopic submucosal dissection (ESD) Esophagus. 2020;17:440–7. doi: 10.1007/s10388-020-00730-z. et al. [DOI] [PubMed] [Google Scholar]
- 34.Hanaoka N, Ishihara R, Takeuchi Y, Uedo N, Higashino K, Ohta T. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy. 2012;44:1007–11. doi: 10.1055/s-0032-1310107. et al. [DOI] [PubMed] [Google Scholar]
- 35.Chu Y, Chen T, Li H, Zhou P, Zhang Y, Chen W. Long-term efficacy and safety of intralesional steroid injection plus oral steroid administration in preventing stricture after endoscopic submucosal dissection for esophageal epithelial neoplasms. Surg Endosc. 2019;33:1244–51. doi: 10.1007/s00464-018-6404-9. et al. [DOI] [PubMed] [Google Scholar]
- 36.Iizuka T, Kikuchi D, Hoteya S, Kaise M. Effectiveness of modified oral steroid administration for preventing esophageal stricture after entire circumferential endoscopic submucosal dissection. Dis Esophagus. 2018. p. 31. [DOI] [PubMed]
- 37.Ishida T, Morita Y, Hoshi N, Yoshizaki T, Ohara Y, Kawara F. Disseminated nocardiosis during systemic steroid therapy for the prevention of esophageal stricture after endoscopic submucosal dissection. Dig Endosc. 2015;27:388–91. doi: 10.1111/den.12317. et al. [DOI] [PubMed] [Google Scholar]
- 38.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Daar AS, Greenwood HL. A proposed definition of regenerative medicine. J Tissue Eng Regen Med. 2007;1:179–84. doi: 10.1002/term.20. [DOI] [PubMed] [Google Scholar]
- 40.Zuercher BF, George M, Escher A, Piotet E, Ikonomidis C, Andrejevic SB. Stricture prevention after extended circumferential endoscopic mucosal resection by injecting autologous keratinocytes in the sheep esophagus. Surg Endosc. 2013;27:1022–8. doi: 10.1007/s00464-012-2509-8. et al. [DOI] [PubMed] [Google Scholar]
- 41.Sakurai T, Miyazaki S, Miyata G, Satomi S, Hori Y. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest Endosc. 2007;66:167–73. doi: 10.1016/j.gie.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 42.Perrod G, Rahmi G, Pidial L, Camilleri S, Bellucci A, Casanova A. Cell Sheet Transplantation for Esophageal Stricture Prevention after Endoscopic Submucosal Dissection in a Porcine Model. PLoS One. 2016;11:e0148249. doi: 10.1371/journal.pone.0148249. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanai N, Yamato M, Ohki T, Yamamoto M, Okano T. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc. 2012;76:873–81. doi: 10.1016/j.gie.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi S, Kanai N, Tanaka N, Maeda M, Hosoi T, Fukai F. Transplantation of epidermal cell sheets by endoscopic balloon dilatation to avoid esophageal re-strictures: initial experience in a porcine model. Endosc Int Open. 2016;4:E1116–23. doi: 10.1055/s-0042-116145. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi R, Yamato M, Murakami D, Kondo M, Ohki T, Sasaki R. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology. 2011;78:311–9. doi: 10.1159/000322575. et al. [DOI] [PubMed] [Google Scholar]
- 46.Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–8. doi: 10.1053/j.gastro.2012.04.050. et al. e582. [DOI] [PubMed] [Google Scholar]
- 47.Jonas E, Sjöqvist S, Elbe P, Kanai N, Enger J, Haas SL. Transplantation of tissue-engineered cell sheets for stricture prevention after endoscopic submucosal dissection of the oesophagus. United European Gastroenterol J. 2016;4:741–53. doi: 10.1177/2050640616631205. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hochberger J, Koehler P, Wedi E, Gluer S, Rothstein RI, Niemann H. Transplantation of mucosa from stomach to esophagus to prevent stricture after circumferential endoscopic submucosal dissection of early squamous cell. Gastroenterology. 2014;146:906–9. doi: 10.1053/j.gastro.2014.01.063. et al. [DOI] [PubMed] [Google Scholar]
- 49.Liao Z, Liao G, Yang X, Peng X, Zhang X, Xie X. Transplantation of autologous esophageal mucosa to prevent stricture after circumferential endoscopic submucosal dissection of early esophageal cancer (with video) Gastrointest Endosc. 2018;88:543–6. doi: 10.1016/j.gie.2018.04.2349. et al. [DOI] [PubMed] [Google Scholar]
- 50.Zhai W, Zhao L, Fan Z. Concerns about transplantation of autologous esophageal mucosa to prevent stricture after circumferential endoscopic submucosal dissection. Gastrointest Endosc. 2018;88:969. doi: 10.1016/j.gie.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Li Z, Dou L, Zhang Y, He S, Zhu J. Autologous esophageal mucosa with polyglycolic acid transplantation and temporary stent implantation can prevent stenosis after circumferential endoscopic submucosal dissection. Ann Transl Med. 2021;9:546. doi: 10.21037/atm-20-6987. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chai N, Zou J, Linghu E, Chai M, Li L, Wang X. Autologous Skin-Grafting Surgery to Prevent Esophageal Stenosis After Complete Circular Endoscopic Submucosal Tunnel Dissection for Superficial Esophageal Neoplasms. Am J Gastroenterol. 2019;114:822–5. doi: 10.14309/ajg.0000000000000169. et al. [DOI] [PubMed] [Google Scholar]
- 53.Aamodt JM, Grainger DW. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016;86:68–82. doi: 10.1016/j.biomaterials.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong C, Qiao F, Chen G, Lv Y. Demineralized and decellularized bone extracellular matrix-incorporated electrospun nanofibrous scaffold for bone regeneration. J Mater Chem B. 2021;9:6881–94. doi: 10.1039/d1tb00895a. [DOI] [PubMed] [Google Scholar]
- 55.Vriend L, Sinkunas V, Camargo CP, van der Lei B, Harmsen MC, van Dongen JA. Extracellular matrix-derived hydrogels to augment dermal wound healing: a systematic review. Tissue Eng Part B Rev 2021.[Online ahead of print] [DOI] [PubMed]
- 56.Hosseinabadi M, Abdolmaleki Z, Beheshtiha SHS. Cardiac aorta-derived extracellular matrix scaffold enhances critical mediators of angiogenesis in isoproterenol-induced myocardial infarction mice. J Mater Sci Mater Med. 2021;32:134. doi: 10.1007/s10856-021-06611-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meder T, Prest T, Skillen C, Marchal L, Yupanqui VT, Soletti L. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen Med. 2021;6:69. doi: 10.1038/s41536-021-00174-8. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nieponice A, McGrath K, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:28996. doi: 10.1016/j.gie.2008.04.022. et al. [DOI] [PubMed] [Google Scholar]
- 59.Schomisch SJ, Yu L, Wu Y, Pauli EM, Cipriano C, Chak A. Commercially available biological mesh does not prevent stricture after esophageal mucosectomy. Endoscopy. 2014;46:144–8. doi: 10.1055/s-0033-1344997. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643–50. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Wijkerslooth L, Vleggaar F, Siersema P. Endoscopic management of difficult or recurrent esophageal strictures. Am J Gastroenterol. 2011;106:2080–91. doi: 10.1038/ajg.2011.348. [DOI] [PubMed] [Google Scholar]
- 62.Kono M, Nagami Y, Fujiwara Y. Easier attachment technique of polyglycolic acid sheet using thin-endoscope for prevention of stricture after esophageal endoscopic submucosal dissection. Dig Endosc. 2021;33:e114–6. doi: 10.1111/den.14008. [DOI] [PubMed] [Google Scholar]
- 63.Ni W, Lin S, Bian S, Xiao M, Wang Y, Yang Y. Biological testing of chitosan-collagen-based porous scaffolds loaded with PLGA/ Triamcinolone microspheres for ameliorating endoscopic dissection-related stenosis in oesophagus. Cell Prolif. 2021;54:e13004. doi: 10.1111/cpr.13004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oumrani S, Barret M, Beuvon F, Nicco C, Chêne C, Batteux F. Prevention of esophageal stricture after circumferential endoscopic submucosal dissection using a modified self-assembling peptide. Dis Esophagus. 2021;34:doaa133. doi: 10.1093/dote/doaa133. et al. [DOI] [PubMed] [Google Scholar]
- 65.Xiang J, Linghu E, Li L, Zou J, Wang X, Chai N. Utility of radial incision and cutting with steroid injection for refractory stricture after endoscopic submucosal dissection for large superficial esophageal squamous cell carcinoma. Surg Endosc. 2021;35:6930–7. doi: 10.1007/s00464-020-08204-0. [DOI] [PubMed] [Google Scholar]
- 66.Sakaguchi Y, Tsuji Y, Shinozaki T, Ohki D, Mizutani H, Minatsuki C. Steroid injection and polyglycolic acid shielding to prevent stricture after esophageal endoscopic submucosal dissection: a retrospective comparative analysis (with video) Gastrointest Endosc. 2020;92:1176–86. doi: 10.1016/j.gie.2020.04.070. et al. e1171. [DOI] [PubMed] [Google Scholar]
- 67.Hikichi T, Nakamura J, Takasumi M, Hashimoto M, Kato T, Kobashi R. Prevention of Stricture after Endoscopic Submucosal Dissection for Superficial Esophageal Cancer: A Review of the Literature. J Clin Med. 2020;10:20. doi: 10.3390/jcm10010020. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honda M, Nakamura T, Hori Y, Shionoya Y, Nakada A, Sato T. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy. 2010;42:1092–5. doi: 10.1055/s-0030-1255741. et al. [DOI] [PubMed] [Google Scholar]
- 69.Nonaka K, Miyazawa M, Ban S, Aikawa M, Akimoto N, Koyama I. Different healing process of esophageal large mucosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol. 2013;13:72. doi: 10.1186/1471-230X-13-72. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawamura Y, Kawada K, Ito T, Saito K, Fujiwara N, Okada T. Histological changes in the human esophagus following triamcinolone injection to prevent esophageal stricture after endoscopic submucosal dissection. Esophagus. 2021;18:594–603. doi: 10.1007/s10388-021-00818-0. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali S, Haque N, Azhar Z, Saeinasab M, Sefat F. Regenerative Medicine of Liver: Promises, Advances and Challenges. Biomimetics (Basel) 2021;6:62. doi: 10.3390/biomimetics6040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vatsa P, Negi R, Ansari UA, Khanna VK, Pant AB. Insights of Extracellular Vesicles of Mesenchymal Stem Cells: a Prospective Cell-Free Regenerative Medicine for Neurodegenerative Disorders. Mol Neurobiol. 2022;59:45974. doi: 10.1007/s12035-021-02603-7. [DOI] [PubMed] [Google Scholar]
- 73.Kent I, Freund MR, Agarwal S, Wexner SD. The application of regenerative medicine in colorectal surgery. Surgery. 2021. [Online ahead of print] [DOI] [PubMed]
- 74.Sid-Otmane C, Perrault LP, Ly HQ. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J Transl Med. 2020;18:336. doi: 10.1186/s12967-020-02504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lakhani A, Sharma E, Kapila A, Khatri K. Known data on applied regenerative medicine in tendon healing. Bioinformation. 2021;17:514–27. doi: 10.6026/97320630017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sjoqvist S, Kasai Y, Shimura D, Ishikawa T, Ali N, Iwata T. Oral keratinocyte-derived exosomes regulate proliferation of fibroblasts and epithelial cells. Biochem Biophys Res Commun. 2019;514:706–12. doi: 10.1016/j.bbrc.2019.04.202. et al. [DOI] [PubMed] [Google Scholar]
- 77.Isch JA, Engum SA, Ruble CA, Davis MM, Grosfeld JL. Patch esophagoplasty using AlloDerm as a tissue scaffold. J Pediatr Surg. 2001;36:266–8. doi: 10.1053/jpsu.2001.20685. [DOI] [PubMed] [Google Scholar]
- 78.Sakurai T, Miyazaki S, Miyata G, Satomi S, Hori Y. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest Endosc. 2007;66:167–73. doi: 10.1016/j.gie.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 79.Lynen Jansen P, Klinge U, Anurov M, Titkova S, Mertens PR, Jansen M. Surgical mesh as a scaffold for tissue regeneration in the esophagus. Eur Surg Res. 2004;36:104–11. doi: 10.1159/000076650. [DOI] [PubMed] [Google Scholar]
- 80.Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–10. doi: 10.1136/gut.2005.088518. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doede T, Bondartschuk M, Joerck C, Schulze E, Goernig M. Unsuccessful alloplastic esophageal replacement with porcine small intestinal submucosa. Artif Organs. 2009;33:328–33. doi: 10.1111/j.1525-1594.2009.00727.x. [DOI] [PubMed] [Google Scholar]
- 82.Chian KS, Leong MF, Kono K. Regenerative medicine for oesophageal reconstruction after cancer treatment. Lancet Oncol. 2015;16:e84–92. doi: 10.1016/S1470-2045(14)70410-3. [DOI] [PubMed] [Google Scholar]
- 83.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. et al. [DOI] [PubMed] [Google Scholar]
- 84.Urbani L, Camilli C, Phylactopoulos DE, Crowley C, Natarajan D, Scottoni F. Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat Commun. 2018;9:4286. doi: 10.1038/s41467-018-06385-w. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Catry J, Luong-Nguyen M, Arakelian L, Poghosyan T, Bruneval P, Domet T. Circumferential Esophageal Replacement by a Tissue-engineered Substitute Using Mesenchymal Stem Cells: An Experimental Study in Mini Pigs. Cell Transplant. 2017;26:1831–9. doi: 10.1177/0963689717741498. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He K, Zhao L, Bu S, Liu L, Wang X, Wang M. Endoscopic mucosal autograft for treating esophageal caustic strictures: preliminary human experience. Endoscopy. 2018;50:1017–21. doi: 10.1055/a-0622-8019. et al. [DOI] [PubMed] [Google Scholar]
- 87.Maeda M, Kanai N, Kobayashi S, Hosoi T, Takagi R, Ohki T. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest Endosc. 2015;82:147–52. doi: 10.1016/j.gie.2015.01.062. et al. [DOI] [PubMed] [Google Scholar]
- 88.Mallis P, Chachlaki P, Katsimpoulas M, Stavropoulos-Giokas C, Michalopoulos E. Optimization of Decellularization Procedure in Rat Esophagus for Possible Development of a Tissue Engineered Construct. Bioengineering (Basel, Switzerland) 2018;6:3. doi: 10.3390/bioengineering6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]


